Accuracy of Tumour-Associated Circulating Endothelial Cells as a Screening Biomarker for Clinically Significant Prostate Cancer
Abstract
:1. Introduction
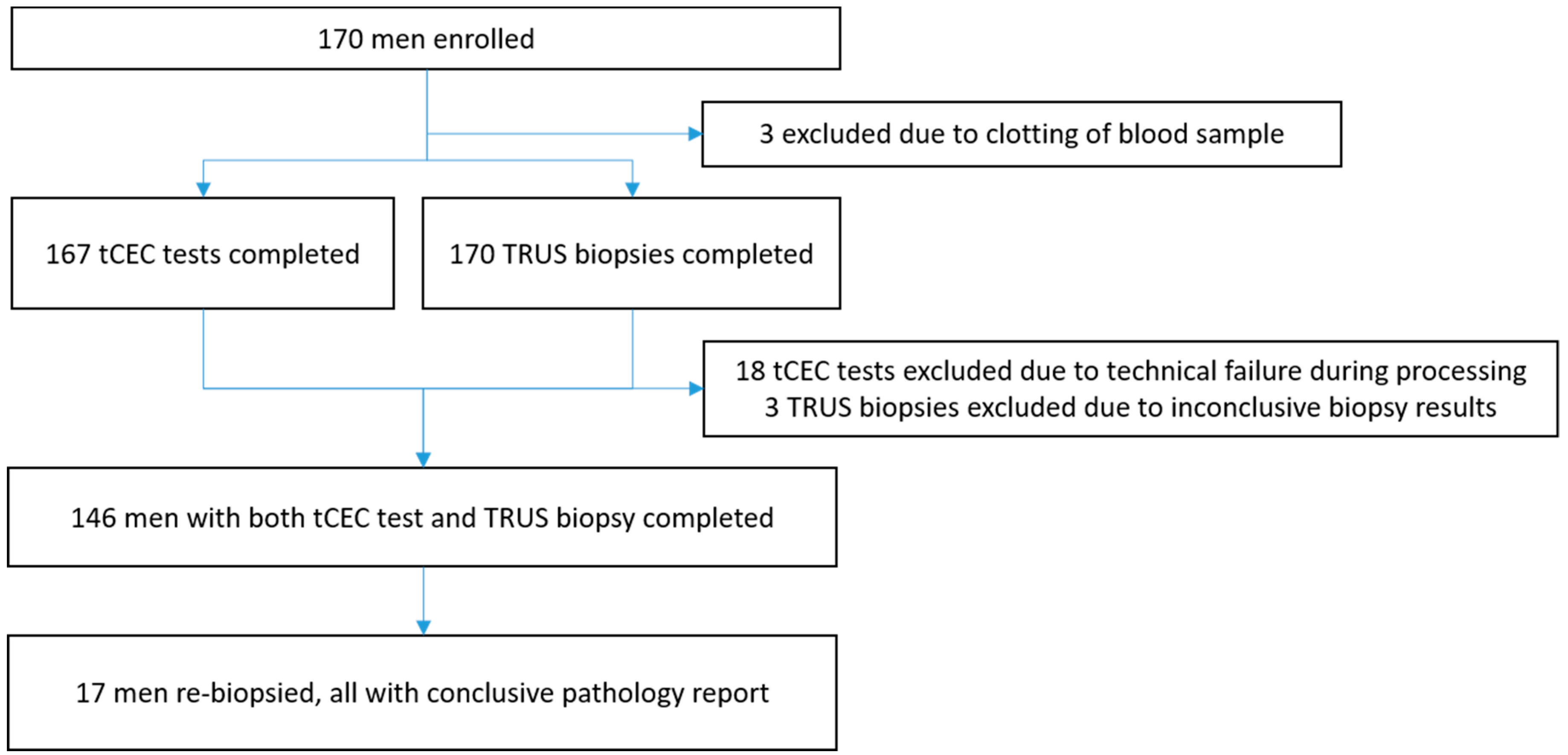
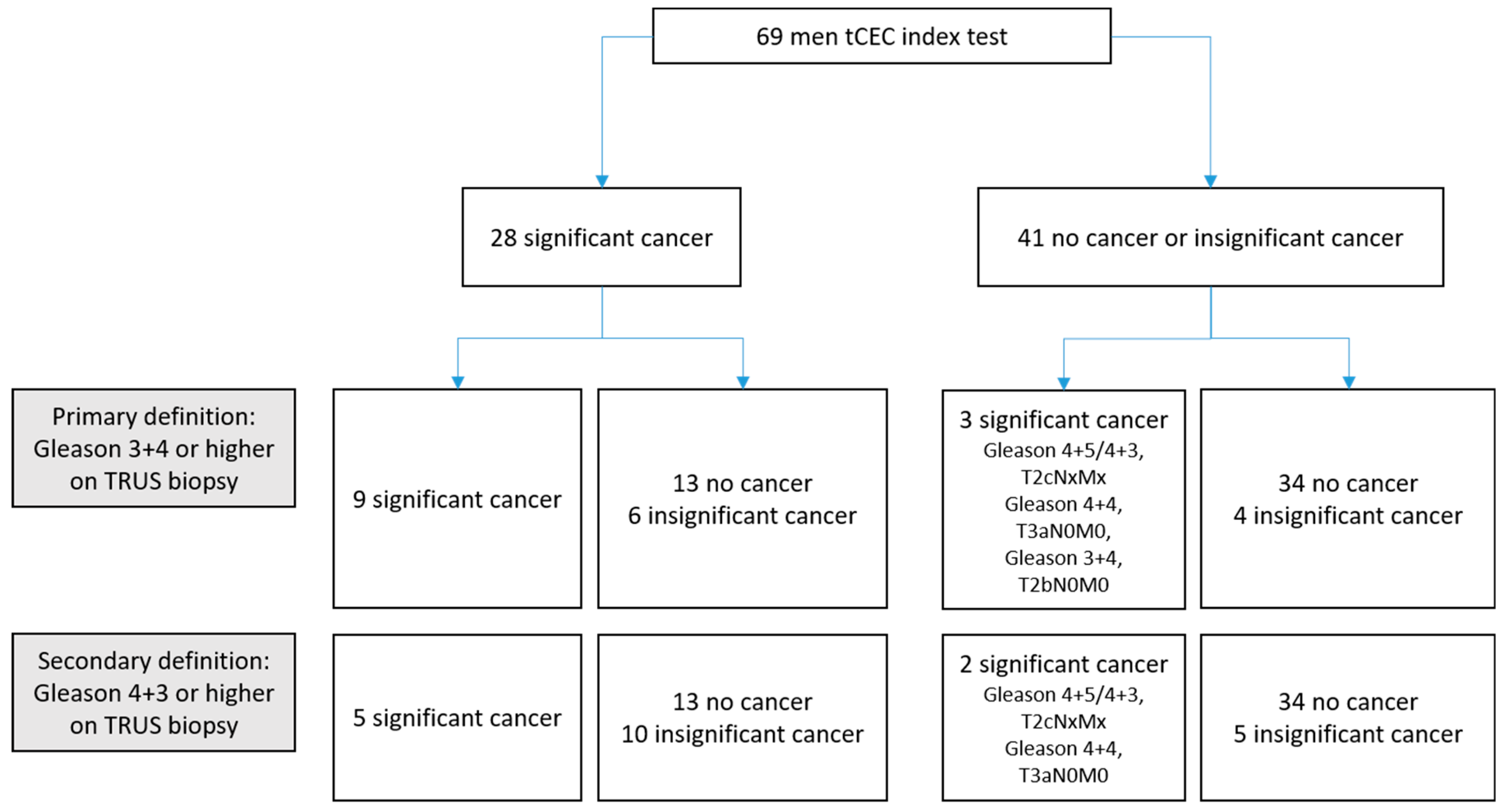
2. Results
3. Discussion
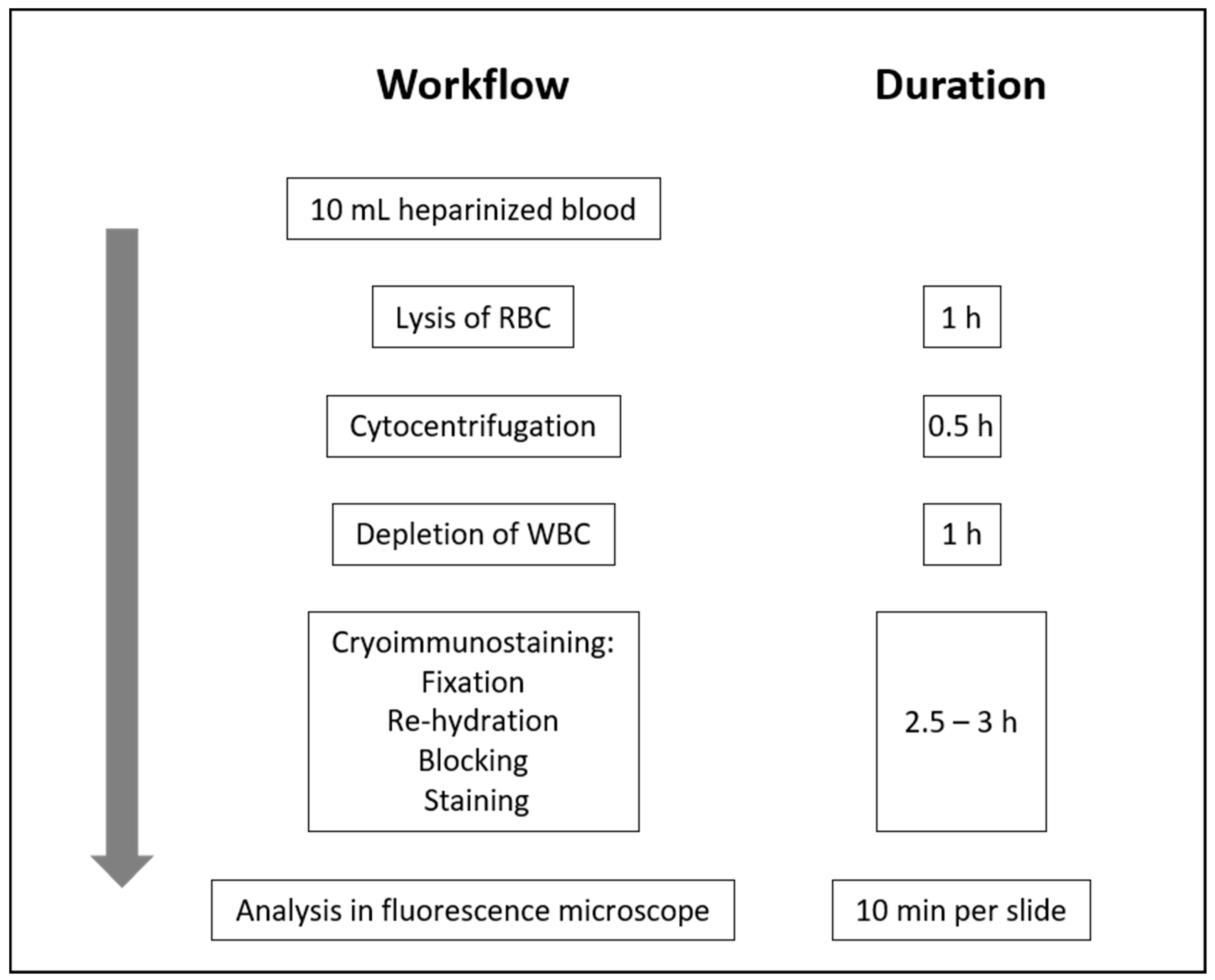
4. Patients and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Cancer Society. Cancer Facts & Figures 2018; American Cancer Society: Atlanta, GA, USA, 2018. [Google Scholar]
- Eckersberger, E.; Finkelstein, J. Screening for prostate cancer: A review of the ERSPC and PLCO trials. Rev. Urol. 2009, 11, 127–133. [Google Scholar] [PubMed]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W.; Kemper, A.R.; et al. Screening for prostate cancer. US Preventive services taskforce recommendation statement. J. Am. Med. Assoc. 2018, 319, 1901–1913. [Google Scholar]
- Saini, S. PSA and beyond: Alternative prostate cancer biomarkers. Cell. Oncol. 2016, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Catalona, W.J. The Prostate Health Index: A new test for the detection of prostate cancer. Ther. Adv. Urol. 2014, 6, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.D.; Dong, Y.; Linder, V.; Zappala, S. Use of the 4Kscore test to predict the risk of aggressive prostate cancer prior to prostate biopsy: Overall cost savings and improved quality of care to the us healthcare system. Rev. Urol. 2017, 19, 1–10. [Google Scholar] [PubMed]
- Soest, R.J.; Tombal, B.; Lolkema, M.P.; Wit, R. Cell-free DNA in Advanced Prostate Cancer: A Biomarker Revolution Under Way? Eur. Urol. 2018, 74, 292–293. [Google Scholar] [CrossRef]
- Sharova, E.G.A.; Marcer, A.; Ruggero, K.; Pinto, F.; Bassi, P.; Zanovello, P.; Zattoni, F.; D’Agostino, D.M.; Iafrate, M.; Ciminale, V. A circulating miRNA assay as a first-line test for prostate cancer screening. Br. J. Cancer 2016, 114, 1362–1366. [Google Scholar] [CrossRef] [Green Version]
- Vickers, A.; Vertosick, E.A.; Sjoberg, D.D.; Roobol, M.J.; Hamdy, F.; Neal, D.; Bjartell, A.; Hugosson, J.; Donovan, J.L.; Villers, A.; et al. Properties of the four kallikrein panel outside the diagnostic grey zone: Meta-analysis of patients with positive digital rectal exam or prostate-specific antigen 10 ng/mL and above. J. Urol. 2017, 197, 607–613. [Google Scholar] [CrossRef]
- Sonn, G.A.; Fan, R.E.; Ghanouni, P.; Wang, N.N.; Brooks, J.D.; Loening, A.M.; Daniel, B.L.; To’o, K.J.; Thong, A.E.; Leppert, J.T. Prostate Magnetic Resonance Imaging Interpretation Varies Substantially Across Radiologists. Eur. Urol. Focus 2018. [Google Scholar] [CrossRef]
- Filson, C.P.; Natarajan, S.; Margolis, D.J.A.; Huang, J.; Lieu, P.; Dorey, F.J.; Reiter, R.E.; Marks, L.S. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 2016, 122, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater, B.A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Cima, I.; Kong, S.L.; Sengupta, D.; Tan, I.B.; Phyo, W.M.; Lee, D.; Hu, M.; Iliescu, C.; Alexander, I.; Goh, W.L.; et al. Tumor-derived circulating endothelial cell clusters in colorectal cancer. Sci. Transl. Med. 2016, 8, 345–389. [Google Scholar] [CrossRef] [PubMed]
- Almog, N.; Klement, G.L. Platelet proteome and tumor dormancy: Can platelets content serve as predictive biomarkers for exit of tumors from dormancy? Cancers 2010, 2, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.C. Tumor endothelial cells. Cold Spring Harb. Perspect. Med. 2012, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, M.C.; Sesterhenn, I.A.; Moul, J.W.; Bauer, J.J.; Connelly, R.R. CD34 immunohistochemical assessment of angiogenesis as a prognostic marker for prostate cancer recurrence after radical prostatectomy. J. Urol. 2006, 160, 459–465. [Google Scholar] [CrossRef]
- Taille, M.D.A.; Bagiella, M.D.E.; Sharir, M.D.S.; Rubin, M.D.M.A.; Burchardt, M.D.T.; Ennis, M.D.R.D.; Buttyan, M.D.R.; Katz, M.D.A.E.; Olsson, M.D.C.A. Microvessel density as a predictor of PSA recurrence after radical prostatectomy. A comparison of CD34 and CD31. Am. J. Clin. Pathol. 2000, 113, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Bono, A.; Celato, N.; Cova, V.; Salvadore, M.; Chinetti, S.; Novario, R. Microvessel density in prostate carcinoma. Prostate Cancer Prostatic Dis. 2002, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, L.; Lin, Q.; Ren, W.; Xu, G. Prognostic value of endoglin-assessed microvessel density in cancer patients: A systematic review and meta-analysis. Oncotarget 2018, 9, 7660–7671. [Google Scholar] [CrossRef]
- Strijbos, M.H.; Gratama, J.W.; Schmitz, P.I.M.; Rao, C.; Onstenk, W.; Doyle, G.V.; Miller, M.C.; Wit, R.; Terstappen, L.W.M.M.; Sleijfer, S. Circulating endothelial cells, circulating tumour cells, tissue factor, endothelin-1 and overall survival in prostate cancer patients treated with docetaxel. Eur. J. Cancer 2010, 46, 2027–2035. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Schölch, S.; Bork, U.; Kahlert, C.; Schneider, M.; Rahbari, M.; Büchler, M.W.; Weitz, J.; Reissfelder, C. Prognostic value of circulating endothelial cells in metastatic colorectal cancer. Oncotarget 2017, 8, 37491–37501. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.C.; Ottinger, A.; Somsri, S.; Sratongno, P.; Pannadaporn, P.; Chimma, P.; Malasit, P.; Pattanapanyasat, K.; Neumann, H.P. Optimized high gradient magnetic separation for isolation of Plasmodium-infected red blood cells. Malar. J. 2010, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Thaicharoen, P. Easy employment and crosstalk-free detection of seven fluorophores in a widefield fluorescence microscope. Methods Protoc. 2018, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. The 2011 Oxford CEBM Levels of Evidence; Oxford Centre for Evidence-Based Medicine: Oxford, UK, 2011. [Google Scholar]
- Lin, P.P.; Gires, O.; Wang, D.D.; Li, L.; Wang, H. Comprehensive in situ co-detection of aneuploid circulating endothelial and tumor cells. Sci. Rep. 2017, 7, 9789. [Google Scholar] [CrossRef]
- Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.W.M.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Gorin, M.A.; Walsh, P.C. Magnetic Resonance Imaging prior to first prostate biopsy–are we there yet? Eur. Urol. 2018, 74, 409–410. [Google Scholar] [CrossRef]
- Mochtar, C.A.; Atmoko, W.; Umbas, R.; Hamid, A.R.A.H. Prostate cancer detection rate in Indonesian men. Asian J. Surg. 2018, 41, 163–169. [Google Scholar] [CrossRef]
- Jang, J.; Kim, Y. Is prostate biopsy essential to diagnose prostate cancer in the older patient with extremely high prostate-specific antigen? Korean J. Urol. 2012, 53, 82–86. [Google Scholar] [CrossRef]
- Rutjes, A.W.S.; Reitsma, J.B.; Vandenbroucke, J.P.; Glas, A.S.; Bossuyt, P.M.M. Case-control and two-gate designs in diagnostic accuracy studies. Clin. Chem. 2005, 51, 1335–1341. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, T.; Ellis, M.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, G.N. Atlas of Exfoliative Cytology; Harvard University Press: Cambridge, UK, 1956. [Google Scholar]
- Lazzeri, M.; Lughezzani, G.; Haese, A.; Mcnicholas, T.; Taille, A.; Buffi, N.M.; Cardone, P.; Hurle, R.; Casale, P.; Bini, V.; et al. Clinical performance of prostate health index in men with tPSA >10ng/ml: Results from a multicentric European study. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 13–415. [Google Scholar] [CrossRef] [PubMed]
- Leisenring, W.; Alonzo, T.; Pepe, M.S. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics 2010, 56, 345–351. [Google Scholar] [CrossRef]
- Moskowitz, C.S.; Pepe, M.S. Comparing the predictive values of diagnostic tests: Sample size and analysis for paired study designs. Clin. Trials 2006, 3, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Davis, C.S.; Soong, S.J. Comparison of predictive values of two diagnostic tests from the same sample of subjects using weighted least squares. Stat. Med. 2006, 25, 2215–2229. [Google Scholar] [CrossRef]
| PSA Level | 146 Men Included | 24 Men Excluded | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men, n | Mean Age | SD | Mean PSA | SD | Men, n | Mean Age | SD | Mean PSA | SD | |
| <10 | 69 | 61.4 | 20.1 | 6.5 | 2.2 | 12 | 69.9 | 8.6 | 5.9 | 2.2 |
| 10–20 | 34 | 66.2 | 13.4 | 14.0 | 2.7 | 7 | 64.4 | 3.0 | 12.1 | 6.5 |
| 20–40 | 13 | 64.5 | 21.1 | 30.3 | 7.3 | 3 | 68.7 | 3.2 | 28.2 | 6.2 |
| 40–60 | 5 | 65.8 | 9.8 | 51.0 | 7.0 | 0 | NA | NA | NA | NA |
| 60–80 | 5 | 69.8 | 7.5 | 69.8 | 4.4 | 1 | 76.0 | NA | 60.7 | NA |
| 80–100 | 0 | NA | NA | NA | NA | 0 | NA | NA | NA | NA |
| >100 | 20 | 69.9 | 8.6 | 1271.9 | 1188.8 | 1 | 73.0 | NA | 1114.0 | NA |
| Total | 146 | 24 | ||||||||
| PSA ng/mL | All PCa | csPCa, Gleason ≥ 3 + 4 | csPCa, Gleason ≥ 4 + 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TRUS Biopsy+ | TRUS Biopsy− | % | TRUS Biopsy+ | TRUS Biopsy− | % | TRUS Biopsy+ | TRUS Biopsy− | % | |
| <10 | 24 | 47 | 34 | 15 | 56 | 21 | 9 | 62 | 13 |
| 10–20 | 15 | 17 | 47 | 12 | 20 | 38 | 8 | 24 | 25 |
| 20–40 | 7 | 6 | 54 | 7 | 6 | 54 | 4 | 9 | 31 |
| 40–60 | 4 | 1 | 80 | 4 | 1 | 80 | 4 | 1 | 80 |
| 60–80 | 5 | 0 | 100 | 5 | 0 | 100 | 3 | 2 | 60 |
| 80–100 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| >100 | 20 | 0 | 100 | 20 | 0 | 100 | 20 | 0 | 100 |
| Total | 75 | 71 | 63 | 83 | 48 | 98 | |||
| A | PSA < 10 ng/mL | ||||
| PSA, % (95% CI) | tCEC, % (95% CI) | Test Ratio * (95% CI) | p Value * | ||
| Primary endpoint, prevalence of all cancer: n = 22 | |||||
| PPV | 32 (21–44) | 54 (34–73) | 2.47 (1.43, 4.24) | 0.0011 | |
| Secondary endpoint, primary definition, prevalence of clinically significant cancer with Gleason score 3 + 4 or higher: n = 12 | |||||
| PPV | 17 (9–28) | 32 (16–52) | 2.25 (1.38, 3.68) | 0.0012 | |
| Secondary endpoint, secondary definition, prevalence of clinically significant cancer with Gleason score 4 + 3 or higher: n = 7 | |||||
| PPV | 10 (4–20) | 18 (6–37) | 1.93 (1.09, 3.40) | 0.0242 | |
| B | PSA 10–20 ng/mL | ||||
| PSA, % (95% CI) | tCEC, % (95% CI) | Test Ratio * (95% CI) | p Value | ||
| Primary endpoint, prevalence of all cancer: n = 16 | |||||
| PPV | 47 (30–65) | 60 (32–84) | 1.69 (0.77, 3.69) | 0.1904 | |
| Secondary endpoint, primary definition, prevalence of clinically significant cancer with Gleason score 3 + 4 or higher: n = 12 | |||||
| PPV | 35 (20–53) | 46 (21–73) | 1.60 (0.77, 3.33) | 0.2050 | |
| Secondary endpoint, secondary definition, prevalence of clinically significant cancer with Gleason score 4 + 3 or higher: n = 8 | |||||
| PPV | 24 (11–42) | 28 (9–56) | 1.18 (0.52, 2.70) | 0.6917 | |
| Class | Cytopathological Diagnosis | Immunocytomorphology |
|---|---|---|
| I | Negative for malignancy | No CD45− cells |
| II | Atypical cells but negative for malignancy | CD45− cells without positive markers (consider plasma cells) CD31+ CD34− VIM− CK− CD45− cells with aneuploidy (consider normal endothelial cells, see Lin et al. [27]) CD45+ cells with atypical nuclei |
| III | Suspicious for malignancy | Less than five single cells: CD31= CD34+ VIM+ CK− CD45− (angiogenic tip cell: tumour-associated vs. inflammatory) More than one large cell: CD31− CD34= VIM+ CK− CD45− (mesenchymal CTC vs. haematopoietic stem cell) Both conditions with or without aneuploidy |
| IV | Strongly suggestive for malignancy | Less than five single cells: CD31− CD34− VIM= CK+ CD45− (epithelial CTC or cell in epithelial-mesenchymal transition) One clump: CD31= CD34+ VIM= CK= CD45− More than one cell with aneuploidy: CD31+ CD34+ VIM+ CK= CD45− More than five single cells without aneuploidy: CD31+ CD34+ VIM+ CK= CD45− One or more large cell with aneuploidy: CD45− |
| V | Conclusive for malignancy | More than one clump: CD31= CD34+ VIM+ CK= CD45− More than five cells: CD31− CD34− VIM= CK+ CD45− (epithelial CTC or cell in epithelial-mesenchymal transition) One or more CD45− cell in atypical mitosis (chromosome missegregation) One or more giant polyploidic cells, CD45− |
| Low-Risk | Intermediate-Risk | High-Risk | |
|---|---|---|---|
| Definition | |||
| PSA <10 ng/mL | PSA 10–20 ng/mL | PSA > 20 ng/mL | any PSA |
| and GS <7 | or GS 7 | or GS >7 | any GS |
| and cT1-2a | or cT2b | or cT2c | cT3–4 or cN+ |
| Localised | Localised | Localised | Locally advanced |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhakdi, S.C.; Suriyaphol, P.; Thaicharoen, P.; Grote, S.T.K.; Komoltri, C.; Chaiyaprasithi, B.; Charnkaew, K. Accuracy of Tumour-Associated Circulating Endothelial Cells as a Screening Biomarker for Clinically Significant Prostate Cancer. Cancers 2019, 11, 1064. https://doi.org/10.3390/cancers11081064
Bhakdi SC, Suriyaphol P, Thaicharoen P, Grote STK, Komoltri C, Chaiyaprasithi B, Charnkaew K. Accuracy of Tumour-Associated Circulating Endothelial Cells as a Screening Biomarker for Clinically Significant Prostate Cancer. Cancers. 2019; 11(8):1064. https://doi.org/10.3390/cancers11081064
Chicago/Turabian StyleBhakdi, Sebastian Chakrit, Prapat Suriyaphol, Ponpan Thaicharoen, Sebastian Tobias Karl Grote, Chulaluk Komoltri, Bansithi Chaiyaprasithi, and Komgrid Charnkaew. 2019. "Accuracy of Tumour-Associated Circulating Endothelial Cells as a Screening Biomarker for Clinically Significant Prostate Cancer" Cancers 11, no. 8: 1064. https://doi.org/10.3390/cancers11081064





