The Impact of Different Sounds on Stress Level in the Context of EEG, Cardiac Measures and Subjective Stress Level: A Pilot Study
Abstract
1. Introduction
2. Stress under the Influence of Music
Review of Previous Research
3. Materials and Methods
3.1. Practical Experiment and Participants
3.2. Measurement Devices
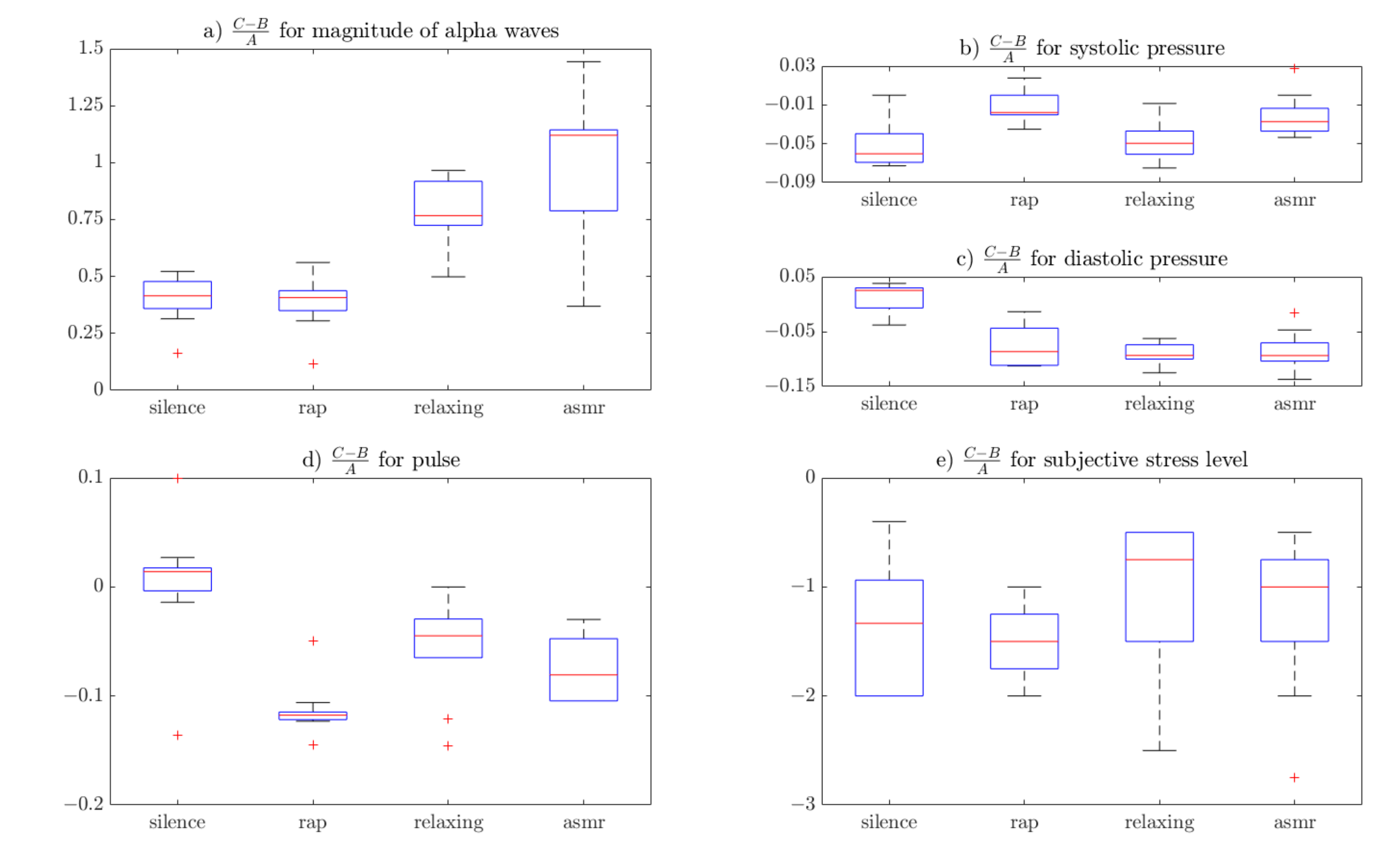
3.3. Numerical Measures: CBA Ratio and the Statistical Analysis
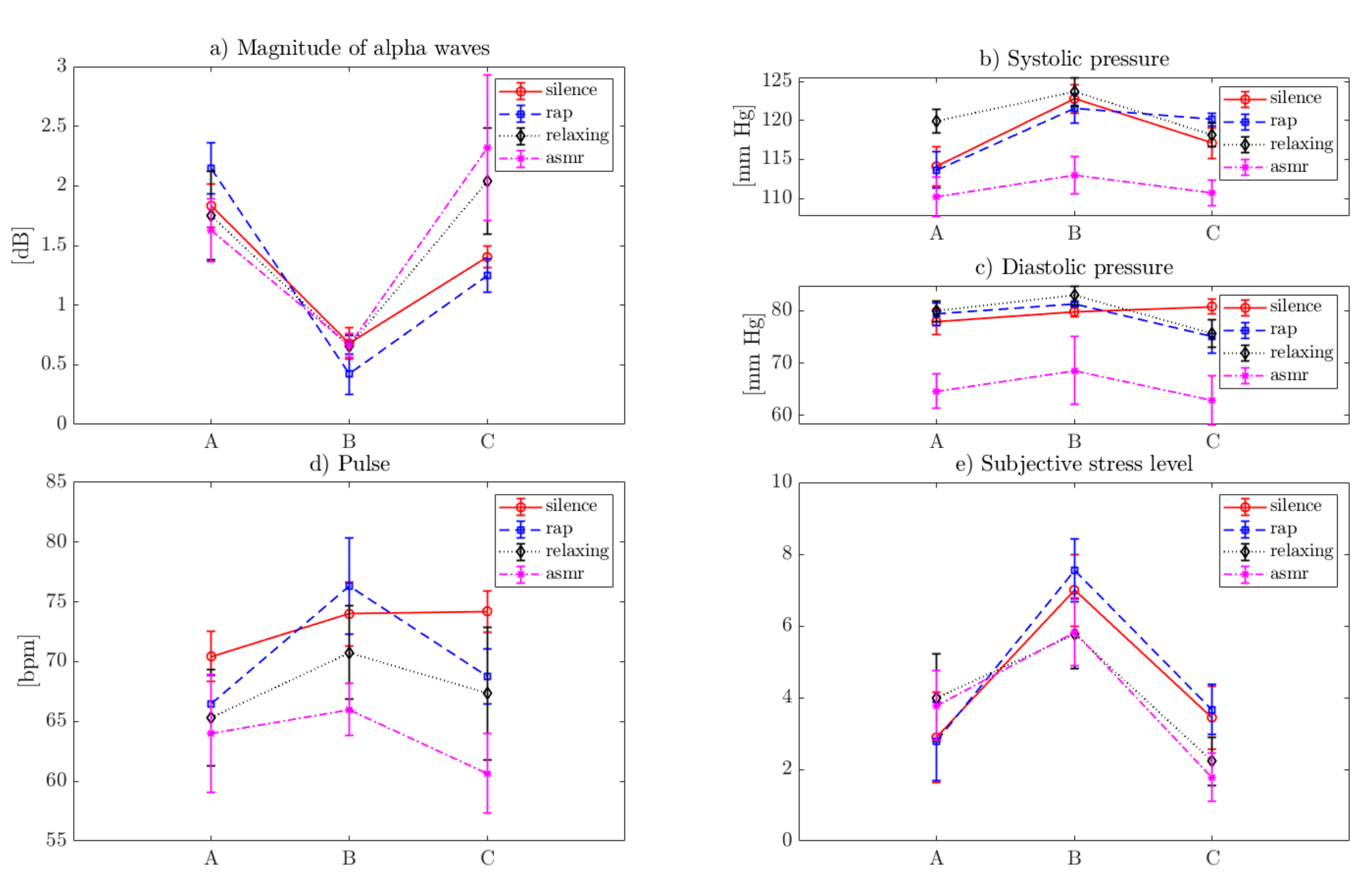
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, J.; Rickson, D.; Jiang, C. The mechanism of music for reducing psychological stress: Music preference as a mediator arts psychotherapy. Arts Psychother. 2016, 48, 62–68. [Google Scholar] [CrossRef]
- De la Torre-Luque, A.; Díaz-Piedra, C.; Buela-Casal, G. Effects of preferred relaxing music after acute stress exposure: A randomized controlled trial. Psychol. Music 2017, 1–19. [Google Scholar] [CrossRef]
- Asif, A.; Majid, M.; Anwar, S.M. Human stress classification using EEG signals in response to music tracks. Comput. Biol. Med. 2019, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.V.; La Marca, R.; Brönnimann, R.; Finkel, L.; Ehlert, U.; Nater, U.M. The effect of music on the human stress response. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R. ECG signatures of psychological stress. J. Electrocardiol. 2015, 48, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Boucsein, W. Electrodermal Activity, 2nd ed.; Springer: New York, NY, USA; Berlin, Germany, 2012; pp. 1–523. [Google Scholar]
- Giggins, O.M.; Persson, U.; Caufield, B. Biofeedback in rehabilitation. J. Neuroeng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef]
- Linnemann, A.; Strahler, J.; Nater, U.M. The stress-reducing effect of music listening varies depending on the social context. Psychoneuroendocrinology 2016, 72, 97–105. [Google Scholar] [CrossRef]
- Lingham, J.; Theorell, T. Self-selected “favourite” stimulative and sedative music listening–how does familiar and preferred music listening affect the body. Nord. J. Music Ther. 2009, 18, 150–166. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Liu, C.; Liu, D.; Ding, X.; Zhou, C. Graph theoretical analysis of EEG functional connectivity during music perception. Brain Res. 2012, 1483, 71–81. [Google Scholar] [CrossRef]
- Daly, I.; Malik, A.; Hwang, F.; Roesch, E.; Weaver, J.; Kirke, A.; Williams, D.; Miranda, E.; Nasuto, S.J. Neural correlates of emotional responses to music: An EEG study. Neurosci. Lett. 2014, 52–57. [Google Scholar] [CrossRef]
- Banerjee, A.; Sanyal, S.; Patranabis, A.; Banerjee, K.; Guhathakurta, T.; Sengupta, R.; Ghosh, D.; Ghose, P. Study on brain dynamics by non-linear analysis of music induced EEG signals. Phys. A 2016, 444, 110–120. [Google Scholar] [CrossRef]
- Phneah, S.W.; Nisar, H. EEG-based alpha neurofeedback training for mood enhancement. Australas. Coll. Phys. Sci. Eng. Med. 2017, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Gooßes, M.; Saliger, J.; Folkerts, A.; Nielsen, J.; Zierer, J.; Schmoll, P.; Niepold, A.; Colbach, L.; Leemhuis, J.; Engels, L.; et al. Feasibility of music-assisted treadmill training in parkinson’s disease patients with and without deep brain stimulation: Insights from an ongoing pilot randomized controlled trial. Front. Neurol. 2020, 790, 1–16. [Google Scholar] [CrossRef]
- Carrière, M.; Larroque, S.K.; Martial, C.; Bahri, M.A.; Aubinet, C.; Perrin, F.; Laureys, S.; Heine, L. An Echo of Consciousness: Brain Function during Preferred Music. Brain Connect. 2020, 10/7, 385–395. [Google Scholar] [CrossRef]
- Wong, A.; Bergen, D.; Nordvall, M.; Allnutt, A.; Bagheri, R. Cardiac autonomic and blood pressure responses to an acute session of battling ropes exercise. Physiol. Behav. 2020, 227. [Google Scholar] [CrossRef]
- Muñoz-López, A.; Naranjo-Orellana, J. Individual versus team heart rate variability responsiveness analyses in a national soccer team during training camps. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soker-Elimaliah, S.; Jennings, C.A.; Hashimi, M.M.; Cassim, T.Z.; Lehrfield, A.; Wagner, J.B. Autistic traits moderate relations between cardiac autonomic activity, interoceptive accuracy, and emotion processing in college students. Int. J. Psychophysiol. 2020, 155, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Demos, I.N. Getting Started with Neurofeedback; W.W. Norton & Company: New York, NY, USA, 2005. [Google Scholar]
- Meyer, P.; Friederich, H.; Zastrow, A. Breathe to ease-Respiratory biofeedback to improve heart rate variability and coping with stress in obese patients: A pilot study. Ment. Health Prevetion 2018, 11, 41–46. [Google Scholar] [CrossRef]
- Dadashi, M.; Birashk, B.; Taremian, F.; Asgarnejad, A.A.; Momtazi, S. Effects of increase in amplitude of occipital alpha & theta brain waves on global functioning level of patients with GAD. Basic Clin. Neurosci. 2015, 6, 14–20. [Google Scholar]
- Paszkiel, S.; Szpulak, P. Methods of acquisition, archiving and biomedical data analysis of brain functioning. In Biomedical Engineering and Neuroscience; Hunek, W., Paszkiel, S., Eds.; Series: Advances in Intelligent Systems and Computing 720; Springer: Berlin/Heidelberg, Germany, 2018; pp. 158–171. [Google Scholar] [CrossRef]
- Zabcikova, M. Measurement of visual and auditory stimuli using EEG headset Emotiv Epoc+. Matec Web Conf. 2019. [Google Scholar] [CrossRef]
- Marcuse, L.; Fields, M.; Yoo, J. Rowan’s Primer of EEG, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–216. [Google Scholar]
- Paszkiel, S. Characteristics of question of blind source separation using Moore-Penrose pseudoinversion for reconstruction of EEG signal. In Automation 2017 Innovations in Automation, Robotics and Measurement Techniques; Szewczyk, R., Zieliński, C., Kaliczyńska, M., Eds.; Series: Advances in Intelligent Systems and Computing 550; Springer: Berlin/Heidelberg, Germany, 2017; pp. 393–400. [Google Scholar] [CrossRef]
- Inman, M. A Rosetta Stone for Brain Waves. PLoS Biol. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Arsenault, R.; Napper, S.; Griebel, P. Models and Methods to Investigate Acute Stress Responses in Cattle. Animals 2015, 5, 1268–1295. [Google Scholar] [CrossRef] [PubMed]
| Hypothalamus | → ← | Central Nervous System | |
| Pituitary | Adrenal Medulla | Peripheral Sympathetic Nerves | |
| Adrenal Cortex | Epinephrine | Norepinephrine | |
| Glucocorticoid | Metabolic Glycolysis | ||
| Metabolic Glycolysis/gluconeogenesis Anabolism/catabolism Lipolysis Insulin signaling# | Immune Regulation of cytokines Stabilization of cytoskeleton Infection Wound healing | ||
| Immune Apoptosis Neutrophil/lymphocyte trafficking Cytokine production Anti-inflammatory responses Pro- inflammatory responses | Behavior Aggressive behaviors Increased heart rate and blood pressure Increased respiration | ||
| Stage | Activity |
|---|---|
| 1. | Filling in the questionnaire, measuring pulse and blood pressure, EEG examination (called A stage for short in the rest of the paper). |
| 2. | Monitoring values achieved by alpha waves until the end of the test. |
| 3. | Putting a subject in a stressful situation. |
| 4. | Filling in the questionnaire, measuring pulse and blood pressure (called B stage for short in the rest of the paper). |
| 5. | Playing a selected sound to the subject. |
| 6. | Filling in the questionnaire, measuring pulse and blood pressure (called C stage for short in the rest of the paper). |
| 7. | The end of the test. |
| Sound Type | A (Prior to Stressor) | B (After the Stressor) | C (After the Sound) | |||
|---|---|---|---|---|---|---|
| Mean | std | Mean | std | Mean | std | |
| Mean alpha wave amplitude value [dB] | ||||||
| Silence | 1.83 | 0.18 | 0.73 | 0.14 | 1.40 | 0.09 |
| Rap | 2.12 | 0.20 | 0.47 | 0.21 | 1.25 | 0.14 |
| Relaxing | 1.48 | 0.42 | 0.66 | 0.09 | 1.67 | 0.60 |
| ASMR | 1.46 | 0.33 | 0.66 | 0.10 | 1.92 | 0.84 |
| Systolic pressure [mm Hg] | ||||||
| Silence | 115.00 | 2.06 | 122.78 | 1.86 | 117.89 | 1.27 |
| Rap | 113.89 | 2.20 | 121.78 | 1.48 | 120.00 | 0.87 |
| Relaxing | 119.89 | 1.54 | 123.22 | 1.72 | 118.22 | 1.30 |
| ASMR | 111.78 | 1.79 | 113.67 | 2.18 | 111.00 | 1.50 |
| Diastolic pressure [mm Hg] | ||||||
| Silence | 77.89 | 2.52 | 80.00 | 1.00 | 80.33 | 1.50 |
| Rap | 79.33 | 2.18 | 81.22 | 0.44 | 75.11 | 3.14 |
| Relaxing | 79.89 | 1.96 | 82.89 | 1.83 | 76.11 | 2.20 |
| ASMR | 69.11 | 5.60 | 76.00 | 8.62 | 67.33 | 6.12 |
| Pulse [bpm] | ||||||
| Silence | 70.44 | 2.07 | 74.00 | 2.65 | 73.11 | 2.26 |
| Rap | 67.89 | 2.89 | 76.22 | 3.96 | 69.67 | 2.92 |
| Relaxing | 65.89 | 4.26 | 71.00 | 3.97 | 67.33 | 5.52 |
| ASMR | 64.22 | 5.09 | 66.56 | 2.51 | 61.33 | 3.71 |
| Subjective stress level [1–10] | ||||||
| Silence | 2.78 | 1.20 | 6.89 | 0.93 | 3.11 | 0.93 |
| Rap | 2.67 | 1.00 | 7.72 | 0.67 | 4.11 | 0.78 |
| Relaxing | 3.83 | 1.17 | 6.00 | 0.83 | 2.22 | 0.67 |
| ASMR | 3.61 | 0.86 | 6.06 | 0.77 | 1.78 | 0.67 |
| Sound Type | Mean Alpha Wave Amplitude Value [dB] | Systolic Pressure [mm Hg] | Diastolic Pressure [mm Hg] | Pulse [bpm] | Subjective stress level [1–10] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | std | Mean | std | Mean | std | Mean | std | Mean | std | |
| Silence | 0.394 | 0.107 | −0.050 | 0.024 | 0.013 | 0.028 | 0.002 | 0.061 | −1.554 | 1.062 |
| Rap | 0.382 | 0.122 | −0.013 | 0.016 | −0.077 | 0.039 | −0.113 | 0.026 | −1.491 | 0.347 |
| Relaxing | 0.781 | 0.152 | −0.046 | 0.021 | −0.090 | 0.019 | −0.055 | 0.047 | −1.054 | 0.737 |
| ASMR | 0.985 | 0.314 | −0.021 | 0.022 | −0.087 | 0.037 | −0.086 | 0.054 | −1.220 | 0.723 |
| Statistical Difference | p-Value | ||||
|---|---|---|---|---|---|
| EEG | Systolic Pressure | Diastolic Pressure | Pulse | Subjective Stress | |
| Kruskal–Wallis | 6.0 × 10−5 | 1.9 × 10−3 | 4.5 × 10−4 | 6.4 × 10−4 | 2.3 × 10−1 |
| Silence—rap | 1.0 × 100 | 7.0 × 10−3 | 5.5 × 10−3 | 3.5 × 10−4 | 9.9 × 10−1 |
| Silence—relaxing | 2.8 × 10−2 | 2.3 × 10−3 | 1.0 × 100 | 3.7 × 10−1 | 6.9 × 10−1 |
| Silence—ASMR | 2.5 × 10−3 | 2.3 × 10−3 | 1.4 × 10−1 | 3.6 × 10−2 | 9.8 × 10−1 |
| Rap—relaxing | 1.3 × 10−2 | 1.0 × 100 | 1.8 × 10−2 | 1.5 × 10−1 | 2.7 × 10−1 |
| Rap—ASMR | 1.1 × 10−3 | 1.0 × 100 | 8.7 × 10−1 | 7.4 × 10−1 | 7.2 × 10−1 |
| Relaxing—ASMR | 9.8 × 10−1 | 1.0 × 100 | 3.0 × 10−1 | 9.2 × 10−1 | 9.9 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszkiel, S.; Dobrakowski, P.; Łysiak, A. The Impact of Different Sounds on Stress Level in the Context of EEG, Cardiac Measures and Subjective Stress Level: A Pilot Study. Brain Sci. 2020, 10, 728. https://doi.org/10.3390/brainsci10100728
Paszkiel S, Dobrakowski P, Łysiak A. The Impact of Different Sounds on Stress Level in the Context of EEG, Cardiac Measures and Subjective Stress Level: A Pilot Study. Brain Sciences. 2020; 10(10):728. https://doi.org/10.3390/brainsci10100728
Chicago/Turabian StylePaszkiel, Szczepan, Paweł Dobrakowski, and Adam Łysiak. 2020. "The Impact of Different Sounds on Stress Level in the Context of EEG, Cardiac Measures and Subjective Stress Level: A Pilot Study" Brain Sciences 10, no. 10: 728. https://doi.org/10.3390/brainsci10100728
APA StylePaszkiel, S., Dobrakowski, P., & Łysiak, A. (2020). The Impact of Different Sounds on Stress Level in the Context of EEG, Cardiac Measures and Subjective Stress Level: A Pilot Study. Brain Sciences, 10(10), 728. https://doi.org/10.3390/brainsci10100728







