Modern Radiotherapy for Pediatric Brain Tumors
Abstract
1. Introduction
2. Physical and Dosimetric Principles
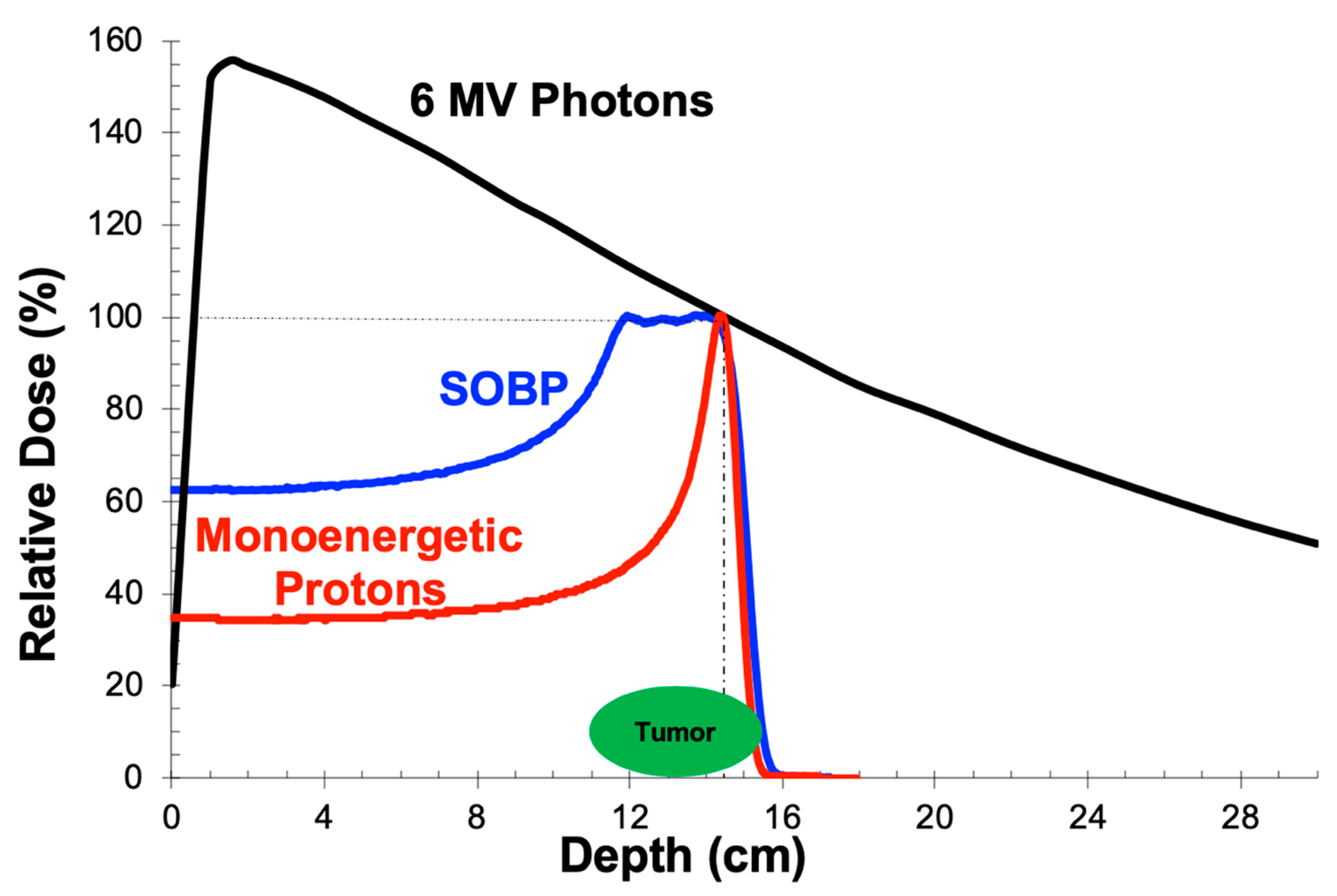
2.1. Depth-Dose Properties: Photons
2.2. Depth-Dose Properties: Protons
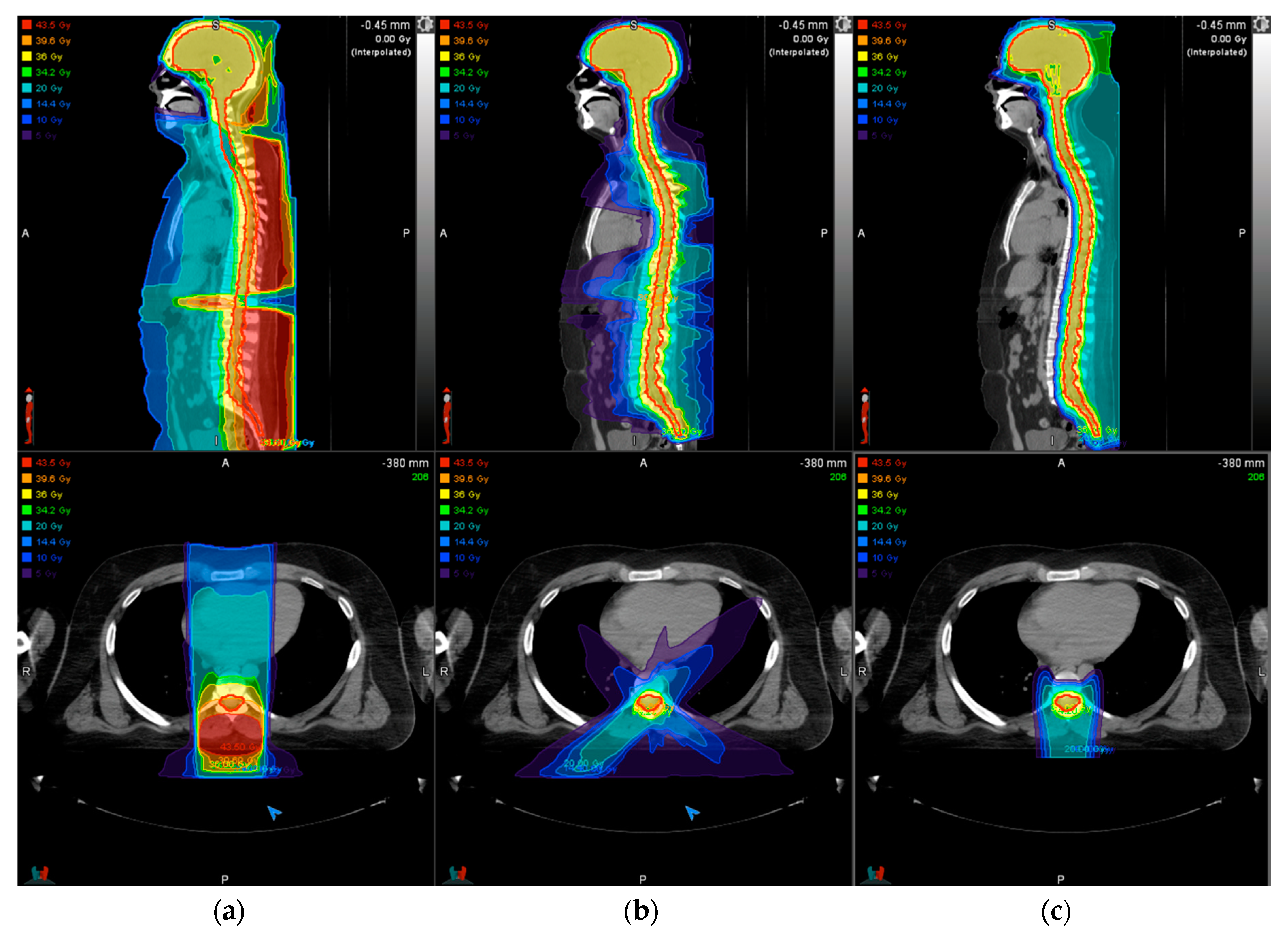
2.3. Treatment Delivery: Photons
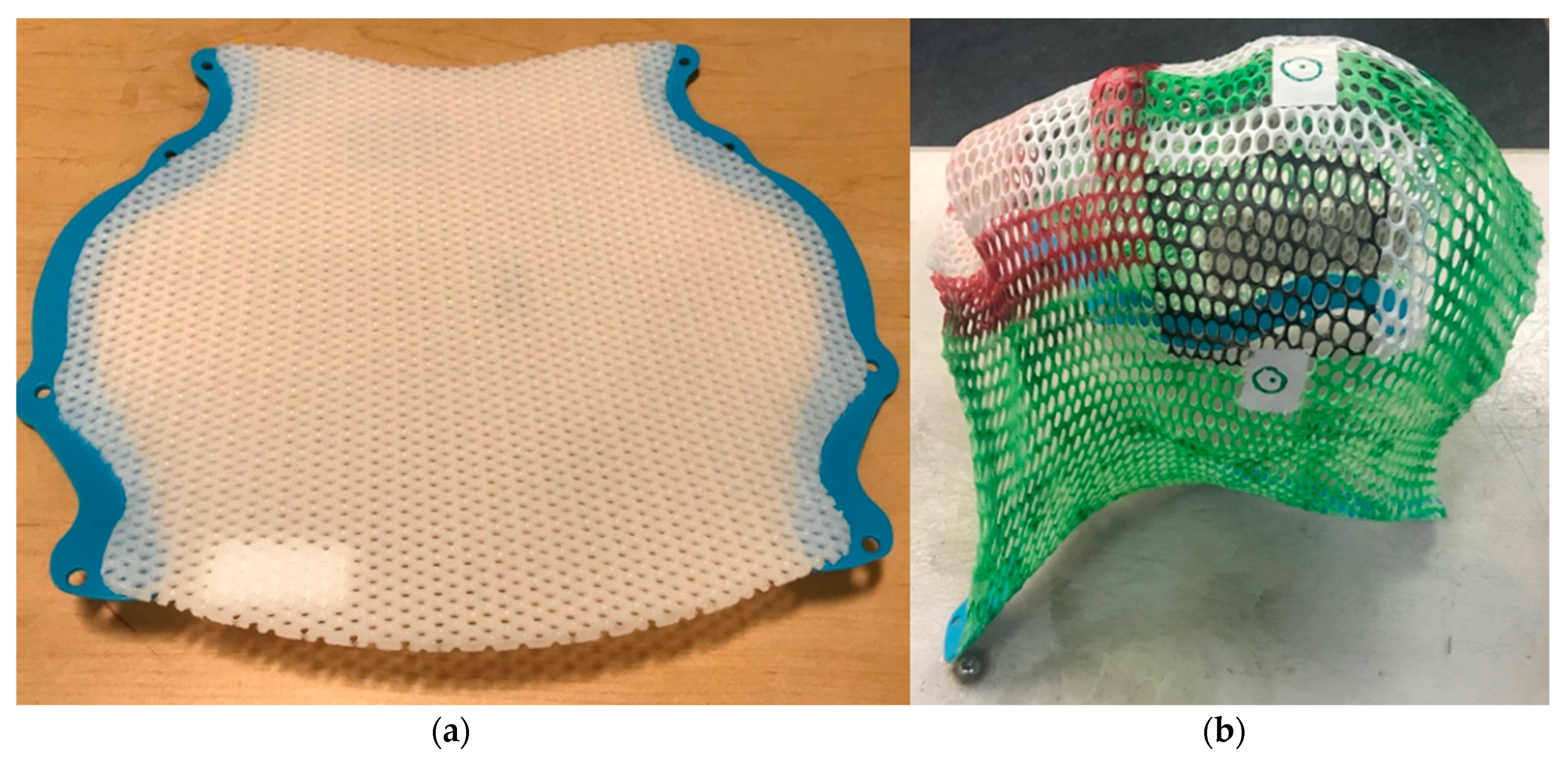
2.4. Treatment Delivery: Protons
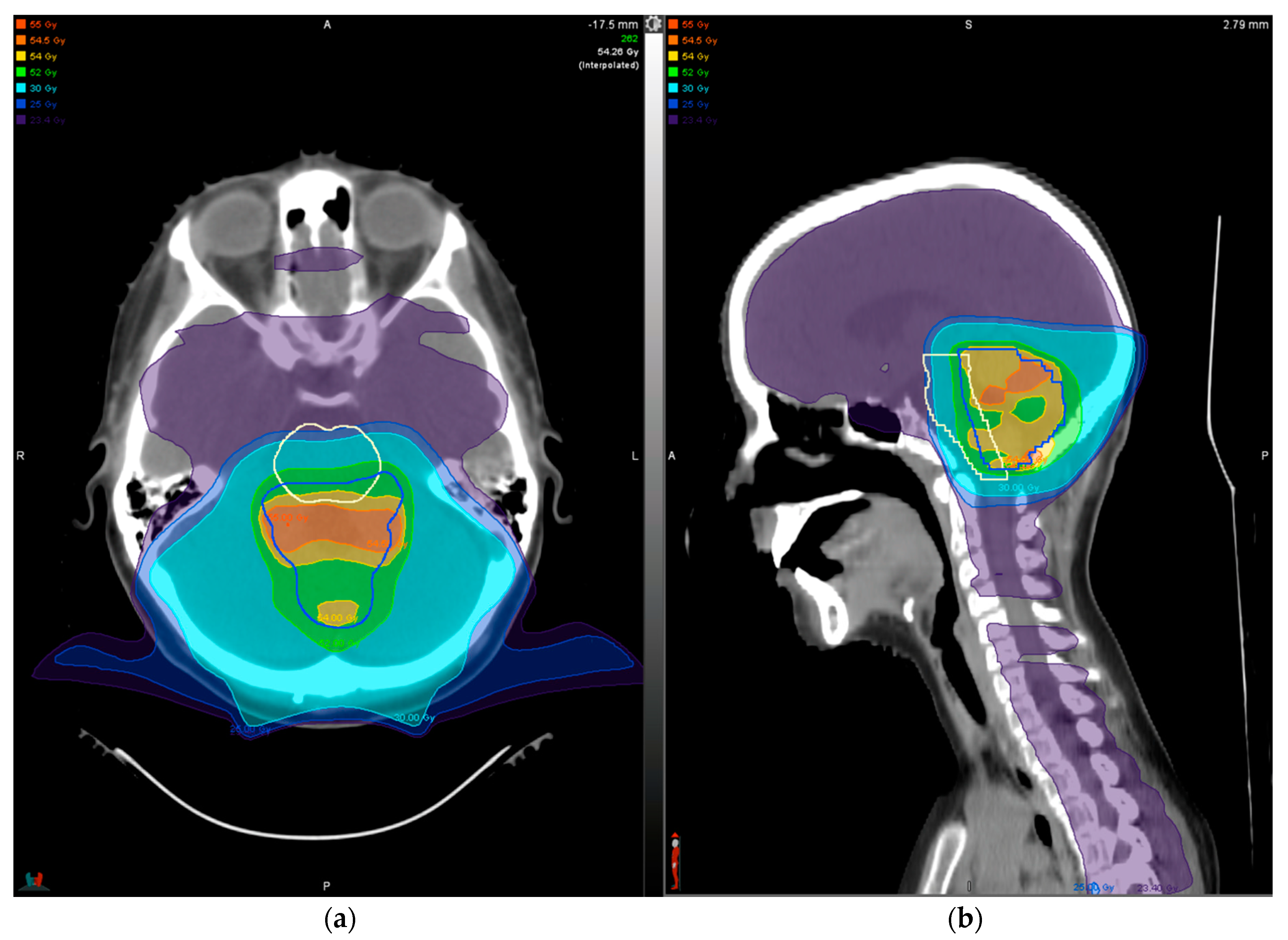
3. Treatment Planning
4. Treatment Outcomes for Select Histologies
4.1. Medulloblastoma
4.2. Ependymoma
4.3. Glioma
5. Late Effects
5.1. Neurocognition
5.2. Neuroendocrine Function
5.3. Hearing
5.4. Cerebrovascular Effects
5.4.1. Moyamoya
5.4.2. Cavernomas and Cerebrovascular Bleeds
5.4.3. Stroke
5.4.4. Post-Treatment Surveillance
5.5. Relative Biologic Effectiveness and Brainstem Toxicity
5.6. Alopecia
5.7. Second Neoplasms
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Howlader, N.N.A.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019; https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019.
- Merchant, T.E.; Conklin, H.M.; Wu, S.; Lustig, R.H.; Xiong, X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 2009, 27, 3691–3697. [Google Scholar] [CrossRef]
- Paulino, A.C.; Lobo, M.; Teh, B.S.; Okcu, M.F.; South, M.; Butler, E.B.; Su, J.; Chintagumpala, M. Ototoxicity after intensity-modulated radiation therapy and cisplatin-based chemotherapy in children with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1445–1450. [Google Scholar] [CrossRef]
- Vatner, R.E.; Niemierko, A.; Misra, M.; Weyman, E.A.; Goebel, C.P.; Ebb, D.H.; Jones, R.M.; Huang, M.S.; Mahajan, A.; Grosshans, D.R.; et al. Endocrine Deficiency As a Function of Radiation Dose to the Hypothalamus and Pituitary in Pediatric and Young Adult Patients With Brain Tumors. J. Clin. Oncol. 2018, 36, 2854–2862. [Google Scholar] [CrossRef]
- Bhakta, N.; Liu, Q.; Ness, K.K.; Baassiri, M.; Eissa, H.; Yeo, F.; Chemaitilly, W.; Ehrhardt, M.J.; Bass, J.; Bishop, M.W.; et al. The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017, 390, 2569–2582. [Google Scholar] [CrossRef]
- Diller, L.; Chow, E.J.; Gurney, J.G.; Hudson, M.M.; Kadin-Lottick, N.S.; Kawashima, T.I.; Leisenring, W.M.; Meacham, L.R.; Mertens, A.C.; Mulrooney, D.A.; et al. Chronic disease in the Childhood Cancer Survivor Study cohort: A review of published findings. J. Clin. Oncol. 2009, 27, 2339–2355. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Meadows, A.T.; Friedman, D.L.; Neglia, J.P.; Mertens, A.C.; Donaldson, S.S.; Stovall, M.; Hammond, S.; Yasui, Y.; Inskip, P.D. Second neoplasms in survivors of childhood cancer: Findings from the Childhood Cancer Survivor Study cohort. J. Clin. Oncol. 2009, 27, 2356–2362. [Google Scholar] [CrossRef]
- Turcotte, L.M.; Whitton, J.A.; Friedman, D.L.; Hammond, S.; Armstrong, G.T.; Leisenring, W.; Robison, L.L.; Neglia, J.P. Risk of Subsequent Neoplasms During the Fifth and Sixth Decades of Life in the Childhood Cancer Survivor Study Cohort. J. Clin. Oncol. 2015, 33, 3568–3575. [Google Scholar] [CrossRef]
- Map of Proton Therapy Centers. Available online: https://www.proton-therapy.org/map/ (accessed on 14 February 2020).
- Chung, C.S.; Yock, T.I.; Nelson, K.; Xu, Y.; Keating, N.L.; Tarbell, N.J. Incidence of second malignancies among patients treated with proton versus photon radiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 46–52. [Google Scholar] [CrossRef]
- Ladra, M.M.; Edgington, S.K.; Mahajan, A.; Grosshans, D.; Szymonifka, J.; Khan, F.; Moteabbed, M.; Friedmann, A.M.; MacDonald, S.M.; Tarbell, N.J.; et al. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiother. Oncol. 2014, 113, 77–83. [Google Scholar] [CrossRef]
- Miralbell, R.; Lomax, A.; Cella, L.; Schneider, U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 824–829. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Fontenot, J.D.; Mahajan, A.; Kornguth, D.; Stovall, M.; Zheng, Y.; Taddei, P.J.; Mirkovic, D.; Mohan, R.; Cox, J.D.; et al. The risk of developing a second cancer after receiving craniospinal proton irradiation. Phys. Med. Biol. 2009, 54, 2277–2291. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H.; Athar, B.S.; Moteabbed, M.; Adams, J.A.; Schneider, U.; Yock, T.I. Assessment of radiation-induced second cancer risks in proton therapy and IMRT for organs inside the primary radiation field. Phys. Med. Biol. 2012, 57, 6047–6061. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.T.; Todd, K.E.; Oakley, H.; Bradley, J.A.; Rotondo, R.L.; Morris, C.G.; Klein, S.; Mendenhall, N.P.; Indelicato, D.J. Reducing Anesthesia and Health Care Cost Through Utilization of Child Life Specialists in Pediatric Radiation Oncology. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Slifer, K.J.; Bucholtz, J.D.; Cataldo, M.D. Behavioral training of motion control in young children undergoing radiation treatment without sedation. J. Pediatr. Oncol. Nurs. 1994, 11, 55–63. [Google Scholar] [CrossRef]
- Kool, M.; Korshunov, A.; Remke, M.; Jones, D.T.; Schlanstein, M.; Northcott, P.A.; Cho, Y.J.; Koster, J.; Schouten-van Meeteren, A.; van Vuurden, D.; et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012, 123, 473–484. [Google Scholar] [CrossRef]
- Gajjar, A.; Bowers, D.C.; Karajannis, M.A.; Leary, S.; Witt, H.; Gottardo, N.G. Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape. J. Clin. Oncol. 2015, 33, 2986–2998. [Google Scholar] [CrossRef]
- Merchant, T.E.; Bendel, A.E.; Sabin, N.D.; Burger, P.C.; Shaw, D.W.; Chang, E.; Wu, S.; Zhou, T.; Eisenstat, D.D.; Foreman, N.K.; et al. Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma. J. Clin. Oncol. 2019, 37, 974–983. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Hielscher, T.; Mack, S.C.; Lassaletta, A.; Lin, T.; Pajtler, K.W.; Jones, D.T.; Luu, B.; Cavalli, F.M.; Aldape, K.; et al. Therapeutic Impact of Cytoreductive Surgery and Irradiation of Posterior Fossa Ependymoma in the Molecular Era: A Retrospective Multicohort Analysis. J. Clin. Oncol. 2016, 34, 2468–2477. [Google Scholar] [CrossRef]
- Oh, K.S.; Hung, J.; Robertson, P.L.; Garton, H.J.; Muraszko, K.M.; Sandler, H.M.; Hamstra, D.A. Outcomes of multidisciplinary management in pediatric low-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e481–e488. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Claassen, D.; al-Shboul, Q.; Janosky, J.E.; Deutsch, M. Low-grade gliomas of the cerebral hemispheres in children: An analysis of 71 cases. J. Neurosurg. 1995, 82, 536–547. [Google Scholar] [CrossRef]
- Wisoff, J.H.; Sanford, R.A.; Heier, L.A.; Sposto, R.; Burger, P.C.; Yates, A.J.; Holmes, E.J.; Kun, L.E. Primary neurosurgery for pediatric low-grade gliomas: A prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery 2011, 68, 1548–1554. [Google Scholar] [CrossRef]
- Merchant, T.E.; Hua, C.H.; Shukla, H.; Ying, X.; Nill, S.; Oelfke, U. Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr. Blood Cancer 2008, 51, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.B.; Indelicato, D.J.; Paulino, A.C.; Hartsell, W.F.; Hill-Kayser, C.E.; Perkins, S.M.; Mahajan, A.; Laack, N.N.; Ermoian, R.P.; Chang, A.L.; et al. An Update From the Pediatric Proton Consortium Registry. Front. Oncol. 2018, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Kasper, H.B.; Raeke, L.; Indelicato, D.J.; Symecko, H.; Hartsell, W.; Mahajan, A.; Hill-Kayser, C.; Perkins, S.M.; Chang, A.L.; Childs, S.; et al. The Pediatric Proton Consortium Registry: A multi-institutional collaboration in US proton centers. Int. J. Part Ther. 2014, 1, 323–333. [Google Scholar] [CrossRef]
- Constine, L.S.; Ronckers, C.M.; Hua, C.H.; Olch, A.; Kremer, L.C.M.; Jackson, A.; Bentzen, S.M. Pediatric Normal Tissue Effects in the Clinic (PENTEC): An International Collaboration to Analyse Normal Tissue Radiation Dose-Volume Response Relationships for Paediatric Cancer Patients. Clin. Oncol. 2019, 31, 199–207. [Google Scholar] [CrossRef]
- Gentile, M.S.; Yeap, B.Y.; Paganetti, H.; Goebel, C.P.; Gaudet, D.E.; Gallotto, S.L.; Weyman, E.A.; Morgan, M.L.; MacDonald, S.M.; Giantsoudi, D.; et al. Brainstem Injury in Pediatric Patients With Posterior Fossa Tumors Treated With Proton Beam Therapy and Associated Dosimetric Factors. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 719–729. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Flampouri, S.; Rotondo, R.L.; Bradley, J.A.; Morris, C.G.; Aldana, P.R.; Sandler, E.; Mendenhall, N.P. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014, 53, 1298–1304. [Google Scholar] [CrossRef]
- Lawenda, B.D.; Gagne, H.M.; Gierga, D.P.; Niemierko, A.; Wong, W.M.; Tarbell, N.J.; Chen, G.T.; Hochberg, F.H.; Loeffler, J.S. Permanent alopecia after cranial irradiation: Dose-response relationship. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 879–887. [Google Scholar] [CrossRef]
- Palma, G.; Taffelli, A.; Fellin, F.; D’Avino, V.; Scartoni, D.; Tommasino, F.; Scifoni, E.; Durante, M.; Amichetti, M.; Schwarz, M.; et al. Modelling the risk of radiation induced alopecia in brain tumor patients treated with scanned proton beams. Radiother. Oncol. 2019, 144, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yock, T.I.; Yeap, B.Y.; Ebb, D.H.; Weyman, E.; Eaton, B.R.; Sherry, N.A.; Jones, R.M.; MacDonald, S.M.; Pulsifer, M.B.; Lavally, B.; et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016, 17, 287–298. [Google Scholar] [CrossRef]
- Greenberger, B.A.; Pulsifer, M.B.; Ebb, D.H.; MacDonald, S.M.; Jones, R.M.; Butler, W.E.; Huang, M.S.; Marcus, K.J.; Oberg, J.A.; Tarbell, N.J.; et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Sharma, S.; Xiong, X.; Wu, S.; Conklin, H. Effect of cerebellum radiation dosimetry on cognitive outcomes in children with infratentorial ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Zureick, A.H.; Evans, C.L.; Niemierko, A.; Grieco, J.A.; Nichols, A.J.; Fullerton, B.C.; Hess, C.B.; Goebel, C.P.; Gallotto, S.L.; Weyman, E.A.; et al. Left hippocampal dosimetry correlates with visual and verbal memory outcomes in survivors of pediatric brain tumors. Cancer 2018, 124, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Moxon-Emre, I.; Bouffet, E.; Taylor, M.D.; Laperriere, N.; Scantlebury, N.; Law, N.; Spiegler, B.J.; Malkin, D.; Janzen, L.; Mabbott, D. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J. Clin. Oncol. 2014, 32, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Ris, M.D.; Walsh, K.; Wallace, D.; Armstrong, F.D.; Holmes, E.; Gajjar, A.; Zhou, T.; Packer, R.J. Intellectual and academic outcome following two chemotherapy regimens and radiotherapy for average-risk medulloblastoma: COG A9961. Pediatr. Blood Cancer 2013, 60, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Kahalley, L.S.; Peterson, R.; Ris, M.D.; Janzen, L.; Okcu, M.F.; Grosshans, D.R.; Ramaswamy, V.; Paulino, A.C.; Hodgson, D.; Mahajan, A.; et al. Superior Intellectual Outcomes After Proton Radiotherapy Compared With Photon Radiotherapy for Pediatric Medulloblastoma. J. Clin. Oncol. 2020, 38, 454–461. [Google Scholar] [CrossRef]
- Hua, C.; Bass, J.K.; Khan, R.; Kun, L.E.; Merchant, T.E. Hearing loss after radiotherapy for pediatric brain tumors: Effect of cochlear dose. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 892–899. [Google Scholar] [CrossRef]
- Nageswara Rao, A.A.; Wallace, D.J.; Billups, C.; Boyett, J.M.; Gajjar, A.; Packer, R.J. Cumulative cisplatin dose is not associated with event-free or overall survival in children with newly diagnosed average-risk medulloblastoma treated with cisplatin based adjuvant chemotherapy: Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2014, 61, 102–106. [Google Scholar] [CrossRef]
- Paulino, A.C.; Mahajan, A.; Ye, R.; Grosshans, D.R.; Fatih Okcu, M.; Su, J.; McAleer, M.F.; McGovern, S.; Mangona, V.A.; Chintagumpala, M. Ototoxicity and cochlear sparing in children with medulloblastoma: Proton vs. photon radiotherapy. Radiother. Oncol. 2018, 128, 128–132. [Google Scholar] [CrossRef]
- Scott, R.M.; Smith, E.R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 2009, 360, 1226–1237. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Robertson, R.; Kinnamon, D.D.; Scott, R.M.; Kieran, M.W.; Turner, C.D.; Chi, S.N.; Goumnerova, L.; Proctor, M.; Tarbell, N.J.; et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology 2007, 68, 932–938. [Google Scholar] [CrossRef]
- Miura, M.; Nakajima, M.; Fujimoto, A.; Kaku, Y.; Kawano, T.; Watanabe, M.; Kuratsu, J.I.; Ando, Y. High prevalence of small vessel disease long after cranial irradiation. J. Clin. Neurosci. 2017, 46, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; Sear, K.; Felton, E.; Tamrazi, B.; Gauvain, K.; Torkildson, J.; Buono, B.D.; Samuel, D.; Haas-Kogan, D.A.; Chen, J.; et al. Presence of cerebral microbleeds is associated with worse executive function in pediatric brain tumor survivors. Neuro Oncol. 2016, 18, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Roongpiboonsopit, D.; Kuijf, H.J.; Charidimou, A.; Xiong, L.; Vashkevich, A.; Martinez-Ramirez, S.; Shih, H.A.; Gill, C.M.; Viswanathan, A.; Dietrich, J. Evolution of cerebral microbleeds after cranial irradiation in medulloblastoma patients. Neurology 2017, 88, 789–796. [Google Scholar] [CrossRef]
- Yates, P.A.; Villemagne, V.L.; Ellis, K.A.; Desmond, P.M.; Masters, C.L.; Rowe, C.C. Cerebral microbleeds: A review of clinical, genetic, and neuroimaging associations. Front. Neurol. 2014, 4, 205. [Google Scholar] [CrossRef]
- Gastelum, E.; Sear, K.; Hills, N.; Roddy, E.; Randazzo, D.; Chettout, N.; Hess, C.; Cotter, J.; Haas-Kogan, D.A.; Fullerton, H.; et al. Rates and characteristics of radiographically detected intracerebral cavernous malformations after cranial radiation therapy in pediatric cancer patients. J. Child Neurol. 2015, 30, 842–849. [Google Scholar] [CrossRef]
- Mueller, S.; Sear, K.; Hills, N.K.; Chettout, N.; Afghani, S.; Gastelum, E.; Haas-Kogan, D.; Fullerton, H.J. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 643–648. [Google Scholar] [CrossRef]
- Bowers, D.C.; Liu, Y.; Leisenring, W.; McNeil, E.; Stovall, M.; Gurney, J.G.; Robison, L.L.; Packer, R.J.; Oeffinger, K.C. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2006, 24, 5277–5282. [Google Scholar] [CrossRef]
- El-Fayech, C.; Haddy, N.; Allodji, R.S.; Veres, C.; Diop, F.; Kahlouche, A.; Llanas, D.; Jackson, A.; Rubino, C.; Guibout, C.; et al. Cerebrovascular Diseases in Childhood Cancer Survivors: Role of the Radiation Dose to Willis Circle Arteries. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Fullerton, H.J.; Stratton, K.; Leisenring, W.; Weathers, R.E.; Stovall, M.; Armstrong, G.T.; Goldsby, R.E.; Packer, R.J.; Sklar, C.A.; et al. Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: A report from the Childhood Cancer Survivor Study. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, H.J.; Stratton, K.; Mueller, S.; Leisenring, W.W.; Armstrong, G.T.; Weathers, R.E.; Stovall, M.; Sklar, C.A.; Goldsby, R.E.; Robison, L.L.; et al. Recurrent stroke in childhood cancer survivors. Neurology 2015, 85, 1056–1064. [Google Scholar] [CrossRef]
- Mayo, C.; Yorke, E.; Merchant, T.E. Radiation associated brainstem injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S36–S41. [Google Scholar] [CrossRef] [PubMed]
- Haas-Kogan, D.; Indelicato, D.; Paganetti, H.; Esiashvili, N.; Mahajan, A.; Yock, T.; Flampouri, S.; MacDonald, S.; Fouladi, M.; Stephen, K.; et al. National Cancer Institute Workshop on Proton Therapy for Children: Considerations Regarding Brainstem Injury. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 152–168. [Google Scholar] [CrossRef] [PubMed]
- McGovern, S.L.; Okcu, M.F.; Munsell, M.F.; Kumbalasseriyil, N.; Grosshans, D.R.; McAleer, M.F.; Chintagumpala, M.; Khatua, S.; Mahajan, A. Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1143–1152. [Google Scholar] [CrossRef]
- Yock, T.I.; Constine, L.S.; Mahajan, A. Protons, the brainstem, and toxicity: Ingredients for an emerging dialectic. Acta Oncol. 2014, 53, 1279–1282. [Google Scholar] [CrossRef]
- Roberts, K.W.; Wan Chan Tseung, H.S.; Eckel, L.J.; Harmsen, W.S.; Beltran, C.; Laack, N.N. Biologic Dose and Imaging Changes in Pediatric Brain Tumor Patients Receiving Spot Scanning Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 664–673. [Google Scholar] [CrossRef]
- Kinahan, K.E.; Sharp, L.K.; Seidel, K.; Leisenring, W.; Didwania, A.; Lacouture, M.E.; Stovall, M.; Haryani, A.; Robison, L.L.; Krull, K.R. Scarring, disfigurement, and quality of life in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor study. J. Clin. Oncol. 2012, 30, 2466–2474. [Google Scholar] [CrossRef]
- Mertens, A.C.; Liu, Q.; Neglia, J.P.; Wasilewski, K.; Leisenring, W.; Armstrong, G.T.; Robison, L.L.; Yasui, Y. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2008, 100, 1368–1379. [Google Scholar] [CrossRef]
- Friedman, D.L.; Whitton, J.; Leisenring, W.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L.; Neglia, J.P. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2010, 102, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Neglia, J.P.; Friedman, D.L.; Yasui, Y.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood cancer survivor study. J. Natl. Cancer Inst. 2001, 93, 618–629. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeNunzio, N.J.; Yock, T.I. Modern Radiotherapy for Pediatric Brain Tumors. Cancers 2020, 12, 1533. https://doi.org/10.3390/cancers12061533
DeNunzio NJ, Yock TI. Modern Radiotherapy for Pediatric Brain Tumors. Cancers. 2020; 12(6):1533. https://doi.org/10.3390/cancers12061533
Chicago/Turabian StyleDeNunzio, Nicholas J., and Torunn I. Yock. 2020. "Modern Radiotherapy for Pediatric Brain Tumors" Cancers 12, no. 6: 1533. https://doi.org/10.3390/cancers12061533
APA StyleDeNunzio, N. J., & Yock, T. I. (2020). Modern Radiotherapy for Pediatric Brain Tumors. Cancers, 12(6), 1533. https://doi.org/10.3390/cancers12061533



