Mass Spectrometric Comparison of HPV-Positive and HPV-Negative Oropharyngeal Cancer
Abstract
1. Introduction
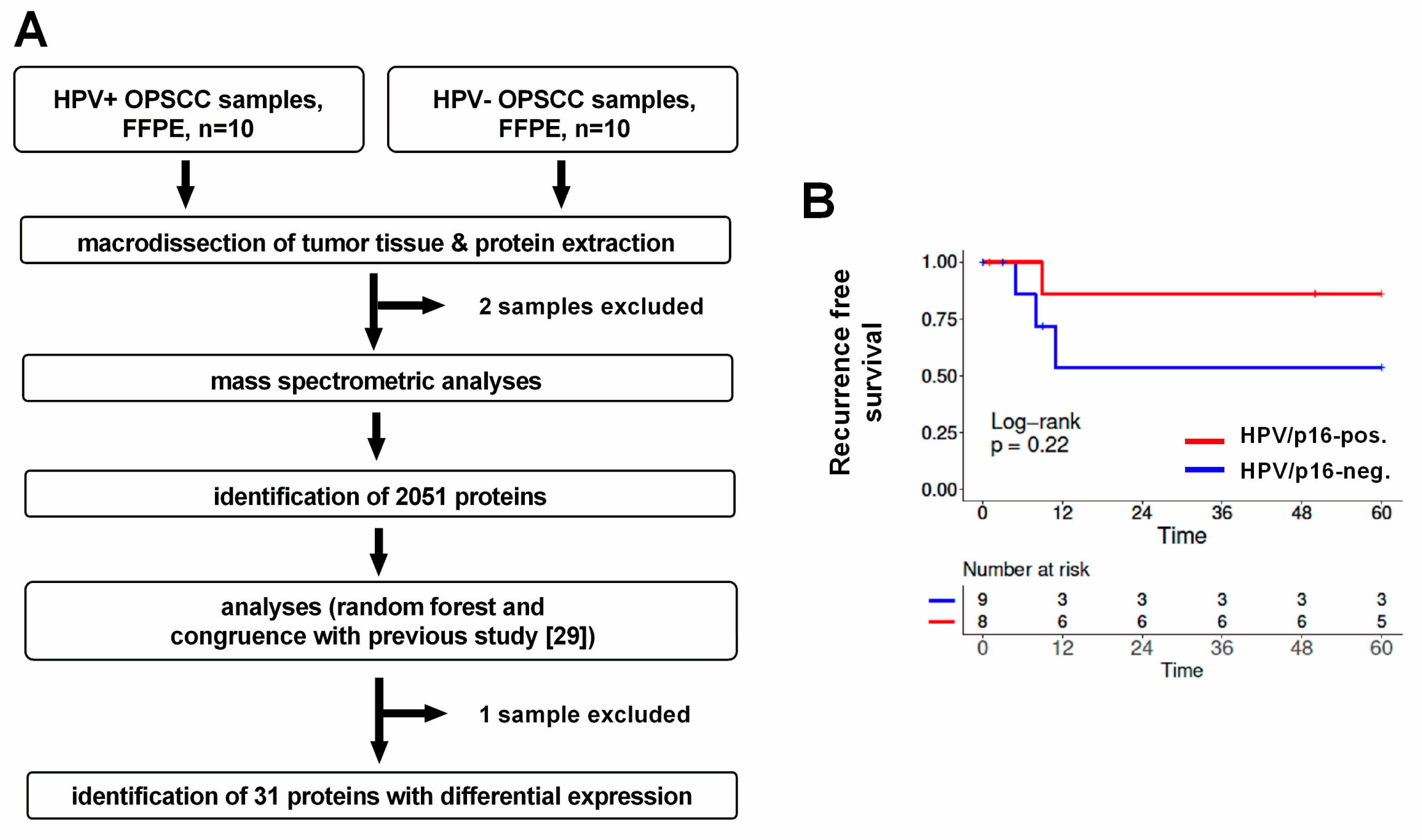
2. Material and Methods
2.1. Patient Characteristics
2.2. Sample Preparation and Processing
2.2.1. Tissue Sectioning and Protein Extraction
2.2.2. Tryptic Digestion and Desalting
2.2.3. LC–MS/MS Analysis
2.3. Peptide Identification and Quantitative Proteomics
2.4. Random Forest Analysis
2.5. Analyses of Identified Proteins
2.6. Tissue Microarray (TMA) Analysis
2.7. Analysis of Patient Survival
3. Results
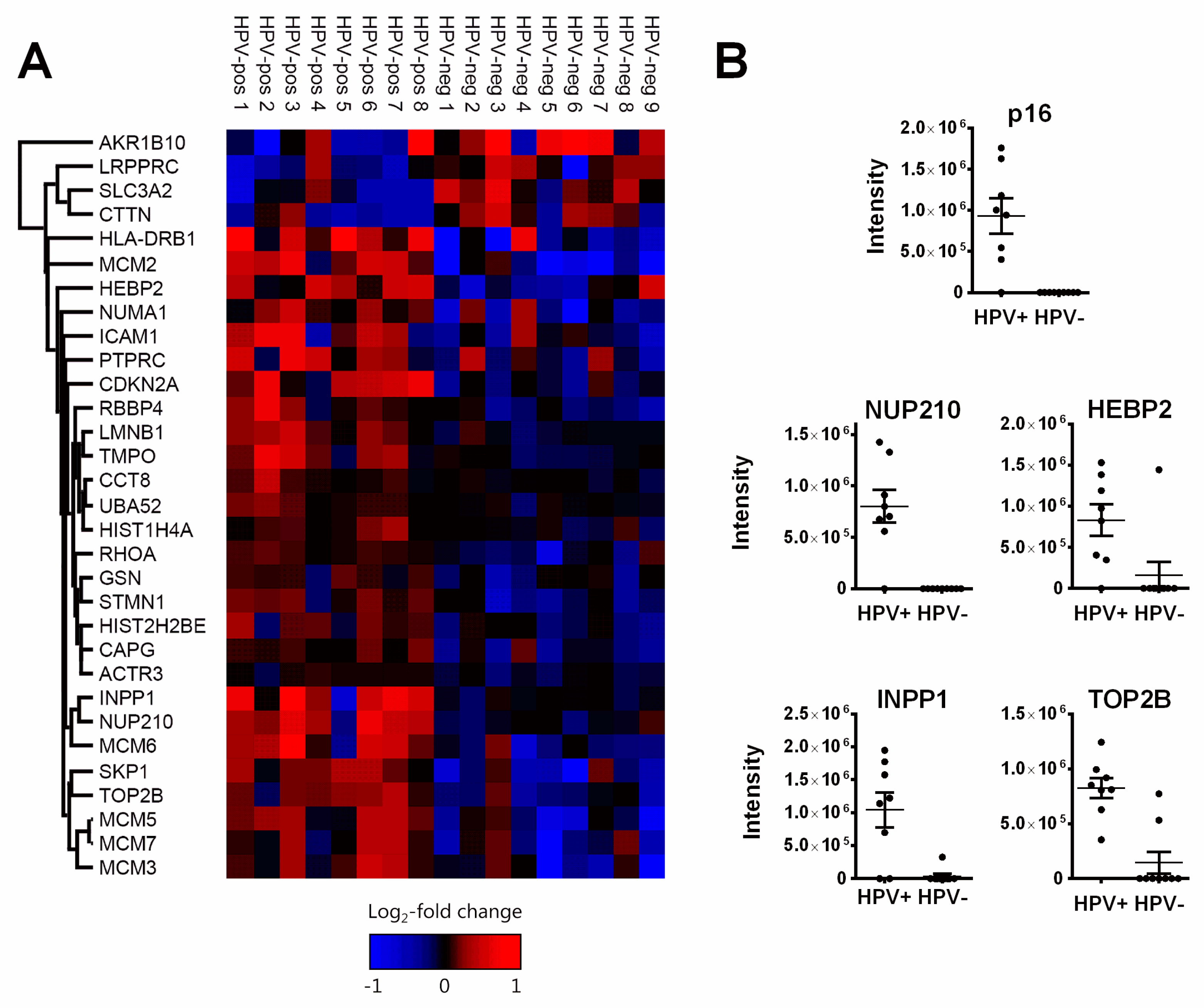
3.1. Pathways and Functions of Identified Proteins
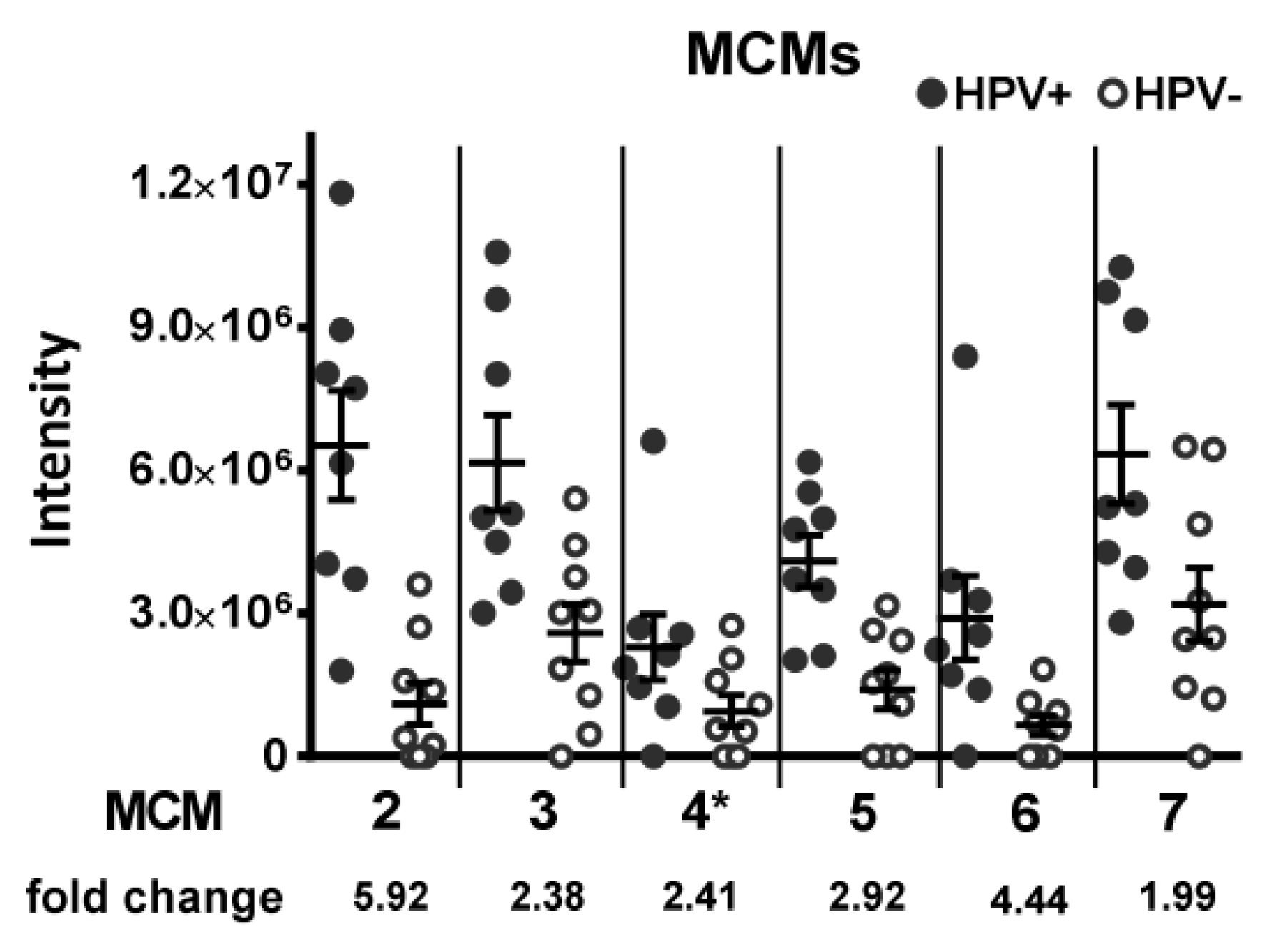
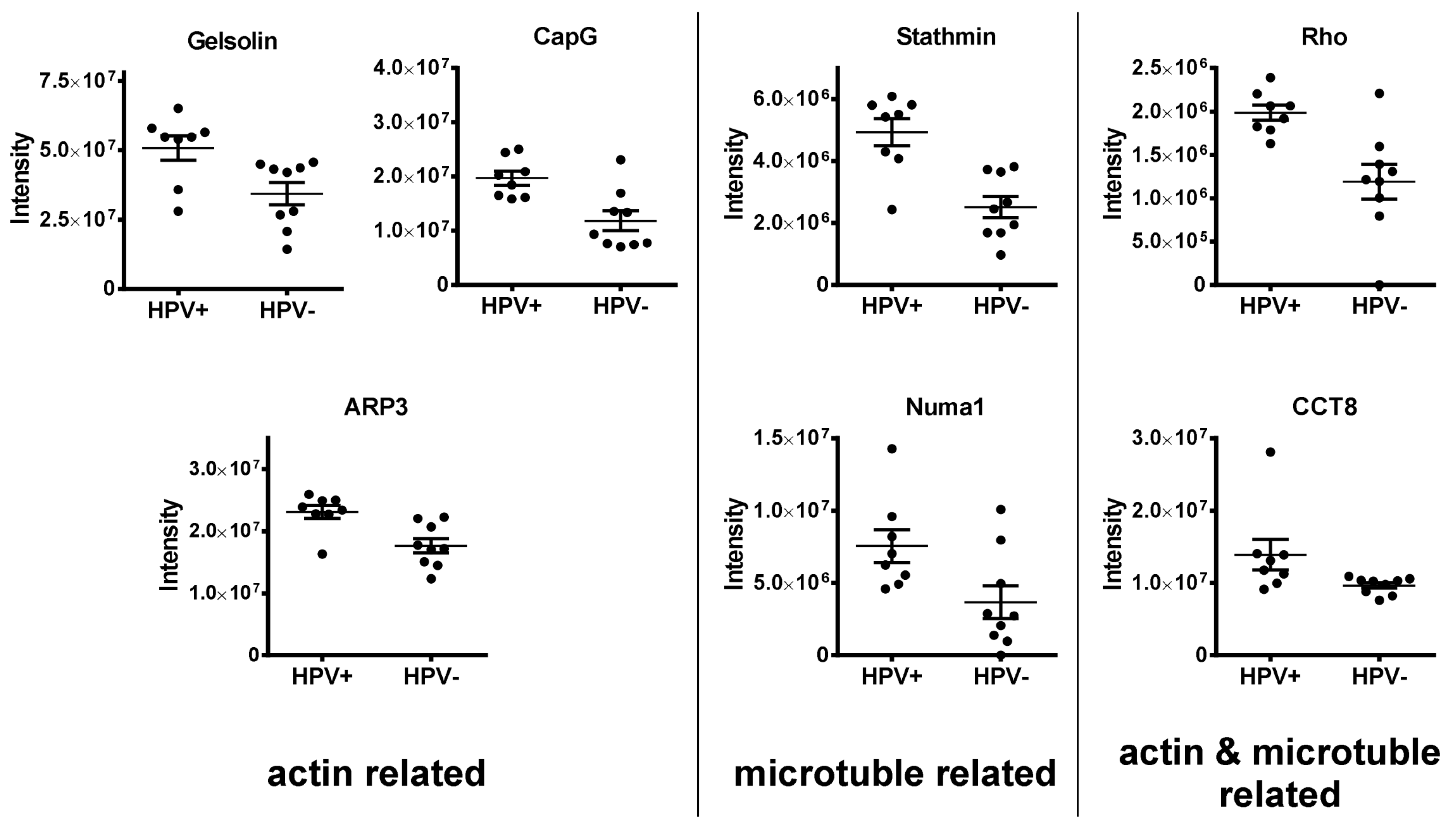
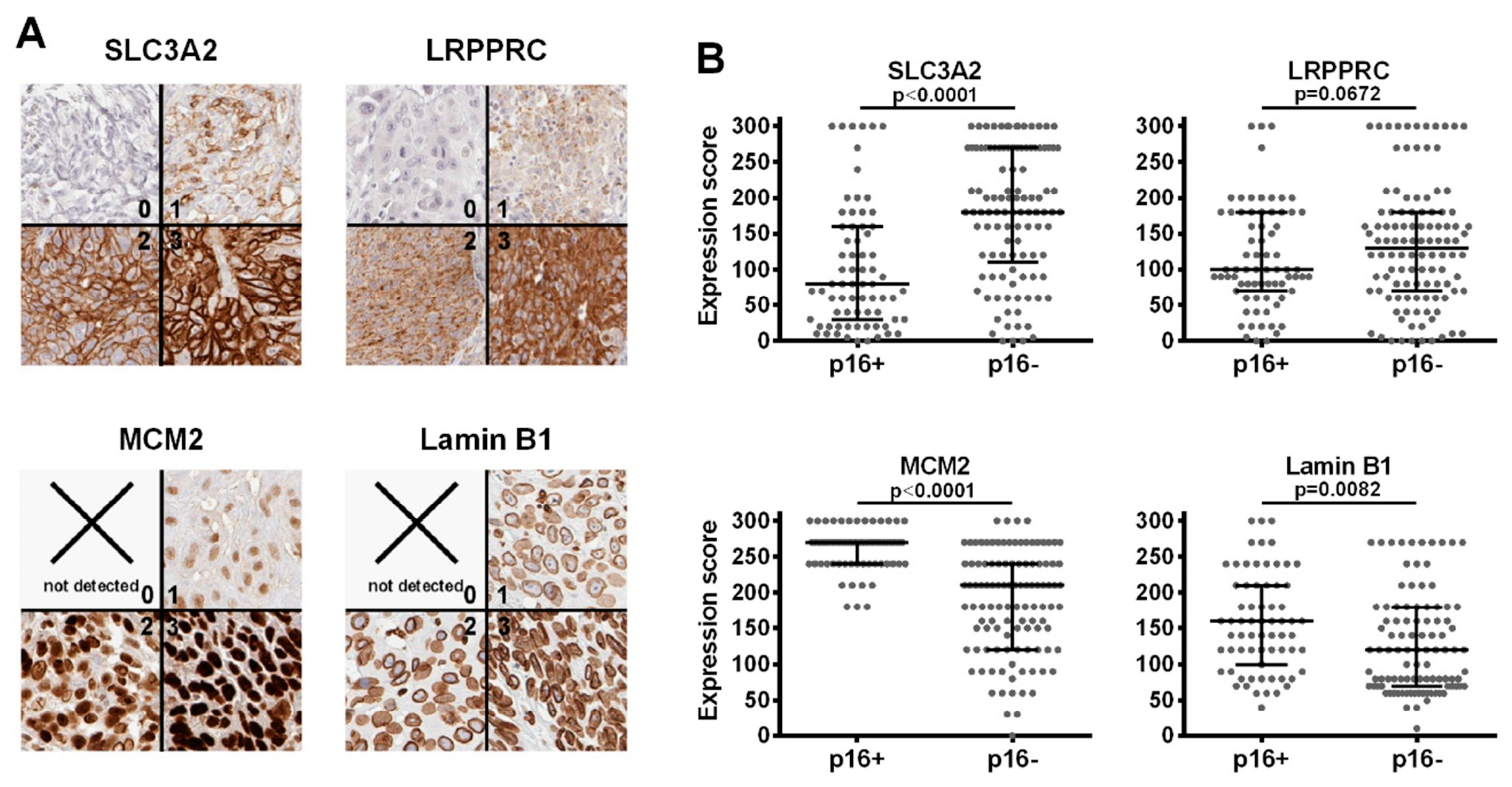
3.1.1. DNA Replication Factors (MCM2/3/5/6/7, RBBP4)
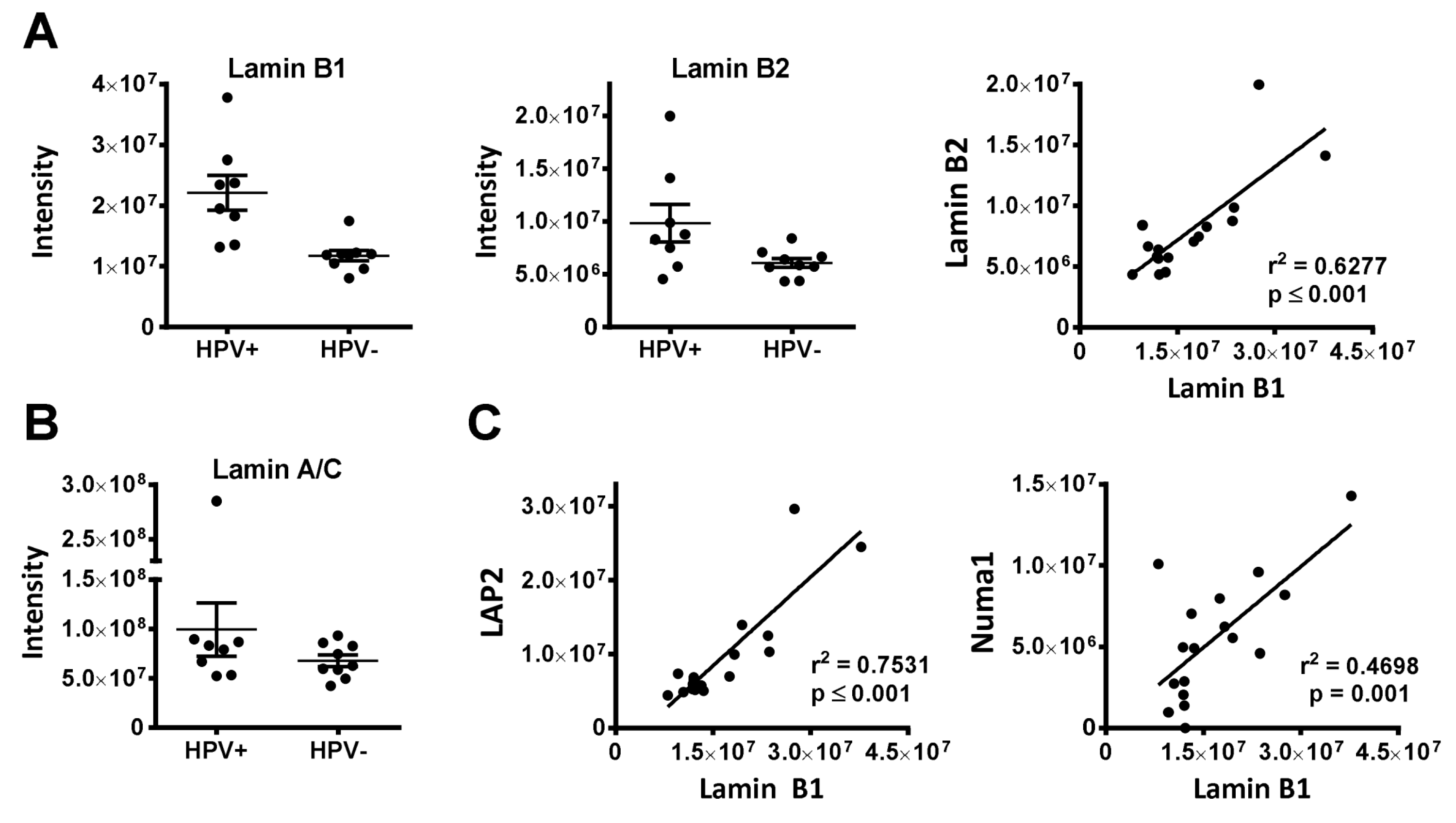
3.1.2. Nuclear Architecture (Lamin B1, LAP2, NUP210, Numa1)
3.1.3. Regulators of the Cytoskeleton (APR3, CAPG, Gelsolin, Stathmin, Numa1, CCT8, RhoA/B/C)
3.2. Immunohistochemistry (IHC)
4. Discussion
4.1. Implications for Tumor Biology
4.2. Limitations of Our Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Conway, D.I.; Brenner, D.R.; McMahon, A.D.; Macpherson, L.M.; Agudo, A.; Ahrens, W.; Bosetti, C.; Brenner, H.; Castellsague, X.; Chen, C.; et al. Estimating and explaining the effect of education and income on head and neck cancer risk: INHANCE consortium pooled analysis of 31 case-control studies from 27 countries. Int. J. Cancer 2015, 136, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Lohaus, F.; Linge, A.; Tinhofer, I.; Budach, V.; Gkika, E.; Stuschke, M.; Balermpas, P.; Rodel, C.; Avlar, M.; Grosu, A.L.; et al. HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: Results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 113, 317–323. [Google Scholar] [CrossRef]
- Lassen, P. The role of Human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010, 95, 371–380. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Huang, S.H.; Perez-Ordonez, B.; Massey, C.; Siu, L.L.; Weinreb, I.; Hope, A.; Kim, J.; Bayley, A.J.; Cummings, B.; et al. Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012, 103, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Balermpas, P.; Michel, Y.; Wagenblast, J.; Seitz, O.; Weiss, C.; Rodel, F.; Rodel, C.; Fokas, E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br. J. Cancer 2014, 110, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Rodel, F.; Martin, D.; Balermpas, P.; Wieland, U.; Winkelmann, R.; Riekmann, T.; Falk, S.; Rodel, C.; Fokas, E. Modulation of radiation sensitivity and antitumor immunity by viral pathogenic factors: Implications for radio-immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.P.; Ma, W.; Santegoets, S.; Goedemans, R.; Ehsan, I.; Jordanova, E.S.; van Ham, V.J.; van Unen, V.; Koning, F.; van Egmond, S.I.; et al. Intratumoral HPV16-Specific T Cells Constitute a Type I-Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin. Cancer Res. 2018, 24, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Gottgens, E.L.; Ostheimer, C.; Span, P.N.; Bussink, J.; Hammond, E.M. HPV, hypoxia and radiation response in head and neck cancer. Br. J. Radiol. 2018. [Google Scholar] [CrossRef]
- Kimple, R.J.; Smith, M.A.; Blitzer, G.C.; Torres, A.D.; Martin, J.A.; Yang, R.Z.; Peet, C.R.; Lorenz, L.D.; Nickel, K.P.; Klingelhutz, A.J.; et al. Enhanced Radiation Sensitivity in HPV-Positive Head and Neck Cancer. Cancer Res. 2013, 73, 4791–4800. [Google Scholar] [CrossRef]
- Rieckmann, T.; Tribius, S.; Grob, T.J.; Meyer, F.; Busch, C.J.; Petersen, C.; Dikomey, E.; Kriegs, M. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 107, 242–246. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. MCP 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. 1D and 2D annotation enrichment: A statistical method integrating quantitative proteomics with complementary high-throughput data. BMC Bioinform. 2012, 13, e12. [Google Scholar] [CrossRef]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Bubendorf, L.; Kononen, J.; Koivisto, P.; Schraml, P.; Moch, H.; Gasser, T.C.; Willi, N.; Mihatsch, M.J.; Sauter, G.; Kallioniemi, O.P. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999, 59, 803–806. [Google Scholar]
- Dancau, A.M.; Simon, R.; Mirlacher, M.; Sauter, G. Tissue microarrays. Methods Mol. Biol. 2010, 576, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version .84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 25 May 2020).
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Sepiashvili, L.; Waggott, D.; Hui, A.; Shi, W.; Su, S.; Ignatchenko, A.; Ignatchenko, V.; Laureano, M.; Huang, S.H.; Xu, W.; et al. Integrated omic analysis of oropharyngeal carcinomas reveals human papillomavirus (HPV)-dependent regulation of the activator protein 1 (AP-1) pathway. Mol. Cell. Proteom. 2014, 13, 3572–3584. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Chakravarthy, A.R.; Walter, V.; Masterson, L.; Feber, A.; Jay, A.; Weinberger, P.M.; McIndoe, R.A.; Forde, C.T.; Chester, K.; et al. Frequent HPV-independent p16/INK4A overexpression in head and neck cancer. Oral Oncol. 2018, 83, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Leman, A.R.; Noguchi, E. The replication fork: Understanding the eukaryotic replication machinery and the challenges to genome duplication. Genes 2013, 4, 1–32. [Google Scholar] [CrossRef]
- Chuang, C.H.; Yang, D.; Bai, G.; Freeland, A.; Pruitt, S.C.; Schimenti, J.C. Post-transcriptional homeostasis and regulation of MCM2-7 in mammalian cells. Nucleic Acids Res. 2012, 40, 4914–4924. [Google Scholar] [CrossRef]
- Ibarra, A.; Schwob, E.; Mendez, J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc. Natl. Acad. Sci. USA 2008, 105, 8956–8961. [Google Scholar] [CrossRef]
- Smith, S.; Stillman, B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 1989, 58, 15–25. [Google Scholar] [CrossRef]
- Verreault, A.; Kaufman, P.D.; Kobayashi, R.; Stillman, B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell 1996, 87, 95–104. [Google Scholar] [CrossRef]
- Radulescu, A.E.; Cleveland, D.W. NuMA after 30 years: The matrix revisited. Trends Cell Biol. 2010, 20, 214–222. [Google Scholar] [CrossRef]
- Abad, P.C.; Lewis, J.; Mian, I.S.; Knowles, D.W.; Sturgis, J.; Badve, S.; Xie, J.; Lelievre, S.A. NuMA influences higher order chromatin organization in human mammary epithelium. Mol. Biol. Cell 2007, 18, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Chandramouly, G.; Abad, P.C.; Knowles, D.W.; Lelievre, S.A. The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J. Cell Sci. 2007, 120, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Salvador Moreno, N.; Liu, J.; Haas, K.M.; Parker, L.L.; Chakraborty, C.; Kron, S.J.; Hodges, K.; Miller, L.D.; Langefeld, C.; Robinson, P.J.; et al. The nuclear structural protein NuMA is a negative regulator of 53BP1 in DNA double-strand break repair. Nucleic Acids Res. 2019, 47, 2703–2715. [Google Scholar] [CrossRef] [PubMed]
- Vidi, P.A.; Liu, J.; Salles, D.; Jayaraman, S.; Dorfman, G.; Gray, M.; Abad, P.; Moghe, P.V.; Irudayaraj, J.M.; Wiesmuller, L.; et al. NuMA promotes homologous recombination repair by regulating the accumulation of the ISWI ATPase SNF2h at DNA breaks. Nucleic Acids Res. 2014, 42, 6365–6379. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.L.; Munger, K. Human papillomavirus E7 protein deregulates mitosis via an association with nuclear mitotic apparatus protein 1. J. Virol. 2009, 83, 1700–1707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oswald, E.; Kirschberg, M.; Aubin, F.; Alonso, A.; Hufbauer, M.; Akgul, B.; Auvinen, E. BetaHPV E6 and E7 colocalize with NuMa in dividing keratinocytes. Virus Genes 2019. [Google Scholar] [CrossRef]
- Chow, K.H.; Factor, R.E.; Ullman, K.S. The nuclear envelope environment and its cancer connections. Nat. Rev. Cancer 2012, 12, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Krishnamoorthy, V.; Parnaik, V.K. E3 ubiquitin ligase RNF123 targets lamin B1 and lamin-binding proteins. FEBS J. 2018, 285, 2243–2262. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Rustum, C.; Hallberg, E. Dynamic properties of nuclear pore complex proteins in gp210 deficient cells. FEBS Lett. 2004, 572, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Scheele, S.; Ekblom, P. Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp. Cell Res. 2004, 292, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Stavru, F.; Nautrup-Pedersen, G.; Cordes, V.C.; Gorlich, D. Nuclear pore complex assembly and maintenance in POM121- and gp210-deficient cells. J. Cell Biol. 2006, 173, 477–483. [Google Scholar] [CrossRef]
- de Las Heras, J.I.; Batrakou, D.G.; Schirmer, E.C. Cancer biology and the nuclear envelope: A convoluted relationship. Semin. Cancer Biol. 2013, 23, 125–137. [Google Scholar] [CrossRef]
- Borlido, J.; Sakuma, S.; Raices, M.; Carrette, F.; Tinoco, R.; Bradley, L.M.; D’Angelo, M.A. Nuclear pore complex-mediated modulation of TCR signaling is required for naive CD4(+) T cell homeostasis. Nat. Immunol. 2018, 19, 594–605. [Google Scholar] [CrossRef]
- Gomez-Cavazos, J.S.; Hetzer, M.W. The nucleoporin gp210/Nup210 controls muscle differentiation by regulating nuclear envelope/ER homeostasis. J. Cell Biol. 2015, 208, 671–681. [Google Scholar] [CrossRef]
- Hohmann, T.; Dehghani, F. The Cytoskeleton-A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef]
- Pollard, T.D. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 451–477. [Google Scholar] [CrossRef]
- Nag, S.; Larsson, M.; Robinson, R.C.; Burtnick, L.D. Gelsolin: The tail of a molecular gymnast. Cytoskeleton 2013, 70, 360–384. [Google Scholar] [CrossRef]
- Silacci, P.; Mazzolai, L.; Gauci, C.; Stergiopulos, N.; Yin, H.L.; Hayoz, D. Gelsolin superfamily proteins: Key regulators of cellular functions. Cell. Mol. Life Sci. 2004, 61, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, L.; Curmi, P.; Sobel, A.; Pantaloni, D.; Carlier, M.F. Stathmin: A tubulin-sequestering protein which forms a ternary T2S complex with two tubulin molecules. Biochemistry 1997, 36, 10817–10821. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.D.; Ridley, A.J. Rho GTPase signaling complexes in cell migration and invasion. J. Cell Biol. 2018, 217, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, H.; Farr, G.W.; Sternlicht, M.L.; Driscoll, J.K.; Willison, K.; Yaffe, M.B. The t-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 9422–9426. [Google Scholar] [CrossRef]
- Digomann, D.; Kurth, I.; Tyutyunnykova, A.; Chen, O.; Lock, S.; Gorodetska, I.; Peitzsch, C.; Skvortsova, I.I.; Negro, G.; Aschenbrenner, B.; et al. The CD98 Heavy Chain Is a Marker and Regulator of Head and Neck Squamous Cell Carcinoma Radiosensitivity. Clin. Cancer Res. 2019, 25, 3152–3163. [Google Scholar] [CrossRef]
- Feral, C.C.; Nishiya, N.; Fenczik, C.A.; Stuhlmann, H.; Slepak, M.; Ginsberg, M.H. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 355–360. [Google Scholar] [CrossRef]
- Cantor, J.M.; Ginsberg, M.H. CD98 at the crossroads of adaptive immunity and cancer. J. Cell Sci. 2012, 125, 1373–1382. [Google Scholar] [CrossRef]
- de la Ballina, L.R.; Cano-Crespo, S.; Gonzalez-Munoz, E.; Bial, S.; Estrach, S.; Cailleteau, L.; Tissot, F.; Daniel, H.; Zorzano, A.; Ginsberg, M.H.; et al. Amino Acid Transport Associated to Cluster of Differentiation 98 Heavy Chain (CD98hc) Is at the Cross-road of Oxidative Stress and Amino Acid Availability. J. Biol. Chem. 2016, 291, 9700–9711. [Google Scholar] [CrossRef]
- El Ansari, R.; Craze, M.L.; Diez-Rodriguez, M.; Nolan, C.C.; Ellis, I.O.; Rakha, E.A.; Green, A.R. The multifunctional solute carrier 3A2 (SLC3A2) confers a poor prognosis in the highly proliferative breast cancer subtypes. Br. J. Cancer 2018, 118, 1115–1122. [Google Scholar] [CrossRef]
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Kawashima, O.; Kamide, Y.; Ishizuka, T.; et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in surgically resectable stage III non-small cell lung cancer. Exp. Med. 2010, 1, 799–808. [Google Scholar] [CrossRef]
- Martens-de Kemp, S.R.; Brink, A.; Stigter-van Walsum, M.; Damen, J.M.; Rustenburg, F.; Wu, T.; van Wieringen, W.N.; Schuurhuis, G.J.; Braakhuis, B.J.; Slijper, M.; et al. CD98 marks a subpopulation of head and neck squamous cell carcinoma cells with stem cell properties. Stem. Cell Res. 2013, 10, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Rietbergen, M.M.; Martens-de Kemp, S.R.; Bloemena, E.; Witte, B.I.; Brink, A.; Baatenburg de Jong, R.J.; Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. Cancer stem cell enrichment marker CD98: A prognostic factor for survival in patients with human papillomavirus-positive oropharyngeal cancer. Eur. J. Cancer 2014, 50, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Linge, A.; Lock, S.; Gudziol, V.; Nowak, A.; Lohaus, F.; von Neubeck, C.; Jutz, M.; Abdollahi, A.; Debus, J.; Tinhofer, I.; et al. Low Cancer Stem Cell Marker Expression and Low Hypoxia Identify Good Prognosis Subgroups in HPV(-) HNSCC after Postoperative Radiochemotherapy: A Multicenter Study of the DKTK-ROG. Clin. Cancer Res. 2016, 22, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Kaira, K.; Shino, M.; Sakakura, K.; Takahashi, K.; Takayasu, Y.; Tominaga, H.; Oriuchi, N.; Nikkuni, O.; Suzuki, M.; et al. CD98 as a novel prognostic indicator for patients with stage III/IV hypopharyngeal squamous cell carcinoma. Head Neck 2015, 37, 1569–1574. [Google Scholar] [CrossRef]
- van der Heijden, M.; Essers, P.B.M.; de Jong, M.C.; de Roest, R.H.; Sanduleanu, S.; Verhagen, C.V.M.; Hamming-Vrieze, O.; Hoebers, F.; Lambin, P.; Bartelink, H.; et al. Biological Determinants of Chemo-Radiotherapy Response in HPV-Negative Head and Neck Cancer: A Multicentric External Validation. Front. Oncol. 2019, 9, 1470. [Google Scholar] [CrossRef]
- Siira, S.J.; Spahr, H.; Shearwood, A.J.; Ruzzenente, B.; Larsson, N.G.; Rackham, O.; Filipovska, A. LRPPRC-mediated folding of the mitochondrial transcriptome. Nat. Commun. 2017, 8, 1532. [Google Scholar] [CrossRef]
- Cui, J.; Wang, L.; Ren, X.; Zhang, Y.; Zhang, H. LRPPRC: A Multifunctional Protein Involved in Energy Metabolism and Human Disease. Front. Physiol. 2019, 10, 595. [Google Scholar] [CrossRef]
- Refolo, G.; Ciccosanti, F.; Di Rienzo, M.; Basulto Perdomo, A.; Romani, M.; Alonzi, T.; Tripodi, M.; Ippolito, G.; Piacentini, M.; Fimia, G.M. Negative Regulation of Mitochondrial Antiviral Signaling Protein-Mediated Antiviral Signaling by the Mitochondrial Protein LRPPRC During Hepatitis C Virus Infection. Hepatology 2019, 69, 34–50. [Google Scholar] [CrossRef]
- Chiang, C.; Pauli, E.K.; Biryukov, J.; Feister, K.F.; Meng, M.; White, E.A.; Munger, K.; Howley, P.M.; Meyers, C.; Gack, M.U. The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Slebos, R.J.; Jehmlich, N.; Brown, B.; Yin, Z.; Chung, C.H.; Yarbrough, W.G.; Liebler, D.C. Proteomic analysis of oropharyngeal carcinomas reveals novel HPV-associated biological pathways. Int. J. Cancer 2013, 132, 568–579. [Google Scholar] [CrossRef]
- Lohavanichbutr, P.; Houck, J.; Fan, W.; Yueh, B.; Mendez, E.; Futran, N.; Doody, D.R.; Upton, M.P.; Farwell, D.G.; Schwartz, S.M.; et al. Genomewide gene expression profiles of HPV-positive and HPV-negative oropharyngeal cancer: Potential implications for treatment choices. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 180–188. [Google Scholar] [CrossRef]
- Pyeon, D.; Newton, M.A.; Lambert, P.F.; den Boon, J.A.; Sengupta, S.; Marsit, C.J.; Woodworth, C.D.; Connor, J.P.; Haugen, T.H.; Smith, E.M.; et al. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res. 2007, 67, 4605–4619. [Google Scholar] [CrossRef] [PubMed]
- Slebos, R.J.; Yi, Y.; Ely, K.; Carter, J.; Evjen, A.; Zhang, X.; Shyr, Y.; Murphy, B.M.; Cmelak, A.J.; Burkey, B.B.; et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin. Cancer Res. 2006, 12, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Iwanaga, R.; Nakamura, M.; Ikeda, M.; Yabuta, N.; Tsuruga, H.; Nojima, H. Cell growth-regulated expression of mammalian MCM5 and MCM6 genes mediated by the transcription factor E2F. Oncogene 1999, 18, 2299–2309. [Google Scholar] [CrossRef]
- Parise, P.; Finocchiaro, G.; Masciadri, B.; Quarto, M.; Francois, S.; Mancuso, F.; Muller, H. Lap2alpha expression is controlled by E2F and deregulated in various human tumors. Cell Cycle 2006, 5, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Dorner, D.; Vlcek, S.; Foeger, N.; Gajewski, A.; Makolm, C.; Gotzmann, J.; Hutchison, C.J.; Foisner, R. Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway. J. Cell Biol. 2006, 173, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.W.; Wang, Y.C.; Hollingsworth, R.E., Jr.; Jones, D.; Ling, N.; Lee, E.Y. A retinoblastoma-binding protein related to a negative regulator of Ras in yeast. Nature 1993, 364, 648–652. [Google Scholar] [CrossRef]
- Schultz, L.E.; Haltom, J.A.; Almeida, M.P.; Wierson, W.A.; Solin, S.L.; Weiss, T.J.; Helmer, J.A.; Sandquist, E.J.; Shive, H.R.; McGrail, M. Epigenetic regulators Rbbp4 and Hdac1 are overexpressed in a zebrafish model of RB1 embryonal brain tumor, and are required for neural progenitor survival and proliferation. Dis. Models Mech. 2018, 11. [Google Scholar] [CrossRef]
- Deng, R.; Hao, J.; Han, W.; Ni, Y.; Huang, X.; Hu, Q. Gelsolin regulates proliferation, apoptosis, migration and invasion in human oral carcinoma cells. Oncol. Lett. 2015, 9, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Ha, Y.J.; Tak, K.H.; Roh, S.A.; Kwon, Y.H.; Kim, C.W.; Yoon, Y.S.; Lee, J.L.; Park, Y.; Kim, S.K.; et al. Opposite functions of GSN and OAS2 on colorectal cancer metastasis, mediating perineural and lymphovascular invasion, respectively. PLoS ONE 2018, 13, e0202856. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.J.; Wu, T.I.; Huang, Y.H.; Chang, T.C.; Wang, C.S.; Tsai, M.M.; Hsu, C.Y.; Tsai, M.H.; Lai, C.H.; Lin, K.H. Overexpression of gelsolin in human cervical carcinoma and its clinicopathological significance. Gynecol. Oncol. 2011, 120, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mann, D.; Sinha, U.K.; Kokot, N.C. The molecular mechanisms of increased radiosensitivity of HPV-positive oropharyngeal squamous cell carcinoma (OPSCC): An extensive review. J. Otolaryngol. Head Neck Surg. 2018, 47, 59. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.Z.; He, J.Z.; Wu, Z.Y.; Ji, X.; Chen, L.Q.; Xu, X.E.; Liao, L.D.; Wu, J.Y.; Li, E.M.; Xu, L.Y. Overexpression of Stathmin 1 correlates with poor prognosis and promotes cell migration and proliferation in oesophageal squamous cell carcinoma. Oncol. Rep. 2017, 38, 3608–3618. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; He, X.; Huang, Q.; Liu, X.; Sun, G.; Guo, J.; Yuan, D.; Yang, L.; Ban, N.; Fan, S.; et al. Overexpression of CCT8 and its significance for tumor cell proliferation, migration and invasion in glioma. Pathol. Res. Pract. 2015, 211, 717–725. [Google Scholar] [CrossRef]
- Van Impe, K.; Bethuyne, J.; Cool, S.; Impens, F.; Ruano-Gallego, D.; De Wever, O.; Vanloo, B.; Van Troys, M.; Lambein, K.; Boucherie, C.; et al. A nanobody targeting the F-actin capping protein CapG restrains breast cancer metastasis. Breast Cancer Res. 2013, 15, R116. [Google Scholar] [CrossRef]
- Yang, X.; Ren, H.; Shao, Y.; Sun, Y.; Zhang, L.; Li, H.; Zhang, X.; Yang, X.; Yu, W.; Fu, J. Chaperonin-containing Tcomplex protein 1 subunit 8 promotes cell migration and invasion in human esophageal squamous cell carcinoma by regulating alpha-actin and beta-tubulin expression. Int. J. Oncol. 2018, 52, 2021–2030. [Google Scholar] [CrossRef]
- Yurong, L.; Biaoxue, R.; Wei, L.; Zongjuan, M.; Hongyang, S.; Ping, F.; Wenlong, G.; Shuanying, Y.; Zongfang, L. Stathmin overexpression is associated with growth, invasion and metastasis of lung adenocarcinoma. Oncotarget 2017, 8, 26000–26012. [Google Scholar] [CrossRef]
- Mileo, A.M.; Abbruzzese, C.; Vico, C.; Bellacchio, E.; Matarrese, P.; Ascione, B.; Federico, A.; Della Bianca, S.; Mattarocci, S.; Malorni, W.; et al. The human papillomavirus-16 E7 oncoprotein exerts antiapoptotic effects via its physical interaction with the actin-binding protein gelsolin. Carcinogenesis 2013, 34, 2424–2433. [Google Scholar] [CrossRef][Green Version]
- Matarrese, P.; Abbruzzese, C.; Mileo, A.M.; Vona, R.; Ascione, B.; Visca, P.; Rollo, F.; Benevolo, M.; Malorni, W.; Paggi, M.G. Interaction between the human papillomavirus 16 E7 oncoprotein and gelsolin ignites cancer cell motility and invasiveness. Oncotarget 2016, 7, 50972–50985. [Google Scholar] [CrossRef][Green Version]
- Weed, S.A.; Karginov, A.V.; Schafer, D.A.; Weaver, A.M.; Kinley, A.W.; Cooper, J.A.; Parsons, J.T. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 2000, 151, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Bracken, A.P.; Brien, G.L.; Verrijzer, C.P. Dangerous liaisons: Interplay between SWI/SNF, NuRD, and Polycomb in chromatin regulation and cancer. Genes Dev. 2019, 33, 936–959. [Google Scholar] [CrossRef]
- Comet, I.; Riising, E.M.; Leblanc, B.; Helin, K. Maintaining cell identity: PRC2-mediated regulation of transcription and cancer. Nat. Rev. Cancer 2016, 16, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Eke, I.; Deuse, Y.; Hehlgans, S.; Gurtner, K.; Krause, M.; Baumann, M.; Shevchenko, A.; Sandfort, V.; Cordes, N. β(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J. Clin. Investig. 2012, 122, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Torres-Roca, J.F.; Eschrich, S.; Zhao, H.; Bloom, G.; Sung, J.; McCarthy, S.; Cantor, A.B.; Scuto, A.; Li, C.; Zhang, S.; et al. Prediction of radiation sensitivity using a gene expression classifier. Cancer Res. 2005, 65, 7169–7176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Tang, W.; Wei, F.; Wang, H.; Liu, J.; Lu, Y.; Cheng, Y.; Bai, X.; Yu, X.; Zhao, W. Radiation-inducible protein RbAp48 contributes to radiosensitivity of cervical cancer cells. Gynecol. Oncol. 2013, 130, 601–608. [Google Scholar] [CrossRef]
- Elia, A.E.; Boardman, A.P.; Wang, D.C.; Huttlin, E.L.; Everley, R.A.; Dephoure, N.; Zhou, C.; Koren, I.; Gygi, S.P.; Elledge, S.J. Quantitative Proteomic Atlas of Ubiquitination and Acetylation in the DNA Damage Response. Mol. Cell 2015, 59, 867–881. [Google Scholar] [CrossRef]
- Li, J.M.; Jin, J. CRL Ubiquitin Ligases and DNA Damage Response. Front. Oncol. 2012, 2, 29. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Zhang, L.; Wu, C.Y.; Rezaeian, A.H.; Chan, C.H.; Li, J.M.; Wang, J.; Gao, Y.; Han, F.; et al. Skp2 E3 ligase integrates ATM activation and homologous recombination repair by ubiquitinating NBS1. Mol. Cell 2012, 46, 351–361. [Google Scholar] [CrossRef]
- Zhang, Q.; Karnak, D.; Tan, M.; Lawrence, T.S.; Morgan, M.A.; Sun, Y. FBXW7 Facilitates Nonhomologous End-Joining via K63-Linked Polyubiquitylation of XRCC4. Mol. Cell 2016, 61, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Elfadl, D.; Hodgkinson, V.C.; Long, E.D.; Scaife, L.; Drew, P.J.; Lind, M.J.; Cawkwell, L. A pilot study to investigate the role of the 26S proteasome in radiotherapy resistance and loco-regional recurrence following breast conserving therapy for early breast cancer. Breast 2011, 20, 334–337. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Yang, H.; Wu, L.; He, Z.; Zhou, S.; Zhao, H.; Fu, Z.; Zhou, F.; Zhou, Y. UBE2D3 gene overexpression increases radiosensitivity of EC109 esophageal cancer cells in vitro and in vivo. Oncotarget 2016, 7, 32543–32553. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.J.; Tak, K.H.; Kim, C.W.; Roh, S.A.; Choi, E.K.; Cho, D.H.; Kim, J.H.; Kim, S.K.; Kim, S.Y.; Kim, Y.S.; et al. PSMB8 as a Candidate Marker of Responsiveness to Preoperative Radiation Therapy in Rectal Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Qutob, O.; Watson, M.B.; Beavis, A.W.; Potts, D.; Welham, K.J.; Garimella, V.; Lind, M.J.; Drew, P.J.; Cawkwell, L. Proteomic identification of putative biomarkers of radiotherapy resistance: A possible role for the 26S proteasome? Neoplasia 2009, 11, 1194–1207. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Wu, L.; Ke, S.; Wang, W.; Yang, L.; Gao, X.; Fang, H.; Yu, H.; Zhong, Y.; Xie, C.; et al. Downregulation of Ubiquitin-conjugating Enzyme UBE2D3 Promotes Telomere Maintenance and Radioresistance of Eca-109 Human Esophageal Carcinoma Cells. J. Cancer 2016, 7, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.H.; Yun, H.S.; Lee, S.J.; Baek, J.H.; Lee, C.W.; Song, J.Y.; Um, H.D.; Park, J.K.; Kim, J.S.; Park, I.C.; et al. Radiosensitizing effect of PSMC5, a 19S proteasome ATPase, in H460 lung cancer cells. Biochem. Biophys. Res. Commun. 2016, 469, 94–100. [Google Scholar] [CrossRef][Green Version]
- Hurst, V.; Shimada, K.; Gasser, S.M. Nuclear Actin and Actin-Binding Proteins in DNA Repair. Trends Cell Biol. 2019, 29, 462–476. [Google Scholar] [CrossRef]
- Schrank, B.R.; Aparicio, T.; Li, Y.; Chang, W.; Chait, B.T.; Gundersen, G.G.; Gottesman, M.E.; Gautier, J. Nuclear ARP2/3 drives DNA break clustering for homology-directed repair. Nature 2018, 559, 61–66. [Google Scholar] [CrossRef]
- Wheeler, A.P.; Ridley, A.J. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp. Cell Res. 2004, 301, 43–49. [Google Scholar] [CrossRef]

| Cohort | p16-Positive | p16-Negative |
|---|---|---|
| Number of patients | 8 | 9 |
| Age, median (range) (p = 0.6993) | 64.9 (59–76) | 66.8 (53–83) |
| Sex (p = 1) | ||
| Male | 6 | 7 |
| Female | 2 | 2 |
| pT classification (p = 0.8756) | ||
| T1 | 4 | 6 |
| T2 | 2 | 2 |
| T3 | 1 | 1 |
| T4 | 1 | 0 |
| pN classification (p = 0.1316) | ||
| N0 | 0 | 4 |
| N1 | 3 | 1 |
| N2 | 4 | 4 |
| N3 | 1 | 0 |
| TNM stage (7th ed.) (p = 0.2467) | ||
| I | 0 | 2 |
| II | 0 | 2 |
| III | 3 | 1 |
| IV | 5 | 4 |
| ECS (p = 1) | ||
| Pos | 3 | 3 |
| Neg | 5 | 6 |
| smoking (p = 0.2941) | ||
| Yes | 5 | 8 |
| No | 3 | 1 |
| No | Gene (Protein) Names | Boruta Score | p-Value | Log2FC (HPV+/−) | p-Value (Sepiashvili et al.) [29] | Log2FC (HPV+/−) (Sepiashvili et al.) |
|---|---|---|---|---|---|---|
| 1 | MCM2 | 200 | 0.000235 | 2.8231 | 0.000573 | 1.1887 |
| 2 | NUP210 | 200 | 0.000261 | 1.3284 | 0.000764 | 1.5418 |
| 3 | LMNB1 (Lamin B1) | 200 | 0.000651 | 0.8628 | 0.002252 | 0.5517 |
| 4 | TOP2B | 200 | 0.004052 | 1.3653 | 0.007912 | 0.6057 |
| 5 | CDKN2A (p16) | 200 | 0.004598 | 1.3982 | 0.000002 | 3.3464 |
| 6 | RHOA; RHOC; RHOB | 200 | 0.012392 | 0.8633 | 0.6526 (RHOA) | 0.0336 (RHOA) |
| 7 | STMN1; STMN2 (Stathmin; Stathmin-2) | 196 | 0.001886 | 1.0479 | 0.0544 (STMN1); 0.0050 (STMN2) | 0.3838 (STMN1) 1.1807 (STMN2) |
| 8 | ACTR3 (ARP3) | 196 | 0.005275 | 0.4038 | 0.042775 | 0.3757 |
| 9 | UBA52; RPS27A; UBB; UBC | 132 | 0.001915 | 0.4820 | 0.0601 (UBA52); 0.0765 (RPS27A) | 0.3174 (UBA52); 0.3108 (RPS27A) |
| 10 | SKP1 | 72 | 0.001183 | 2.0275 | 0.015037 | 2.1586 |
| 11 | HEBP2 | 46 | 0.056098 | 0.8142 | 0.186598 | 0.4573 |
| 12 | HLA-DRB1; HLA-DRB5 | 40 | 0.002618 | 2.6530 | 0.006311 | 0.8931 |
| 13 | CAPG | 34 | 0.002681 | 0.8428 | 0.000616 | 0.7927 |
| 14 | HIST1H4A | 34 | 0.011118 | 0.5163 | 0.076559 | 0.2950 |
| 15 | HIST2H2BE; HIST1H2BB; HIST1H2BO; HIST1H2BJ; HIST3H2BB (Histone H2B, multiple types) | 24 | 0.010950 | 0.7303 | 0.1806 (HIST1H2BB); 0.1988 (HIST1H2BJ); 0.1244 (HIST3H2BB) | 0.2649 (HIST1H2BB); 0.2754 (HIST1H2BJ); 0.3035 (HIST3H2BB) |
| 16 | INPP1 | 20 | 0.041938 | 1.2062 | n. d. | n. d. |
| 17 | MCM5 | 18 | 0.002374 | 1.5963 | 0.005306 | 1.3743 |
| 18 | LRPPRC | 18 | 0.085066 | −1.1076 | 0.060111 | −0.5621 |
| 19 | MCM6 | 16 | 0.007168 | 1.5859 | 0.000426 | 1.2566 |
| 20 | CCT8 | 14 | 0.022356 | 0.4512 | 0.463460 | 0.1661 |
| 21 | TMPO (LAP2) | 12 | 0.005542 | 1.0096 | 0.016226 | 1.0534 |
| 22 | RBBP4;RBBP7 | 10 | 0.003404 | 1.1455 | 0.0707 (RBBP4) 0.9507 (RBBP7) | −0.4031 (RBBP4) −0.0165 (RBBP7) |
| 23 | SLC3A2 | 10 | 0.006046 | −1.5631 | 0.317565 | −0.4671 |
| 24 | GSN (Gelsolin) | 10 | 0.027809 | 0.6165 | 0.029810 | 0.3432 |
| 25 | CTTN (Cortactin) | 1 | 0.015119 | −1.2674 | 0.000396 | −1.2752 |
| 26 | MCM3 | 0 | 0.014093 | 1.5990 | 0.000023 | 1.3652 |
| 27 | NUMA1 | 0 | 0.016250 | 1.5247 | 0.000340 | 1.1813 |
| 28 | MCM7 | 0 | 0.028008 | 1.2418 | 0.000820 | 1.0757 |
| 29 | AKR1B10 | 0 | 0.035758 | −2.6277 | 0.042775 | −1.0294 |
| 30 | ICAM1 | 0 | 0.037439 | 1.2982 | 0.000168 | 3.5303 |
| 31 | PTPRC | 0 | 0.042037 | 1.0681 | 0.012870 | 1.0362 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurlitzer, M.; Möckelmann, N.; Kriegs, M.; Vens, M.; Omidi, M.; Hoffer, K.; von Bargen, C.; Möller-Koop, C.; Witt, M.; Droste, C.; et al. Mass Spectrometric Comparison of HPV-Positive and HPV-Negative Oropharyngeal Cancer. Cancers 2020, 12, 1531. https://doi.org/10.3390/cancers12061531
Wurlitzer M, Möckelmann N, Kriegs M, Vens M, Omidi M, Hoffer K, von Bargen C, Möller-Koop C, Witt M, Droste C, et al. Mass Spectrometric Comparison of HPV-Positive and HPV-Negative Oropharyngeal Cancer. Cancers. 2020; 12(6):1531. https://doi.org/10.3390/cancers12061531
Chicago/Turabian StyleWurlitzer, Marcus, Nikolaus Möckelmann, Malte Kriegs, Maren Vens, Maryam Omidi, Konstantin Hoffer, Clara von Bargen, Christina Möller-Koop, Melanie Witt, Conrad Droste, and et al. 2020. "Mass Spectrometric Comparison of HPV-Positive and HPV-Negative Oropharyngeal Cancer" Cancers 12, no. 6: 1531. https://doi.org/10.3390/cancers12061531
APA StyleWurlitzer, M., Möckelmann, N., Kriegs, M., Vens, M., Omidi, M., Hoffer, K., von Bargen, C., Möller-Koop, C., Witt, M., Droste, C., Oetting, A., Petersen, H., Busch, C.-J., Münscher, A., Schlüter, H., Clauditz, T. S., & Rieckmann, T. (2020). Mass Spectrometric Comparison of HPV-Positive and HPV-Negative Oropharyngeal Cancer. Cancers, 12(6), 1531. https://doi.org/10.3390/cancers12061531






