An Electrochemical Aptasensor for Accurate and Sensitive Detection of Exosomes Based on Dual-Probe Recognition and Hybridization Chain Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Apparatus
2.2. Preparation of Exosome–HCR Complexes
2.3. Fabrication of the Electrochemical Aptasensor
3. Results
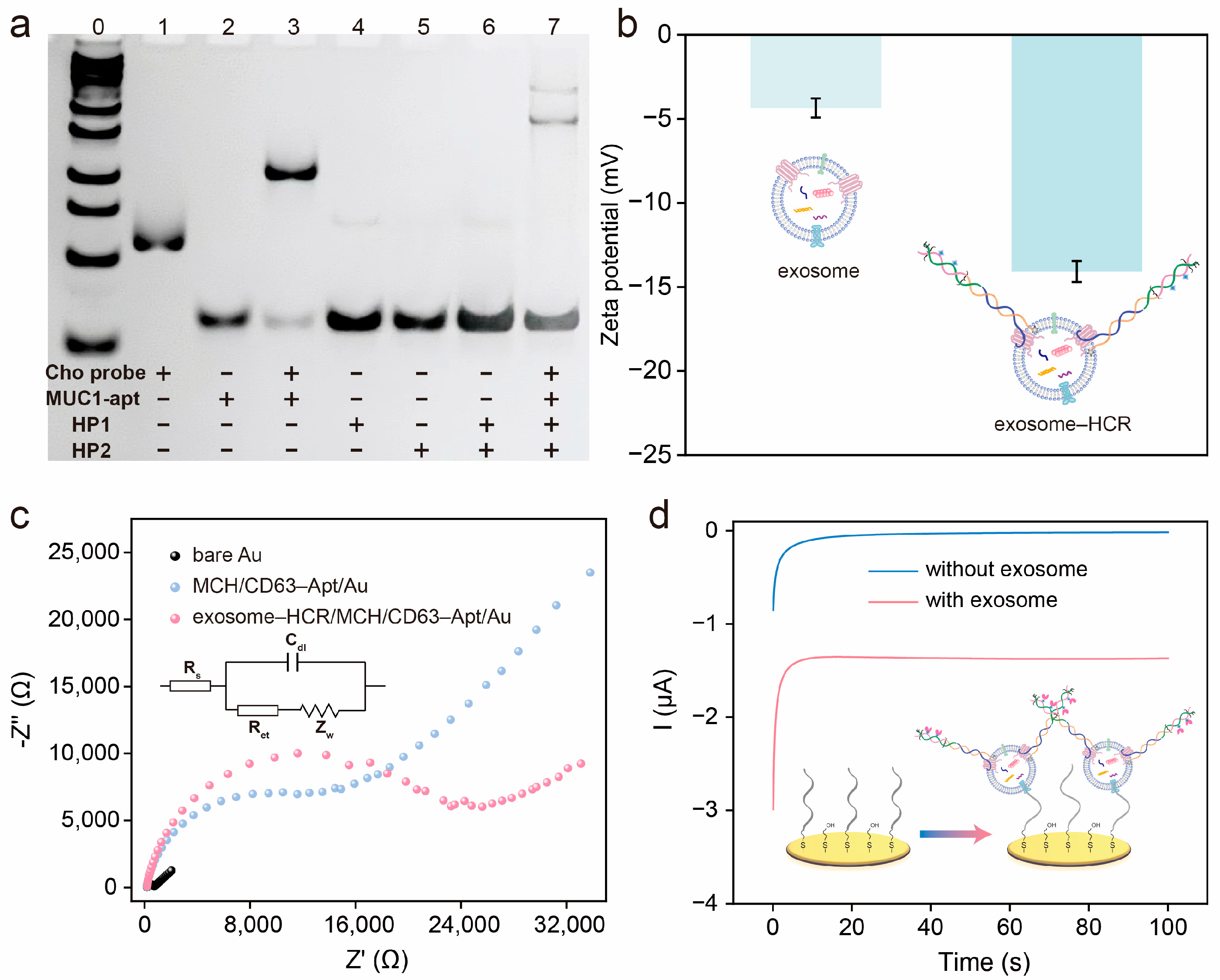
3.1. Design Principle of the Electrochemical Aptasensor
3.2. Characterization of Exosomes
3.3. Feasibility of the Electrochemical Aptasensor for Exosome Detection
3.4. Optimization of Experimental Conditions
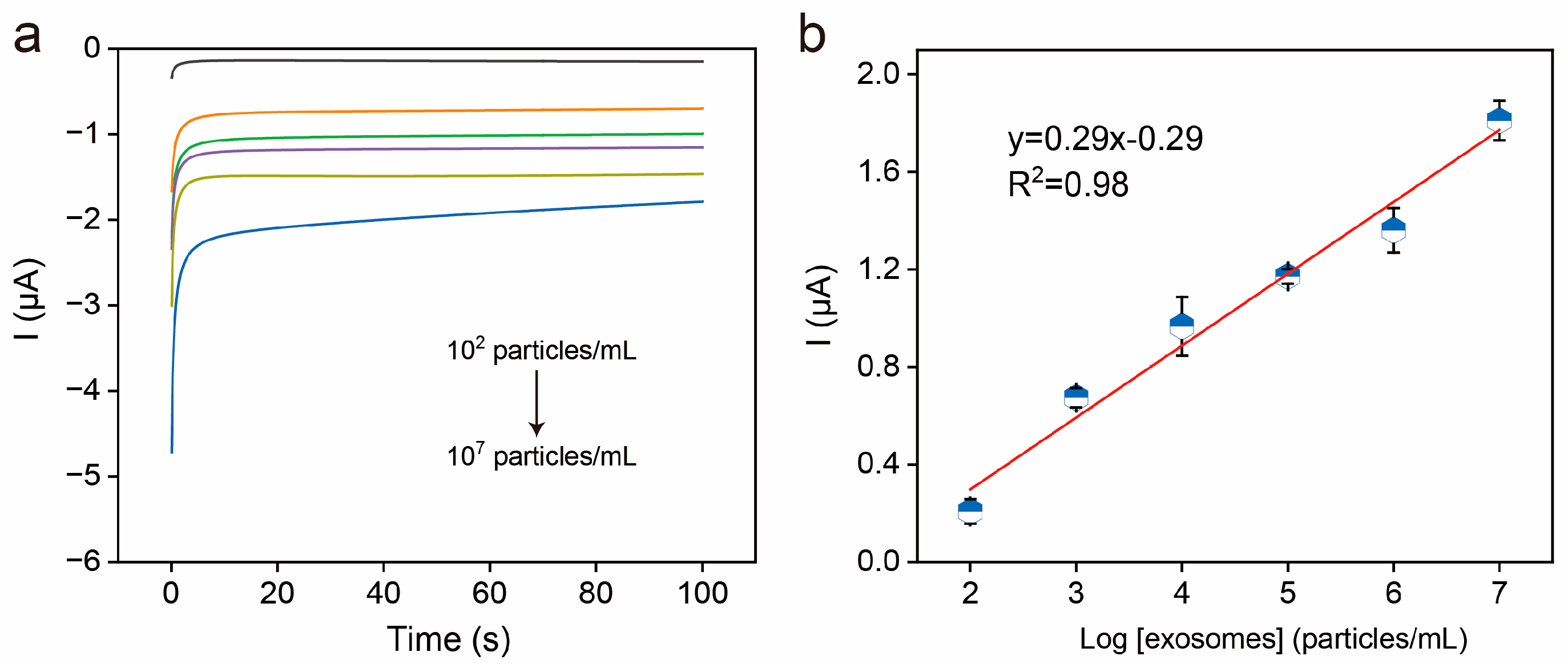
3.5. Electrochemical Detection of Exosomes
3.6. Selectivity, Anti-Interference, Reproducibility, and Stability of the Electrochemical Aptasensor
3.7. Detection of Exosome in Serum Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Wang, B.; Lai, H.; Li, S.; You, Q.; Fang, Y.; Li, Q.; Liu, Y. Long Non-Coding RNA Crala Is Associated with Poor Response to Chemotherapy in Primary Breast Cancer. Thorac. Cancer 2017, 8, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, R.; Dastgiri, S.; de Bock, G.H.; Sanaat, Z.; Ranjkesh, M.; Jabbaripour, P.; Pashaee, S. The First Screening Experiences of Breast Cancer in Northwest of Iran. J. Clin. Oncol. 2018, 36, e13551. [Google Scholar] [CrossRef]
- Sebastião, A.I.; Simões, G.; Oliveira, F.; Mateus, D.; Falcão, A.; Carrascal, M.A.; Gomes, C.; Neves, B.; Cruz, M.T. Dendritic Cells in Triple-Negative Breast Cancer: From Pathophysiology to Therapeutic Applications. Cancer Treat. Rev. 2025, 133, 102884. [Google Scholar] [CrossRef]
- Sadeghi, M.; Sadeghi, S.; Naghib, S.M.; Garshasbi, H.R. A Comprehensive Review on Electrochemical Nano Biosensors for Precise Detection of Blood-Based Oncomarkers in Breast Cancer. Biosensors 2023, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour-Haratbar, A.; Boraei, S.B.; Zare, Y.; Rhee, K.Y.; Park, S.-J. Graphene-Based Electrochemical Biosensors for Breast Cancer Detection. Biosensors 2023, 13, 80. [Google Scholar] [CrossRef]
- Moroni, S.; Casettari, L.; Lamprou, D.A. 3D and 4D Printing in the Fight against Breast Cancer. Biosensors 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Cao, Y. CA 15-3 Elevation in U.S. Women without Breast Cancer. J. Clin. Oncol. 2018, 38, e13609. [Google Scholar] [CrossRef]
- Bae, S.Y.; Lim, W.; Jeong, J.; Lee, S.; Choi, J.; Park, H.; Jung, Y.S.; Jung, S.P.; Nam, S. The Prognostic Significance of Preoperative Tumour Marker (CEA, CA 15-3) Elevation in Breast Cancer Patients. Ann. Oncol. 2019, 30, v93. [Google Scholar] [CrossRef]
- Zhou, Q.; Pan, Y.; Liang, Q.; Yang, J.; Zhu, S.; Shi, H.; Li, G. Peptide-Guided Assembly of Silver Nanoparticles for the Diagnosis of HER2-Positive Breast Cancer. Anal. Chem. 2024, 96, 19304–19311. [Google Scholar] [CrossRef]
- Li, H.; Tie, X.J. Exploring Research Progress in Studying Serum Exosomal miRNA-21 as a Molecular Diagnostic Marker for Breast Cancer. Clin. Transl. Oncol. 2024, 26, 2166–2171. [Google Scholar] [CrossRef]
- Tokumaru, Y.; Oshi, M.; Katsuta, E.; Murthy, V.; Matsuhashi, N.; Futamura, M.; Yoshida, K.; Takabe, K. Low Expression of microRNA-195 Is a Poor Prognostic Marker for ER-Positive Breast Cancer Patients. J. Clin. Oncol. 2021, 39, e12576. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Fan, X.; Zhang, P.; Wang, P.; Cheng, S.; Zhang, J. MicroRNA-125b as a Tumor Suppressor by Targeting MMP11 in Breast Cancer. Thorac. Cancer 2020, 11, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yu, X.; Zeng, T.; Fu, Z.; Zhao, Y.; Nie, B.; Zhao, J.; Yin, Y.; Li, G. Molecular Characterization of Exosomes for Subtype-Based Diagnosis of Breast Cancer. J. Am. Chem. Soc. 2022, 144, 13475–13486. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Fan, Y.; Pionneau, C.; Cocozza, F.; Boëlle, P.-Y.; Chardonnet, S.; Charrin, S.; Théry, C.; Zimmermann, P.; Rubinstein, E. Differential Proteomics Argues against a General Role for CD9, CD81 or CD63 in the Sorting of Proteins into Extracellular Vesicles. J. Extracel. l Vesicles 2023, 12, 12352. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, S.; Luo, X.; Xian, Y.; Zhang, C. Polymerase-Driven Logic Signal Amplification for the Detection of Small Extracellular Vesicle Surface Proteins and the Identification of Breast Cancer. Anal. Chem. 2023, 95, 10330–10336. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Q.; Huang, Z.; Xiong, H.; Yang, B.; Chen, H.; Kong, J. Aptamer-Initiated Catalytic Hairpin Assembly Fluorescence Assay for Universal, Sensitive Exosome Detection. Anal. Chem. 2022, 94, 5723–5728. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Li, Y.; Hai, X.; Bi, S. A Colorimetric and Photothermal Dual-Mode Biosensing Platform Based on Nanozyme-Functionalized Flower-Like DNA Structures for Tumor-Derived Exosome Detection. Talanta 2023, 258, 124456. [Google Scholar] [CrossRef]
- Wang, Y.; Jie, H.; Ye, H.; Zhang, Y.; Li, N.; Zhuang, J. Methylene Blue-Stained Single-Stranded DNA Aptamers as a Highly Efficient Electronic Switch for Quasi-Reagentless Exosomes Detection: An Old Dog with New Tricks. Anal. Chem. 2023, 95, 18166–18173. [Google Scholar] [CrossRef]
- Gong, L.; Chen, B.; Tong, Y.; Luo, Y.; Zhu, D.; Chao, J.; Wang, L.; Su, S. Two-in-One Sensing Platform for the Detection of Exosomes Based on Molybdenum Disulfide-Based Nanozymes. Sensor. Actuat. B Chem. 2024, 398, 134686. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Chiu, N.-F.; Hsiao, Y.-S.; Lai, Y.-J.; Nanda, H.S. Advances in Nanoplasmonic Biosensors: Optimizing Performance for Exosome Detection Applications. Biosensors 2024, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, T.; Chen, W.; Chen, G.; Xu, L.; Zhang, L.; Zhang, X.; Sun, W.; Lan, J.; Lin, X.; et al. A Dual-Modal Aptasensor Based on a Multifunctional Acridone Derivate for Exosomes Detection. Anal. Chim. Acta 2022, 1191, 339279. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, L.; Wang, Z.; Irfan, M.; Qu, F. Recent Advances of Aptasensors for Exosomes Detection. Biosens. Bioelectron. 2020, 160, 112213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, S.; Tang, X.; Feng, L.; Ding, Z.; Chen, Z.; Luo, X.; Deng, R.; Sheng, J.; Xie, S.; et al. DNA Four-Way Junction-Driven Dual-Rolling Circle Amplification Sandwich-Type Aptasensor for Ultra-Sensitive and Specific Detection of Tumor-Derived Exosomes. Biosens. Bioelectron. 2024, 246, 115841. [Google Scholar] [CrossRef]
- He, Y.; Zeng, X.; Xiong, Y.; Shen, C.; Huang, K.; Chen, P. Portable Aptasensor Based on Parallel Rolling Circle Amplification for Tumor-Derived Exosomes Liquid Biopsy. Adv. Sci. 2024, 11, 2403371. [Google Scholar] [CrossRef]
- Abramowicz, A.; Widlak, P.; Pietrowska, M. Proteomic Analysis of Exosomal Cargo: The Challenge of High Purity Vesicle Isolation. Mol. Biosyst. 2016, 12, 1407–1419. [Google Scholar] [CrossRef]
- Ercole, F.; Whittaker, M.R.; Quinn, J.F.; Davis, T.P. Cholesterol Modified Self-Assemblies and Their Application to Nanomedicine. Biomacromolecules 2015, 16, 1886–1914. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, Q.; He, M.; Chen, B.; Chen, G.; Yin, X.; Kang, Q.; Xu, Y.; Hu, B. Sensitive Detection of Exosomes by Gold Nanoparticles Labeling Inductively Coupled Plasma Mass Spectrometry Based on Cholesterol Recognition and Rolling Circle Amplification. Anal. Chim. Acta 2022, 1212, 339938. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, C.; Mei, Q.; Zhang, H.; Zhang, W.; Su, D.; Fu, W.; Luo, Y. Aptamer-Cholesterol-Mediated Proximity Ligation Assay for Accurate Identification of Exosomes. Anal. Chem. 2020, 92, 5411–5418. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Cai, M.-D.; Sun, L.-L.; Hua, R.-N. A Rapid and Facile Separation–Detection Integrated Strategy for Exosome Profiling Based on Boronic Acid-Directed Coupling Immunoaffinity. Anal. Chem. 2021, 93, 16059–16067. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, J.; Xu, H.; Yuan, K.; Aryee, A.A.; Zhang, C.; Meng, H.; Qu, L.; Li, Z. Split-Aptamer Mediated Regenerable Temperature-Sensitive Electrochemical Biosensor for the Detection of Tumour Exosomes. Anal. Chim. Acta 2022, 1219, 340027. [Google Scholar] [CrossRef]
- Pallares-Rusiñol, A.; Moura, S.L.; Martí, M.; Pividori, M.I. Electrochemical Genosensing of Overexpressed Gapdh Transcripts in Breast Cancer Exosomes. Anal. Chem. 2023, 95, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, L.; Deng, Y.; Wang, M.; Peng, Y.; Yang, J.; Li, G. A Simple and Sensitive Method for Exosome Detection Based on Steric Hindrance-Controlled Signal Amplification. Chem. Commun. 2020, 56, 13768–13771. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, L.; Sang, X.; Jiang, X.; Wang, H.; Qin, P.; Huang, L. Colorimetric Aptasensor Based on Spherical Nucleic Acid-Induced Hybridization Chain Reaction for Sensitive Detection of Exosomes. Talanta 2023, 258, 124453. [Google Scholar] [CrossRef]
- Pan, H.; Dong, Y.; Gong, L.; Zhai, J.; Song, C.; Ge, Z.; Su, Y.; Zhu, D.; Chao, J.; Su, S.; et al. Sensing Gastric Cancer Exosomes with MoS2-Based SERS Aptasensor. Biosens. Bioelectron. 2022, 215, 114553. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Liu, W.; Zhang, K.; Zhao, H.; Zhang, H.; Zhang, Z. A Simple, Specific and “on-off” Type MUC1 Fluorescence Aptasensor Based on Exosomes for Detection of Breast Cancer. Sensor. Actuat. B Chem. 2018, 276, 552–559. [Google Scholar] [CrossRef]




| Methods | Linear Range (Particles/mL) | Limit of Detection (Particles/mL) | Detection Time (Min) | Ref. |
|---|---|---|---|---|
| Electrochemistry | 102−107 | 20 | 360 | [25] |
| Fluorescence | 102−104 | 30 | 328 | [26] |
| Electrochemistry | 2.0 × 106−4.0 × 108 | 1.5 × 106 | 30 | [32] |
| Electrochemistry | 105−4.0 × 107 | 4 × 105 | 206 | [33] |
| Fluorescence | 1.66 × 106−1.66 × 109 | 4.8 × 105 | 250 | [34] |
| Colorimetry | 5 × 104−1.0 × 106 | 5 × 104 | 150 | [35] |
| Surface-enhanced Raman scattering | 5.5 × 104−5.5 × 108 | 1.7 × 104 | 60 | [36] |
| Electrochemistry | 102−107 | 45 | 135 | This work |
| Sample | Added (Particles/mL) | Detected (Particles/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 1.00 × 102 | 1.02 × 102 | 102.00 | 9.89 |
| 2 | 1.00 × 104 | 1.07 × 104 | 107.00 | 5.27 |
| 3 | 1.00 × 107 | 0.97 × 107 | 97.00 | 9.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Li, J.; Gao, M.; Dong, Y.; Luo, Y.; Su, S. An Electrochemical Aptasensor for Accurate and Sensitive Detection of Exosomes Based on Dual-Probe Recognition and Hybridization Chain Reaction. Biosensors 2025, 15, 302. https://doi.org/10.3390/bios15050302
Ma H, Li J, Gao M, Dong Y, Luo Y, Su S. An Electrochemical Aptasensor for Accurate and Sensitive Detection of Exosomes Based on Dual-Probe Recognition and Hybridization Chain Reaction. Biosensors. 2025; 15(5):302. https://doi.org/10.3390/bios15050302
Chicago/Turabian StyleMa, Haojie, Jie Li, Mengjia Gao, Yan Dong, Yi Luo, and Shao Su. 2025. "An Electrochemical Aptasensor for Accurate and Sensitive Detection of Exosomes Based on Dual-Probe Recognition and Hybridization Chain Reaction" Biosensors 15, no. 5: 302. https://doi.org/10.3390/bios15050302
APA StyleMa, H., Li, J., Gao, M., Dong, Y., Luo, Y., & Su, S. (2025). An Electrochemical Aptasensor for Accurate and Sensitive Detection of Exosomes Based on Dual-Probe Recognition and Hybridization Chain Reaction. Biosensors, 15(5), 302. https://doi.org/10.3390/bios15050302






