Prediction of Human Inhibition Brain Function with Inter-Subject and Intra-Subject Variability
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
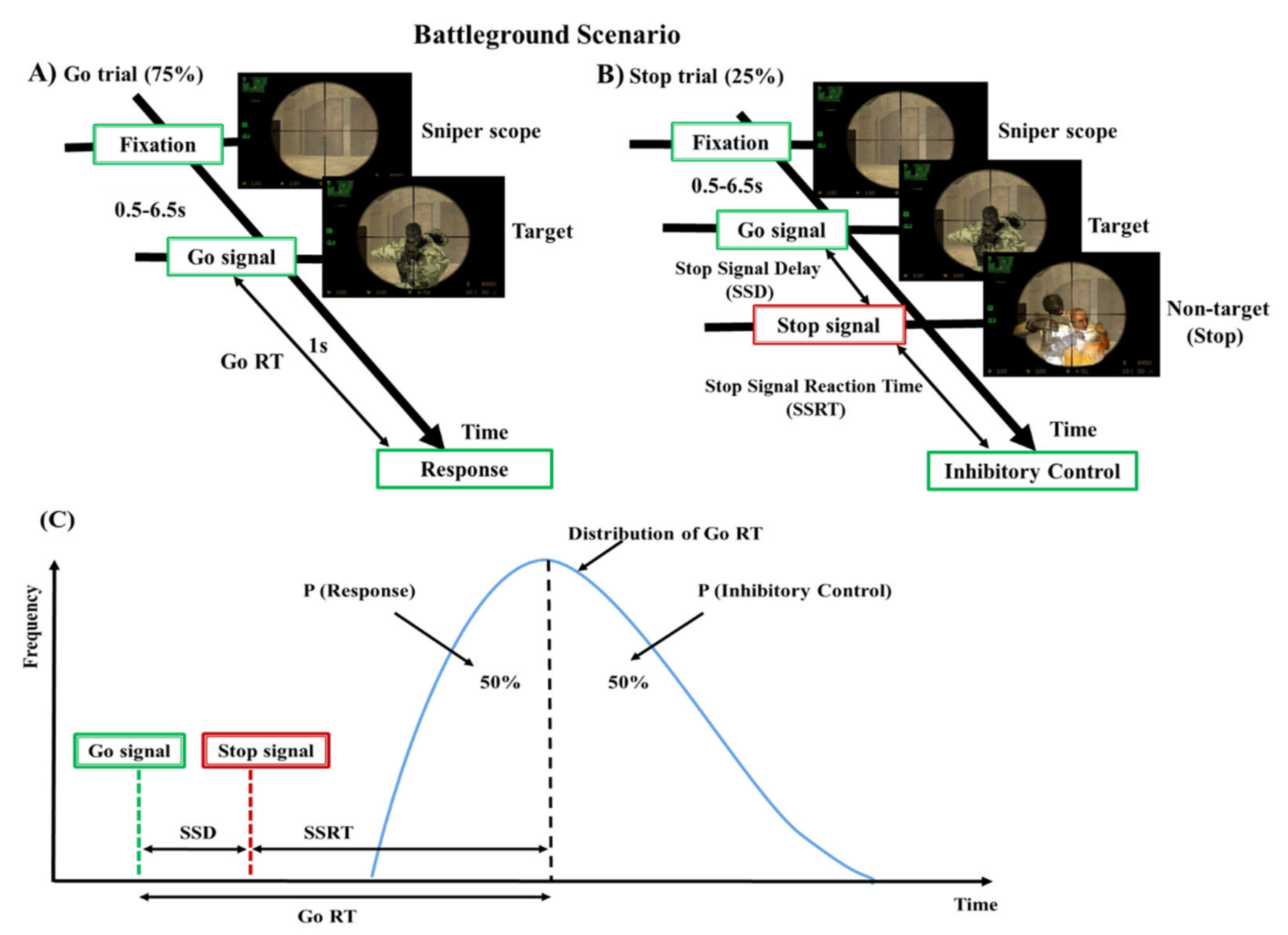
2.2. Experimental Design
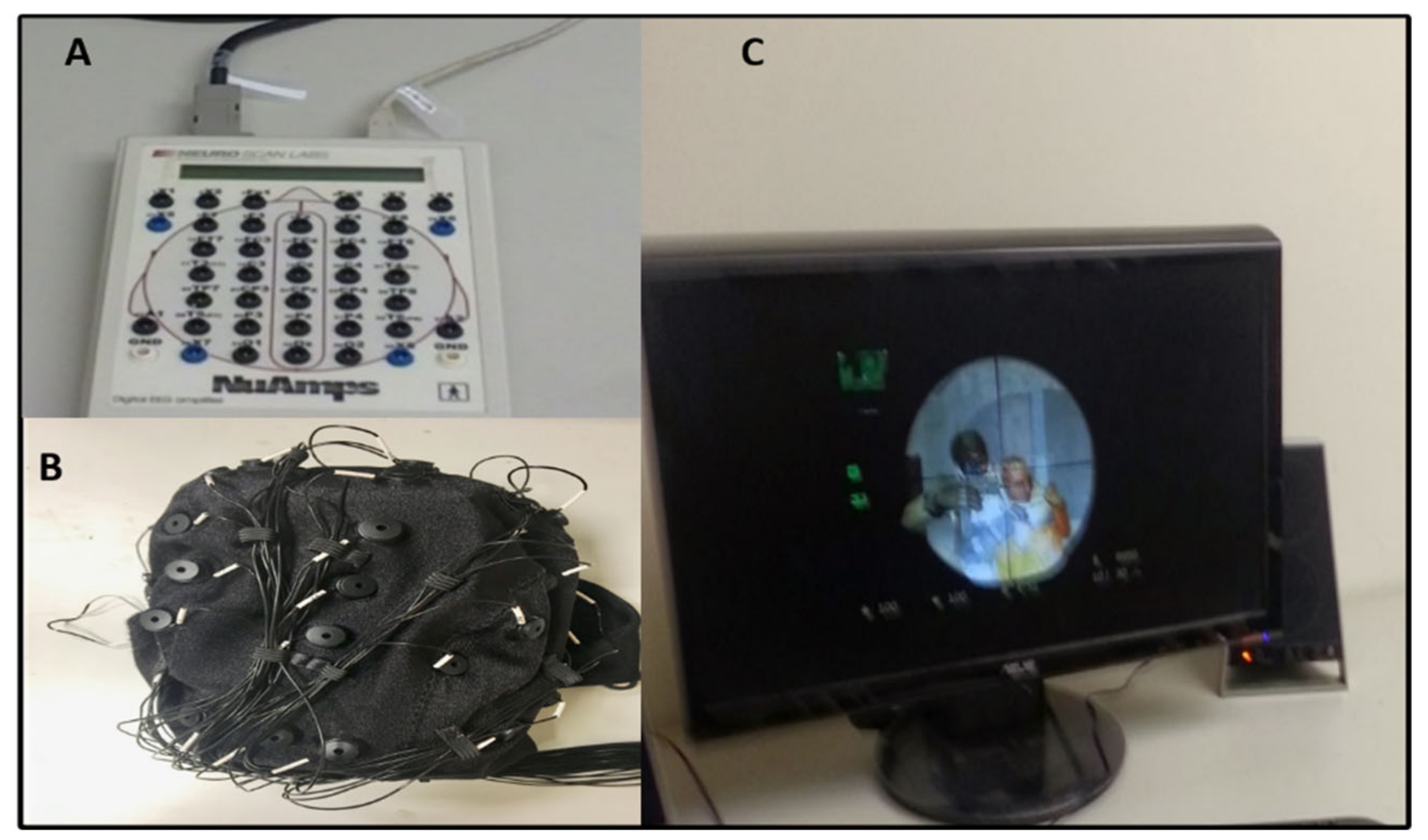
2.3. Acquisition of Electroencephalography (EEG) Signals
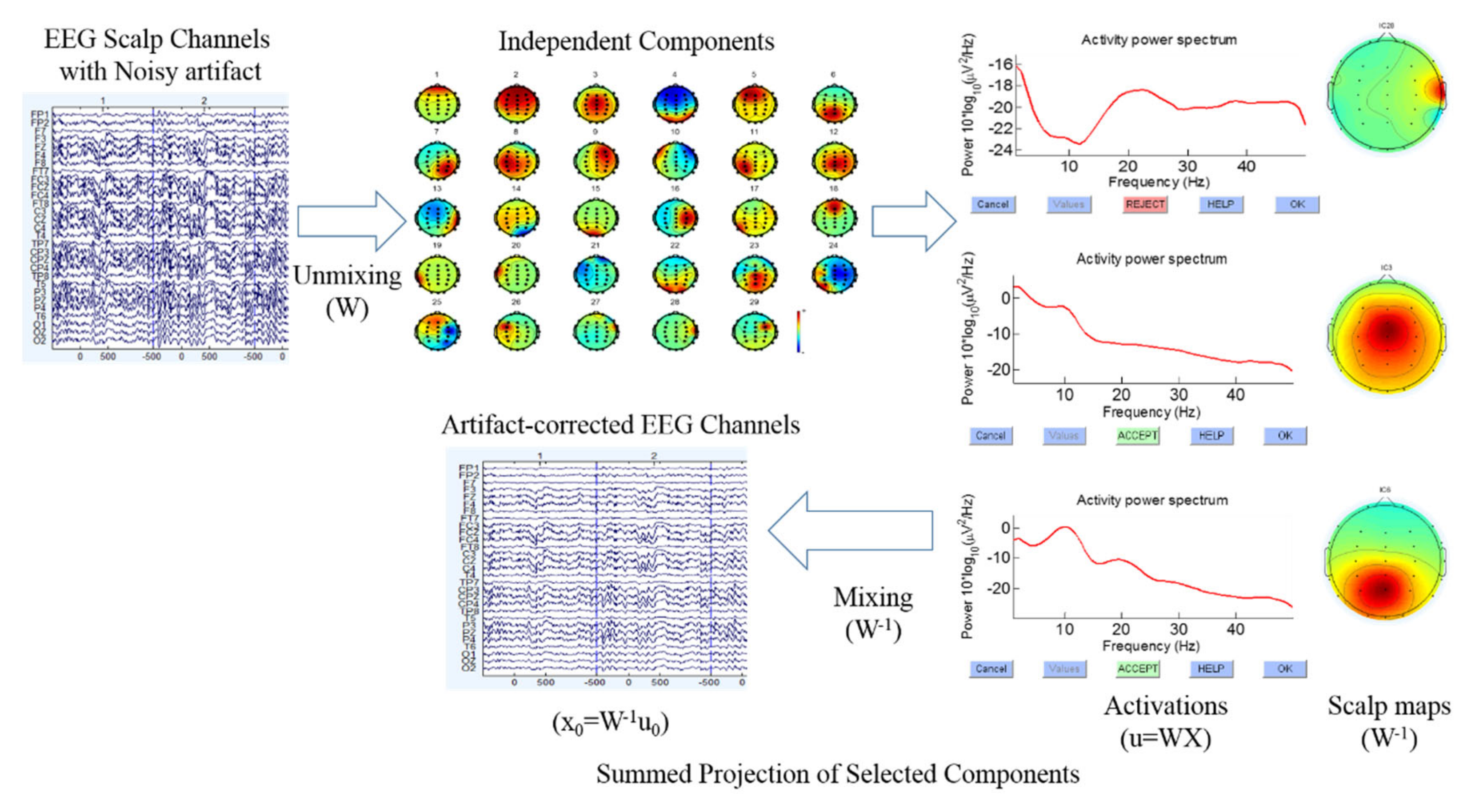
2.4. Independent Component Analysis (ICA) and Back-Projection
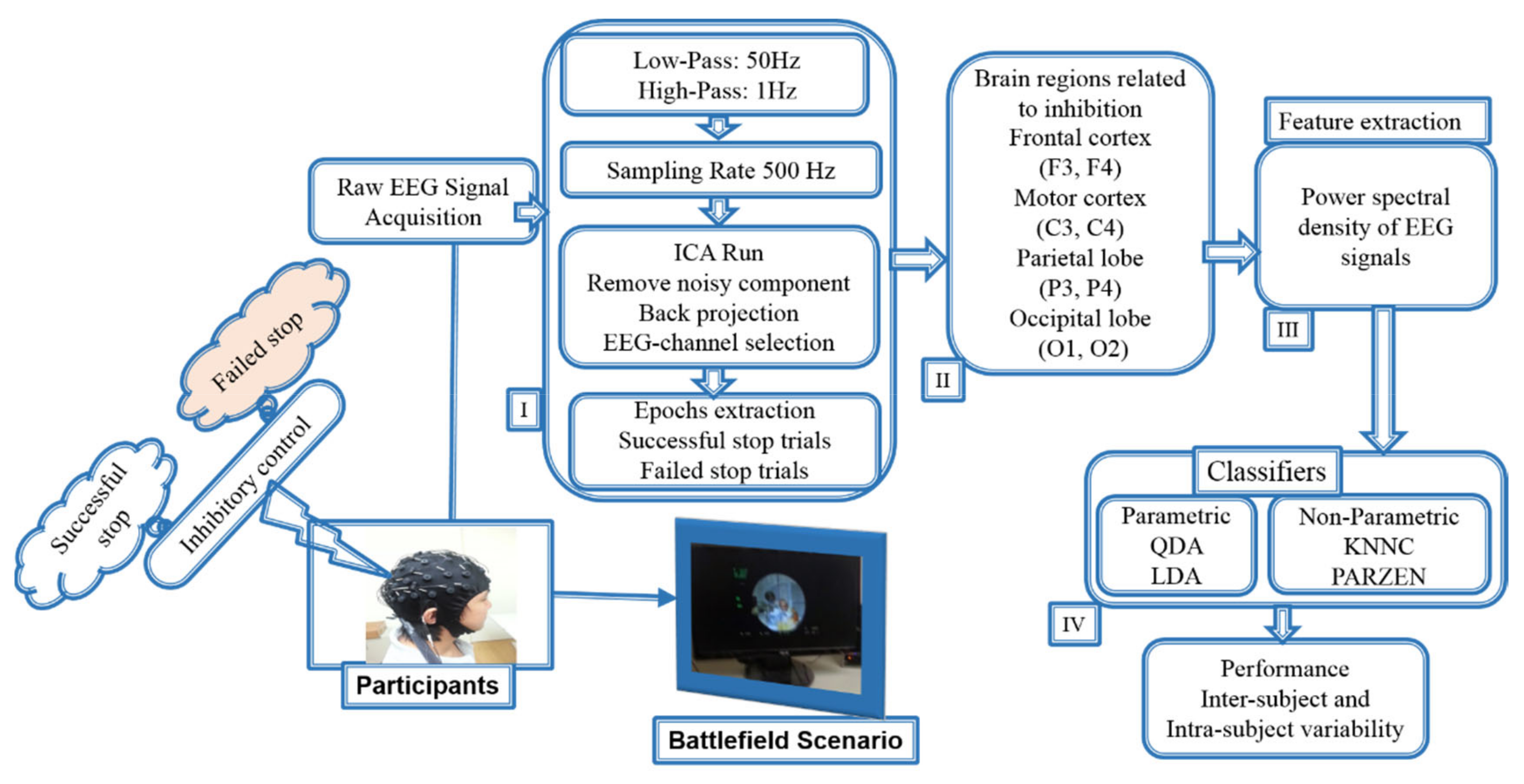
2.5. Analysis and Epoch Extraction of EEG Signals
2.6. Power Spectral Density (PSD) Analysis
2.7. Machine Learning Classifiers: Nonparametric (KNNC and PARZEN) and Parametric (LDA and QDA)
2.7.1. K-Nearest-Neighbor Classifier (KNNC)
2.7.2. Parzen Density-Based Classifier (PARZENDC)
2.7.3. Linear Discriminant Analysis (LDA) Classifier
2.7.4. Quadratic Discriminant Analysis (QDA) Classifier
2.8. EEG Features Extraction and Selection Method
2.9. Statistical Analysis
3. Results
3.1. Behavioral Results
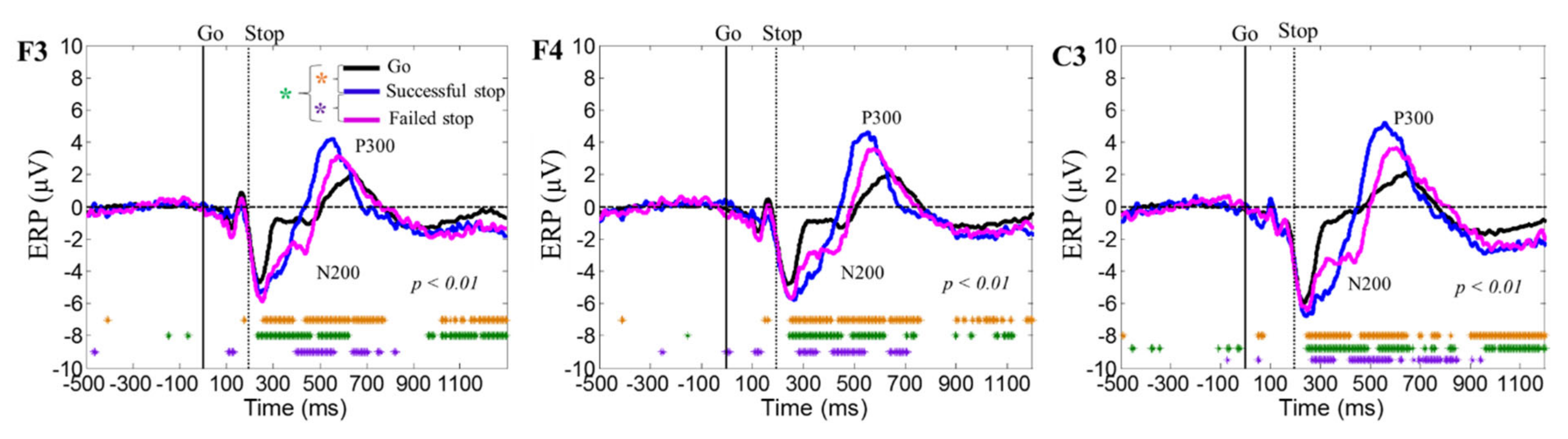
3.2. Event-Related Potential (ERP) Results
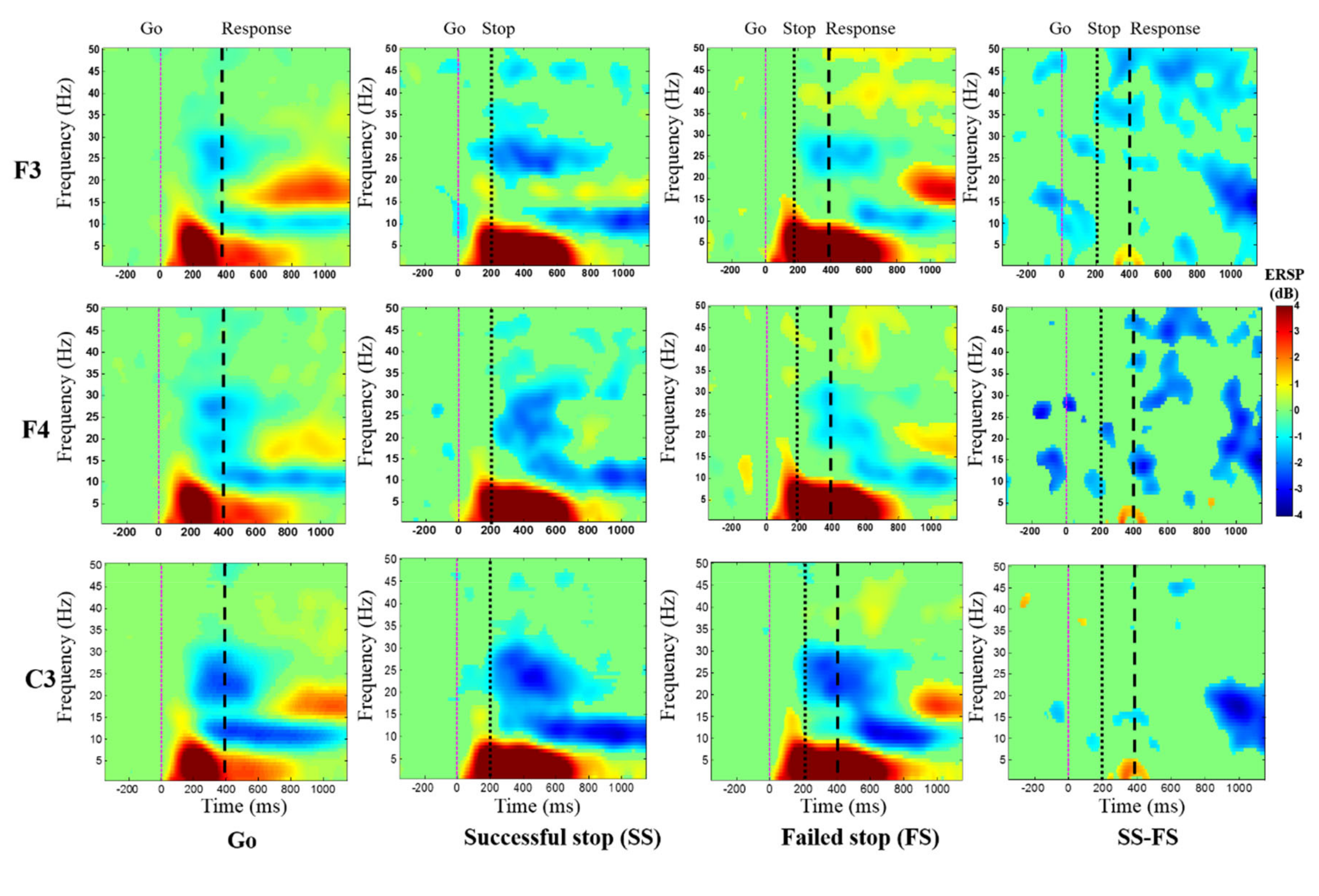
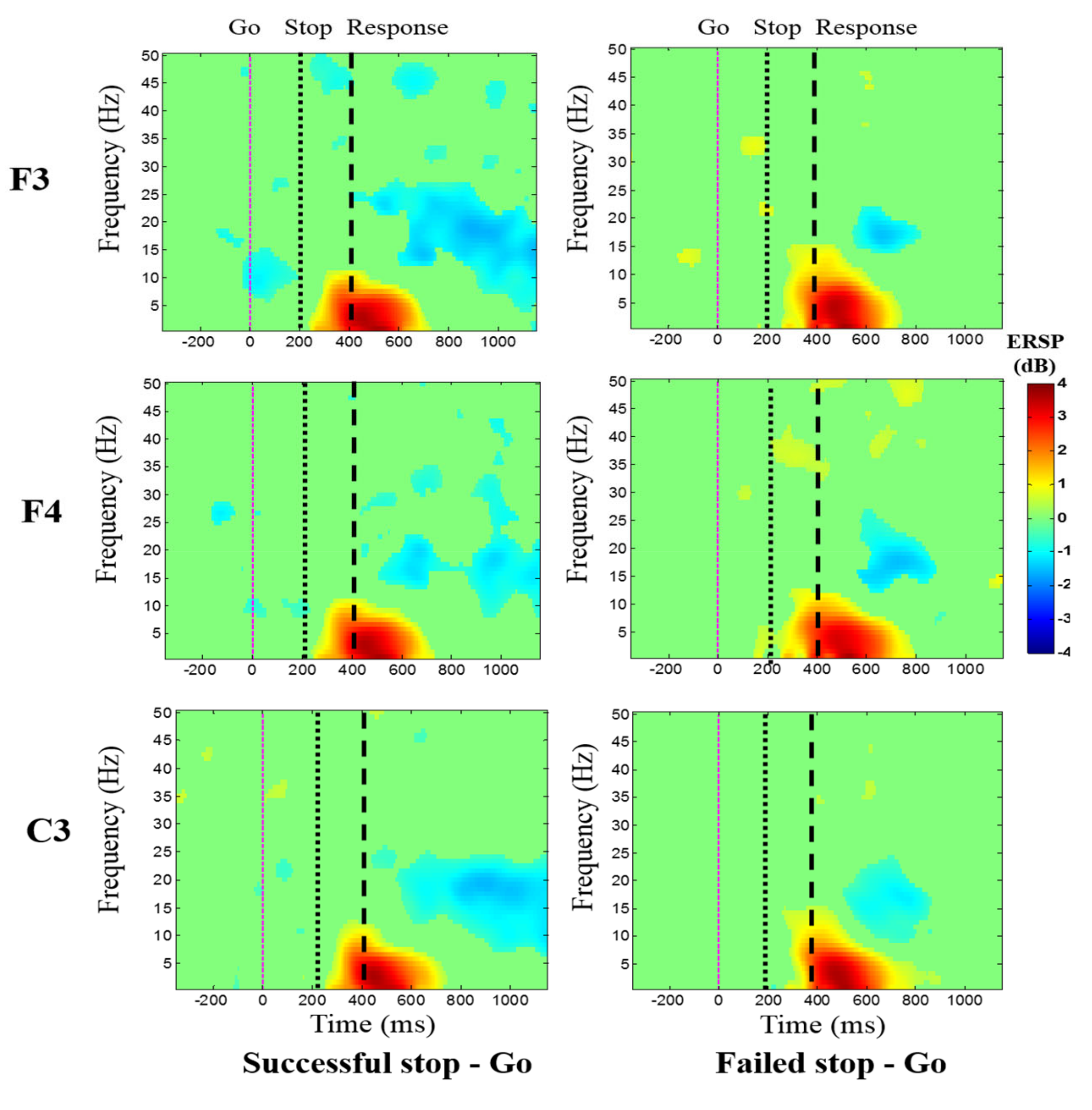
3.3. Event-Related Spectral Perturbation (ERSP) Results
3.4. Power Spectral Density (PSD) of Inter- and Intra-Subject Variability under Human Inhibition
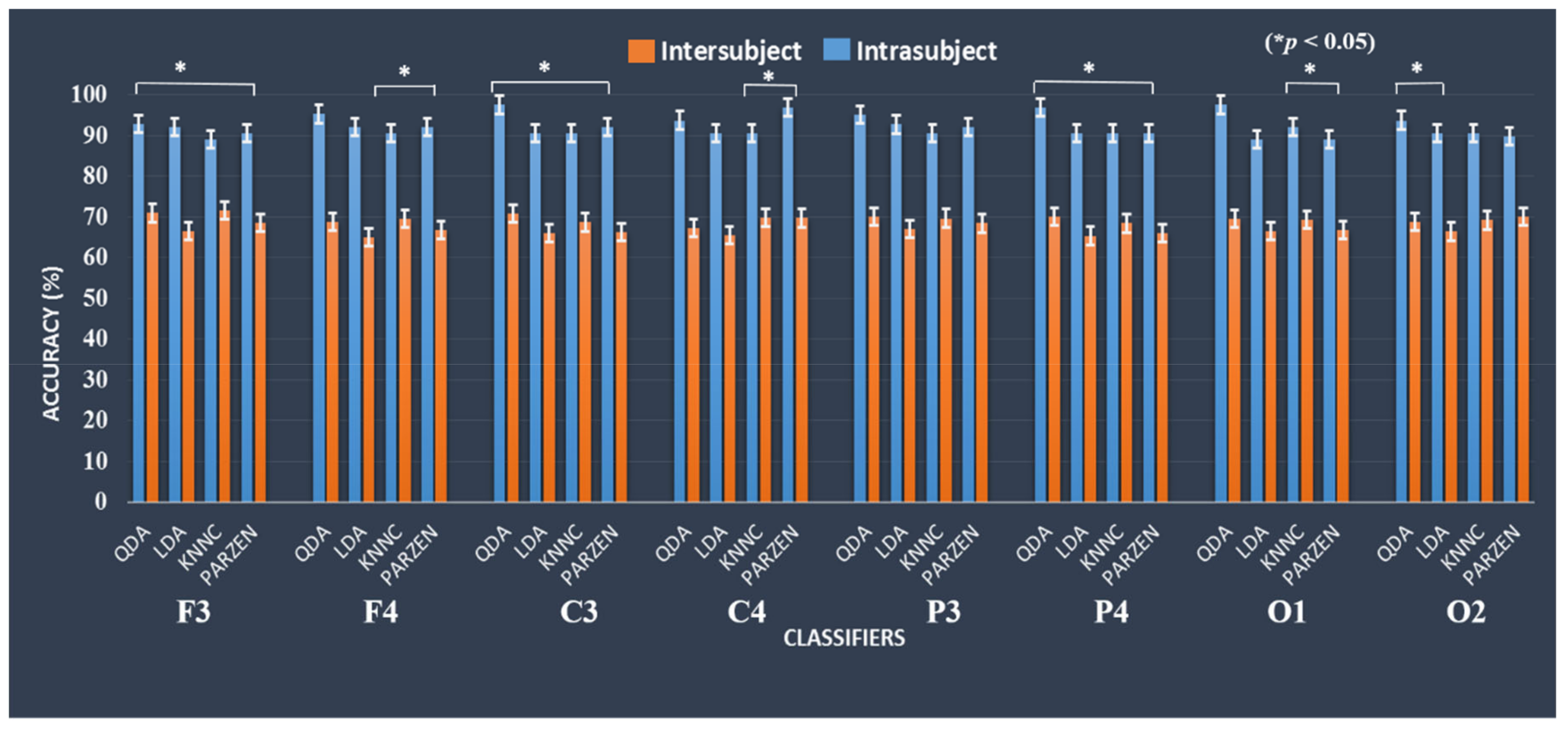
3.5. Classification System Performance using the EEG Frequency Spectrum (1–50 Hz) as Input in the Machine Learning Approaches with Inter- and Intra-Subject Variability
3.6. Comparison of Classification Model Performance between Inter-Subject and Intra-Subject Variability using the EEG Frequency Spectrum (1–50 Hz) as Input in the Machine Learning Approaches
4. Discussion
4.1. Neural Activity Change with Inter-Subject and Intra-Subject Variability
4.2. Relationship between Inter- and Intra-Subject Variability
4.3. Application and Future Works
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EEG | Electroencephalography |
| QDA | Quadratic discriminant analysis |
| LDA | Linear discriminant analysis |
| KNNC | K-nearest neighbor classifier |
| PARZEN | Parzen density-based |
| PSD | Power spectral density |
| ADHD | Attention deficit hyperactivity disorder |
| OCD | Obsessive-compulsive disorder |
| SST | Stop signal task |
| GNG | Go/no-go |
| IFC | Inferior frontal cortex |
| Pre-SMA | Presupplementary motor area |
| ERP | Event-related potential |
| SSD | Stop signal delay |
| SSRT | Stop signal reaction time |
| RT | Reaction time |
| SG | Successful go |
| SS | Successful stop |
| FS | Failed stop |
| IIR | Infinite impulse response |
| ICA | Independent component analysis |
| ERSP | Event-related spectral perturbation |
| LOOCV | Leave-one-out cross validation |
| SD | Standard deviation |
References
- Aron, A.R. The neural basis of inhibition in cognitive control. Neuroscientist 2007, 13, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.D.; Garavan, H.; Bellgrove, M.A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009, 33, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Logan, G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008, 12, 11418–11424. [Google Scholar]
- Verbruggen, F.; Logan, G.D. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci. Biobehav. Rev. 2009, 33, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Lappin, J.; Eriksen, C. Use of delayed signal to stop a visual reaction-time response. J. Exp. Psychol. 1966, 72, 805–811. [Google Scholar] [CrossRef]
- Logan, G.D.; Van Zandt, T.; Verbruggen, F.; Wagenmakers, E.J. On the ability to inhibit thought and action: General and special theories of an act of control. Psychol. Rev. 2014, 121, 66–95. [Google Scholar] [CrossRef]
- Tabu, H.; Tatsuya, M.; Toshihiko, A.; Ryosuke, T.; Hidenao, F. Common inhibitory prefrontal activation during inhibition of hand and foot responses. NeuroImage 2012, 59, 3373–3378. [Google Scholar] [CrossRef]
- Huster, R.J.; Enriquez-Geppert, S.; Lavallee, C.F.; Falkenstein, M.; Herrmann, C.S. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int. J. Psychophysiol. 2013, 87, 217–233. [Google Scholar] [CrossRef]
- Pawliczek, C.M.; Derntl, B.; Kellermann, T.; Kohn, N.; Gur, R.C.; Habel, U. Inhibitory control and trait aggression: Neural and behavioral insights using the emotional stop signal task. NeuroImage 2013, 79, 264–274. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Laumann, T.O.; Gordon, E.M.; Adeyemo, B.; Snyder, A.Z.; Joo, S.J.; Chen, M.-Y.; Gilmore, A.W.; McDermott, K.B.; Nelson, S.M.; Dosenbach, N.U.F. Functional system and areal organization of a highly sampled individual human brain. Neuron 2015, 87, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Wang, D.; Fox, M.D.; Yeo, B.T.T.; Sepulcre, J.; Sabuncu, M.R.; Shafee, R.; Lu, J.; Liu, H. Individual variability in functional connectivity architecture of the human brain. Neuron 2013, 77, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [PubMed]
- Ko, L.-W.; Shih, Y.-C.; Chikara, R.K.; Chuang, Y.-T.; Chang, E.C. Neural Mechanisms of Inhibitory Response in a Battlefield Scenario: A Simultaneous fMRI-EEG Study. Front. Hum. Neurosci. 2016, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Chikara, R.K.; Chang, E.C.; Lu, Y.C.; Lin, D.S.; Lin, C.T.; Ko, L.W. Monetary Reward and Punishment to Response Inhibition Modulate Activation and Synchronization Within the Inhibitory Brain Network. Front. Hum. Neurosci. 2018, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Mishkin, M.; Ungerleider, L.G. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav. Brain Res. 1982, 6, 57–77. [Google Scholar] [CrossRef]
- Fogassi, L.; Luppino, G. Motor functions of the parietal lobe. Curr. Opin. Neurobiol. 2005, 15, 626–631. [Google Scholar] [CrossRef]
- Logan, G.D.; Cowan, W.B.; Davis, K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984, 10, 275–291. [Google Scholar] [CrossRef]
- Chikara, R.K.; Ko, L.-W. Modulation of the Visual to Auditory Human Inhibitory Brain Network: An EEG Dipole Source Localization Study. Brain Sci. 2019, 9, 216. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; Westerfield, M.; Townsend, J.; Courchesne, E.; Sejnowski, T.J. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. 2000, 111, 1745–1758. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; McKeown, M.J.; Bell, A.J.; Lee, T.W.; Sejnowski, T.J. Imaging brain dynamics using independent component analysis. Proc. IEEE 2001, 89, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.J.; Sejnowski, T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995, 7, 1129–1159. [Google Scholar] [CrossRef] [PubMed]
- Makeig, S.; Jung, T.P.; Bell, A.J.; Ghahremani, D.; Sejnowski, T.J. Blind separation of auditory event-related brain responses into independent components. Proc. Natl. Acad. Sci. USA 1997, 94, 10979. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Ko, L.W.; Lin, K.L.; Liang, S.F.; Kuo, B.C.; Chung, I.F.; Van, L.D. Classification of Driver’s Cognitive Responses Using Nonparametric Single-Trial EEG Analysis. In Proceedings of the 2007 IEEE International Symposium on Circuits and Systems, New Orleans, LA, USA, 27–30 May 2007. [Google Scholar]
- Subasi, A.; Ismail, G.M. EEG signal classification using PCA, ICA, LDA and support vector machines. Expert Syst. Appl. 2010, 37, 8659–8666. [Google Scholar] [CrossRef]
- Oweis, R.J.; Hamdi, N.; Ghazali, A.; Lwissy, K. A Comparison Study on Machine Learning Algorithms Utilized in P300-based BCI. J. Health Med. Inf. 2013, 4, 126. [Google Scholar] [CrossRef]
- Shen, H.; Chou, K.-C. Using optimized evidence-theoretic K-nearest neighbor classifier and pseudo-amino acid composition to predict membrane protein types. Biochem. Biophys. Res. Commun. 2005, 334, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Duan, F.; Solé-Casals, J.; Dinarès-Ferran, J.; Cichocki, A.; Yang, Z.; Sun, Z. A Novel Deep Learning Approach with Data Augmentation to Classify Motor Imagery Signals. IEEE Access 2019, 7, 15945. [Google Scholar] [CrossRef]
- Parzen, E. On estimation of a probability density function and mode. Ann. Math. Stat. 1962, 33, 1065–1076. [Google Scholar] [CrossRef]
- Iman, R.L.; Conover, W. A distribution-free approach to inducing rank correlation among input variables. Commun. Stat. Simul. Comput. 1982, 11, 311–334. [Google Scholar] [CrossRef]
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Chan, H.-P.; Wei, D.; Helvie, M.A.; Sahiner, B.; Adler, D.D.; Goodsitt, M.M.; Petrick, N. Computer-aided classification of mammographic masses and normal tissue: Linear discriminant analysis in texture feature space. Phys. Med. Biol. 1995, 40, 857. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Tsuruoka, S.; Kimura, F.; Miyake, Y. Increasing the Feature size in handwritten Numeral Recognition to improve accuracy. Syst. Comput. Jpn. 1995, 26, 35–44. [Google Scholar] [CrossRef]
- Harmony, T.; Alba, A.; Marroqun, J.; González-Frankenberger, B. Time-frequency-topographic analysis of induced power and synchrony of EEG signals during a Go/No-Go task. Int. J. Psychophysiol. 2009, 71, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Palva, S.; Palva, J.M. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007, 30, 150–158. [Google Scholar] [CrossRef]
- Swann, N.; Poizner, H.; Houser, M.; Gould, S.; Greenhouse, I.; Cai, W.; Strunk, J.; George, J.; Aron, A.R. Deep Brain Stimulation of the Subthalamic Nucleus Alters the Cortical Profile of Response Inhibition in the Beta Frequency Band: A Scalp EEG Study in Parkinson’s Disease. J. Neurosci. 2011, 186, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, M.L.; Pong-Wong, R.; Spiliopoulou, A.; Hayward, C.; Rudan, I.; Campbell, H.; Wright, A.F.; Wilson, J.F.; Agakov, F.; Navarro, P. Application of high-dimensional feature selection: Evaluation for genomic prediction in man. Sci. Rep. 2015, 5, 10312. [Google Scholar] [CrossRef] [PubMed]
- Efron, B.; Gong, G. A leisurely look at the bootstrap the jackknife and cross-validation. Am. Stat. 1983, 37, 36–48. [Google Scholar]
- Macnamara, B.N.; Hambrick, D.Z.; Oswald, F.L. Deliberate practice and performance in music, games, sports, education, and professions: A meta-analysis. Psychol. Sci. 2014, 25, 1608–1618. [Google Scholar] [CrossRef]
- Guerrieri, R.; Nederkoorn, C.; Jansen, A. Disinhibition is easier learned than inhibition. The effects of (dis)inhibition training on food intake. Appetite 2012, 59, 96–99. [Google Scholar] [CrossRef]
- Houben, K.; Nederkoorn, C.; Wiers, R.W.; Jansen, A. Resisting temptation: Decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug Alcohol Depend. 2011, 116, 132–136. [Google Scholar] [CrossRef]
- Verbruggen, F.; Adams, R.; Chambers, C.D. Proactive motor control reduces monetary risk taking in gambling. Psychol. Sci. 2012, 23, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Manuel, A.L.; Bernasconi, F.; Spierer, L. Plastic modifications within inhibitory control networks induced by practicing a stop-signal task: An electrical neuroimaging study. Cortex 2013, 49, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Berkman, E.T.; Kahn, L.E.; Merchant, J.S. Training induced changes in inhibitory control network activity. J. Neurosci. 2014, 34, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Asvestas, P.A.; Ventouras, E.; Karanasiou, I.; Matsopoulos, G.K. Classification of Event-Related Potentials associated with response errors in actors. In Proceedings of the 8th IEEE International Conference on BioInformatics and BioEngineering, Athens, Greece, 8–10 October 2008; pp. 1–6. [Google Scholar]
- Vasios, C.E.; Ventouras, E.M.; Matsopoulos, G.K.; Karanasiou, I.; Asvestas, P.; Uzunoglu, N.K.; Van Schie, H.T.; de Bruijn, E.R. Classification of event-related potentials associated with response errors in actors and observers based on autoregressive modeling. Open Med. Inform. J. 2009, 3, 32–43. [Google Scholar] [CrossRef]
- Cecotti, H.; Eckstein, M.P.; Giesbrecht, B. Single-Trial Classification of Event-Related Potentials in Rapid Serial Visual Presentation Tasks Using Supervised Spatial Filtering. IEEE Trans. Neural Netw. Learn. Syst. 2014, 25, 2030–2042. [Google Scholar] [CrossRef]
- Bissett, P.G.; Logan, G.D. Balancing cognitive demands: Control adjustments in the stop-signal paradigm. J. Exp. Psychol. Learn. Mem. Cogn. 2011, 37, 392–404. [Google Scholar] [CrossRef]
- Chikazoe, J.; Jimura, K.; Hirose, S.; Yamashita, K.I.; Miyashita, Y.; Konishi, S. Preparation to inhibit a response complements response inhibition during performance of a stop signal task. J. Neurosci. 2009, 29, 15870–15877. [Google Scholar] [CrossRef]
- Chikara, R.K.; Komarov, O.; Ko, L.W. Neural signature of event-related N200 and P300 modulation in parietal lobe during human response inhibition. Int. J. Comput. Biol. Drug Des. 2018, 11, 171–182. [Google Scholar] [CrossRef]
- Chikara, R.K.; Lo, W.-C.; Ko, L.-W. Exploration of Brain Connectivity during Human Inhibitory Control Using Inter-Trial Coherence. Sensors 2020, 20, 1722. [Google Scholar] [CrossRef]
- Leotti, L.A.; Wager, T.D. Motivational influences on response inhibition measures. J. Exp. Psychol. Hum. Percept. Perform. 2010, 36, 430–447. [Google Scholar] [CrossRef]

| Subject | Go Trial (75%) | Stop Trial (25%) | |||
|---|---|---|---|---|---|
| ID | RT (ms) | SG Ratio (%) | SSD (ms) | SSRT (ms) | SS Ratio (%) |
| 1 | 446 | 94 | 183 | 263 | 48 |
| 2 | 376 | 99 | 172 | 204 | 49 |
| 3 | 381 | 95 | 167 | 214 | 51 |
| 4 | 346 | 93 | 167 | 179 | 29 |
| 5 | 378 | 90 | 260 | 118 | 54 |
| 6 | 416 | 88 | 198 | 218 | 54 |
| 7 | 382 | 84 | 184 | 198 | 48 |
| 8 | 350 | 98 | 167 | 183 | 27 |
| 9 | 366 | 86 | 166 | 200 | 38 |
| 10 | 420 | 94 | 167 | 253 | 10 |
| 11 | 366 | 82 | 215 | 151 | 49 |
| 12 | 346 | 93 | 219 | 127 | 49 |
| 13 | 338 | 96 | 167 | 171 | 33 |
| 14 | 350 | 95 | 166 | 184 | 49 |
| 15 | 352 | 94 | 234 | 118 | 52 |
| 16 | 336 | 87 | 193 | 143 | 51 |
| 17 | 382 | 89 | 167 | 215 | 46 |
| 18 | 434 | 85 | 290 | 144 | 57 |
| 19 | 376 | 93 | 249 | 127 | 35 |
| 20 | 368 | 92 | 167 | 201 | 21 |
| (Average ± SD) | 375 ± 30 * | 91 ± 4 | 195 ± 36 * | 180 ± 41 * | 43 ± 12 |
| Subject | F3 | F4 | ||||||
|---|---|---|---|---|---|---|---|---|
| ID | QDA | LDA | KNNC | PARZEN | QDA | LDA | KNNC | PARZEN |
| 1 | 73.46 | 65.30 | 77.55 | 65.30 | 87.75 | 57.14 | 55.10 | 63.26 |
| 2 | 67.21 | 65.57 | 68.85 | 60.65 | 49.18 | 63.93 | 62.29 | 63.93 |
| 3 | 58.82 | 54.90 | 60.78 | 52.94 | 60.78 | 58.82 | 62.74 | 56.86 |
| 4 | 73.07 | 73.00 | 73.00 | 75.00 | 73.07 | 73.00 | 86.53 | 71.15 |
| 5 | 72.00 | 72.00 | 68.00 | 74.00 | 50.00 | 64.00 | 70.00 | 62.00 |
| 6 | 75.60 | 65.85 | 78.04 | 70.73 | 56.09 | 65.85 | 51.21 | 70.73 |
| 7 | 64.91 | 63.15 | 56.14 | 63.15 | 52.63 | 66.66 | 66.67 | 64.91 |
| 8 | 73.46 | 73.47 | 71.42 | 73.46 | 75.51 | 73.46 | 87.75 | 75.52 |
| 9 | 68.51 | 62.96 | 61.11 | 66.66 | 66.67 | 62.96 | 72.22 | 64.81 |
| 10 | 90.62 | 92.18 | 89.06 | 90.63 | 95.31 | 92.19 | 90.62 | 92.19 |
| 11 | 59.25 | 59.26 | 57.40 | 62.96 | 85.18 | 51.85 | 59.25 | 61.11 |
| 12 | 65.21 | 67.39 | 65.21 | 56.52 | 73.91 | 58.69 | 60.86 | 60.87 |
| 13 | 79.06 | 72.09 | 83.72 | 74.41 | 67.44 | 67.40 | 69.76 | 67.43 |
| 14 | 64.28 | 64.29 | 66.66 | 69.04 | 71.42 | 57.14 | 71.43 | 71.41 |
| 15 | 92.85 | 59.52 | 80.95 | 71.42 | 78.57 | 61.90 | 66.66 | 54.76 |
| 16 | 61.81 | 49.09 | 56.36 | 54.54 | 67.27 | 63.63 | 74.54 | 56.36 |
| 17 | 58.62 | 67.24 | 75.86 | 56.89 | 51.72 | 58.62 | 55.17 | 51.72 |
| 18 | 66.00 | 62.00 | 80.00 | 74.00 | 58.00 | 58.10 | 77.00 | 72.00 |
| 19 | 73.46 | 67.34 | 77.55 | 79.59 | 75.51 | 65.30 | 71.42 | 75.52 |
| 20 | 81.63 | 75.51 | 85.71 | 79.59 | 81.64 | 79.59 | 79.58 | 79.59 |
| Accuracy (Avg. ± SD) | 70.99 ± 9.40 | 66.60 ± 8.61 | 71.66 ± 9.81 | 68.57 ± 9.38 | 68.88 ± 12.98 | 65.01 ± 8.88 | 69.54 ± 10.74 | 66.80 ± 9.33 |
| Subject | C3 | C4 | ||||||
|---|---|---|---|---|---|---|---|---|
| ID | QDA | LDA | KNNC | PARZEN | QDA | LDA | KNNC | PARZEN |
| 1 | 77.55 | 73.46 | 73.47 | 73.46 | 63.26 | 63.27 | 65.30 | 71.42 |
| 2 | 77.04 | 65.57 | 63.93 | 67.21 | 49.18 | 55.73 | 63.93 | 59.01 |
| 3 | 66.66 | 54.90 | 66.66 | 66.67 | 64.70 | 64.71 | 68.62 | 72.54 |
| 4 | 73.07 | 73.00 | 69.23 | 71.15 | 75.00 | 73.07 | 84.61 | 76.92 |
| 5 | 76.00 | 58.00 | 56.00 | 60.00 | 68.00 | 66.00 | 70.00 | 66.00 |
| 6 | 58.53 | 58.54 | 56.09 | 53.65 | 48.78 | 56.09 | 60.97 | 51.21 |
| 7 | 57.89 | 70.17 | 59.64 | 70.18 | 66.66 | 63.15 | 68.42 | 64.91 |
| 8 | 69.38 | 73.46 | 85.71 | 73.46 | 73.46 | 77.55 | 81.63 | 77.56 |
| 9 | 72.22 | 68.51 | 75.92 | 72.22 | 75.92 | 64.81 | 59.25 | 74.07 |
| 10 | 90.63 | 90.62 | 90.63 | 92.18 | 93.75 | 90.62 | 90.60 | 96.87 |
| 11 | 90.74 | 62.96 | 64.81 | 55.55 | 55.56 | 62.96 | 57.40 | 57.41 |
| 12 | 71.73 | 60.86 | 76.08 | 60.86 | 65.21 | 54.34 | 54.35 | 56.52 |
| 13 | 67.44 | 69.76 | 62.79 | 72.09 | 69.76 | 67.44 | 81.39 | 79.06 |
| 14 | 57.14 | 57.15 | 69.04 | 52.38 | 71.42 | 57.14 | 78.57 | 69.04 |
| 15 | 97.61 | 57.14 | 71.42 | 61.90 | 92.85 | 59.52 | 71.42 | 69.04 |
| 16 | 61.81 | 58.18 | 54.54 | 58.18 | 54.54 | 74.54 | 69.09 | 76.36 |
| 17 | 50.00 | 65.51 | 65.51 | 60.34 | 60.34 | 62.06 | 56.89 | 51.72 |
| 18 | 58.00 | 60.00 | 66.00 | 60.00 | 52.00 | 54.00 | 58.00 | 74.00 |
| 19 | 63.26 | 63.27 | 67.34 | 63.26 | 67.34 | 63.26 | 69.38 | 65.30 |
| 20 | 81.63 | 79.59 | 79.60 | 81.64 | 79.59 | 79.60 | 85.71 | 85.72 |
| Accuracy (Avg. ± SD) | 70.91 ± 12.27 | 66.03 ± 8.74 | 68.72 ± 9.28 | 66.31 ± 9.55 | 67.36 ± 12.20 | 65.49 ± 9.21 | 69.77 ± 10.50 | 69.73 ± 11.06 |
| Subject | P3 | P4 | ||||||
|---|---|---|---|---|---|---|---|---|
| ID | QDA | LDA | KNNC | PARZEN | QDA | LDA | KNNC | PARZEN |
| 1 | 73.46 | 71.42 | 61.22 | 61.23 | 59.18 | 73.46 | 65.30 | 69.38 |
| 2 | 62.29 | 65.57 | 72.13 | 63.93 | 65.57 | 72.13 | 60.65 | 68.85 |
| 3 | 64.70 | 56.86 | 62.74 | 56.86 | 70.58 | 58.82 | 66.66 | 66.67 |
| 4 | 76.92 | 73.07 | 78.84 | 76.92 | 80.76 | 73.07 | 78.84 | 75.00 |
| 5 | 58.00 | 54.00 | 72.00 | 62.00 | 52.00 | 52.00 | 72.00 | 58.00 |
| 6 | 60.97 | 53.65 | 73.17 | 65.85 | 51.21 | 48.78 | 73.17 | 68.29 |
| 7 | 75.43 | 77.19 | 71.92 | 77.19 | 57.89 | 61.40 | 68.42 | 57.89 |
| 8 | 77.55 | 75.51 | 79.59 | 85.71 | 73.46 | 73.47 | 79.59 | 77.55 |
| 9 | 66.66 | 68.51 | 68.52 | 68.51 | 66.67 | 61.11 | 66.66 | 70.37 |
| 10 | 93.75 | 92.18 | 90.62 | 92.19 | 96.87 | 90.62 | 90.61 | 90.62 |
| 11 | 77.77 | 51.85 | 68.51 | 62.96 | 79.62 | 62.96 | 53.70 | 55.55 |
| 12 | 65.21 | 73.91 | 67.39 | 63.04 | 93.47 | 58.69 | 69.56 | 67.39 |
| 13 | 72.09 | 74.41 | 72.00 | 72.09 | 72.09 | 79.06 | 76.74 | 74.41 |
| 14 | 57.14 | 61.90 | 59.52 | 59.53 | 54.76 | 54.76 | 66.66 | 61.90 |
| 15 | 95.23 | 59.52 | 64.28 | 66.67 | 92.85 | 61.90 | 54.76 | 57.14 |
| 16 | 70.90 | 67.27 | 69.09 | 58.18 | 63.63 | 58.18 | 61.81 | 45.45 |
| 17 | 50.00 | 55.17 | 44.82 | 56.89 | 58.62 | 62.06 | 56.89 | 55.17 |
| 18 | 54.00 | 64.00 | 64.00 | 66.00 | 62.00 | 62.00 | 62.00 | 60.00 |
| 19 | 61.22 | 63.26 | 75.51 | 71.42 | 61.22 | 63.26 | 65.30 | 59.18 |
| 20 | 87.75 | 81.63 | 77.55 | 81.63 | 87.75 | 79.59 | 79.59 | 81.63 |
| Accuracy (Avg. ± SD) | 70.05 ± 12.19 | 67.04 ± 10.27 | 69.67 ± 9.11 | 68.44 ± 9.61 | 70.01 ± 13.87 | 65.36 ± 10.11 | 68.44 ± 9.12 | 66.02 ± 10.33 |
| Subject | O1 | O2 | ||||||
|---|---|---|---|---|---|---|---|---|
| ID | QDA | LDA | KNNC | PARZEN | QDA | LDA | KNNC | PARZEN |
| 1 | 61.22 | 63.26 | 59.18 | 61.22 | 65.30 | 69.38 | 67.34 | 67.35 |
| 2 | 59.01 | 59.00 | 50.18 | 59.01 | 55.73 | 54.09 | 47.54 | 60.65 |
| 3 | 64.70 | 60.78 | 66.66 | 62.74 | 54.90 | 54.90 | 60.78 | 62.74 |
| 4 | 69.23 | 71.15 | 75.00 | 75.00 | 73.07 | 73.07 | 80.76 | 84.61 |
| 5 | 58.00 | 62.00 | 62.00 | 50.00 | 62.00 | 68.00 | 72.00 | 64.00 |
| 6 | 63.41 | 63.41 | 70.73 | 63.41 | 73.17 | 60.97 | 73.17 | 60.97 |
| 7 | 64.91 | 59.64 | 57.89 | 57.89 | 56.14 | 57.89 | 66.66 | 64.91 |
| 8 | 73.47 | 73.46 | 79.59 | 77.55 | 83.67 | 85.71 | 81.63 | 85.71 |
| 9 | 64.81 | 62.96 | 68.51 | 70.37 | 57.40 | 62.96 | 61.11 | 61.11 |
| 10 | 96.87 | 89.06 | 92.18 | 89.06 | 93.75 | 90.62 | 90.62 | 89.06 |
| 11 | 77.77 | 59.25 | 61.11 | 64.81 | 59.25 | 51.85 | 59.25 | 55.55 |
| 12 | 58.69 | 73.91 | 76.08 | 67.93 | 80.43 | 71.73 | 80.43 | 82.60 |
| 13 | 74.41 | 79.06 | 72.09 | 76.74 | 72.09 | 67.44 | 67.44 | 74.41 |
| 14 | 61.90 | 73.80 | 69.04 | 73.80 | 64.28 | 69.04 | 66.66 | 69.04 |
| 15 | 97.61 | 54.76 | 66.66 | 69.04 | 78.57 | 59.52 | 64.28 | 71.42 |
| 16 | 58.18 | 63.63 | 67.27 | 54.54 | 63.63 | 63.63 | 52.72 | 63.63 |
| 17 | 62.06 | 63.79 | 68.96 | 62.06 | 60.34 | 58.62 | 65.51 | 60.34 |
| 18 | 66.00 | 56.00 | 70.00 | 62.00 | 64.00 | 66.00 | 72.00 | 70.00 |
| 19 | 77.55 | 61.22 | 69.38 | 59.18 | 71.42 | 61.22 | 65.30 | 63.26 |
| 20 | 81.63 | 79.59 | 83.67 | 79.59 | 85.71 | 81.63 | 89.79 | 89.79 |
| Accuracy (Avg. ± SD) | 69.57 ± 11.49 | 66.48 ± 8.80 | 69.30 ± 9.11 | 66.79 ± 9.34 | 68.74 ± 10.86 | 66.41 ± 10.08 | 69.24 ± 10.90 | 70.05 ± 10.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikara, R.K.; Ko, L.-W. Prediction of Human Inhibition Brain Function with Inter-Subject and Intra-Subject Variability. Brain Sci. 2020, 10, 726. https://doi.org/10.3390/brainsci10100726
Chikara RK, Ko L-W. Prediction of Human Inhibition Brain Function with Inter-Subject and Intra-Subject Variability. Brain Sciences. 2020; 10(10):726. https://doi.org/10.3390/brainsci10100726
Chicago/Turabian StyleChikara, Rupesh Kumar, and Li-Wei Ko. 2020. "Prediction of Human Inhibition Brain Function with Inter-Subject and Intra-Subject Variability" Brain Sciences 10, no. 10: 726. https://doi.org/10.3390/brainsci10100726
APA StyleChikara, R. K., & Ko, L.-W. (2020). Prediction of Human Inhibition Brain Function with Inter-Subject and Intra-Subject Variability. Brain Sciences, 10(10), 726. https://doi.org/10.3390/brainsci10100726




