Optimizing Whole-Cell Biosensors for the Early Detection of Crop Infections: A Proof-of-Concept Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Bacterial Strains and Growth Conditions
2.4. Inoculation of Whole Tubers with Pectobacterium Brasiliense
2.5. Preparation of Calcium Alginate Tablets for Bacterial Immobilization
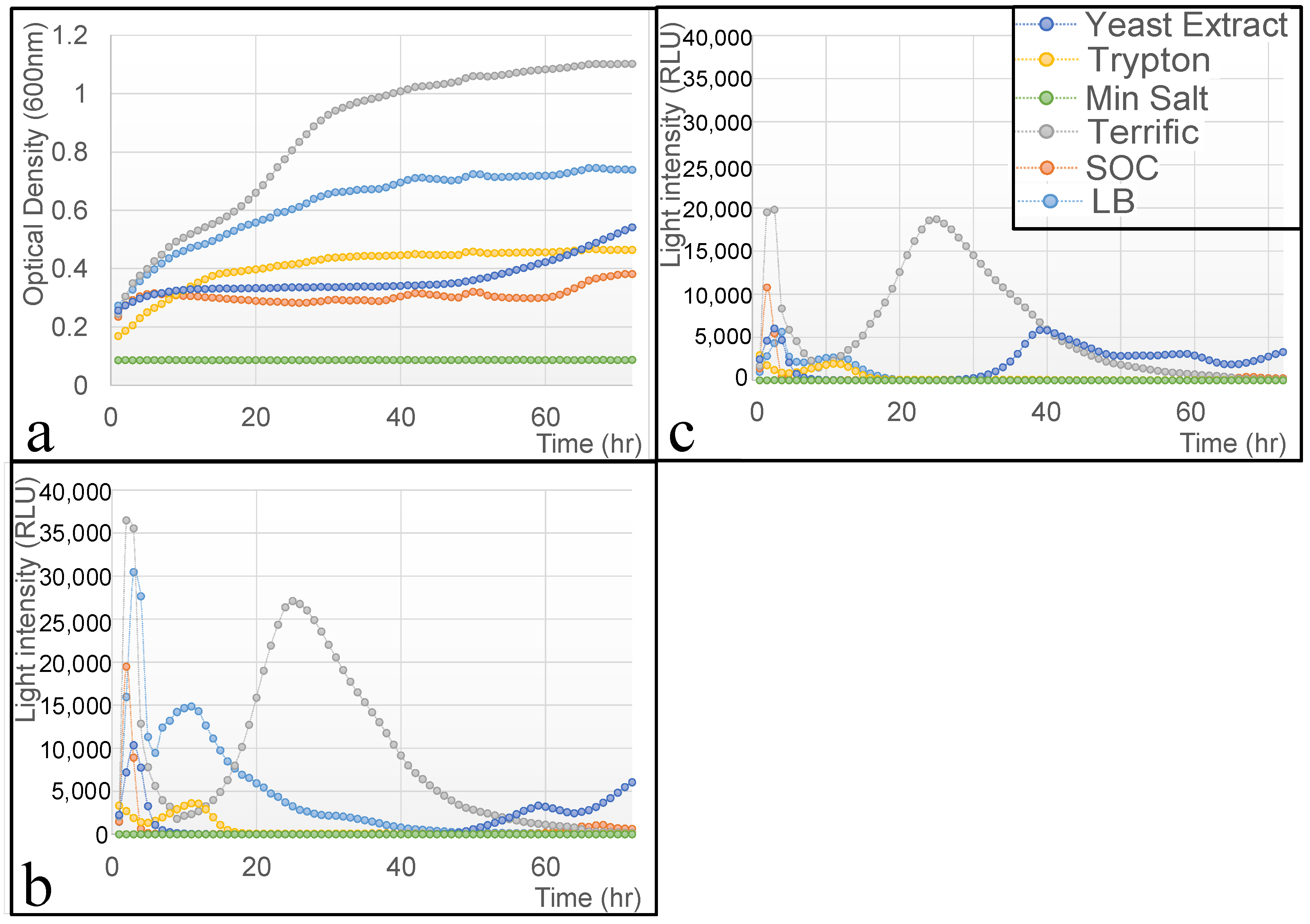
2.6. Evaluation of Effect of the Medium on the Cells’ Viability and Functionality
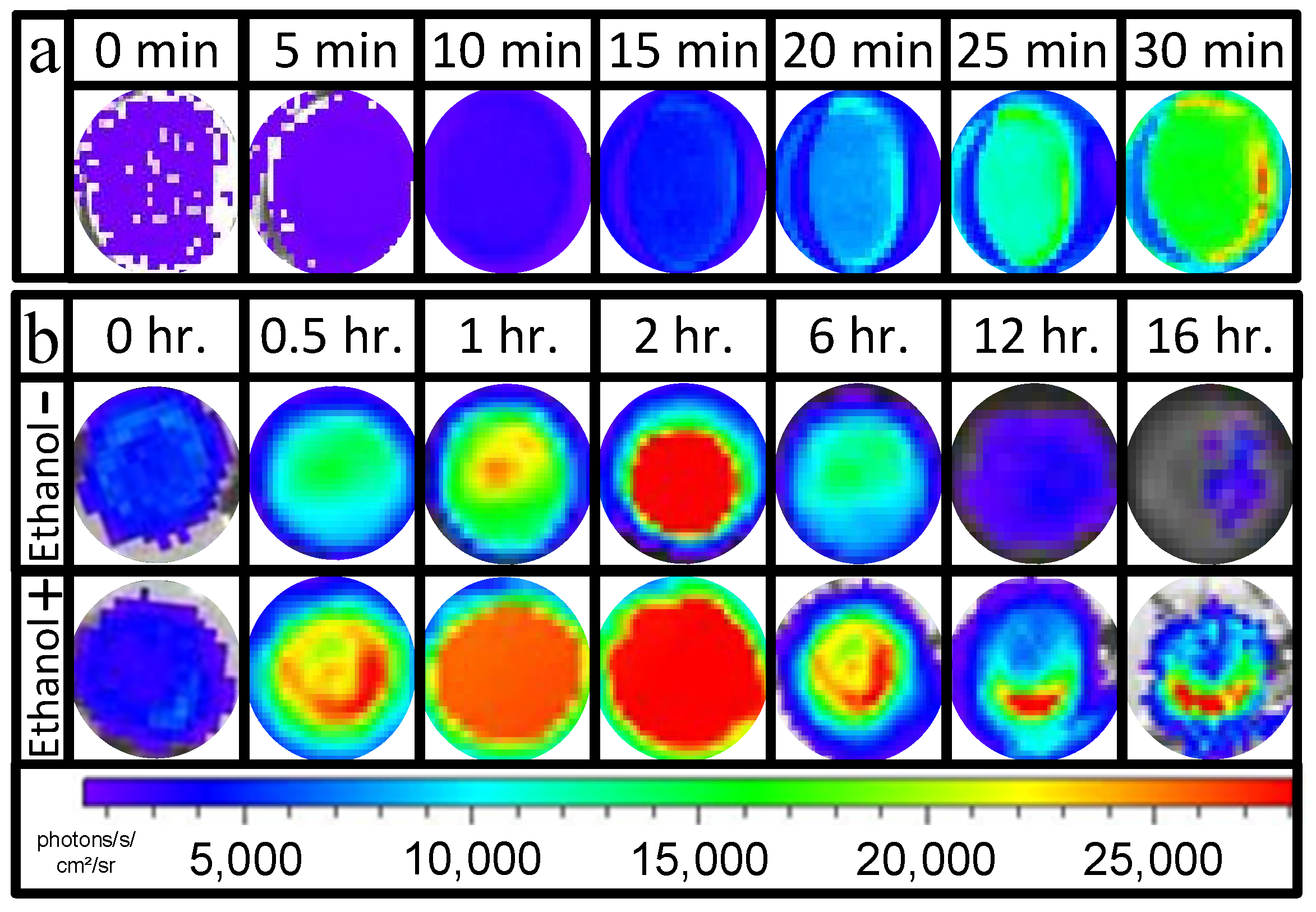
2.7. Evaluation Impact of Polymerization Processes on Bacterial Uniformity in the Membrane
2.8. Evaluation of the Effect of Storage Temperature and Nutrient Supply on Bacterial Viability and Activity
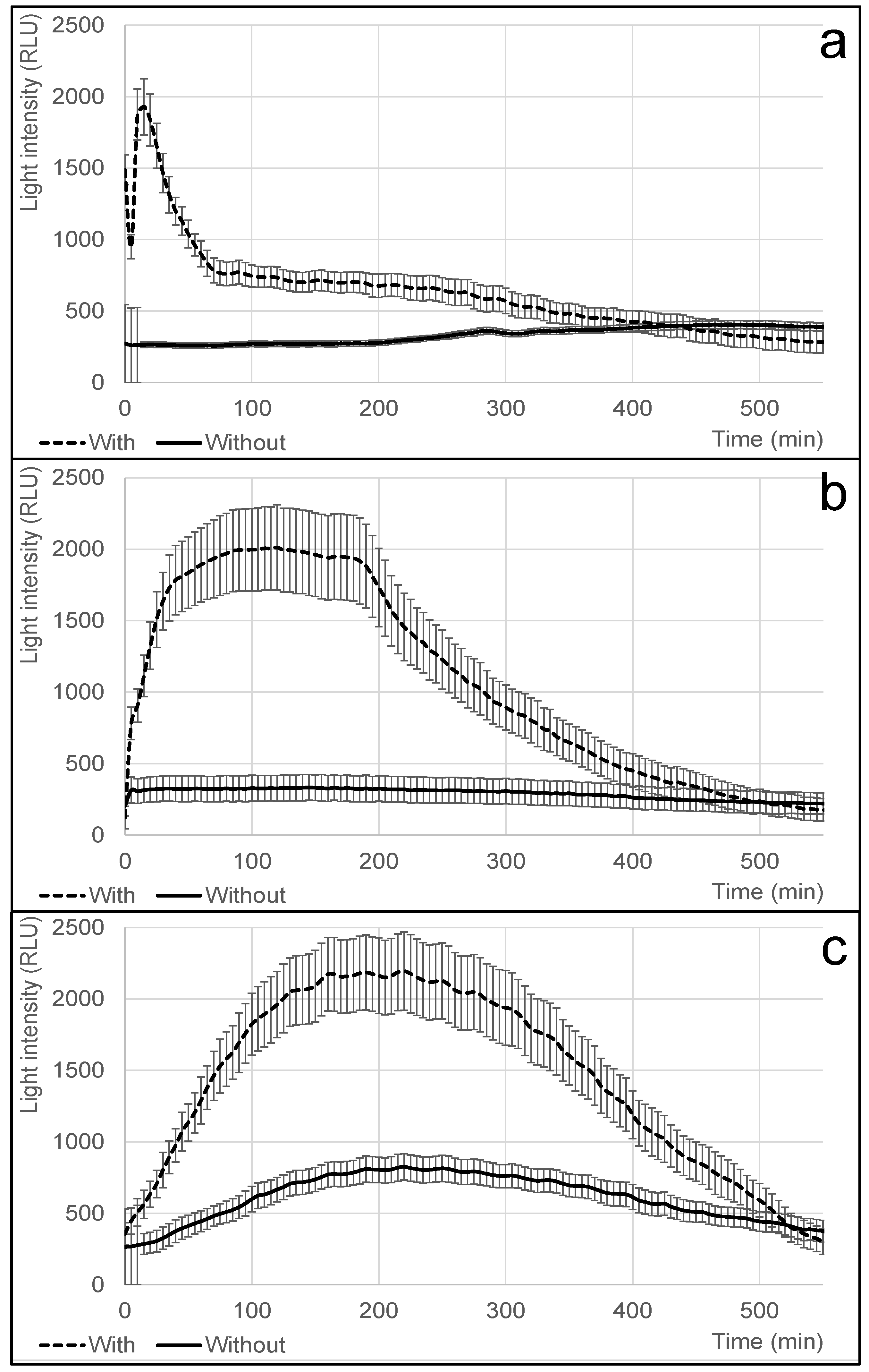
2.9. Assessing the Effect of Environmental Temperature on Whole-Cell-Based Sensor Functionality
2.10. Temperature and Distance Impact on Bioreporters’ Response Times for the Detection of Pectobacterium Brasiliense in Potatoes
2.11. Assessing the Whole Cells’ Sensitivity
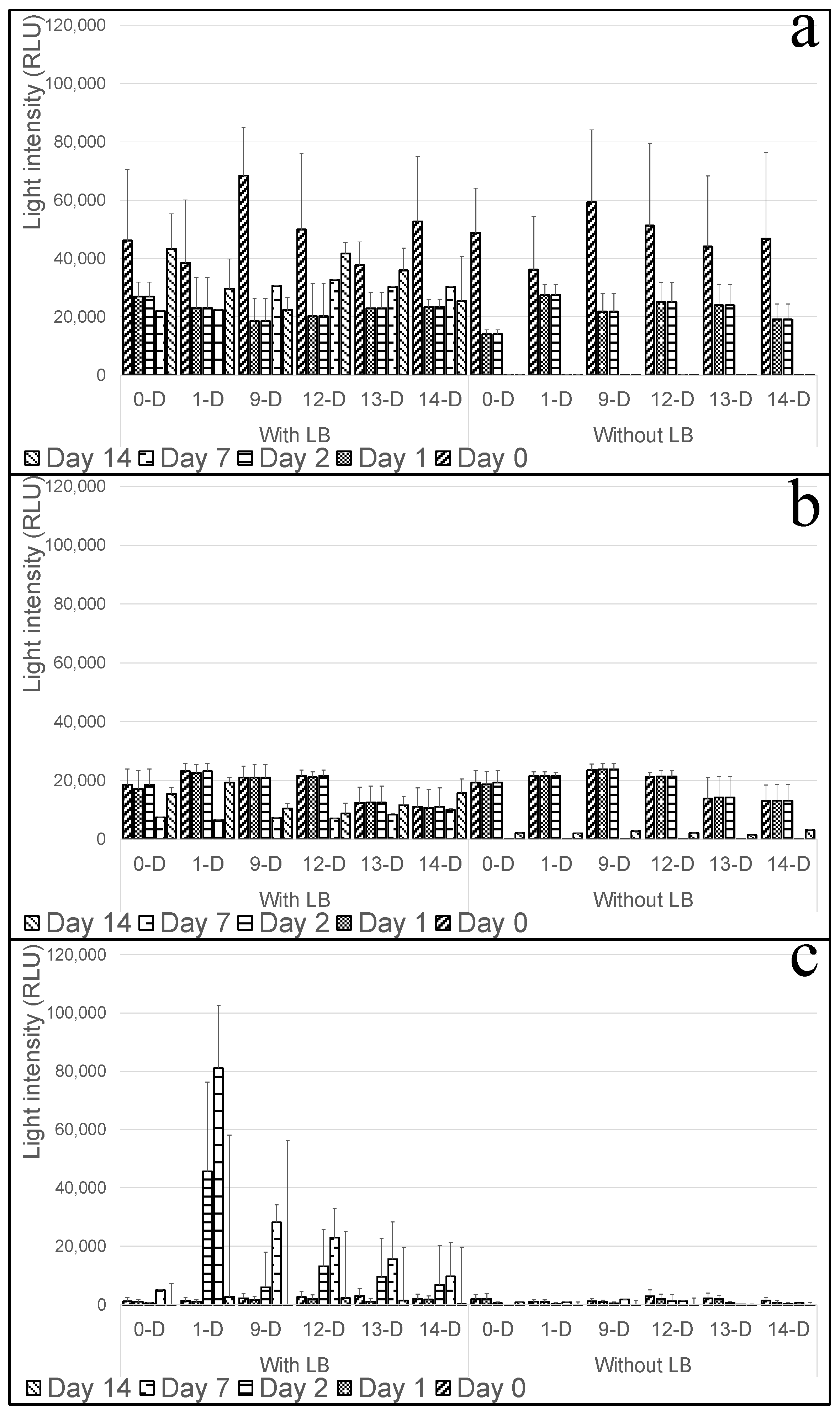
2.12. Assessing the Capabilities of the Whole Cell-Based Sensor for the Determination of Infection in the Stored Tubers
2.13. Data Analysis
3. Results and Discussion
3.1. Evaluation of the Effect of Polymerization Processes on Bacterial Uniformity
3.2. Effect of the Nutrient Source on Bacterial Viability and Activity
3.3. Effect of Temperature and Nutrient Supply on Bacterial Functionality
3.4. Effect of Temperature on Bioreporter Response and Sensor Performance
3.5. Assessment of Temperature’s Impact on Bioreporters’ Response Times for the Detection of Pectobacterium in Potatoes
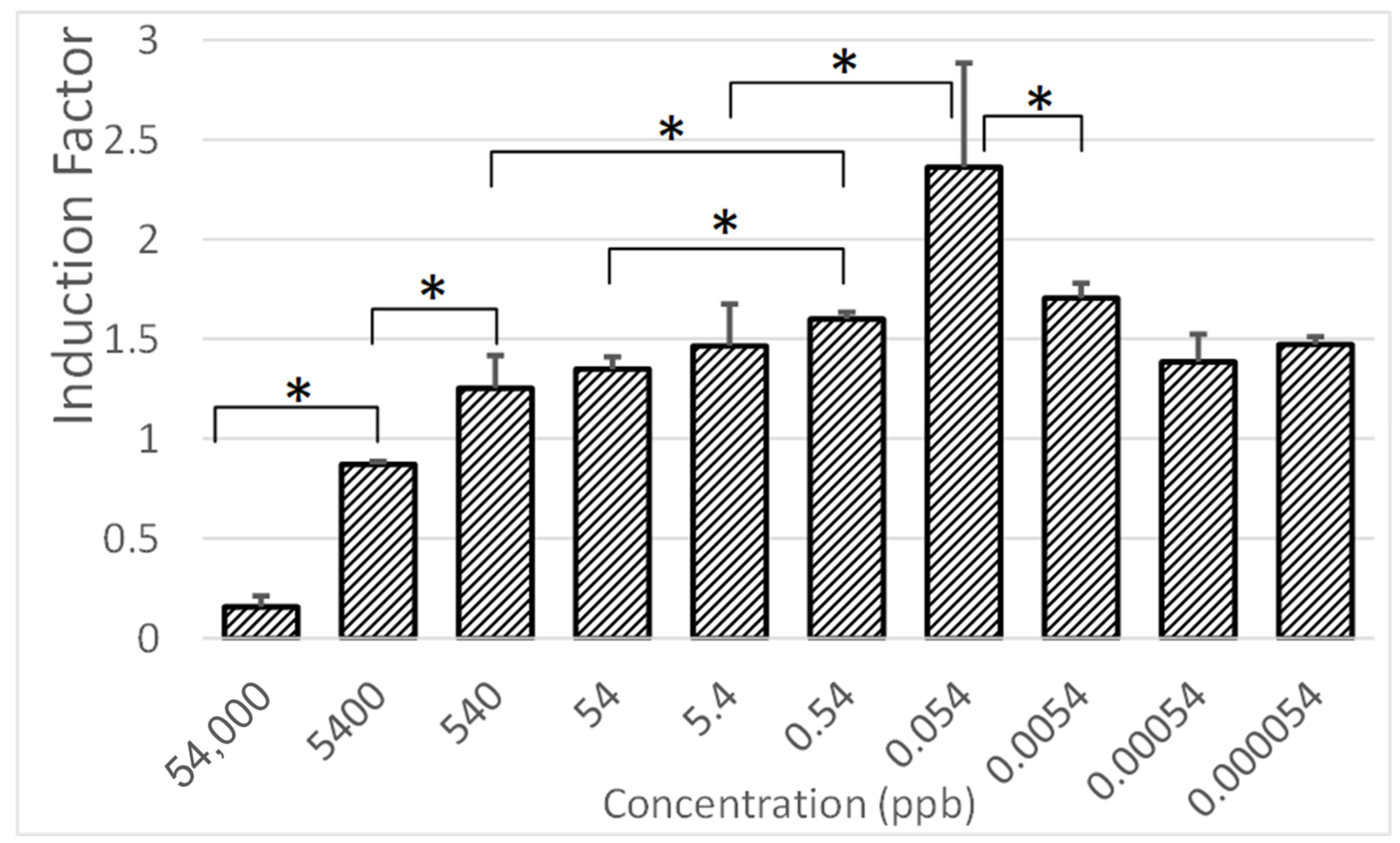
3.6. Sensitivity Assessment of the Bioreporter System
3.7. Evaluation of Whole-Cell-Based Sensors’ Capacity for Real-Time and Continuous Monitoring of Pectobacterium Infection in Potato Tubers
3.8. Summary and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gatto, A.; Chepeliev, M. Reducing global food loss and waste could improve air quality and lower the risk of premature mortality. Environ. Res. Lett. 2024, 19, 014080. [Google Scholar] [CrossRef]
- Veltman, B.; Harpaz, D.; Melamed, S.; Tietel, Z.; Tsror, L.; Eltzov, E. Whole-cell bacterial biosensor for volatile detection from Pectobacterium-infected potatoes enables early identification of potato tuber soft rot disease. Talanta 2022, 247, 123545. [Google Scholar] [CrossRef] [PubMed]
- Voet, D.; Voet, J.G.; Pratt, C.W. Principles of Biochemistry; Wiley: New York, NY, USA, 2008; Volume 4. [Google Scholar]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. Plant VOC emissions: Making use of the unavoidable. Trends Ecol. Evol. 2004, 19, 402–404. [Google Scholar] [CrossRef] [PubMed]
- McLafferty, F.W.; Turecek, F. Interpretation of Mass Spectra; University Science Books: Herndon, VA, USA, 1993. [Google Scholar]
- Grob, R.L.; Barry, E.F. Modern Practice of Gas Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Tothill, I.E. Biosensors developments and potential applications in the agricultural diagnosis sector. Comput. Electron. Agric. 2001, 30, 205–218. [Google Scholar] [CrossRef]
- Veltman, B.; Harpaz, D.; Sadeh, A.; Eltzov, E. Whole-cell bacterial biosensor applied to identify the presence of Thaumatotibia leucotreta larva in citrus fruits by volatile sensing. Food Control 2024, 160, 110388. [Google Scholar] [CrossRef]
- Veltman, B.; Ma, J.N.; Harpaz, D.; Xing, F.G.; Eltzov, E. Genetically engineered bacterial strains constructed as a whole-cell biosensor for specific volatiles identification of infected potato tubers with a soft rot disease. Sens. Actuators B Chem. 2023, 387, 133788. [Google Scholar] [CrossRef]
- Tsror, L.; Erlich, O.; Hazanovsky, M.; Ben Daniel, B.; Zig, U.; Lebiush, S. Detection of Dickeya spp. latent infection in potato seed tubers using PCR or ELISA and correlation with disease incidence in commercial field crops under hot-climate conditions. Plant Pathol. 2012, 61, 161–168. [Google Scholar] [CrossRef]
- Harpaz, D.; Zoabi, K.; Eltzov, E. Characterization and optimization of bioluminescent bacterial cells immobilization process in calcium alginate hydrogel tablets. J. Appl. Microbiol. 2023, 134, lxad070. [Google Scholar] [CrossRef]
- Caetano, L.A.; Almeida, A.J.; Gonçalves, L.M.D. Effect of Experimental Parameters on Alginate/Chitosan Microparticles for BCG Encapsulation. Mar. Drugs 2016, 14, 90. [Google Scholar] [CrossRef]
- Nussinovitch, A. Polymer Macro-and Micro-Gel Beads: Fundamentals and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Zhou, Y.; Martins, E.; Groboillot, A.; Champagne, C.P.; Neufeld, R.J. Spectrophotometric quantification of lactic bacteria in alginate and control of cell release with chitosan coating. J. Appl. Microbiol. 1998, 84, 342–348. [Google Scholar] [CrossRef]
- Yamada, M.; Seki, M. Multiphase Microfluidic Processes to Produce Alginate-Based Microparticles and Fibers. J. Chem. Eng. Jpn. 2018, 51, 318–330. [Google Scholar] [CrossRef]
- Luo, X.; Song, H.; Yang, J.; Han, B.; Feng, Y.; Leng, Y.; Chen, Z. Encapsulation of Escherichia coli strain Nissle 1917 in a chitosan―Alginate matrix by combining layer-by-layer assembly with CaCl2 cross-linking for an effective treatment of inflammatory bowel diseases. Colloids Surf. B Biointerfaces 2020, 189, 110818. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.B.; Mitchell, R.J.; Kim, B.C. Whole-cell-based biosensors for environmental biomonitoring and application. Biomanufacturing 2004, 87, 269–305. [Google Scholar]
- Gu, M.B.; Dhurjati, P.S.; Van Dyk, T.K.; LaRossa, R.A. A Miniature Bioreactor for Sensing Toxicity Using Recombinant Bioluminescent Escherichia coli Cells. Biotechnol. Prog. 1996, 12, 393–397. [Google Scholar] [CrossRef]
- Eltzov, E.; Slobodnik, V.; Ionescu, R.E.; Marks, R.S. On-line biosensor for the detection of putative toxicity in water contaminants. Talanta 2015, 132, 583–590. [Google Scholar] [CrossRef]
- Eltzov, E.; Marks, R.S.; Voost, S.; Wullings, B.A.; Heringa, M.B. Flow-through real time bacterial biosensor for toxic compounds in water. Sens. Actuators B Chem. 2009, 142, 11–18. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Peterson, M.L. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef]
- Setianto, W.; Wibowo, T.; Yohanes, H.; Illaningtyas, F.; Anggoro, D. Synthesis of glycerol mono-laurate from lauric acid and glycerol for food antibacterial additive. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 10–11 October 2016; p. 012046. [Google Scholar]
- Igbokwe, V.C.; Ball, V.; Benzaamia, N.-O.; Gree, S.; Hellé, S.; Soubirou-Blot, J.; Nardin, C.; Ploux, L. Sucrose and Glycerol Additives: A Way to Tune the Biological and Physicochemical Properties of Agarose Hydrogels? Macromol. Mater. Eng. 2024, 309, 2400150. [Google Scholar] [CrossRef]
- Jia, K.; Eltzov, E.; Marks, R.S.; Ionescu, R.E. Bioluminescence enhancement through an added washing protocol enabling a greater sensitivity to carbofuran toxicity. Ecotoxicol. Environ. Saf. 2013, 96, 61–66. [Google Scholar] [CrossRef]
- Jia, K.; Eltzov, E.; Toury, T.; Marks, R.S.; Ionescu, R.E. A lower limit of detection for atrazine was obtained using bioluminescent reporter bacteria via a lower incubation temperature. Ecotoxicol. Environ. Saf. 2012, 84, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Jana, S.; Bhattacharjee, A.; Dutta, T.K.; Chauhan, A. Chapter 9— Escherichia coli biofilms. In Application of Biofilms in Applied Microbiology; Shah, M.P., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 153–171. [Google Scholar]
- De Los Rios, P.; Barducci, A. Hsp70 chaperones are non-equilibrium machines that achieve ultra-affinity by energy consumption. eLife 2014, 3, e02218. [Google Scholar] [CrossRef] [PubMed]


| Medium | Key Components | Growth Rate | Bioluminescence (RLUs) | Stability Over Time | Notes |
|---|---|---|---|---|---|
| LB | Tryptone, Yeast Extract, and NaCl | Moderate | Stable and selective | Long-term viability | The best balance for biosensor applications |
| SOC | Tryptone, Yeast Extract, Glucose, MgCl2, and MgSO4 | High (initially) | Rapid decline | Short-lived stability | Likely osmotic stress effects |
| Terrific Broth | Tryptone, Yeast Extract, Glycerol, and Phosphate buffer | Highest | Unwanted background luminescence | Unstable | Glycerol induces non-specific bioluminescence |
| Tryptone | Tryptone only | Low | Weak response | Limited viability | Lacks essential cofactors for stable metabolism |
| Yeast Extract | Yeast Extract only | Low | Delayed induction | Short-term stability | Nutrient-limited growth |
| Minimal Salt | Inorganic salts, with no carbon source | No growth | No response | Not viable | Insufficient for bacterial maintenance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanger, N.; Eltzov, E. Optimizing Whole-Cell Biosensors for the Early Detection of Crop Infections: A Proof-of-Concept Study. Biosensors 2025, 15, 300. https://doi.org/10.3390/bios15050300
Zanger N, Eltzov E. Optimizing Whole-Cell Biosensors for the Early Detection of Crop Infections: A Proof-of-Concept Study. Biosensors. 2025; 15(5):300. https://doi.org/10.3390/bios15050300
Chicago/Turabian StyleZanger, Nadav, and Evgeni Eltzov. 2025. "Optimizing Whole-Cell Biosensors for the Early Detection of Crop Infections: A Proof-of-Concept Study" Biosensors 15, no. 5: 300. https://doi.org/10.3390/bios15050300
APA StyleZanger, N., & Eltzov, E. (2025). Optimizing Whole-Cell Biosensors for the Early Detection of Crop Infections: A Proof-of-Concept Study. Biosensors, 15(5), 300. https://doi.org/10.3390/bios15050300






