Simple Summary
Certain immune cells, namely T cells, of cancer patients can be genetically manipulated to express so-called chimeric antigen receptors (CARs), which enables these cells to kill the tumor cells after recognition by the receptor. This therapy is very successful in the treatment of hematologic tumors such as lymphoma or leukemia. However, tumors growing as a solid mass are less susceptible to this kind of treatment. This review summarizes known data of all clinical trials using this therapy against solid tumors that are registered at clinicaltrials.gov.
Abstract
CAR-T cells showed great potential in the treatment of patients with hematologic tumors. However, the clinical efficacy of CAR-T cells against solid tumors lags behind. To obtain a comprehensive overview of the landscape of CAR-T cell clinical trials against this type of cancer, this review summarizes all the 196 studies registered at clinicaltrials.gov. Special focus is on: (1) geographical distribution; (2) targeted organs, tumor entities, and antigens; (3) CAR transfer methods, CAR formats, and extra features introduced into the T cells; and (4) patient pretreatments, injection sites, and safety measurements. Finally, the few data on clinical outcome are reported. The last assessment of clinicaltrials.gov for the data summarized in this paper was on 4 August 2020.
1. Introduction
T cells reprogrammed with a tumor specificity via the expression of a chimeric antigen receptor (CAR-T cells) are increasingly used in the adoptive cellular therapy of cancer. The advantage of transferring a CAR, in contrast to normal T-cell receptors (TCRs), is that a CAR can recognize the tumor in an MHC-independent way. The CAR concept was originally developed by Zelig Eshhar (Weizmann Institute of Science, Rehovot, Israel) in the late 1980s [1,2]. Most CARs are created by assembling a tumor-antigen-binding, antibody-derived single chain Fv (scFv) and the intracellular part of the CD3ζ chain linked in cis with one or several co-stimulatory domains [3], however many other formats exist (see below). This modular composition allows for T-cell activation in response to antigens located on the surface of malignant cells by binding of the scFv and subsequent signaling through the CD3ζ chain and co-stimulatory domains [3].
CD19-specific CAR-T cells induced impressive clinical regressions of leukemias or lymphomas in several clinical trials [4,5,6]. This resulted in the approval by the FDA and EMA of Kymriah® (Tisagenlecleucel, Basel, Switzerland), for the treatment of B-cell acute lymphoblastic leukemia (ALL), and Yescarta® (Axicabtagen-Ciloleucel, Santa Monica, CA, USA), for the treatment of aggressive B-cell non-Hodgkin lymphoma [3].
Most clinical trials focus on the elimination of such hematologic tumors; the development of CAR-T cells against solid tumors lags behind (reviewed in [7,8,9,10]). This is due in part to the lack of suitable tumor-specific antigens that can be targeted by CAR-T cells, causing potential on-target/off-tumor toxicity due to the accidental killing of non-malignant bystander cells co-expressing the target antigen [11,12].
This review gives a comprehensive overview of all clinical trials applying CAR-T cells against solid tumors registered at clinicaltrials.gov, irrespective of their status (i.e., withdrawn; suspended; terminated; completed; active, but not recruiting; recruiting; not yet recruiting). The search terms used at clinicaltrials.gov were: “CAR”, “T-body”, “designer T cells”, and “NKR-2”. Just the search term “CAR” already resulted in 1500 found studies, from which CAR-T cell trials against hematologic tumors, long-term follow-up, and retrospective studies, and trials not related to chimeric antigen receptors (e.g., automotive-related studies) were filtered out. To date (last assessment: 4 August 2020), there are 196 CAR-T cell trials against solid tumors registered.
Looking at the geographical distribution of the registered clinical trials, it is clear that most of these trials are performed in China (n = 99; 50.0%; Figure 1), followed by the USA (n = 85; 42.9%; Figure 1), and only very few trials are taking place in Europe, Australia, and the rest of Asia (all together responsible for n = 14; 7.1%; Figure 1).
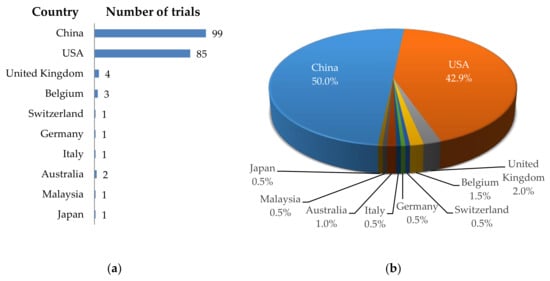
Figure 1.
Schematic overview of the geographical distribution of clinical trials using CAR-T cells against solid tumors. (a) Number of clinical trials per country; (b) Proportional distribution of clinical trials per country. Data was extracted from clinicaltrials.gov.
Data considering (1) targeted antigen, (2) targeted tumor, (3) CAR format, (4) transfer method of the CAR into the T cells, (5) additionally introduced qualities of the CAR-T cells, (6) number of cells applied, (7) patient pretreatment, (8) clinical outcome, (9) adverse events, and several other parameters are summarized in the following chapters. Additional information on e.g., clinical outcome of the trials and adverse events was gathered through literature search on pubmed.ncbi.nlm.nih.gov [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30].
2. CAR-T Cell Clinical Trials against Solid Tumors—Organs, Tumor Entities, Antigens
2.1. Targeted Organs
Many different solid tumors (see Section 2.2) are targeted in a total of 20 organs (Figure 2). Especially the tumors in the brain/CNS, liver, pancreas, and lung are targeted in many clinical trials (n = 45, 43, 38, and 36, respectively). This might represent the high medical need and/or the absence of effective alternative therapies (i.e., not CAR-T therapies) for tumors in these organs. In total 51 clinical trials target several organs (Figure 2), mostly because the antigen targeted by the CAR-T cells (see Section 2.3) is expressed on tumors in different organs (e.g., epidermal growth factor receptor (EGFR), natural killer group 2D (NKG2D)-ligands, human epidermal growth factor receptor 2 (HER2), mucin 1 (MUC1), and carcinoembryonic antigen (CEA)).
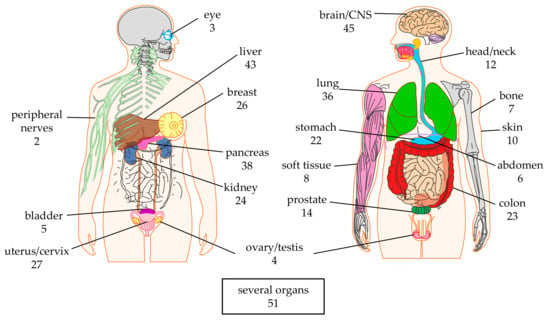
Figure 2.
Schematic overview of the organs targeted by CAR-T cells against solid tumors. The numbers indicate the number of clinical trials targeting this organ. Data was extracted from clinicaltrials.gov. The Motifolio Scientific Illustration Toolkit was used for the generation of this figure.
2.2. Targeted Tumor Entities
As can be seen in Figure 3, there are 57 different tumor entities targeted by CAR-T cells registered at clinicaltrials.gov. Nine different tumor entities were described in the brain, six in the liver, and five in the lung (Figure 3). Unfortunately, many registered clinical trials did not exactly specify which tumor entity was targeted. These files just indicated the organ (e.g., “brain”; not specifying which type of tumor) (Figure 3). Furthermore, 34 registered trials just indicated “solid tumor” (Figure 3). The four most targeted tumor entities are pancreatic cancer (n = 34), gastric cancer (n = 22), ovarian cancer (n = 21), and colorectal cancer (n = 20) (Figure 3). This does not reflect the world-wide cancer incidence. In 2018, the top 3 of cancer types newly diagnosed for both sexes was: (1) lung cancer (12.3%), (2) breast cancer (12.3%), and (3) colorectal cancer (10.6%) [31,32,33]. This could be caused by local difference in cancer incidence (e.g., in China, gastric cancer is the third most diagnosed cancer after lung cancer and colorectal cancer, and even the second most common cause of cancer-related death [34], and might therefore have a higher interest in performing clinical trials targeting this cancer entity). Indeed, of the 22 clinical trials targeting gastric cancer, 15 were/are performed in China.
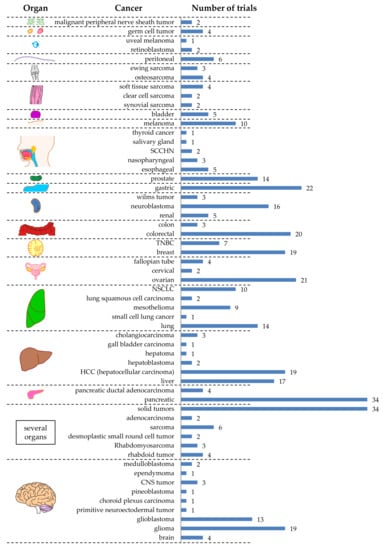
Figure 3.
Schematic overview of the tumor entities targeted by CAR-T cells against solid tumors grouped by organ. The numbers indicate the number of clinical trials targeting this tumor. Data was extracted from clinicaltrials.gov. The Motifolio Scientific Illustration Toolkit was used for the generation of parts of this figure.
At which tumor stage the CAR-T cells are applied, i.e., at an early stage (e.g., only primary tumor present), or at a late stage with several (distant) metastases, can have an impact on the effectiveness of CAR-T-cell therapy. However, most registered trials do not provide information on the exact treated tumor stage. One can hypothesize that the therapy would most probably be more effective at lower tumor burden.
2.3. Targeted Antigens
Ideal target antigens on solid tumors unify three essential attributes: (i) uniform presence on the surface of malignant cells reducing the risk for antigen-negative escape variants; (ii) absent expression on non-malignant host cells precluding on-target/off-tumor activity, which harbors the potential for severe, potentially lethal, side-effects [12]; and (iii) crucial role as an oncogenic driver in cancer cells, which may compound antigen-shutdown due to the selective survival advantage conferred on malignant cells. Co-expression on by-stander cells maintaining the tumor-microenvironment—such as tumor-associated vasculature, fibroblasts, and macrophages—represents another beneficial trait.
The registered CAR-T cell studies at clinicaltrials.gov target 44 different antigens on solid tumors (Table 1). Table 2 shows a more detailed overview of which antigen is targeted on which tumor. Sixteen clinical trials target several antigens at the same time [35,36], and two clinical trials do not disclose the targeted antigen (Table 1). The top six targeted antigens that are expressed on solid tumors in many different organs are (1) EGFR [37,38,39,40,41] (14 different organs), (2) NKG2D-ligands [42,43] (11 different organs), (3) HER2 [12,44,45,46,47,48,49,50] (11 different organs), (4) B7-H3 (10 different organs), (5) MUC1 (9 different organs), and (6) CEA [51,52,53,54,55,56,57,58] (9 different organs) (Table 1).

Table 1.
Summary of antigens expressed by solid tumors in different organs targeted by. CAR-T cells in trials registered at clinicaltrials.gov.

Table 2.
Summary of antigens targeted on different tumors by CAR-T cells in trials registered at clinicaltrials.gov.
EGFR and HER2 are members of the ErbB family of receptor tyrosine kinases (i.e., EGFR (ErbB-1), HER2/(neu) (ErbB-2), Her 3 (ErbB-3), and Her 4 (ErbB-4). Mutations in EGFR lead to its overexpression, which results in its constant activation and uncontrolled cell division in many different cancers (e.g., non-small cell lung cancer (NSCLC), colorectal cancer, pancreatic cancer, etc.) [59,60,61]. HER2 functions similarly and is overexpressed mainly in breast cancer, but also in other cancer types like ovarian cancer, glioma, and many more [62,63,64].
The NKG2D-ligands MIC-A, MIC-B, and the ULBPs 1, 2, 3, 4, 5, 6 are induced-self proteins, which are upregulated on stressed, infected, and transformed cells. These ligands can be recognized by the NKG2D receptor expressed on NK cells, NKT cells, γ/δ T cells, and activated CD8+ αβ T cells [65,66]. Colorectal cancers, ovarian cancers, and other cancers [67,68,69,70] express higher levels of NKG2D-ligands and can be targeted by CAR-T cells incorporating NKG2D in the chimeric receptor.
B7-H3 (i.e., CD276, or B7 homolog 3) is a co-stimulatory molecule for T cells and is for example expressed on activated dendritic cells and monocytes. T cells stimulated by B7-H3 proliferate and differentiate into cytotoxic T cells and selectively secrete IFNγ when TCR signaling and B7-H3 co-stimulation are combined [71]. It has only a limited expression on healthy tissues [72,73]. However, B7-H3 is overexpressed on neuroblastomas, where it inhibits recognition and killing of the tumor cells by NK cells [74]. Furthermore, CD276 is overexpressed on several other tumors—such as pancreatic ductal adenocarcinoma (PDAC), prostate cancer, ovarian cancer, lung cancer, and clear cell renal carcinoma—and on tumor-associated vasculature and stroma fibroblasts [73,75,76,77,78,79,80,81,82,83,84,85].
Mucin 1 (MUC1) is a highly glycosylated membrane protein expressed on the surface of epithelial cells in intestine, stomach, lung, eye, and other organs, where it inhibits pathogens from reaching the cell membrane by binding them to oligosaccharides [86,87]. MUC1 is overexpressed on colorectal, breast, ovarian, lung, and pancreatic cancers [88,89,90].
Carcinoembryonic antigen (CEA, also CEACAM5) is a glycoprotein which is widely expressed during fetal development and on some adult tissues (e.g., epithelium of the colon, stomach, and esophagus) [91]. In normal epithelial cells of the lung and gastrointestinal tract, CEA has an apical polarity and is facing the lumen and cannot be recognized by CAR-T cells [92]. Its function and signaling in normal tissue are still not fully understood [93]. CEA is overexpressed in colorectal, pancreatic, gastric, lung, and breast carcinoma where it plays a role in metastasis of the tumor [94]. In carcinomas, CEA has lost its apical polarity and is even partly shed, resulting in an increased serum level [95]. At this stage, CEA expressed on tumor cells can be targeted by CAR-T cells [96].
These six antigens are not perfectly tumor specific. When looking and mRNA and/or protein expression on BioGPS (www.biogps.org [97]), The Human Protein Atlas (www.proteinatlas.org [98]), and Expression Atlas (www.ebi.ac.uk/gxa/), all of these antigens are expressed to some extend on some normal tissues. It is very hard to find a target antigen for CARs on solid tumors that is not expressed on healthy tissue, and the use of CAR-T cells is a double-edged sword, because the potency of these cells can also turn against the patient [99]. It can never be excluded that some rare but important cell type in healthy tissue expresses the antigen. A case highlighting the lethal potential associated with on-target/off-tumor toxicity was shared by investigators from the NCI. Shortly after infusing T cells expressing an HER2-specific CAR to a patient with metastatic colon cancer, clinical symptoms of acute respiratory distress syndrome were observed necessitating mechanical ventilation [12]. Unfortunately, the patient died 5 days later [12]. The cause of death was assumed to be on-target/off-tumor toxicity elicited by low levels of HER2 on epithelial cells in the lung. Remarkably, the CAR was derived from the FDA-approved monoclonal antibody trastuzumab, which has been widely used without the occurrence of severe pulmonary toxicities [100]. This justifies the addition of safety measurements when using CAR-T cells in patients (see Section 4.3).
3. CAR-T Cell Clinical Trials against Solid Tumors—CAR Transfer Methods, CAR Formats, Extra Features
3.1. Transfer Methods to Introduce the CAR into T Cells
To introduce the chimeric antigen receptors into T cells, several methods can be used (Figure 4). Most clinical trials use a viral transfer method (retroviral or lentiviral) to stably introduce the CAR. During this procedure, a CAR encoding gene is transported by the virus into the T cell, where it is stably integrated into the genomic DNA. The offspring of these transduced cells will all carry the CAR gene and can express the receptor on its cell surface. Some disadvantages of viral transduction are the random integration into the host cell’s genome, which can result in destruction or activation of some genes (i.e., insertional mutagenesis), and the introduction of viral material/genes. This method can cause problems in CAR-T cell treated patients. Lamers et al. described, for example, the development of immune responses to the receptor-encoding transgene and the retroviral vector [101]. As can be seen in Figure 4, lentiviral [102] and retroviral [103,104] transduction was mostly used for the transfer of the CAR (i.e., n = 46; 23.5%, and n = 44; 22.4%, respectively). Unfortunately, most clinical trial registrations do not clearly indicate which transfer method is used (i.e., transduction/transfection? n = 9, genetically engineered/modified? n = 7, virally transduced? n = 1, or no indication at all (unknown) n = 80; Figure 4). Some clinical trials use a non-viral gene delivery system or a transfer method integrating the CAR gene into a specified site (i.e., sleeping beauty transposon system [103,105,106,107], PiggyBac transposon system [103,107], CRISPR-Cas9 [108], or transfection of DNA or RNA [109]). The latter two methods do not result in an integration of the CAR-encoding gene into the host cell’s genome, which has certain advantages (highlighted in Section 4.3 for mRNA transfection).
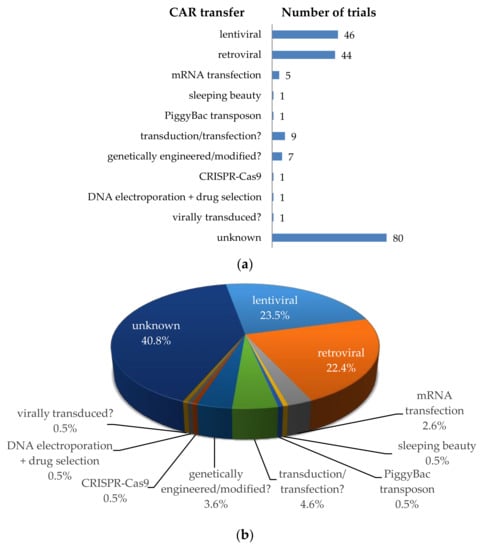
Figure 4.
Schematic overview of the methods used for CAR transfer into T cells. (a) Number of clinical trials using a specific transfer method; (b) Proportional distribution of clinical trials per transfer method. Data was extracted from clinicaltrials.gov.
The time needed for the production of the CAR-T-cell product is highly variable and can even be patient dependent. Most registered clinical trials do not provide details on the production time. However, it is usually several weeks.
3.2. CAR Formats; the Classical and the More Exotic Models
3.2.1. CARs: The Classical Models
Since the first CAR concept was presented by Zelig Eshhar in 1989 [1,2], several generations of CARs were developed. The classical CAR always incorporates an antibody-based scFv, which binds to the tumor antigen. In first generation CARs, this scFv is linked via a flexible linker and transmembrane domain to either the intracellular signaling domain of FcεRIγ or CD3ζ [110]. In clinicaltrials.gov there are indeed one trial registered using the first signaling domain and nine trials using CD3ζ (Figure 5). Most registered trials, however, use a second generation CAR [110] incorpora-ting a co-stimulatory domain. Co-stimulation is mostly provided by CD28 or 4-1BB domains [3].
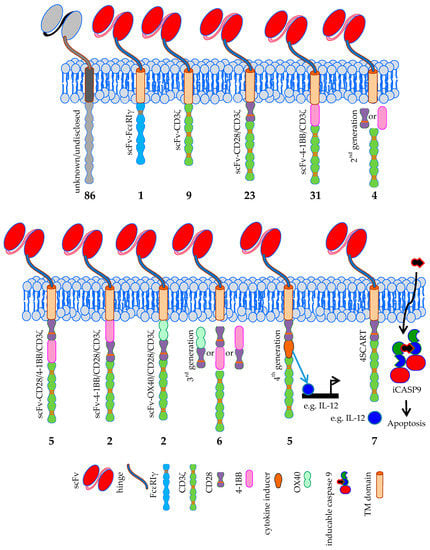
Figure 5.
Schematic overview of the classical CAR formats used in clinical trials treating solid tumors. The number of clinical trials using a specific CAR format is indicated. Data was extracted from clinicaltrials.gov. TM = transmembrane domain, iCASP9 = inducible caspase 9, 4SCART = fourth generation safety CAR-T cells. The Motifolio Scientific Illustration Toolkit was used for the generation of parts of this figure.
Physiologically, CD28 co-stimulation promotes the production of IL-2, -6, -10, and further interleukins, as well as cell cycle progression, survival, differentiation, and cytolytic functions [111]. Many studies that employed CARs with a CD28 signaling domain observed potent and quick anti-tumor effector functions. However, these were short-lived and associated with limited cell persistence in vivo when compared to, e.g., the 4-1BB signaling domain. Notwithstanding, it was shown that human CD8+ CAR-T cells containing a CD28 co-stimulatory domain differentiate towards both a central-memory and effector-memory type [112,113,114,115,116,117,118,119]. The transmembrane domain of CD28 used in many CARs as a connector between extra- and intra-cellular domains is associated with improved expression of these CARs on the surface [120,121], but might also cause tonic CAR signaling [122,123] and thereby lead to Fas-dependent activation-induced cell death (AICD) in CAR-T cells, possibly explaining the observed limited cell persistence [113]. Clinical trials confirmed the preclinical findings that CD28 supports strong but short-lived anti-tumor efficacy [124,125].
Physiological 4-1BB signaling in T cells enhances cell cycle progression and proliferation, cytokine secretion, cytolytic potential, and inhibits clonal deletion and AICD [126,127]. CARs containing 4-1BB as a signaling domain allowed for a more robust cell activation, as well as an increased persistence in vivo, and 4-1BB co-stimulation promotes differentiation of CAR-T cells towards a central-memory phenotype [4,112,113,114,115,116,117,118,128,129]. However, 4-1BB co-stimulated CARs showed a slower onset of cytotoxicity, but longer durability and accumulation of CAR cells over time [128]. Potent anti-tumor efficacy and very long persistence of 4-1BB-containing CAR-T cells in patients were also reported in clinical trials [4,130].
CD28 is incorporated in CARs used in 23 trials [131,132], and 4-1BB co-stimulation is used in 31 CAR-T cell trials (Figure 5). Four trials just indicate that second generation CARs are used, but do not specify which co-stimulatory domain is included (Figure 5). In 86 registered trials, the used CAR format is not disclosed (Figure 5).
The third generation CARs [111] used in the registered clinical trials incorporate combinations of CD28/4-1BB, 4-1BB/CD28, or OX40/CD28 co-stimulatory domains (Figure 5) [133,134]. Six trials mention the use of third generation CARs but do not indicate the exact co-stimulatory domains used, or their order in the chimeric molecules (Figure 5). Fourth generation CARs (also known as “T cells redirected for universal cytokine-mediated killing” (TRUCKs)) are in principle second generation CARs with the extra feature that they can induce the production of e.g., cytokines in a very restricted local fashion [135]. The effects induced depend on the cytokines that are secreted: e.g., IL-12 can activate an innate immune response in the tumor [136], causes less susceptibility to Treg suppression [137], and increases cytokine secretion and expansion [138,139], and IL-15 increases the anti-tumor activity of the CAR-T cells [140]. A variant of this format is the 4SCART (fourth generation safety CAR-T) which additionally incorporates an inducible caspase 9 as a safety measurement (will be described in detail in Section 4.3). Five registered trials rely on the fourth generation CAR format, while seven trials use the 4SCART format (Figure 5).
3.2.2. CARs: The More Exotic Models
A group of 19 clinical trials in total use alternative binding moieties instead of a scFv directed against an antigen expressed on the cell surface of the tumor (Figure 6).
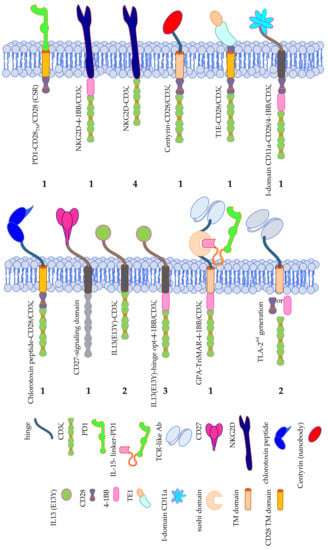
Figure 6.
Schematic overview of the exotic CAR formats used in clinical trials treating solid tumors. The number of clinical trials using a specific CAR format is indicated. Data was extracted from clinicaltrials.gov. TM = transmembrane domain, PD1 = programmed cell death protein 1, IL13 (E13Y) = mutated IL-13 optimized to bind IL-13Rα2, TE1 = promiscuous ErbB ligand derived from EGF and TGFα. The Motifolio Scientific Illustration Toolkit was used for the generation of parts of this figure.
One trial applies a so-called chimeric switch receptor (CSR), consisting of PD1 as extracellular domain and CD28 transmembrane and intracellular signaling domain (Figure 6). As tumors often express PD-L1 on their surface to activate the inhibitory PD1 receptor on T cells to circumvent/inhibit an anti-tumor T-cell response, the CSR will turn the inhibitory into an activation signal induced by CD28 [141].
Several trials target NKG2D ligands by linking NKG2D to either CD3ζ (4 trials), or 4-1BB/CD3ζ (1 trial) (Figure 6) [43]. This strategy is very attractive, since NKG2D binds a plethora of ligands (MIC-A, MIC-B, and the ULBPs 1, 2, 3, 4, 5, 6), that are induced-self proteins, which are upregulated on stressed, infected, and transformed cells, as already described above, and therefore can be used for many types of cancer.
Poseida Therapeutics, Inc., together with the City of Hope Comprehensive Cancer Center, the Sarah Cannon Research Institute at HealthONE, and the Memorial Sloan Kettering Cancer Center, are performing a phase 1/2 trial with CAR-T cells in which the binding moiety consists of a nanobody (named Centyrin) specific for PSMA linked to the signaling domains of CD28 and CD3ζ (Figure 6).
The T1E-CD28/CD3ζ CAR is coupling a promiscuous ErbB ligand derived from EGF and TGFα to a fused CD28/CD3ζ endodomain (Figure 6). This CAR can bind several ErbB2 dimers (i.e., HER2, HER3, and EGFR) and therefore can target several tumors [142,143,144].
AffyImmune Therapeutics, Inc., together with the Weill Medical College of Cornell University is clinically testing AIC100 CAR-T cells. The binding moiety of this CAR consists of the I-domain of CD11a of LFA-1, which binds to ICAM1 on tumor cells. Intracellularly CD28, 4-1BB, and CD3ζ domains facilitate signaling (Figure 6).
Originally used as an imaging agent to guide glioblastoma resection surgery, and to carry different therapeutics to these tumors, a 36-amino acid long peptide of chlorotoxin, a component of scorpion venom, was linked to the transmembrane and intracellular domains of CD28 and signaling domain of CD3ζ to form a CAR (Figure 6). Preclinical testing showed that chlorotoxin binds to a greater proportion of patient tumors, and cells within these tumors, while ignoring non-tumor cells in the brain and other organs, and that this binding to its ligand is not so much influenced by tumor heterogeneity compared to other antigens such as IL13Rα2, HER2, and EGFR [145]. Therefore, the City of Hope Medical Center, together with the National Cancer Institute is now testing this CAR in a clinical trial against recurrent glioblastoma and recurrent malignant glioma.
CD70-CD27 interactions are important for the regulation of adaptive immunity. CD70 shows a restricted expression on non-malignant cells, but is expressed on some solid tumors (e.g., on renal carcinoma, pancreatic cancer, breast cancer, melanoma, and ovarian cancer) and is implicated in tumor escape from immunosurveillance [146,147,148,149,150]. In a clinical trial performed by the NCI, a CAR consisting of CD27, linked to undisclosed intracellular signaling domains is used (Figure 6).
Five clinical trials use a mutated form of IL-13, which is optimized for binding to IL-13Rα2, either linked to the signaling domain of CD3ζ alone, or a combination of 4-BB and CD3ζ signaling domains for the treatment of patients with glioblastoma, glioma, or melanoma (Figure 6) [151,152,153,154].
A highly complex CAR for the treatment of melanoma patients was developed by Timmune Biotech Inc., and used in a clinical trial by the Second Affiliated Hospital of Hainan Medical University. This so-called GPA-TriMAR binds to a peptide derived from the melanoma-associated antigen gp100, presented in HLA-A2 through a TCR-like antibody [155,156] (Figure 6). This might increase the tumor specificity, but nullifies the advantage of a CAR that it can bind to a native cell-surface tumor antigen, which does not need to be processed and presented in an HLA-context and is therefore not dependent on the HLA-type of the patient. The other two extracellular subunits are a sushi domain, which can bind IL-15, and an IL-15-linker-PD1 construct (Figure 6). The latter two subunits are supposed to stimulate the innate immune system. The GPA-TriMAR is linked to the intracellular signaling domains of 4-1BB and CD3ζ [157].
Finally, two clinical trials use a TCR-like antibody (TLA) as binding moiety linked to a second generation intracellular domain (i.e., either CD28/CD3ζ, or 4-1BB/CD3ζ) (Figure 6) [158]. Both trials target a peptide of alphafetoprotein (AFP) presented in HLA-A2 in HCC patients. The two different CARs were both developed by the companies Eureka Therapeutics Inc. and Aeon Therapeutics (Shanghai, China) Co., Ltd. providing only limited published data [158].
3.3. Add-Ons; T-Cell Populations Used for Transfer or Extra Features Introduced into CAR-T Cells
3.3.1. T-Cell Populations Used for CAR-T-Cell Therapy
In many clinical trials, certain T-cell populations are used for the CAR introduction. For example, T cells specific for VZV, EBV, adenovirus, CMV, or multivirus-specific T cells are used. The idea behind it is that these T cells can be stimulated via their endogenous virus-specific TCR to proliferate and therefore increase their persistence and number. Epitopes of latent viruses like EBV or CMV are constantly presented and stimulate the CAR-T cells. Another strategy is to use virus vaccination to boost T-cell proliferation (like VZV vaccination, or oncolytic adenovirus injected intratumorally). Some trials used directly vaccine-specific T cells to induce this proliferation [46,159,160,161]. To increase persistence of the CAR-T cells, one can alternatively use memory T cells for the transfer of the CAR [162,163].
Most trials use autologous patient T cells to introduce the CARs. However, there might be certain situations making the use of allogeneic T cells necessary (e.g., not enough T cells can be isolated/expanded from the patient, or CAR-T cell therapy is performed after allogeneic stem cells transplantation). Furthermore, the use of allogeneic T cells that are genetically modified (see below) in such a way that they are not recognized by the endogenous immune system, or can harm healthy tissue of the patient, is very attractive, and can generate an off-the-shelf therapy for many different patients. Alternatively, the endogenous TCR of γ/δ-T cells does not recognize peptides presented in HLA molecules [164,165] and therefore they do not induce graft-versus-host disease after CAR-T-cell transfer in HLA-mismatched patients [166]. This allows the use of γ/δ-T cells for the generation of CAR-T cells from healthy donors, which are not impaired by tumor- or therapy-related immunosuppression [167,168], and the application of CAR-T cells in a multitude of patients, irrespective of their T-cell numbers and HLA-type. An additional positive effect is that γ/δ-T cells have an intrinsic anti-tumor activity [169], potentiating the adoptive T-cell therapy against tumors. Moreover, the number of CAR-γ/δ-T cells can be boosted in vivo by systemic administration of zoledronic acid [170].
3.3.2. Extra Features Introduced into CAR-T Cells
Several resistance mechanisms to CAR-T-cell therapy in solid tumors may play a role in the observed lower effectiveness compared to CAR-T cells in hematologic malignancies [171,172,173,174]. For example, the tumor microenvironment can be hostile for CAR-T cells (e.g., unfavorable pH or oxygen levels) or unfavorable electrolyte or cytokine concentrations, inhibiting an effective immune response [175,176,177]. Additionally, the homing of CAR-T cells can be hampered in solid tumors [172]. Furthermore, solid tumors can induce inhibitory receptors on CAR-T cells like PD1 and CTLA-4, making the CAR-T cells exhausted. The patient’s own immune cells can even attack the CAR-T cells e.g., by antibody production [178,179]. To overcome these resistance mechanisms, several strategies were developed, like induced expression of cytokines, expression of constitutively active or dominant negative cytokine receptors, expression of homing receptors, prevention of anti-CAR antibody production, or blocking of PD1/CTLA-4. All are described in more detail below.
An often-used strategy to improve the effectiveness of CAR-T cells is to equip them with the ability to secrete cytokines like IL-12, IL-15, IL-21, IL-7, or combinations thereof (Figure 7). In two trials performed by the Second Affiliated Hospital of Guangzhou Medical University and the Sixth Affiliated Hospital of Wenzhou Medical University in China, the used CAR-T cells produce IL-7 and CCL19 [180,181,182,183]. IL-7 is known for its positive effects on T-cell survival [184], and CCL19 is a chemokine attracting other endogenous immune cells, like dendritic cells, B cells, and central memory T cells [185,186,187] to the tumor site. IL-12, IL-15, and IL-21 are all cytokines known to stimulate immune cells. The fact that these cytokines are produced very locally is an advantage, since some of them can have toxic effects when applied systemically [135].

Figure 7.
Schematic overview of the extra features introduced into CAR-T cells used in clinical trials treating solid tumors. The number of clinical trials using a specific feature is indicated. Data was extracted from clinicaltrials.gov. 4αβ = chimeric cytokine receptor containing the IL-4Rα ectodomain coupled to the IL-2Rβ endodomain, mbIL-15 = membrane-bound IL-15, caIL-7R = constitutively active IL-7 receptor, dnTGFβR = dominant negative TGFβ receptor. The Motifolio Scientific Illustration Toolkit was used for the generation of parts of this figure.
Some trials take this even a step further and use CAR-T cells co-expressing a constitutively active IL-7 receptor [188], or a membrane-bound form of IL-15 [189] (Figure 7). Others introduce a chimeric cytokine receptor containing the IL-4Rα ectodomain coupled to the IL-2Rβ endodomain (4αβ) resulting in a robust expansion of CAR-T cells after IL-4 binding, a cytokine with several pathophysiologic and therapeutic links to cancer [190] (Figure 7). TGFβ is known to have an immunosuppressive effect, and many tumors, especially prostate cancer, secrete TGFβ and thereby promote metastasis and neoangiogenesis and suppress T cells [191,192]. In vitro and in vivo models showed that blocking TGFβ signaling in T cells by using a dominant-negative TGFβ receptor II (i.e., a truncated form which lacks the intracellular domain necessary for downstream signaling) [193] resulted in an increased ability to infiltrate, proliferate, and mediate anti-tumor responses [194]. Therefore, this dnTGFβR was co-introduced next to a CAR specific for PSMA in T cells to treat prostate cancer patients [195] (Figure 7).
CAR-T cells can also be engineered to express homing molecules to target these cells to specific tissue locations. A clinical trial performed by the Sun Yat-sen University, Guangzhou, China in collaboration with Bio-gene Technology Co., Ltd., Guangzhou, China uses CAR-T cells specific for EGFR which were additionally transduced with the lymphoid follicle homing molecule CXCR5 [40]. Alternatively, to circumvent problems with homing of CAR-T cells to the tumor site, these cells can be directly infused into the tumor [55,154,196,197].
In total, five clinical trials co-introduce an anti-CD19 CAR next to the tumor-antigen-specific CAR into T cells. This extra CAR is directed against CD19 positive B cells which can produce antibodies, and these cells will be lysed. That means that CD19-positive cells that might produce anti-CAR antibodies, e.g., because the tumor-antigen-specific CAR is based on a murine scFv, will also be destroyed. This will increase the persistence of the anti-tumor CAR-T cells in the patients (Figure 7). Additionally, lymphodepleting chemotherapy will also prevent graft rejection.
The anti-tumor activity of T cells can be inhibited by various tumor-associated immunosuppressive ligands like PD1 and CTLA-4 [198]. Several strategies are used in clinical trials to prevent inhibition of the CAR-T cells by PD1 or CTLA-4 interactions between tumor cells and CAR-T cells. Four clinical trials knockout the PD1 gene in the CAR-T cells (Figure 7), preventing the interaction of this molecule with PD-L1 expressed on tumor cells. Furthermore, several studies introduce genes into CAR-T cells encoding blocking anti-PD1, anti-PD1 and anti-CTLA-4, or anti-PD-L1 antibodies, which after secretion by the CAR-T cells result in the same prevention of inhibition by the tumor cells (Figure 7). One study introduced a gene encoding an anti-PD1 nanobody with the same purpose (not shown). Moreover, some trials combined CAR-T cell therapy with anti-PD1, anti-CTLA-4 antibodies ([197,199], NCT03980288, NCT03726515, NCT01822652, NCT04003649 (see Table S1)).
As already described above, the use of allogeneic CAR-T cells has certain advantages. Therefore, several trials use T cells that are genetically modified in such a way that they are not recognized by the endogenous immune system, or can harm healthy tissue of the patient by knocking out the endogenous TCR and/or β2M, the latter resulting in the absence of MHC class I expression on the cell surface (Figure 7). This approach can lead to an off-the-shelf therapy using allogeneic T cells for many different patients.
4. CAR-T Cell Clinical Trials against Solid Tumors—Patient Pretreatments, Injection Sites, Safety Measurements, Clinical Outcomes
4.1. Treatments of Patients before CAR-T-Cell Transfer
To provide the best conditions for the introduced CAR-T cells, it is common to perform a lymphodepleting pretreatment of the patients. This is based on results obtained after transfer of tumor infiltrating lymphocytes (TILs) and CD19-directed CAR-T cells. In these studies, it was shown that lymphodepleting or conditioning chemotherapy administered prior to T-cell infusion clearly improve persistence and efficacy of these T cells [200], for example by reducing the number of suppressive cells, or removing competing sink cells, making IL-7 and IL-15 cytokines available for T cell expansion [201].
Different types of lymphodepleting or conditioning chemotherapies were also performed in the CAR-T-cell clinical trials against solid tumors (Figure 8). Mostly, the classical non-myeloablative lymphodepleting regimen with cyclophosphamide and fludarabine [202] was performed (n = 59, 28.2%; Figure 8), but also schedules with cyclophosphamide or fludarabine as single agent are described (n = 21 and n = 2, respectively; Figure 8). Other chemotherapies include: paclitaxel + cyclophosphamide, Temozolomide [203], or bis-1-nitrosourea + etoposide + arabinoside + cyclophosphamide (b + e + a + c) (Figure 8). A total of 93 trials do not clearly state if preconditioning is performed (unknown, n = 75), or what kind of preconditioning is performed (lymphodepleting pretreatment, n = 14; chemotherapy, n = 4) (Figure 8). In the registered clinical trials that describe the timing of lymphodepletion, the interval between lymphodepletion and CAR-T-cell application is mostly performed 3–5 days before the infusion of CAR-T cells, for 2–4 days. Twenty-five trials explicitly mention that no lymphodepletion is executed (Figure 8).

Figure 8.
Schematic overview of the pretreatments used before CAR-T cells were applied in patients. (a) Number of clinical trials using a specific pretreatment; (b) Proportional distribution of clinical trials per pretreatment. Data was extracted from clinicaltrials.gov.
4.2. Injection Sites for CAR-T Cell Application
Most clinical trials apply the CAR-T cells by injecting them intravenously (n = 105; Figure 9) counting on the correct homing of the T cells to the tumor. However, there are also other sites possible, especially if one wants to apply the CAR-T cells very locally in the tumor or at the resection site. For example, trials treating brain tumors use intracranial (n = 2), intracavity (n = 3), or intracerebral (n = 6) injection, or inject into the ventricular system (n = 8) (Figure 9). Nineteen clinical trials indicated intratumoral injection, and nine use intraperitoneal injection (Figure 9). Local treatment of liver or pancreas cancers with CAR-T cells can be achieved by transcatheter arterial infusion (TAI; n = 3), intrahepatic artery injection (n = 10), pancreatic artery (n = 1), or pancreatic venous (n = 1) injection (Figure 9). A total of 56 clinical trials do not indicate the site of CAR-T-cell application (Figure 9).
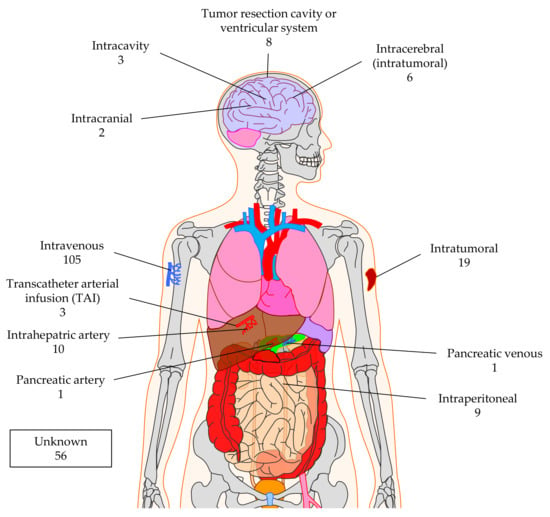
Figure 9.
Schematic overview of the injection sites used to apply CAR-T cells against solid tumors. The numbers indicate the number of clinical trials using this injection site. Data was extracted from clinicaltrials.gov. The Motifolio Scientific Illustration Toolkit was used for the generation of this figure.
4.3. Safety Measurements to Control Negative Effects of CAR-T Cells in the Patient
As already mentioned above, most antigens targeted in clinical trials with CAR-T cells against solid tumors are not perfectly tumor specific. It can be that the antigens are expressed to some extend on normal healthy tissues, and there an on-target/off-tumor toxicity due to the accidental killing of non-malignant bystander cells co-expressing the target antigen can be induced by the CAR-T cells [11]. To be able to shut-off the CAR-T cells as soon as toxicity is noticed in the patient, several strategies were developed (Figure 10). Rimiducid (AP1903) and rapamycin are molecules that are able to induce dimerization of constructs containing inducible caspase 9, which are co-introduced with the CAR into the T cells as a suicide switch. After dimerization, the caspase 9 induces apoptosis of the CAR-T cells and thereby the unwanted/unexpected T-cell activities are eliminated [204,205]. This kind of suicide switch is used in 17 clinical trials (Figure 10) performed with mostly fourth generation safety CAR-T cells (4SCART). Interestingly, rimiducid (AP1903) is also used to provide multimerization of an inducible co-stimulatory molecule based on MyD88 and CD40 (iMC) into T cells, which allows for the selective activation of adoptively transferred T cells in vivo resulting in enhanced anti-tumor activity in solid tumors (Figure 10). Removal of rimiducid will switch off this co-stimulation again [206,207].
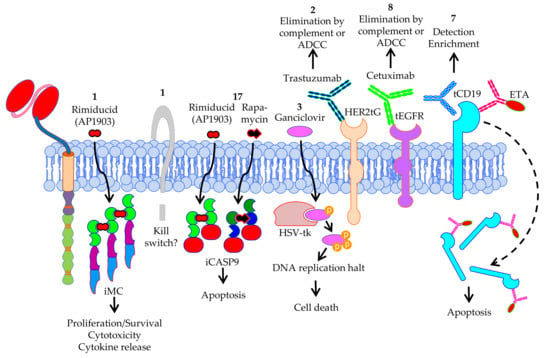
Figure 10.
Schematic overview of safety measurements used in the treatment with CAR-T cells against solid tumors. The numbers indicate the number of clinical trials using this safety measurement. Data was extracted from clinicaltrials.gov. The Motifolio Scientific Illustration Toolkit was used for the generation of this figure.
One of the oldest suicide switches used is the herpes simplex virus-thymidine kinase/ganciclovir (HSV-tk/GCV) strategy. Mechanistically, HSV-tk phosphorylates GCV and the resulting triphosphate form is incorporated by DNA polymerases into the DNA, leading to chain termination and cell death [208]. HSV-tk/GCV also induces apoptosis [209]. A disadvantage of using HSV-tk/GCV is that it can be immunogenic in immunocompetent patients causing a limited persistence of HSV-tk transduced cells [210]. Nevertheless, three clinical trials still use the HSV-tk/GCV strategy (Figure 10) [211].
Furthermore, several trimmed molecules are used for selection and/or depletion of CAR-T cells, like truncated HER2 (HER2tG), truncated EGFR (tEGFR), and truncated CD19 (tCD19) (Figure 10). Trastuzumab (Herceptin®) binds to HER2tG [212] and is used in two clinical trials for the elimination via complement or antibody-dependent cell-mediated cytotoxicity (ADCC) of CAR-T cells in case of on-target/off-tumor reactions (Figure 10). Cetuximab is used for the ablation of tEGFR-expressing CAR-T cells in eight clinical trials (Figure 10) via the same mechanisms [213]. Seven clinical trials use truncated CD19 either as selection marker for CAR-positive T cells [214], or as marker for elimination using an anti-CD19 antibody conjugated to pseudomonas toxin (CD19-ETA’) [215] (Figure 10). One clinical trial is using an undisclosed ‘kill switch’ as safety measurement (Figure 10).
A special safety measurement to circumvent prolonged autoimmunity induced by an on-target/off-tumor reaction of the CAR is the introduction of the CAR by mRNA electroporation (n = 5, 2.6%; Figure 4). We have previously demonstrated that transient transfection of T cells with CARs using mRNA electroporation might be an effective and safe tool in cancer immunotherapy [121,216,217,218,219,220]. The electroporation procedure is based on complex physicochemical mechanisms leading to plasma membrane perforation upon application of electric fields allowing for subsequent entry of mRNA into the cytosol [221]. Using RNA-transfected CAR-T cells offers the advantage that the receptor expression is temporally restricted (Figure 11), rendering potential off-target and on-target/off-tumor toxicity transient as well. The CAR-RNA transfer strategy is especially attractive in phase 0/1 clinical trials exploring new tumor antigens for CAR-T-cell therapy with an unknown clinical safety profile.
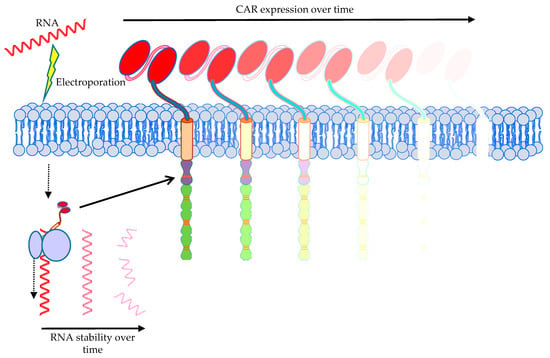
Figure 11.
Schematic representation of introduction of a CAR by mRNA electroporation. Indicated are the low stability of the introduced mRNA over time and the transient expression of the CAR on the T-cell surface. The Motifolio Scientific Illustration Toolkit was used for the generation of this figure.
The mRNA transfection strategy for CARs proposed by us quite some time ago [109] has in the meantime been applied by others in clinical trials. In patients with solid tumors c-MET was used as a CAR-target antigen on breast cancer and melanoma [222], (NCT01837602; NCT03060356) and mesothelin as a CAR-target antigen on mesothelioma, pancreatic cancer, and ovarian cancer [223,224,225], (NCT03608618; NCT01897415; NCT01355965). RNA transfection was even explored with non-solid tumors using CD19 and CD123 as target antigen [226], (NCT02277522; NCT02624258; NCT02623582). The mRNA-CAR-T cells in these studies were well tolerated [222], the cells migrated to primary and metastatic tumor sites, showed a clinical anti-tumor activity, and showed no evidence of on-target/off-tumor toxicity against normal tissues [223]. After local application, c-MET-CAR-T cells induced necrosis within the tumor. Importantly, some of the injected c-MET-CAR-T cells entered the blood stream and could be monitored in the circulation for a short time [222].
The clinical trials published by Beatty et al. and Maus et al., using mesothelin as antigen, showed a cytokine release syndrome (CRS) in one mesothelioma patient resulting in adverse events (anaphylaxis, cardiac arrest, respiratory failure, disseminated intravenous coagulation) within minutes of completing the third infusion [223,225]. In contrast, in pancreatic cancer patients no cytokine release syndrome and no dose-limiting toxicities, but actually stable disease in two of six patients were seen [224]. When using RNA-CAR-T cells, robust proliferation and persistence are not so important, making lymphodepletion unnecessary, as the transient receptor expression per se necessitates repetitive injections. Unlike most of the trials registered in clinicaltrials.gov which use virally transduced cells, which have to be applied only once, presuming that these cells will proliferate upon tumor-antigen recognition, making repeated applications unnecessary, RNA-transfected cells will lose CAR expression (Figure 11) and have to be replenished from the outside to maintain cytolytic pressure on the tumor.
The possible reason for the severe adverse events in the patient described above by Maus et al. and Beatty et al. [223,225], was that the CAR was based on a murine antibody and the adverse event was caused by IgE antibodies specific for the scFv in the CAR (i.e., a human anti-mouse antibody (HAMA) response), subsequently causing a CRS after an additional injection of CAR-T cells. These antibodies were probably induced by the intermittent dosing schedule of the CAR-T cells [223,225]. After the first two injections of RNA CAR-T cells on day 0 and day 7, the third injection was given after a long waiting period on day 49. This is sufficient time to complete an isotype switch from IgG to IgE. Therefore, rapid repetition of infusions seems to be best to prevent isotype switching if a HAMA response is induced.
Although the transient expression of a CAR on the cell surface of T cells by electroporation with mRNA can be an advantage if an on-target/off-tumor response is induced by the CAR-T cells, it can also be a disadvantage for the applicability. It has to be carefully monitored whether the infused CAR-T cells reach their tumor target in time before the CAR expression is too low for an effective anti-tumor response. This might be circumvented by local infusion of these CAR-T cells at the tumor site, as it is performed in several clinical trials (NCT01355965 [223,225], NCT01897415 [224], NCT03608618, NCT01837602 [222]). Furthermore, the necessary repetitive application of mRNA-transfected CAR-T cells harbors some hazards, like elaborately described above (e.g., possible isotype switch of a HAMA response [223,225]). Additionally, this repetitive application necessitates the production and storage of several batches of CAR-T cells, which might be cumbersome. Moreover, an anti-tumor CAR-T-cell memory will not be induced in patients treated with mRNA-transfected CAR-T cells, which might be a problem if the tumor is not completely eradicated and can reoccur. To draw final conclusions on the applicability of mRNA-transfected CAR-T cells, analysis in several clinical trials is necessary. To mitigate safety concerns, another promising strategy is the initial use of repetitive injections of RNA-transfected CAR-T cells to probe for toxicity, and in the case of no serious side-effects, switch to permanently transfected CAR-T cells.
4.4. Clinical Outcomes and Adverse Events of CAR-T-Cell Therapy of Solid Tumors
4.4.1. Clinical Outcomes
Of 42 clinical trials using CAR-T cells against solid tumors registered at clinicaltrials.gov the clinical outcome could be retrieved from either clinicaltrials.gov, or through literature search on pubmed.ncbi.nlm.nih.gov (see Table S1; also including data on number of injected cells, trial phase, (estimated) patient number, trial status, principle investigator, and references) [25,197,199,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247]. Some clinical outcomes were found in abstracts of ASCO meetings published in the Journal of Clinical Oncology (Table S1). Of the 375 treated patients listed in publications reporting on clinical outcome, 13 had a complete response, 35 had a partial response, 4 had a mixed response, 121 had a stable disease, 109 had a progressive disease, 8 had no evidence of disease, 5 were not evaluable, and of 80 patients the clinical outcome was not disclosed. This data is summarized in Table S2.
4.4.2. Adverse Events
In total, 28 clinical trials described in this review also reported on adverse events (Table S1). The adverse events were quite diverse (Figure 12). Some adverse events were very local, and this could be explained by looking at the tumor site (e.g., seizure when treating glioblastoma, or abdominal pain when treating tumors in the liver). However, there were also more general adverse events, for example: fever, fatigue, nausea/vomiting, respirator toxicity/dyspnea, etc. (Figure 12). Although only five clinical trials directly reported on serum cytokine release or cytokine release syndrome (CRS) (Figure 12), this is probably an underestimation. For acute lymphoblastic leukemia (ALL) it is described that in 77% of the patients treated with CD19-CAR-T cells, CRS is prevalent [6]. It is reported that in these patients the clinical manifestations of CRS include a plethora of symptoms including mild fever with headache and myalgia, but also high fever, hypotension, acute respiratory distress syndrome, disseminated intravascular coagulation, organ failure, and death. Furthermore, elevated values for C-reactive protein (CRP) and IL-6, and signs of multi-organ failure, deranged coagulation parameters, and cytopenias are described [248]. The listed adverse events in Figure 12, and the data on all reported adverse events summarized in detail in Table S1, together with the description of the symptoms of CRS in ALL patients, suggests that more cases of cytokine release syndrome were induced by the treatment of solid tumors with CAR-T cells.
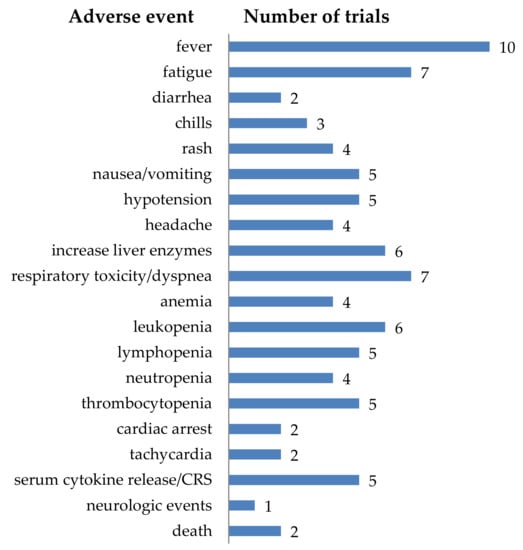
Figure 12.
Schematic overview of the adverse events (all grades) described during the treatment of solid tumors with CAR-T cells. The number of clinical trials reporting a specific adverse event is indicated. Data was extracted from clinicaltrials.gov and literature search on pubmed.ncbi.nlm.nih.gov.
Another adverse event described for CAR-T-cell therapy is CAR-induced neurotoxicity [249], however, the mechanism of this neurotoxicity is not clear yet. Symptoms of neurotoxicity include transient cognitive impairments, hallucinations, and delirium, but also encephalopathy and seizures [249]. The above described seizure in the treatment of glioblastoma patients (NCT02209376; Table S1) might therefore also be a sign for neurotoxicity. Furthermore, one trial describes neurologic events (NCT00730613; Table S1), and in another trial one of the adverse events were olfactory auras (NCT02208362; Table S1), which both might indicate neurotoxicity.
5. Conclusions
This summarizing review on all the clinical trials using CAR-T cells against solid tumors registered at clinicaltrials.gov shows that many strategies are followed using many different CAR formats, application routes, and extra features introduced into the T cells. This probably indicates that the ideal strategy for treating solid tumors with CAR-T cells has not been found yet. This can also be seen in the clinical outcomes of the trials that reported on this; only 52 of 375 patients responded. Notwithstanding, the use of CAR-T cells in the treatment of solid tumors bears great opportunities, and further development and clinical testing is necessary to be able to respond to the high medical need for a treatment of such cancers.
Future clinical trials should be focused on testing new CAR formats. This not only includes testing new extracellular antigen-binding domains, but also formats increasing the safety of CAR-T-cell usage (e.g., bi-specific CARs, or split CARs) [250], and new intracellular signaling domains [251]. Furthermore, new vehicles for CARs [251,252] hold great promise for broadening the applicability. For example, the possibility of the off-the-shelf use of CAR-NK cells [253] or allogeneic CAR-T cells [254] can reduce costs for CAR-cell therapy and make it affordable for many more patients.
Furthermore, antigens which are more tumor specific should be found to prevent on-target/off-tumor reactions. Promising in this area are antigens expressed on the tumor stroma, which can also be targeted by CAR-T cells [255]. Targeting multiple antigens by one CAR-T cell (i.e., expression of different CARs specific for different antigens on one cell) can increase the tumor specificity and lessen the risk of off-target effects, and when intracellular signaling modules are split between the CARs, this can even increase the safety profile of the CAR-T cells. Additionally, the generation of antigen-loss variants of the tumors is less likely.
Moreover, combination therapies of CAR-T cells with various small molecules and monoclonal antibodies to circumvent tumor escape and increase anti-tumor activity are already clinically tested in many hematologic tumors (reviewed in detail in [171]). Such combinations also hold great promise for the treatment of solid tumors and need to be tested in clinical trials in the near future.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/9/2567/s1, Table S1: Characteristics of all clinical trials using CAR-T cells against solid tumors. Data was collected from clinicaltrials.gov and literature search on pubmed.ncbi.nlm.nih.gov; Table S2: Summary of clinical trials reporting on clinical outcome. Data was collected from clinicaltrials.gov and literature search on pubmed.ncbi.nlm.nih.gov.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation), trilateral grant SCHA 1247/3-1.
Acknowledgments
The author acknowledge support by the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding program Open Access Publishing.
Conflicts of Interest
The author declares no conflict of interest.
References
- Gross, G.; Gorochov, G.; Waks, T.; Eshhar, Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transpl. Proc. 1989, 21, 127–130. [Google Scholar]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Go, W.Y. CAR T-Cell Therapy in large B-cell lymphoma. N. Engl. J. Med. 2018, 378, 1065. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jager, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Han, W. Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment. Protein Cell 2017, 8, 896–925. [Google Scholar] [CrossRef]
- Han, S.; Latchoumanin, O.; Wu, G.; Zhou, G.; Hebbard, L.; George, J.; Qiao, L. Recent clinical trials utilizing chimeric antigen receptor T cells therapies against solid tumors. Cancer Lett. 2017, 390, 188–200. [Google Scholar] [CrossRef]
- Yeku, O.; Li, X.; Brentjens, R.J. Adoptive T-cell therapy for solid tumors. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 193–204. [Google Scholar] [CrossRef]
- Arabi, F.; Torabi-Rahvar, M.; Shariati, A.; Ahmadbeigi, N.; Naderi, M. Antigenic targets of CAR T Cell Therapy. A retrospective view on clinical trials. Exp. Cell Res. 2018, 369, 1–10. [Google Scholar] [CrossRef]
- Lamers, C.H.J.; Sleijfer, S.; Steenbergen, S.V.; Elzakker, P.V.; Krimpen, B.V.; Groot, C.; Vulto, A.; Bakker, M.D.; Oosterwijk, E.; Debets, R.; et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol. Ther. 2013, 21, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, P.; Petrocca, F. Regional delivery of chimeric antigen receptor (CAR) T-cells for cancer therapy. Cancers 2017, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, F.; Yang, J.; Zhao, C.; Chu, Y. New chimeric antigen receptor design for solid tumors. Front. Immunol. 2017, 8, 1934. [Google Scholar] [CrossRef]
- Heyman, B.; Yang, Y. Chimeric antigen receptor T cell therapy for solid tumors: Current status, obstacles and future strategies. Cancers 2019, 11, 191. [Google Scholar] [CrossRef]
- Titov, A.; Valiullina, A.; Zmievskaya, E.; Zaikova, E.; Petukhov, A.; Miftakhova, R.; Bulatov, E.; Rizvanov, A. Advancing CAR T-Cell therapy for solid tumors: Lessons learned from lymphoma treatment. Cancers 2020, 12, 125. [Google Scholar] [CrossRef]
- Yu, W.L.; Hua, Z.C. Chimeric Antigen Receptor T-cell (CAR T) Therapy for hematologic and solid malignancies: Efficacy and safety-a systematic review with meta-analysis. Cancers 2019, 11, 47. [Google Scholar] [CrossRef]
- Essand, M.; Loskog, A.S. Genetically engineered T cells for the treatment of cancer. J. Intern. Med. 2013, 273, 166–181. [Google Scholar] [CrossRef]
- Mirzaei, H.R.; Rodriguez, A.; Shepphird, J.; Brown, C.E.; Badie, B. chimeric antigen receptors T cell therapy in solid tumor: Challenges and clinical applications. Front. Immunol. 2017, 8, 1850. [Google Scholar] [CrossRef]
- Yu, S.; Li, A.; Liu, Q.; Li, T.; Yuan, X.; Han, X.; Wu, K. Chimeric antigen receptor T cells: A novel therapy for solid tumors. J. Hematol. Oncol. 2017, 10, 78. [Google Scholar] [CrossRef]
- Springuel, L.; Lonez, C.; Alexandre, B.; Cutsem, E.V.; Machiels, J.H.; Eynde, M.V.D.; Prenen, H.; Hendlisz, A.; Shaza, L.; Carrasco, J.; et al. Chimeric antigen receptor-T cells for targeting solid tumors: Current challenges and existing strategies. BioDrugs 2019, 33, 515–537. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Huang, K.; Zhang, Y.; Kupfer, G.; Zhao, Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J. Hematol. Oncol. 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Zhou, W.L.; Huang, Y.; Liang, X.; Jiang, L.; Yang, X.; Sun, J.; Li, Z.; Han, W.D.; et al. Genetically engineered T cells for cancer immunotherapy. Signal. Transduct Target. 2019, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, A.; Barden, M.; Abken, H. The growing world of CAR T cell trials: A systematic review. Cancer Immunol. Immunother. 2016, 65, 1433–1450. [Google Scholar] [CrossRef]
- Shi, D.; Shi, Y.; Kaseb, A.O.; Qi, X.; Zhang, Y.; Chi, J.; Lu, Q.; Gao, H.; Jiang, H.; Wang, H.; et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: Results of phase I Trials. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Fucà, G.; Reppel, L.; Landoni, E.; Savoldo, B.; Dotti, G. Enhancing chimeric antigen receptor T-cell efficacy in solid tumors. Clin. Cancer Res. 2020, 26, 2444–2451. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, X.; Saw, P.E.; Wu, W.; Huang, H.; Chen, J.; Nie, Y. Chimeric antigen receptor T cells in solid tumors: A war against the tumor microenvironment. Sci. China Life Sci. 2020, 63, 180–205. [Google Scholar] [CrossRef]
- Akhavan, D.; Alizadeh, D.; Wang, D.; Weist, M.R.; Shepphird, J.K.; Brown, C.E. CAR T cells for brain tumors: Lessons learned and road ahead. Immunol. Rev. 2019, 290, 60–84. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019, 25, 1341–1355. [Google Scholar] [CrossRef]
- Holzinger, A.; Abken, H. CAR T cells targeting solid tumors: Carcinoembryonic antigen (CEA) proves to be a safe target. Cancer Immunol. Immunother. 2017, 66, 1505–1507. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in 2020, 70, 313, doi:10.3322/caac.21609. [Google Scholar] [CrossRef] [PubMed]
- WCRF/AICR. Worldwide Cancer Data; Global Cancer Statistics for the Most Common Cancers. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data (accessed on 4 August 2020).
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 4 August 2020).
- Feng, R.M.; Zong, Y.N.; Cao, S.M.; Xu, R.H. Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019, 39, 22. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Lai, Y.; Li, J.; Qin, L.; Xu, Y.; Zhao, R.; Li, B.; Lin, S.; Wang, S.; Wu, Q.; et al. PSCA and MUC1 in non-small-cell lung cancer as targets of chimeric antigen receptor T cells. Oncoimmunology 2017, 6, e1284722. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.C.; Zeng, Z.; Huang, Y.N.; Deng, Y.C.; Fu, G.H. Clinical significance of TM4SF1 as a tumor suppressor gene in gastric cancer. Cancer Med. 2018, 7, 2592–2600. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Jia, H.; Han, W. Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef]
- Feng, K.; Guo, Y.; Dai, H.; Wang, Y.; Li, X.; Jia, H.; Han, W. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci. China Life Sci. 2016, 59, 468–479. [Google Scholar] [CrossRef]
- Feng, K.C.; Guo, Y.L.; Liu, Y.; Dai, H.R.; Wang, Y.; Lv, H.Y.; Huang, J.H.; Yang, Q.M.; Han, W.D. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J. Hematol. Oncol. 2017, 10, 4. [Google Scholar] [CrossRef]
- Pampusch, M.S.; Skinner, P.J. Transduction and expansion of primary T cells in nine days with maintenance of central memory phenotype. J. Vis. Exp. 2020. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Patrizio, M.P.; Magagnoli, F.; Luppi, S.; Serra, M. An update on emerging drugs in osteosarcoma: Towards tailored therapies? Expert Opin. Emerg. Drugs 2019, 24, 153–171. [Google Scholar] [CrossRef]
- Hendlisz, A.P. 33rd Annual Meeting & Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2018): Washington, DC, USA, 7–11 November 2018. J. Immunother. Cancer 2018, 6, 114. [Google Scholar] [CrossRef]
- Lonez, C.; Verma, B.; Hendlisz, A.; Aftimos, P.; Awada, A.; Neste, E.V.D.; Catala, G.; Machiels, J.H.; Piette, F.; Brayer, J.B.; et al. Study protocol for THINK: A multinational open-label phase I study to assess the safety and clinical activity of multiple administrations of NKR-2 in patients with different metastatic tumour types. BMJ Open 2017, 7, e017075. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2018, 9, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Badhiwala, J.; Decker, W.K.; Berens, M.E.; Bhardwaj, R.D. Clinical trials in cellular immunotherapy for brain/CNS tumors. Expert Rev. Neurother. 2013, 13, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Wakefield, A.; Ghazi, A.; Ashoori, A.; Diouf, O.; Gerken, C.; Landi, D.; et al. Autologous HER2 CMV bispecific CAR T cells are safe and demonstrate clinical benefit for glioblastoma in a Phase I trial. J. Immunother. Cancer 2015, 3. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef]
- Navai, S.A.; Derenzo, C.; Joseph, S.; Sanber, K.; Byrd, T.; Zhang, H.; Mata, M.; Gerken, C.; Shree, A.; Mathew, P.R.; et al. Abstract LB-147: Administration of HER2-CAR T cells after lymphodepletion safely improves T cell expansion and induces clinical responses in patients with advanced sarcomas. Cancer Res. 2019, 79, LB-147. [Google Scholar] [CrossRef]
- Hegde, M.; DeRenzo, C.C.; Zhang, H.; Mata, M.; Gerken, C.; Shree, A.; Yi, Z.; Brawley, V.; Dakhova, O.; Wu, M.-F.; et al. Expansion of HER2-CAR T cells after lymphodepletion and clinical responses in patients with advanced sarcoma. J. Clin. Oncol. 2017, 35, 10508. [Google Scholar] [CrossRef]
- Katz, S.C.; Burga, R.A.; McCormack, E.; Wang, L.J.; Mooring, W.; Point, G.R.; Khare, P.D.; Thorn, M.; Ma, Q.; Stainken, B.F.; et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin. Cancer Res. 2015, 21, 3149–3159. [Google Scholar] [CrossRef]
- Saied, A.; Licata, L.; Burga, R.A.; Thorn, M.; McCormack, E.; Stainken, B.F.; Assanah, E.O.; Khare, P.D.; Davies, R.; Espat, N.J.; et al. Neutrophil:lymphocyte ratios and serum cytokine changes after hepatic artery chimeric antigen receptor-modified T-cell infusions for liver metastases. Cancer Gene 2014, 21, 457–462. [Google Scholar] [CrossRef]
- Appelbaum, J.S.; Pinto, N.; Orentas, R.J. Chapter 11 - Promising Chimeric Antigen Receptors for Non-B-Cell Hematological Malignancies, Pediatric Solid Tumors, and Carcinomas. In Chimeric Antigen Receptor T-Cell Therapies for Cancer; Lee, D.W., Shah, N.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–163. [Google Scholar]
- Zhang, C.; Wang, Z.; Yang, Z.; Wang, M.; Li, S.; Li, Y.; Zhang, R.; Xiong, Z.; Wei, Z.; Shen, J.; et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA(+) Metastatic Colorectal Cancers. Mol. Ther. 2017, 25, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Hardaway, J.; Prince, E.; Guha, P.; Cunetta, M.; Moody, A.; Wang, L.J.; Armenio, V.; Espat, N.J.; Junghans, R.P. HITM-SIR: Phase Ib trial of intraarterial chimeric antigen receptor T-cell therapy and selective internal radiation therapy for CEA(+) liver metastases. Cancer Gene 2020, 27, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Prince, E.; Cunetta, M.; Guha, P.; Moody, A.; Armenio, V.; Wang, L.J.; Espat, N.J.; Junghans, R.P. Abstract CT109: HITM-SIR: Phase Ib trial of CAR-T hepatic artery infusions and selective internal radiation therapy for liver metastases. Cancer Res. 2017, 77, CT109. [Google Scholar] [CrossRef]
- Moroz, K. Anti-CEA CAR-T Demonstrates Significant Therapeutic Effect in Pancreatic Cancer Patients With Liver Metastases. Available online: https://www.interventionaloncology360.com/news/anti-cea-car-t-demonstrates-significant-therapeutic-effect-pancreatic-cancer-patients-liver (accessed on 4 August 2020).
- Thistlethwaite, F.C.; Gilham, D.E.; Guest, R.D.; Rothwell, D.G.; Pillai, M.; Burt, D.J.; Byatte, A.J.; Kirillova, N.; Valle, J.W.; Sharma, S.K.; et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 2017, 66, 1425–1436. [Google Scholar] [CrossRef]
- Purba, E.R.; Saita, E.I.; Maruyama, I.N. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The “Rotation Model”. Cells 2017, 6, 13. [Google Scholar] [CrossRef]
- Huang, H.S.; Nagane, M.; Klingbeil, C.K.; Lin, H.; Nishikawa, R.; Ji, X.D.; Huang, C.M.; Gill, G.N.; Wiley, H.S.; Cavenee, W.K. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J. Biol. Chem. 1997, 272, 2927–2935. [Google Scholar] [CrossRef]
- Shtiegman, K.; Kochupurakkal, B.S.; Zwang, Y.; Pines, G.; Starr, A.; Vexler, A.; Citri, A.; Katz, M.; Lavi, S.; Ben-Basat, Y.; et al. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene 2007, 26, 6968–6978. [Google Scholar] [CrossRef]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pr. 2012, 2012, 743193. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Zhang, J.G.; Kruse, C.A.; Driggers, L.; Hoa, N.; Wisoff, J.; Allen, J.C.; Zagzag, D.; Newcomb, E.W.; Jadus, M.R. Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. J. NeuroOncol. 2008, 88, 65–76. [Google Scholar] [CrossRef]
- Ogasawara, K.; Lanier, L.L. NKG2D in NK and T cell-mediated immunity. J. Clin. Immunol. 2005, 25, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Obeidy, P.; Sharland, A.F. NKG2D and its ligands. Int. J. Biochem. Cell Biol. 2009, 41, 2364–2367. [Google Scholar] [CrossRef] [PubMed]
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef] [PubMed]
- López-Soto, A.; Huergo-Zapico, L.; Acebes-Huerta, A.; Villa-Alvarez, M.; Gonzalez, S. NKG2D signaling in cancer immunosurveillance. Int. J. Cancer 2015, 136, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Mandai, M.; Hamanishi, J.; Matsumura, N.; Suzuki, A.; Yagi, H.; Yamaguchi, K.; Baba, T.; Fujii, S.; Konishi, I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: High expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol. Immunother. 2009, 58, 641–652. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Xin, J.; Wang, J.; Yao, C.; Zhang, Z. Role of NKG2D and its ligands in cancer immunotherapy. Am. J. Cancer Res. 2019, 9, 2064–2078. [Google Scholar]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Hofmeyer, K.A.; Ray, A.; Zang, X. The contrasting role of B7-H3. Proc. Natl. Acad. Sci. USA 2008, 105, 10277–10278. [Google Scholar] [CrossRef]
- Seaman, S.; Zhu, Z.; Saha, S.; Zhang, X.M.; Yang, M.Y.; Hilton, M.B.; Morris, K.; Szot, C.; Morris, H.; Swing, D.A.; et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell 2017, 31, 501–515.e8. [Google Scholar] [CrossRef]
- Castriconi, R.; Dondero, A.; Augugliaro, R.; Cantoni, C.; Carnemolla, B.; Sementa, A.R.; Negri, F.; Conte, R.; Corrias, M.V.; Moretta, L.; et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc. Natl. Acad. Sci. USA 2004, 101, 12640–12645. [Google Scholar] [CrossRef]
- Inamura, K.; Yokouchi, Y.; Kobayashi, M.; Sakakibara, R.; Ninomiya, H.; Subat, S.; Nagano, H.; Nomura, K.; Okumura, S.; Shibutani, T.; et al. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer 2017, 103, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Loos, M.; Hedderich, D.M.; Friess, H.; Kleeff, J. B7-h3 and its role in antitumor immunity. Clin. Dev. Immunol. 2010, 2010, 683875. [Google Scholar] [CrossRef] [PubMed]
- Loos, M.; Hedderich, D.M.; Ottenhausen, M.; Giese, N.A.; Laschinger, M.; Esposito, I.; Kleeff, J.; Friess, H. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer 2009, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular pathways: Targeting B7-H3 (CD276) for human cancer immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431. [Google Scholar] [CrossRef]
- Yamato, I.; Sho, M.; Nomi, T.; Akahori, T.; Shimada, K.; Hotta, K.; Kanehiro, H.; Konishi, N.; Yagita, H.; Nakajima, Y. Clinical importance of B7-H3 expression in human pancreatic cancer. Br. J. Cancer 2009, 101, 1709–1716. [Google Scholar] [CrossRef]
- Benzon, B.; Zhao, S.G.; Haffner, M.C.; Takhar, M.; Erho, N.; Yousefi, K.; Hurley, P.; Bishop, J.L.; Tosoian, J.; Ghabili, K.; et al. Correlation of B7-H3 with androgen receptor, immune pathways and poor outcome in prostate cancer: An expression-based analysis. Prostate Cancer Prostatic Dis. 2017, 20, 28–35. [Google Scholar] [CrossRef]
- Parker, A.S.; Heckman, M.G.; Sheinin, Y.; Wu, K.J.; Hilton, T.W.; Diehl, N.N.; Pisansky, T.M.; Schild, S.E.; Kwon, E.D.; Buskirk, S.J. Evaluation of B7-H3 expression as a biomarker of biochemical recurrence after salvage radiation therapy for recurrent prostate cancer. Int. J. Radiat Oncol. Biol. Phys. 2011, 79, 1343–1349. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, H.; Ye, D.; Dai, B.; Zhu, Y.; Shi, G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. Onco Targets 2013, 6, 1667–1673. [Google Scholar] [CrossRef]
- Roth, T.J.; Sheinin, Y.; Lohse, C.M.; Kuntz, S.M.; Frigola, X.; Inman, B.A.; Krambeck, A.E.; McKenney, M.E.; Karnes, R.J.; Blute, M.L.; et al. B7-H3 ligand expression by prostate cancer: A novel marker of prognosis and potential target for therapy. Cancer Res. 2007, 67, 7893–7900. [Google Scholar] [CrossRef]
- Zang, X.; Sullivan, P.S.; Soslow, R.A.; Waitz, R.; Reuter, V.E.; Wilton, A.; Thaler, H.T.; Arul, M.; Slovin, S.F.; Wei, J.; et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod. Pathol. 2010, 23, 1104–1112. [Google Scholar] [CrossRef]
- Zang, X.; Thompson, R.H.; Al-Ahmadie, H.A.; Serio, A.M.; Reuter, V.E.; Eastham, J.A.; Scardino, P.T.; Sharma, P.; Allison, J.P. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA 2007, 104, 19458–19463. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; McAuley, J. The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation. Front. Cell Infect. Micro. Biol. 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.E.; Wheeler, K.M.; Ribbeck, K. Mucins and their role in shaping the functions of mucus barriers. Annu. Rev. Cell Dev. Biol. 2018, 34, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Gautam, S.K.; Cannon, A.; Thompson, C.; Hall, B.R.; Aithal, A.; Banerjee, K.; Jain, M.; Solheim, J.C.; Kumar, S.; et al. Cancer-associated mucins: Role in immune modulation and metastasis. Cancer Metastasis Rev. 2019, 38, 223–236. [Google Scholar] [CrossRef]
- Guo, M.; You, C.; Dou, J. Role of transmembrane glycoprotein mucin 1 (MUC1) in various types of colorectal cancer and therapies: Current research status and updates. Biomed. Pharm. 2018, 107, 1318–1325. [Google Scholar] [CrossRef]
- Taylor-Papadimitriou, J.; Burchell, J.M.; Graham, R.; Beatson, R. Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 2018, 46, 659–668. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Nap, M.; Mollgard, K.; Burtin, P.; Fleuren, G.J. Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumour Biol. 1988, 9, 145–153. [Google Scholar] [CrossRef]
- Han, Z.W.; Lyv, Z.W.; Cui, B.; Wang, Y.Y.; Cheng, J.T.; Zhang, Y.; Cai, W.Q.; Zhou, Y.; Ma, Z.W.; Wang, X.W.; et al. The old CEACAMs find their new role in tumor immunotherapy. Investig. New Drugs 2020. [Google Scholar] [CrossRef]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef]
- Yan, Z.; Deng, X.; Chen, M.; Xu, Y.; Ahram, M.; Sloane, B.F.; Friedman, E. Oncogenic c-Ki-ras but not oncogenic c-Ha-ras up-regulates CEA expression and disrupts basolateral polarity in colon epithelial cells. J. Biol. Chem. 1997, 272, 27902–27907. [Google Scholar] [CrossRef] [PubMed]
- Nolan, K.F.; Yun, C.O.; Akamatsu, Y.; Murphy, J.C.; Leung, S.O.; Beecham, E.J.; Junghans, R.P. Bypassing immunization: Optimized design of “designer T cells” against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA. Clin. Cancer Res. 1999, 5, 3928–3941. [Google Scholar] [PubMed]
- Wu, C.; Orozco, C.; Boyer, J.; Leglise, M.; Goodale, J.; Batalov, S.; Hodge, C.L.; Haase, J.; Janes, J.; Huss, J.W., 3rd; et al. BioGPS: An extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009, 10, R130. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Casucci, M.; Hawkins, R.E.; Dotti, G.; Bondanza, A. Overcoming the toxicity hurdles of genetically targeted T cells. Cancer Immunol. Immunother. 2015, 64, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Lamers, C.H.; Willemsen, R.; Elzakker, P.v.; Steenbergen-Langeveld, S.v.; Broertjes, M.; Oosterwijk-Wakka, J.; Oosterwijk, E.; Sleijfer, S.; Debets, R.; Gratama, J.W. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 2011, 117, 72–82. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Vargas, J.E.; Chicaybam, L.; Stein, R.T.; Tanuri, A.; Delgado-Cañedo, A.; Bonamino, M.H. Retroviral vectors and transposons for stable gene therapy: Advances, current challenges and perspectives. J. Transl. Med. 2016, 14, 288. [Google Scholar] [CrossRef]
- Schambach, A.; Morgan, M. Retroviral vectors for cancer gene therapy. Recent Results Cancer Res. 2016, 209, 17–35. [Google Scholar] [CrossRef]
- Magnani, C.F.; Tettamanti, S.; Alberti, G.; Pisani, I.; Biondi, A.; Serafini, M.; Gaipa, G. Transposon-based CAR T cells in acute leukemias: Where are we going? Cells 2020, 9, 1337. [Google Scholar] [CrossRef] [PubMed]
- Hudecek, M.; Ivics, Z. Non-viral therapeutic cell engineering with the Sleeping Beauty transposon system. Curr. Opin. Genet. Dev. 2018, 52, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Tipanee, J.; Driessche, T.V.D.; Chuah, M.K. Transposons: Moving forward from preclinical studies to clinical trials. Hum. Gene 2017, 28, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Birkholz, K.; Hombach, A.; Krug, C.; Reuter, S.; Kershaw, M.; Kampgen, E.; Schuler, G.; Abken, H.; Schaft, N.; Dorrie, J. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene 2009, 16, 596–604. [Google Scholar] [CrossRef]
- Holzinger, A.; Abken, H. CAR T Cells: A Snapshot on the growing options to design a CAR. Hemasphere 2019, 3, e172. [Google Scholar] [CrossRef]
- Boomer, J.S.; Green, J.M. An enigmatic tail of CD28 signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a002436. [Google Scholar] [CrossRef]
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009, 17, 1453–1464. [Google Scholar] [CrossRef]
- Kawalekar, O.U.; O’Connor, R.S.; Fraietta, J.A.; Guo, L.; McGettigan, S.E.; Posey, A.D., Jr.; Patel, P.R.; Guedan, S.; Scholler, J.; Keith, B.; et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 2016, 44, 380–390. [Google Scholar] [CrossRef]
- Roselli, E.; Frieling, J.S.; Thorner, K.; Ramello, M.C.; Lynch, C.C.; Abate-Daga, D. CAR-T engineering: Optimizing signal transduction and effector mechanisms. BioDrugs 2019, 33, 647–659. [Google Scholar] [CrossRef]
- Hombach, A.A.; Holzinger, A.; Abken, H. The weal and woe of costimulation in the adoptive therapy of cancer with chimeric antigen receptor (CAR)-redirected T cells. Curr. Mol. Med. 2013, 13, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Brentjens, R.; Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Redeker, A.; Arens, R. Improving adoptive T cell therapy: The particular role of T Cell costimulation, cytokines, and post-transfer vaccination. Front. Immunol. 2016, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Weinkove, R.; George, P.; Dasyam, N.; McLellan, A.D. Selecting costimulatory domains for chimeric antigen receptors: Functional and clinical considerations. Clin. Transl. Immunol. 2019, 8, e1049. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wei, R.; Ma, Q.; Shi, L.; He, F.; Shi, Z.; Jin, T.; Xie, R.; Wei, B.; Chen, J.; et al. In vivo expansion and antitumor activity of coinfused CD28- and 4-1BB-Engineered CAR-T cells in patients with B Cell leukemia. Mol. Ther. 2018, 26, 976–985. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, M.R.; Sentman, C.L. An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. J. Immunol. 2012, 189, 2290–2299. [Google Scholar] [CrossRef]
- Krug, C.; Birkholz, K.; Paulus, A.; Schwenkert, M.; Schmidt, P.; Hoffmann, N.; Hombach, A.; Fey, G.; Abken, H.; Schuler, G.; et al. Stability and activity of MCSP-specific chimeric antigen receptors (CARs) depend on the scFv antigen-binding domain and the protein backbone. Cancer Immunol. Immunother. 2015, 64, 1623–1635. [Google Scholar] [CrossRef]
- Frigault, M.J.; Lee, J.; Basil, M.C.; Carpenito, C.; Motohashi, S.; Scholler, J.; Kawalekar, O.U.; Guedan, S.; McGettigan, S.E.; Posey, A.D., Jr.; et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol. Res. 2015, 3, 356–367. [Google Scholar] [CrossRef]
- Long, A.H.; Haso, W.M.; Shern, J.F.; Wanhainen, K.M.; Murgai, M.; Ingaramo, M.; Smith, J.P.; Walker, A.J.; Kohler, M.E.; Venkateshwara, V.R.; et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015, 21, 581–590. [Google Scholar] [CrossRef]
- Park, J.H.; Riviere, I.; Gonen, M.; Wang, X.; Senechal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Davila, M.L.; Bouhassira, D.C.; Park, J.H.; Curran, K.J.; Smith, E.L.; Pegram, H.J.; Brentjens, R. Chimeric antigen receptors for the adoptive T cell therapy of hematologic malignancies. Int. J. Hematol. 2014, 99, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Cannons, J.L.; Choi, Y.; Watts, T.H. Role of TNF receptor-associated factor 2 and p38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. J. Immunol. 2000, 165, 6193–6204. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Nam, K.O.; Park, S.J.; Kwon, B.S. 4-1BB enhances CD8+ T cell expansion by regulating cell cycle progression through changes in expression of cyclins D and E and cyclin-dependent kinase inhibitor p27kip1. Eur. J. Immunol. 2003, 33, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Condomines, M.; Stegen, S.J.C.v.d.; Perna, F.; Kloss, C.C.; Gunset, G.; Plotkin, J.; Sadelain, M. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015, 28, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.A.; June, C.H. The principles of engineering immune cells to treat cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef]
- Fraietta, J.A.; Lacey, S.F.; Orlando, E.J.; Pruteanu-Malinici, I.; Gohil, M.; Lundh, S.; Boesteanu, A.C.; Wang, Y.; O’Connor, R.S.; Hwang, W.T.; et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018, 24, 563–571. [Google Scholar] [CrossRef]
- Petrausch, U.; Schuberth, P.C.; Hagedorn, C.; Soltermann, A.; Tomaszek, S.; Stahel, R.; Weder, W.; Renner, C. Re-directed T cells for the treatment of fibroblast activation protein (FAP)-positive malignant pleural mesothelioma (FAPME-1). BMC Cancer 2012, 12, 615. [Google Scholar] [CrossRef]
- Schuberth, P.C.; Hagedorn, C.; Jensen, S.M.; Gulati, P.; Broek, M.v.d.; Mischo, A.; Soltermann, A.; Jüngel, A.; Marroquin Belaunzaran, O.; Stahel, R.; et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J. Transl. Med. 2013, 11, 187. [Google Scholar] [CrossRef]
- Ceppi, F.; Rivers, J.; Annesley, C.; Pinto, N.; Park, J.R.; Lindgren, C.; Mgebroff, S.; Linn, N.; Delaney, M.; Gardner, R.A. Lymphocyte apheresis for chimeric antigen receptor T-cell manufacturing in children and young adults with leukemia and neuroblastoma. Transfusion 2018, 58, 1414–1420. [Google Scholar] [CrossRef]
- Li, W.; Guo, L.; Rathi, P.; Marinova, E.; Gao, X.; Wu, M.F.; Liu, H.; Dotti, G.; Gottschalk, S.; Metelitsa, L.S.; et al. Redirecting T cells to Glypican-3 with 4-1BB zeta chimeric antigen receptors results in Th1 polarization and potent antitumor activity. Hum. Gene 2017, 28, 437–448. [Google Scholar] [CrossRef]
- Chmielewski, M.; Hombach, A.A.; Abken, H. Of CARs and TRUCKs: Chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev. 2014, 257, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Kopecky, C.; Hombach, A.A.; Abken, H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011, 71, 5697–5706. [Google Scholar] [CrossRef] [PubMed]
- Pegram, H.J.; Lee, J.C.; Hayman, E.G.; Imperato, G.H.; Tedder, T.F.; Sadelain, M.; Brentjens, R.J. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012, 119, 4133–4141. [Google Scholar] [CrossRef] [PubMed]
- Koneru, M.; Purdon, T.J.; Spriggs, D.; Koneru, S.; Brentjens, R.J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 2015, 4, e994446. [Google Scholar] [CrossRef]
- Koneru, M.; O’Cearbhaill, R.; Pendharkar, S.; Spriggs, D.R.; Brentjens, R.J. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J. Transl. Med. 2015, 13, 102. [Google Scholar] [CrossRef]
- Xu, A.; Bhanumathy, K.K.; Wu, J.; Ye, Z.; Freywald, A.; Leary, S.C.; Li, R.; Xiang, J. IL-15 signaling promotes adoptive effector T-cell survival and memory formation in irradiation-induced lymphopenia. Cell Bio. Sci. 2016, 6, 30. [Google Scholar] [CrossRef]
- Liu, X.; Ranganathan, R.; Jiang, S.; Fang, C.; Sun, J.; Kim, S.; Newick, K.; Lo, A.; June, C.H.; Zhao, Y.; et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016, 76, 1578–1590. [Google Scholar] [CrossRef]
- Davies, D.M.; Foster, J.; Stegen, S.J.V.D.; Parente-Pereira, A.C.; Chiapero-Stanke, L.; Delinassios, G.J.; Burbridge, S.E.; Kao, V.; Liu, Z.; Bosshard-Carter, L.; et al. Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol. Med. 2012, 18, 565–576. [Google Scholar] [CrossRef]
- Schalkwyk, M.C.v.; Papa, S.E.; Jeannon, J.P.; Guerrero Urbano, T.; Spicer, J.F.; Maher, J. Design of a phase I clinical trial to evaluate intratumoral delivery of ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Hum. Gene Clin. Dev. 2013, 24, 134–142. [Google Scholar] [CrossRef]
- Papa, S.; Schalkwyk, M.v.; Maher, J. Clinical Evaluation of ErbB-Targeted CAR T-Cells, Following Intracavity Delivery in Patients with ErbB-Expressing Solid Tumors. In Gene Therapy of Solid Cancers: Methods and Protocols; Walther, W., Stein, U., Eds.; Springer: New York, NY, USA, 2015; pp. 365–382. [Google Scholar]
- Wang, D.; Starr, R.; Chang, W.C.; Aguilar, B.; Alizadeh, D.; Wright, S.L.; Yang, X.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Law, C.L.; Gordon, K.A.; Toki, B.E.; Yamane, A.K.; Hering, M.A.; Cerveny, C.G.; Petroziello, J.M.; Ryan, M.C.; Smith, L.; Simon, R.; et al. Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody-drug conjugates. Cancer Res. 2006, 66, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Jilaveanu, L.B.; Sznol, J.; Aziz, S.A.; Duchen, D.; Kluger, H.M.; Camp, R.L. CD70 expression patterns in renal cell carcinoma. Hum. Pathol. 2012, 43, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Jung, G.; Radovanovic, I.; Beier, C.; Steinbach, J.P.; Rimner, A.; Huang, H.; Schulz, J.B.; Ohgaki, H.; Aguzzi, A.; et al. Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res. 2002, 62, 2592–2599. [Google Scholar] [PubMed]
- Claus, C.; Riether, C.; Schürch, C.; Matter, M.S.; Hilmenyuk, T.; Ochsenbein, A.F. CD27 signaling increases the frequency of regulatory T cells and promotes tumor growth. Cancer Res. 2012, 72, 3664–3676. [Google Scholar] [CrossRef]
- Diegmann, J.; Junker, K.; Gerstmayer, B.; Bosio, A.; Hindermann, W.; Rosenhahn, J.; von Eggeling, F. Identification of CD70 as a diagnostic biomarker for clear cell renal cell carcinoma by gene expression profiling, real-time RT-PCR and immunohistochemistry. Eur. J. Cancer 2005, 41, 1794–1801. [Google Scholar] [CrossRef]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and safety of IL13Rα2-Redirected chimeric antigen receptor CD8+ T Cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef]
- Yaghoubi, S.S.; Jensen, M.C.; Satyamurthy, N.; Budhiraja, S.; Paik, D.; Czernin, J.; Gambhir, S.S. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat. Clin. Pr. Oncol. 2009, 6, 53–58. [Google Scholar] [CrossRef]
- Keu, K.V.; Witney, T.H.; Yaghoubi, S.; Rosenberg, J.; Kurien, A.; Magnusson, R.; Williams, J.; Habte, F.; Wagner, J.R.; Forman, S.; et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of glioblastoma after chimeric antigen receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, L.; Cui, H.; Wang, X.; Zhang, G.; Ma, J.; Han, H.; He, W.; Wang, W.; Zhao, Y.; et al. Anti-melanoma activity of T cells redirected with a TCR-like chimeric antigen receptor. Sci. Rep. 2014, 4, 3571. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, R.; Zhu, X.; Wang, L.; Ma, J.; Han, H.; Wang, X.; Zhang, G.; He, W.; Wang, W.; et al. Retargeting NK-92 for anti-melanoma activity by a TCR-like single-domain antibody. Immunol. Cell Biol. 2013, 91, 615–624. [Google Scholar] [CrossRef]
- Timmune. TriCAR-T Technology. Available online: http://www.timmune.com/services/show.php?lang=en&id=142 (accessed on 4 August 2020).
- Eureka Therapeutics. Eureka Therapeutics Achieves Regression of Metastatic Liver Cancer using ET140202 T-cell Therapy. Available online: https://www.eurekatherapeutics.com/media/press-releases/090518/ (accessed on 4 August 2020).
- Tanaka, M.; Tashiro, H.; Omer, B.; Lapteva, N.; Ando, J.; Ngo, M.; Mehta, B.; Dotti, G.; Kinchington, P.R.; Leen, A.M.; et al. Vaccination Targeting native receptors to enhance the function and proliferation of chimeric antigen receptor (CAR)-modified T Cells. Clin. Cancer Res. 2017, 23, 3499–3509. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Yvon, E.; Myers, G.D.; Rossig, C.; Russell, H.V.; Diouf, O.; Liu, E.; et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011, 118, 6050–6056. [Google Scholar] [CrossRef]
- Pule, M.A.; Savoldo, B.; Myers, G.D.; Rossig, C.; Russell, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008, 14, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Berger, C.; Wong, C.W.; Forman, S.J.; Riddell, S.R.; Jensen, M.C. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 2011, 117, 1888–1898. [Google Scholar] [CrossRef]
- Wang, X.; Naranjo, A.; Brown, C.E.; Bautista, C.; Wong, C.W.; Chang, W.C.; Aguilar, B.; Ostberg, J.R.; Riddell, S.R.; Forman, S.J.; et al. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. J. Immunother. 2012, 35, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.T.; Beckman, E.M.; Bukowski, J.F.; Tanaka, Y.; Band, H.; Bloom, B.R.; Golan, D.E.; Brenner, M.B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity 1995, 3, 495–507. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morita, C.T.; Tanaka, Y.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 1995, 375, 155–158. [Google Scholar] [CrossRef]
- Oevermann, L.; Lang, P.; Feuchtinger, T.; Schumm, M.; Teltschik, H.M.; Schlegel, P.; Handgretinger, R. Immune reconstitution and strategies for rebuilding the immune system after haploidentical stem cell transplantation. Ann. N. Y. Acad. Sci. 2012, 1266, 161–170. [Google Scholar] [CrossRef]
- Baitsch, L.; Baumgaertner, P.; Devêvre, E.; Raghav, S.K.; Legat, A.; Barba, L.; Wieckowski, S.; Bouzourene, H.; Deplancke, B.; Romero, P.; et al. Exhaustion of tumor-specific CD8⁺ T cells in metastases from melanoma patients. J. Clin. Investig. 2011, 121, 2350–2360. [Google Scholar] [CrossRef]
- Öhrmalm, L.; Smedman, C.; Wong, M.; Broliden, K.; Tolfvenstam, T.; Norbeck, O. Decreased functional T lymphocyte-mediated cytokine responses in patients with chemotherapy-induced neutropenia. J. Intern. Med. 2013, 274, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Winter, M.C.; Cameron, D.; Bell, R.; Dodwell, D.; Keane, M.M.; Gil, M.; Ritchie, D.; Passos-Coelho, J.L.; Wheatley, D.; et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: Exploratory evidence for direct anti-tumour activity in breast cancer. Br. J. Cancer 2010, 102, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Reshef, R. Revving the CAR–Combination strategies to enhance CAR T cell effectiveness. Blood Rev. 2020. [Google Scholar] [CrossRef]
- Donnadieu, E.; Dupré., L.; Pinho, L.G.; Cotta-de-Almeida, V. Surmounting the obstacles that impede effective CAR T cell trafficking to solid tumors. J. Leukoc. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, U.; Yalniz, F.F.; Srour, S.A.; Rezvani, K.; Singh, H.; Olson, A.; Blumenschein, G., Jr.; Hong, D.S.; Shpall, E.J.; Kebriaei, P. Chimeric Antigen Receptor Therapy: How Are We Driving in Solid Tumors? Biol. Blood Marrow Transplant. 2020. [Google Scholar] [CrossRef]
- Lesch, S.; Benmebarek, M.R.; Cadilha, B.L.; Stoiber, S.; Subklewe, M.; Endres, S.; Kobold, S. Determinants of response and resistance to CAR T cell therapy. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Schurich, A.; Magalhaes, I.; Mattsson, J. Metabolic regulation of CAR T cell function by the hypoxic microenvironment in solid tumors. Immunotherapy 2019, 11, 335–345. [Google Scholar] [CrossRef]
- Scharping, N.E.; Delgoffe, G.M. Tumor microenvironment metabolism: A new checkpoint. for anti-tumor immunity. Vaccines 2016, 4, 46. [Google Scholar] [CrossRef]
- Beatty, G.L.; Moon, E.K. Chimeric antigen receptor T cells are vulnerable to immunosuppressive mechanisms present within the tumor microenvironment. Oncoimmunology 2014, 3, e970027. [Google Scholar] [CrossRef]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Gladney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.C.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol. Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Hege, K.M.; Bergsland, E.K.; Fisher, G.A.; Nemunaitis, J.J.; Warren, R.S.; McArthur, J.G.; Lin, A.A.; Schlom, J.; June, C.H.; Sherwin, S.A. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J. Immunother. Cancer 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Kano, Y.; Nagai, T.; Okuyama, N.; Sakoda, Y.; Tamada, K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018, 36, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Wang, L.S.; Scholler, J.; Monslow, J.; Avery, D.; Newick, K.; O’Brien, S.; Evans, R.A.; Bajor, D.J.; Clendenin, C.; et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015, 75, 2800–2810. [Google Scholar] [CrossRef]
- Wang, L.C.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014, 2, 154–166. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, X.; Chen, S.; Lai, Y.; Wei, X.; Li, B.; Lin, S.; Wang, S.; Wu, Q.; Liang, Q.; et al. Anti-GPC3-CAR T cells suppress the growth of tumor cells in patient-derived xenografts of hepatocellular carcinoma. Front. Immunol. 2016, 7, 690. [Google Scholar] [CrossRef]
- Pearson, C.; Silva, A.; Seddon, B. Exogenous amino acids are essential for interleukin-7 induced CD8 T cell growth. PLoS ONE 2012, 7, e33998. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Finch, R.A.; Jäger, D.; Muller, W.A.; Sartorelli, A.C.; Randolph, G.J. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell 2000, 103, 757–768. [Google Scholar] [CrossRef]
- Reif, K.; Ekland, E.H.; Ohl, L.; Nakano, H.; Lipp, M.; Förster, R.; Cyster, J.G. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 2002, 416, 94–99. [Google Scholar] [CrossRef]
- Bromley, S.K.; Thomas, S.Y.; Luster, A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 2005, 6, 895–901. [Google Scholar] [CrossRef]
- Shum, T.; Omer, B.; Tashiro, H.; Kruse, R.L.; Wagner, D.L.; Parikh, K.; Yi, Z.; Sauer, T.; Liu, D.; Parihar, R.; et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017, 7, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Hurton, L.V.; Singh, H.; Najjar, A.M.; Switzer, K.C.; Mi, T.; Maiti, S.; Olivares, S.; Rabinovich, B.; Huls, H.; Forget, M.A.; et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl. Acad. Sci. USA 2016, 113, E7788–E7797. [Google Scholar] [CrossRef]
- Wilkie, S.; Burbridge, S.E.; Chiapero-Stanke, L.; Pereira, A.C.; Cleary, S.; Stegen, S.J.v.d.; Spicer, J.F.; Davies, D.M.; Maher, J. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J. Biol. Chem. 2010, 285, 25538–25544. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.M.; Massagué, J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Travis, M.A.; Sheppard, D. TGF-β activation and function in immunity. Annu. Rev. Immunol. 2014, 32, 51–82. [Google Scholar] [CrossRef]
- Wieser, R.; Attisano, L.; Wrana, J.L.; Massagué, J. Signaling activity of transforming growth factor beta type II receptors lacking specific domains in the cytoplasmic region. Mol. Cell Biol. 1993, 13, 7239–7247. [Google Scholar] [CrossRef]
- Gorelik, L.; Flavell, R.A. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat. Med. 2001, 7, 1118–1122. [Google Scholar] [CrossRef]
- Kloss, C.C.; Lee, J.; Zhang, A.; Chen, F.; Melenhorst, J.J.; Lacey, S.F.; Maus, M.V.; Fraietta, J.A.; Zhao, Y.; June, C.H. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 2018, 26, 1855–1866. [Google Scholar] [CrossRef]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.C.; Lu, L.; Zheng, Z.; et al. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef]
- Adusumilli, P.S.; Zauderer, M.G.; Rusch, V.W.; O’Cearbhaill, R.; Zhu, A.; Ngai, D.; McGee, E.; Chintala, N.; Messinger, J.; Cheema, W.; et al. Regional delivery of mesothelin-targeted CAR T cells for pleural cancers: Safety and preliminary efficacy in combination with anti-PD-1 agent. J. Clin. Oncol. 2019, 37, 2511. [Google Scholar] [CrossRef]
- Schietinger, A.; Philip, M.; Krisnawan, V.E.; Chiu, E.Y.; Delrow, J.J.; Basom, R.S.; Lauer, P.; Brockstedt, D.G.; Knoblaugh, S.E.; Hämmerling, G.J.; et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 2016, 45, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Heczey, A.; Louis, C.U.; Savoldo, B.; Dakhova, O.; Durett, A.; Grilley, B.; Liu, H.; Wu, M.F.; Mei, Z.; Gee, A.; et al. CAR T Cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 2017, 25, 2214–2224. [Google Scholar] [CrossRef] [PubMed]
- Brentjens, R.J.; Rivière, I.; Park, J.H.; Davila, M.L.; Wang, X.; Stefanski, J.; Taylor, C.; Yeh, R.; Bartido, S.; Borquez-Ojeda, O.; et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011, 118, 4817–4828. [Google Scholar] [CrossRef]
- Gattinoni, L.; Finkelstein, S.E.; Klebanoff, C.A.; Antony, P.A.; Palmer, D.C.; Spiess, P.J.; Hwang, L.N.; Yu, Z.; Wrzesinski, C.; Heimann, D.M.; et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005, 202, 907–912. [Google Scholar] [CrossRef]
- Dudley, M.E.; Wunderlich, J.R.; Yang, J.C.; Sherry, R.M.; Topalian, S.L.; Restifo, N.P.; Royal, R.E.; Kammula, U.; White, D.E.; Mavroukakis, S.A.; et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005, 23, 2346–2357. [Google Scholar] [CrossRef]
- Suryadevara, C.M.; Desai, R.; Abel, M.L.; Riccione, K.A.; Batich, K.A.; Shen, S.H.; Chongsathidkiet, P.; Gedeon, P.C.; Elsamadicy, A.A.; Snyder, D.J.; et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology 2018, 7, e1434464. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, M.; Philip, B.; Traynor-White, C.; Davis, C.G.; Onuoha, S.; Cordoba, S.; Thomas, S.; Pule, M. A Rapamycin-Activated Caspase 9-Based Suicide Gene. Mol. Ther. 2018, 26, 1266–1276. [Google Scholar] [CrossRef]
- Di Stasi, A.; Tey, S.K.; Dotti, G.; Fujita, Y.; Kennedy-Nasser, A.; Martinez, C.; Straathof, K.; Liu, E.; Durett, A.G.; Grilley, B.; et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011, 365, 1673–1683. [Google Scholar] [CrossRef]
- Foster, A.E.; Mahendravada, A.; Shinners, N.P.; Chang, W.C.; Crisostomo, J.; Lu, A.; Khalil, M.; Morschl, E.; Shaw, J.L.; Saha, S.; et al. Regulated expansion and survival of chimeric antigen receptor-modified T cells using small molecule-dependent inducible MyD88/CD40. Mol. Ther. 2017, 25, 2176–2188. [Google Scholar] [CrossRef]
- Becerra, C.R.; Manji, G.A.; Kim, D.W.; Gardner, O.; Malankar, A.; Shaw, J.; Blass, D.; Yi, X.; Foster, A.E.; Woodard, P. Ligand-inducible, prostate stem cell antigen (PSCA)-directed GoCAR-T cells in advanced solid tumors: Preliminary results with cyclophosphamide (Cy) ± fludarabine (Flu) lymphodepletion (LD). J. Clin. Oncol. 2019, 37, 2536. [Google Scholar] [CrossRef]
- Moolten, F.L. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: Paradigm for a prospective cancer control strategy. Cancer Res. 1986, 46, 5276–5281. [Google Scholar] [PubMed]
- Beltinger, C.; Fulda, S.; Kammertoens, T.; Meyer, E.; Uckert, W.; Debatin, K.M. Herpes simplex virus thymidine kinase/ganciclovir-induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc. Natl. Acad. Sci. USA 1999, 96, 8699–8704. [Google Scholar] [CrossRef] [PubMed]
- Traversari, C.; Marktel, S.; Magnani, Z.; Mangia, P.; Russo, V.; Ciceri, F.; Bonini, C.; Bordignon, C. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood 2007, 109, 4708–4715. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Digiusto, D.L.; Slovak, M.; Wright, C.; Naranjo, A.; Wagner, J.; Meechoovet, H.B.; Bautista, C.; Chang, W.C.; Ostberg, J.R.; et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007, 15, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Grier, A.; Gardner, R.; Delaney, C.; Jensen, M.C. Multiplexed Engineering of CD19CAR T Cells for Post-Transplant Consolidative Immunotherapy. Blood 2016, 128, 1159. [Google Scholar] [CrossRef]
- Wang, X.; Chang, W.-C.; Wong, C.W.; Colcher, D.; Sherman, M.; Ostberg, J.R.; Forman, S.J.; Riddell, S.R.; Jensen, M.C. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011, 118, 1255–1263. [Google Scholar] [CrossRef]
- Budde, L.E.; Berger, C.; Lin, Y.; Wang, J.; Lin, X.; Frayo, S.E.; Brouns, S.A.; Spencer, D.M.; Till, B.G.; Jensen, M.C.; et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS ONE 2013, 8, e82742. [Google Scholar] [CrossRef]
- Budde, L.E.; Mardiros, A.; Chang, W.-C.; Wang, X.; Berger, C.; Brown, C.; Riddell, S.R.; Press, O.W.; Forman, S.J. Truncated cell-surface CD19 as a conditional suicide switch for adoptive T Cell immunotherapy. Blood 2013, 122, 1660. [Google Scholar] [CrossRef]
- Harrer, D.C.; Simon, B.; Fujii, S.I.; Shimizu, K.; Uslu, U.; Schuler, G.; Gerer, K.F.; Hoyer, S.; Dorrie, J.; Schaft, N. RNA-transfection of gamma/delta T cells with a chimeric antigen receptor or an alpha/beta T-cell receptor: A safer alternative to genetically engineered alpha/beta T cells for the immunotherapy of melanoma. BMC Cancer 2017, 17, 551. [Google Scholar] [CrossRef]
- Dorrie, J.; Babalija, L.; Hoyer, S.; Gerer, K.F.; Schuler, G.; Heinzerling, L.; Schaft, N. BRAF and MEK Inhibitors influence the function of reprogrammed t cells: Consequences for adoptive T-Cell therapy. Int. J. Mol. Sci. 2018, 19, 289. [Google Scholar] [CrossRef]
- Harrer, D.C.; Schuler, G.; Dorrie, J.; Schaft, N. CSPG4-Specific CAR T cells for high-risk childhood B cell precursor Leukemia. Int. J. Mol. Sci. 2019, 20, 2764. [Google Scholar] [CrossRef] [PubMed]
- Uslu, U.; Schuler, G.; Dorrie, J.; Schaft, N. Combining a chimeric antigen receptor and a conventional T-cell receptor to generate T cells expressing two additional receptors (TETARs) for a multi-hit immunotherapy of melanoma. Exp. Derm. 2016, 25, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, M.; Marz, J.; Kummer, M.; Schuler, G.; Dorrie, J.; Schuler-Thurner, B.; Schaft, N. Clinical-Scale production of CAR-T cells for the treatment of melanoma patients by mRNA transfection of a CSPG4-specific CAR under full GMP compliance. Cancers 2019, 11, 1198. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A Review on electroporation-based intracellular delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef]
- Tchou, J.; Zhao, Y.; Levine, B.L.; Zhang, P.J.; Davis, M.M.; Melenhorst, J.J.; Kulikovskaya, I.; Brennan, A.L.; Liu, X.; Lacey, S.F.; et al. Safety and Efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol. Res. 2017, 5, 1152–1161. [Google Scholar] [CrossRef]
- Beatty, G.L.; Haas, A.R.; Maus, M.V.; Torigian, D.A.; Soulen, M.C.; Plesa, G.; Chew, A.; Zhao, Y.; Levine, B.L.; Albelda, S.M.; et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014, 2, 112–120. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Hara, M.H.; Lacey, S.F.; Torigian, D.A.; Nazimuddin, F.; Chen, F.; Kulikovskaya, I.M.; Soulen, M.C.; McGarvey, M.; Nelson, A.M.; et al. Activity of mesothelin-specific chimeric antigen receptor t cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 2018, 155, 29–32. [Google Scholar] [CrossRef]
- Maus, M.V.; Haas, A.R.; Beatty, G.L.; Albelda, S.M.; Levine, B.L.; Liu, X.; Zhao, Y.; Kalos, M.; June, C.H. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 2013, 1, 26–31. [Google Scholar] [CrossRef]
- Svoboda, J.; Rheingold, S.R.; Gill, S.I.; Grupp, S.A.; Lacey, S.F.; Kulikovskaya, I.; Suhoski, M.M.; Melenhorst, J.J.; Loudon, B.; Mato, A.R.; et al. Nonviral RNA chimeric antigen receptor-modified T cells in patients with Hodgkin lymphoma. Blood 2018, 132, 1022–1026. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 2018, 7, e1440169. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, B.; Li, Z.; Li, J.; Wang, H.; Chen, L.; Jiang, H.; Wu, M.; Xiao, J.; Peng, X.; et al. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. J. Clin. Oncol. 2019, 37, 2509. [Google Scholar] [CrossRef]
- Morgan, R.A.; Johnson, L.A.; Davis, J.L.; Zheng, Z.; Woolard, K.D.; Reap, E.A.; Feldman, S.A.; Chinnasamy, N.; Kuan, C.T.; Song, H.; et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum. Gene 2012, 23, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.V. Designing CAR T cells for glioblastoma. Oncoimmunology 2015, 4, e1048956. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Boesteanu, A.C.; Binder, Z.A.; Xu, C.; Reid, R.A.; Rodriguez, J.L.; Cook, D.R.; Thokala, R.; Blouch, K.; McGettigan-Croce, B.; et al. CheckpoInt. Blockade Reverses Anergy in IL-13Rα2 Humanized scFv-Based CAR T Cells to Treat Murine and Canine Gliomas. Mol. Oncolytics 2018, 11, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Scholler, J.; Ohkuri, T.; Kosaka, A.; Patel, P.R.; McGettigan, S.E.; Nace, A.K.; Dentchev, T.; Thekkat, P.; Loew, A.; et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci. Transl. Med. 2015, 7, 275ra222. [Google Scholar] [CrossRef]
- Kershaw, M.H.; Westwood, J.A.; Parker, L.L.; Wang, G.; Eshhar, Z.; Mavroukakis, S.A.; White, D.E.; Wunderlich, J.R.; Canevari, S.; Rogers-Freezer, L.; et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006, 12, 6106–6115. [Google Scholar] [CrossRef]
- Lee, D.W.; Barrett, D.M.; Mackall, C.; Orentas, R.; Grupp, S.A. The future is now: Chimeric antigen receptors as new targeted therapies for childhood cancer. Clin. Cancer Res. 2012, 18, 2780–2790, Correction in 2017, 23, 611, doi:10.1158/1078-0432.Ccr-16-2684. [Google Scholar] [CrossRef]
- Gargett, T.; Yu, W.; Dotti, G.; Yvon, E.S.; Christo, S.N.; Hayball, J.D.; Lewis, I.D.; Brenner, M.K.; Brown, M.P. GD2-specific CAR T Cells Undergo Potent Activation and Deletion Following Antigen Encounter but can be Protected From Activation-induced Cell Death by PD-1 Blockade. Mol. Ther. 2016, 24, 1135–1149. [Google Scholar] [CrossRef]
- Stroncek, D.F.; Lee, D.W.; Ren, J.; Sabatino, M.; Highfill, S.; Khuu, H.; Shah, N.N.; Kaplan, R.N.; Fry, T.J.; Mackall, C.L. Elutriated lymphocytes for manufacturing chimeric antigen receptor T cells. J. Transl. Med. 2017, 15, 59. [Google Scholar] [CrossRef]
- Straathof, K.; Flutter, B.; Wallace, R.; Thomas, S.; Cheung, G.; Collura, A.; Gileadi, T.; Barton, J.; Wright, G.; Inglott, S.; et al. Abstract CT145: A cancer research UK phase I trial of anti-GD2 chimeric antigen receptor (CAR) transduced T-cells (1RG-CART) in patients with relapsed or refractory neuroblastoma. Cancer Res. 2018, 78, CT145. [Google Scholar] [CrossRef]
- Zhai, B.; Shi, D.; Gao, H.; Qi, X.; Jiang, H.; Zhang, Y.; Chi, J.; Ruan, H.; Wang, H.; Ru, Q.C.; et al. A phase I study of anti-GPC3 chimeric antigen receptor modified T cells (GPC3 CAR-T) in Chinese patients with refractory or relapsed GPC3+ hepatocellular carcinoma (r/r GPC3+ HCC). J. Clin. Oncol. 2017, 35, 3049. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Stashwick, C.; Plesa, G.; Morgan, M.A.; Porter, D.; Maus, M.V.; June, C.H. Possible compartmental cytokine release syndrome in a patient with recurrent ovarian cancer after treatment with mesothelin-targeted CAR-T Cells. J. Immunother. 2017, 40, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Tanyi, J.L.; Haas, A.R.; Beatty, G.L.; Stashwick, C.J.; O’Hara, M.H.; Morgan, M.A.; Porter, D.L.; Melenhorst, J.J.; Plesa, G.; Lacey, S.F.; et al. Anti-mesothelin chimeric antigen receptor T cells in patients with epithelial ovarian cancer. J. Clin. Oncol. 2016, 34, 5511. [Google Scholar] [CrossRef]
- Prasad, S.; Adusumilli, M.G.Z.; Valerie, W.; Rusch, R.E.; Amy, Z.; Daniel, A.; Ngai, E.; Navin, K.; Chintala, J.C.; Elizabeth, F.; et al. CT036—A Phase I Clinical Trial of Malignant Pleural Disease Treated with Regionally Delivered Autologous Mesothelin-Targeted Car T Cells: Safety and Efficacy. Available online: https://www.abstractsonline.com/pp8/#!/6812/presentation/9837 (accessed on 4 August 2020).
- You, F.; Jiang, L.; Zhang, B.; Lu, Q.; Zhou, Q.; Liao, X.; Wu, H.; Du, K.; Zhu, Y.; Meng, H.; et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci. China Life Sci. 2016, 59, 386–397. [Google Scholar] [CrossRef]
- Junghans, R.P.; Ma, Q.; Rathore, R.; Gomes, E.M.; Bais, A.J.; Lo, A.S.; Abedi, M.; Davies, R.A.; Cabral, H.J.; Al-Homsi, A.S.; et al. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: Possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate 2016, 76, 1257–1270. [Google Scholar] [CrossRef]
- Ma, Q.; Safar, M.; Holmes, E.; Wang, Y.; Boynton, A.L.; Junghans, R.P. Anti-prostate specific membrane antigen designer T cells for prostate cancer therapy. Prostate 2004, 61, 12–25. [Google Scholar] [CrossRef]
- Specht, J.M.; Lee, S.; Turtle, C.J.; Berger, C.; Baladrishnan, A.; Srivastava, S.; Voillet, V.; Veatch, J.; Gooley, T.; Mullane, E.; et al. Abstract CT131: A phase I study of adoptive immunotherapy for advanced ROR1+ malignancies with defined subsets of autologous T cells expressing a ROR1-specific chimeric antigen receptor (ROR1-CAR). Cancer Res. 2018, 78, CT131. [Google Scholar] [CrossRef]
- Specht, J.M.L.S.; Turtle, C.; Berger, C.; Balakrishnan, A.; Srivastava, S.; Viollet, V.; Veatch, J.; Gooley, T.; Mullane, E.; Chaney, C.N.; et al. A Phase I Study of Adoptive Immunotherapy for Ror1+ Advanced Triple Negative Breast Cancer (Tnbc) with Defined Subsets of Autologous T Cells Expressing A Ror1-Specific Chimeric Antigen Receptor (ROR1-CAR). Available online: https://www.abstracts2view.com/sabcs/view.php?nu=SABCS18L_1227 (accessed on 4 August 2020).
- Shimabukuro-Vornhagen, A.; Godel, P.; Subklewe, M.; Stemmler, H.J.; Schlosser, H.A.; Schlaak, M.; Kochanek, M.; Boll, B.; Bergwelt-Baildon, M.S.v. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef]
- Perales, M.A.; Kebriaei, P.; Kean, L.S.; Sadelain, M. Building a safer and faster CAR: Seatbelts, airbags and crispr. Biol. Blood Marrow Transplant. 2017. [Google Scholar] [CrossRef]
- Stoiber, S.; Cadilha, B.L.; Benmebarek, M.R.; Lesch, S.; Endres, S.; Kobold, S. Limitations in the design of chimeric antigen receptors for cancer therapy. Cells 2019, 8, 472. [Google Scholar] [CrossRef]
- Sievers, N.M.; Dörrie, J.; Schaft, N. CARs: Beyond T Cells and T Cell-derived signaling domains. Int. J. Mol. Sci. 2020, 21, 3525. [Google Scholar] [CrossRef] [PubMed]
- Harrer, D.C.; Dorrie, J.; Schaft, N. Chimeric antigen receptors in different cell types: New vehicles join the race. Hum. Gene 2018, 29, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Montagner, I.M.; Penna, A.; Fracasso, G.; Carpanese, D.; Dalla Pieta, A.; Barbieri, V.; Zuccolotto, G.; Rosato, A. Anti-PSMA CAR-engineered NK-92 Cells: An Off-the-shelf cell therapy for prostate cancer. Cells 2020, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Cutsem, E.V.; Machiels, J.; Eynde, M.V.D.; Prenen, H.; Hendlisz, A.; Shaza, L.; Carrasco, J.; Canon, J.; Sotiropoulou, P.; Breman, E.; et al. SO-009-Phase 1 studies assessing the safety and clinical activity of autologous and allogeneic NKG2D-based CAR-T therapy in metastatic colorectal cancer. Ann. Oncol. 2019, 30, iv124–iv125. [Google Scholar] [CrossRef]
- Santoro, S.P.; Kim, S.; Motz, G.T.; Alatzoglou, D.; Li, C.; Irving, M.; Powell, D.J., Jr.; Coukos, G. T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol. Res. 2015, 3, 68–84. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).