Bridging Blood and Skin: Biomarker Profiling in Dermal Interstitial Fluid (dISF) for Minimally Invasive Diagnostics
Abstract
1. Introduction
2. Materials and Methods
2.1. Disf Collector, Skin Damage Assessment and Study Plan
2.2. Total Protein Content, HSA, and IgG Quantification
2.3. Meso Scale Discovery (MSD)
2.4. Olink 48 Target Cytokine Panel
3. Results
3.1. dISF Collector Performance
3.2. OCT Skin Damage Assessment
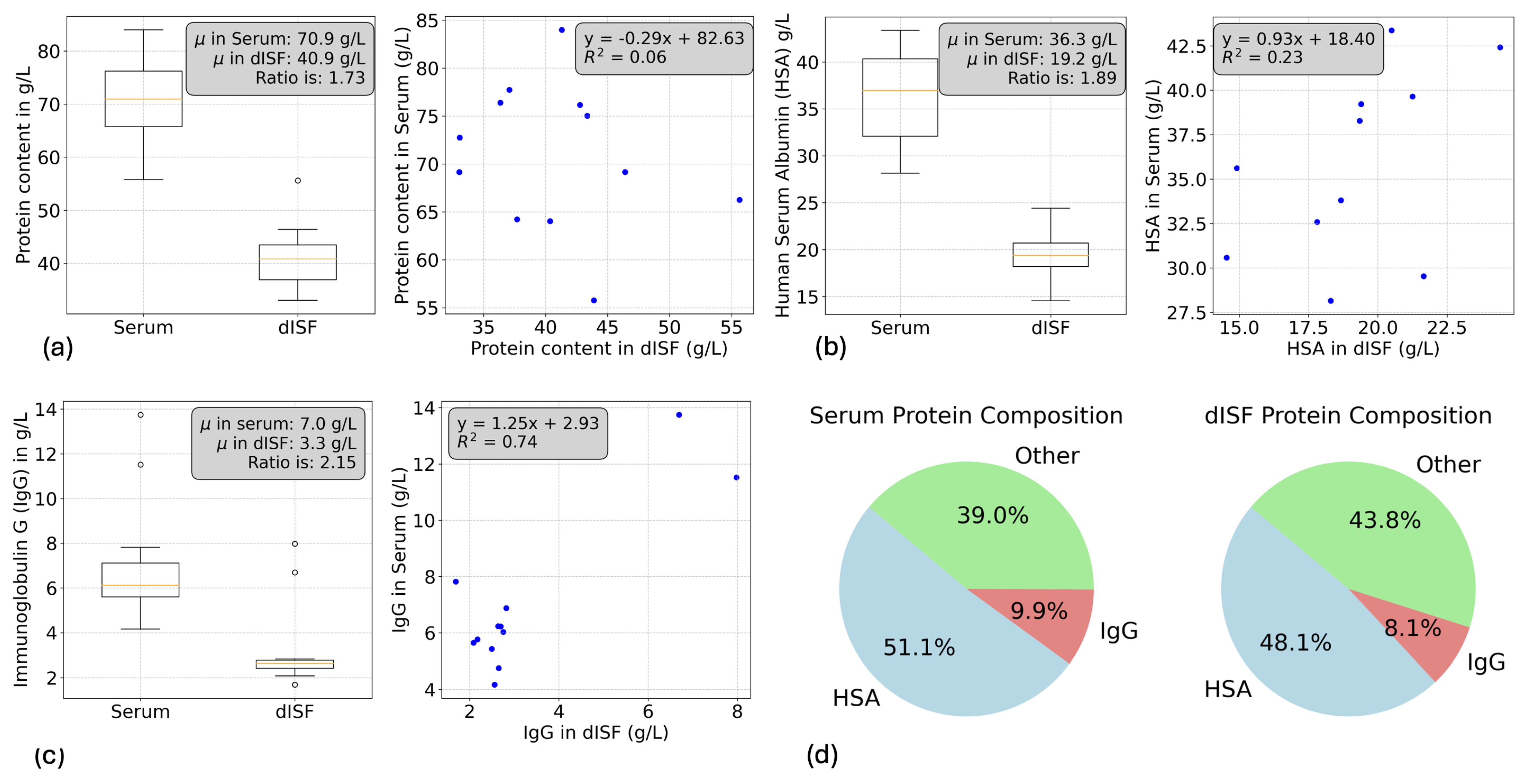
3.3. Albumin, Immunoglobulin G (IgG) and Total Protein
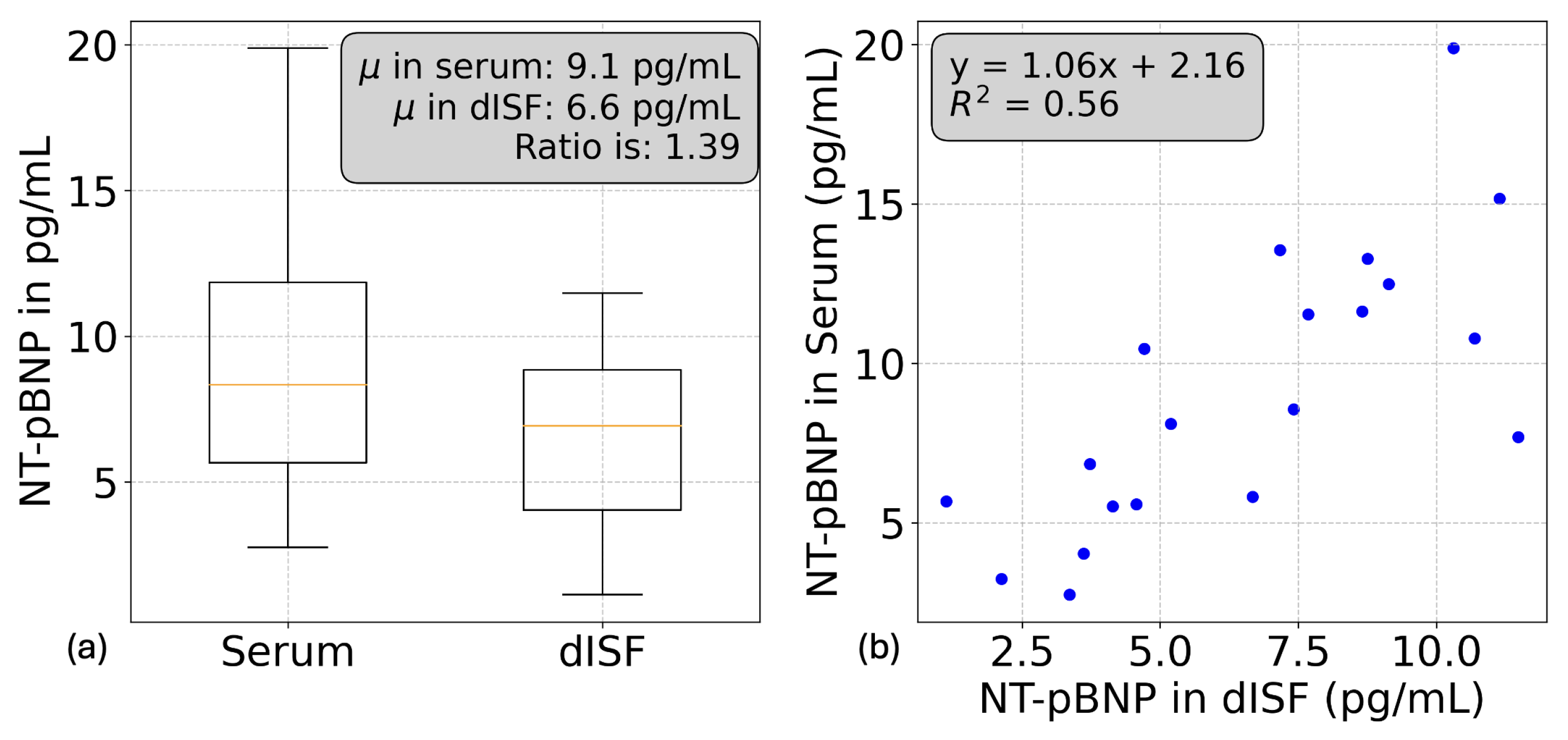
3.4. Baseline Inflammatory Profile and NT-pBNP
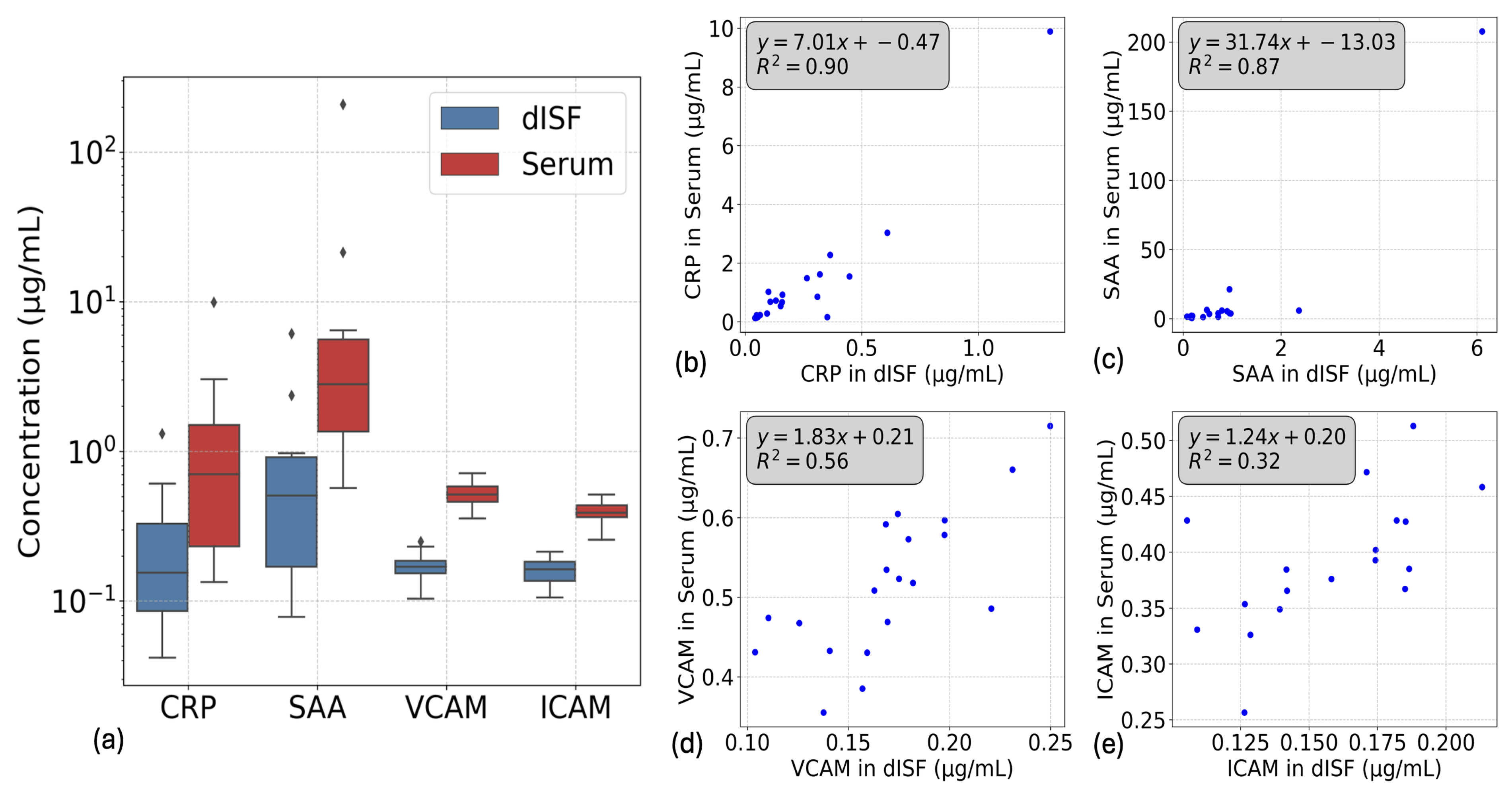
3.4.1. V-Plex Injury Panel and NT-pBNP
3.4.2. OLink Target 48 Cytokine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| dISF | Dermal Interstitial Fluid |
| SBF | Suction Blister Fluid |
| HSA | Human Serum Albumin |
| IgG | Immunoglobulin G |
| CRP | C-Reactive Protein |
| SAA | Serum Amyloid A |
| VCAM | Vascular Cell Adhesion Molecule 1 |
| ICAM | Intercellular Adhesion Molecule 1 |
| NT-pBNP | N-terminal pro b-type natriuretic peptide |
| CGMs | Continuous Glucose Monitors |
| PEA | Proximity Extension Assay |
| MSD | Meso Scale Discovery |
| OCT | Optical Coherence Tomography |
| BCA | BiCinchoninic acid Assay |
References
- Friedel, M.; Thompson, I.A.P.; Kasting, G.; Polsky, R.; Cunningham, D.; Soh, H.T.; Heikenfeld, J. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. 2023, 7, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mao, S.; Chen, S.; Ma, J.; Jaffrezic-Renault, N.; Guo, Z. Opportunities and challenges of microneedle electrochemical sensors for interstitial fluid detection. TrAC Trends Anal. Chem. 2024, 180, 117891. [Google Scholar] [CrossRef]
- Rodbard, D. Continuous glucose monitoring: A review of successes, challenges, and opportunities. Diabetes Technol. Ther. 2016, 18, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res. Clin. Pract. 2017, 133, 178–192. [Google Scholar] [CrossRef]
- Samant, P.P.; Niedzwiecki, M.M.; Raviele, N.; Tran, V.; Mena-Lapaix, J.; Walker, D.I.; Felner, E.I.; Jones, D.P.; Miller, G.W.; Prausnitz, M.R. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 2020, 12, eaaw0285. [Google Scholar] [CrossRef]
- Haslene-Hox, H.; Oveland, E.; Berg, K.C.; Kolmannskog, O.; Woie, K.; Salvesen, H.B.; Tenstad, O.; Wiig, H. A new method for isolation of interstitial fluid from human solid tumors applied to proteomic analysis of ovarian carcinoma tissue. PLoS ONE 2011, 6, e19217. [Google Scholar] [CrossRef]
- Kiistala, U. Suction blister device for separation of viable epidermis from dermis. J. Investig. Dermatol. 1968, 50, 129–137. [Google Scholar] [CrossRef]
- Schmidt, S.; Banks, R.; Kumar, V.; Rand, K.H.; Derendorf, H. Clinical microdialysis in skin and soft tissues: An update. J. Clin. Pharmacol. 2008, 48, 351–364. [Google Scholar] [CrossRef]
- Bodenlenz, M.; Tiffner, K.I.; Raml, R.; Augustin, T.; Dragatin, C.; Birngruber, T.; Schimek, D.; Schwagerle, G.; Pieber, T.R.; Raney, S.G.; et al. Open flow microperfusion as a dermal pharmacokinetic approach to evaluate topical bioequivalence. Clin. Pharmacokinet. 2017, 56, 91–98. [Google Scholar] [CrossRef]
- Sieg, A.; Guy, R.H.; Delgado-Charro, M.B. Electroosmosis in transdermal iontophoresis: Implications for noninvasive and calibration-free glucose monitoring. Biophys. J. 2004, 87, 3344–3350. [Google Scholar] [CrossRef]
- Saifullah, K.M.; Rad, Z.F. Sampling dermal interstitial fluid using microneedles: A review of recent developments in sampling methods and microneedle-based biosensors. Adv. Mater. Interfaces 2023, 10, 2201763. [Google Scholar] [CrossRef]
- Miller, P.R.; Taylor, R.M.; Tran, B.Q.; Boyd, G.; Glaros, T.; Chavez, V.H.; Polsky, R. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun. Biol. 2018, 1, 173. [Google Scholar] [CrossRef] [PubMed]
- Sjöbom, U.; Christenson, K.; Hellström, A.; Nilsson, A.K. Inflammatory markers in suction blister fluid: A comparative study between interstitial fluid and plasma. Front. Immunol. 2020, 11, 597632. [Google Scholar] [CrossRef]
- Li, Y.; Long, Y.; Chen, X.; Wang, T.; Guo, J.; Jin, L.; Hou, Z. Cytokine patterns in the blister fluid and plasma of patients with fracture blisters. Int. Immunopharmacol. 2023, 123, 110738. [Google Scholar] [CrossRef]
- Monedeiro, F.; Ehall, B.; Tiffner, K.; Eberl, A.; Svehlikova, E.; Prietl, B.; Birngruber, T. Characterization of Inflammatory Mediators and Metabolome in Interstitial Fluid Collected with Dermal Open Flow Microperfusion before and at the End of Dupilumab Treatment in Atopic Dermatitis. J. Proteome Res. 2024, 23, 3496–3514. [Google Scholar] [CrossRef]
- Tehrani, F.; Teymourian, H.; Wuerstle, B.; Kavner, J.; Patel, R.; Furmidge, A.; Wang, J. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 2022, 6, 1214–1224. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive microneedle-based biosensor array for simultaneous lactate and glucose monitoring in artificial interstitial fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef]
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Wang, J. Microneedle-based detection of ketone bodies along with glucose and lactate: Toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal. Chem. 2019, 92, 2291–2300. [Google Scholar] [CrossRef]
- Clark, V.L.; Kruse, J.A. Clinical methods: The history, physical, and laboratory examinations. JAMA 1990, 264, 2808–2809. [Google Scholar]
- Mishra, V.; Heath, R.J. Structural and biochemical features of human serum albumin essential for eukaryotic cell culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef]
- Rutili, G.; Arfors, K.E. Protein concentration in interstitial and lymphatic fluids from the subcutaneous tissue. Acta Physiol. Scand. 1977, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, H.O.; Reed, R.K.; Aukl, K. Mechanisms regulating interstitial fluid volume. Lymphology 1978, 11, 165–169. [Google Scholar] [PubMed]
- Young, B.; Gleeson, M.; Cripps, A.W. C-reactive protein: A critical review. Pathology 1991, 23, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.K.; Rubin, K. Transcapillary exchange: Role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc. Res. 2010, 87, 211–217. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprunger, Y.; Longo, J.; Saeidi, A.; Ionescu, A.M. Bridging Blood and Skin: Biomarker Profiling in Dermal Interstitial Fluid (dISF) for Minimally Invasive Diagnostics. Biosensors 2025, 15, 301. https://doi.org/10.3390/bios15050301
Sprunger Y, Longo J, Saeidi A, Ionescu AM. Bridging Blood and Skin: Biomarker Profiling in Dermal Interstitial Fluid (dISF) for Minimally Invasive Diagnostics. Biosensors. 2025; 15(5):301. https://doi.org/10.3390/bios15050301
Chicago/Turabian StyleSprunger, Yann, Johan Longo, Ali Saeidi, and Adrian M. Ionescu. 2025. "Bridging Blood and Skin: Biomarker Profiling in Dermal Interstitial Fluid (dISF) for Minimally Invasive Diagnostics" Biosensors 15, no. 5: 301. https://doi.org/10.3390/bios15050301
APA StyleSprunger, Y., Longo, J., Saeidi, A., & Ionescu, A. M. (2025). Bridging Blood and Skin: Biomarker Profiling in Dermal Interstitial Fluid (dISF) for Minimally Invasive Diagnostics. Biosensors, 15(5), 301. https://doi.org/10.3390/bios15050301




