Hydroxytyrosyl Eicosapentaenoate as a Potential Antioxidant for Omega-3 Fatty Acids: Improved Synthesis and Comparative Evaluation with Other Natural Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedures
2.2.1. Synthesis of Hydroxytyrosyl Acetate (HT-Ac, 2)
2.2.2. Synthesis of Hydroxytyrosyl Eicosapentaenoate (HT-EPA, 3)
2.3. Antioxidant Activity Determinations
2.3.1. Ferric Reducing Antioxidant Power (FRAP) Assay
2.3.2. ABTS Assay
2.3.3. Rancimat® Test
2.4. Statistical Analysis
3. Results
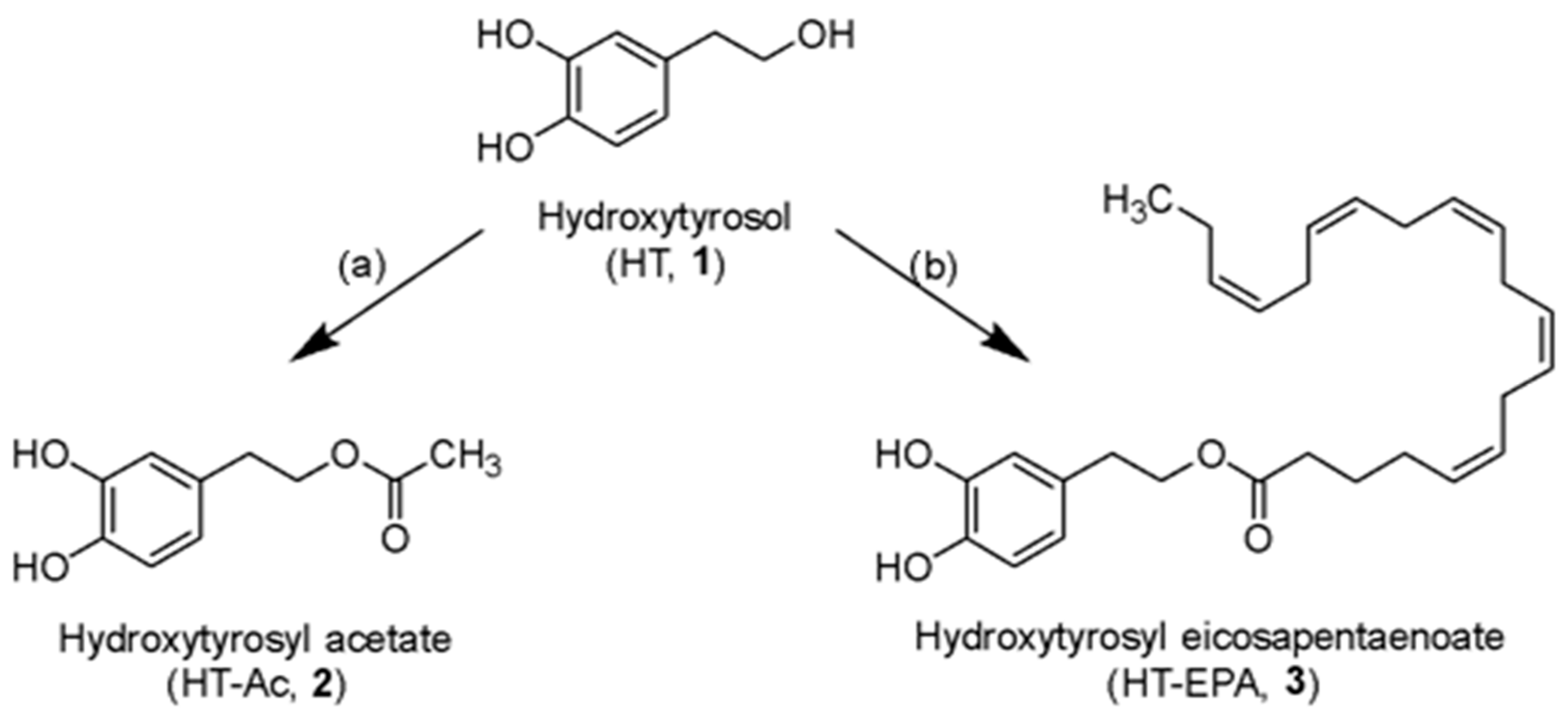
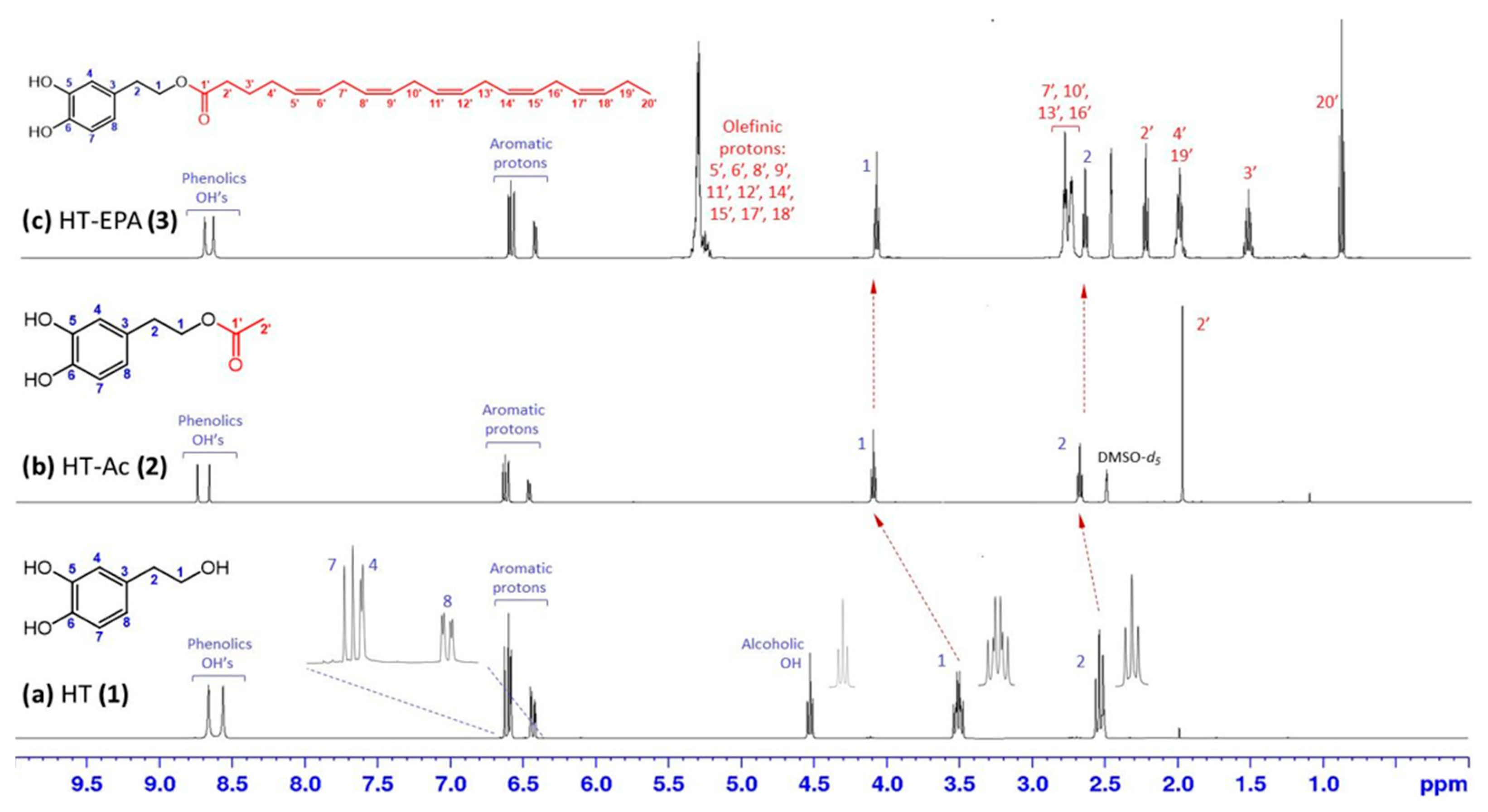
3.1. Preparation and Characterization of HT Esters
3.2. Antioxidant Activity Assays
3.2.1. FRAP Assay
3.2.2. ABTS Assay
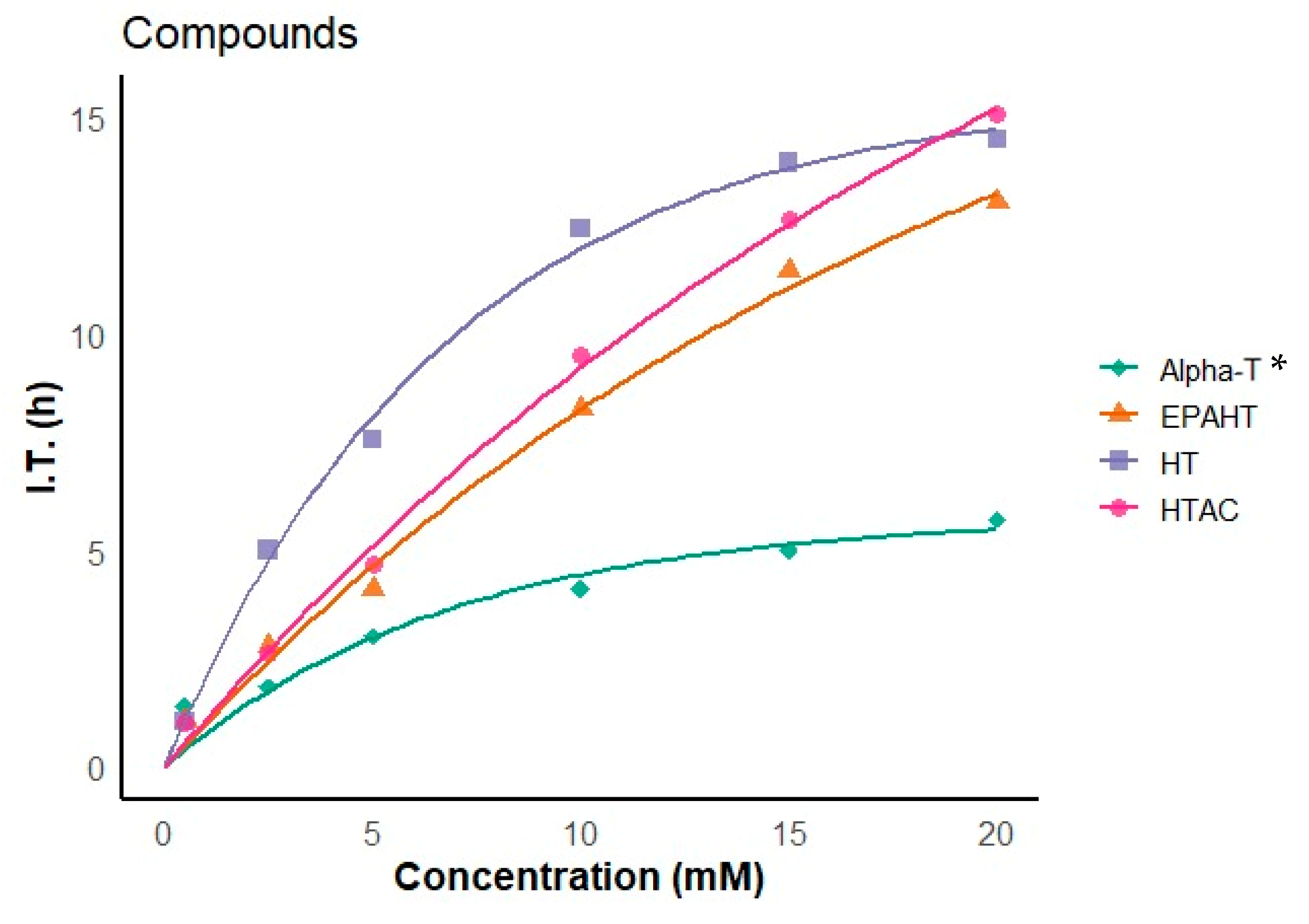
3.2.3. Rancimat Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| AcOEt | Ethyl Acetate |
| APCI | Atmospheric Pressure Chemical Ionization |
| BHT | Butylated Hydroxytoluene |
| CALB | Candida antarctica lipase B |
| COSY | Correlation Spectroscopy |
| CVDs | Cardiovascular Diseases |
| DHA | Docosahexaenoic acid |
| DMSO-d6 | Hexadeuterated Dimethyl Sulfoxide |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EPA | Eicosapentaenoic Acid |
| Et-EPA | Ethyl Eicosapentaenoate |
| ESI | Electrospray Ionization |
| EVOO | Extra Virgin Olive Oil |
| FRAP | Ferric Reducing Antioxidant Power |
| HESI | Heated Electrospray Ionization |
| HMBC | Heteronuclear Multiple Bond Correlation |
| HSQC | Heteronuclear Single Quantum Coherence |
| HT | Hydroxytyrosol |
| HT-Ac | Hydroxytyrosyl Acetate |
| HT-EPA | Hydroxytyrosyl Eicosapentaenoate |
| IT | Induction Time |
| LDL | Low-Density Lipoprotein |
| MeTHF | 2-Methyl Tetrahydrofuran |
| NMR | Nuclear Magnetic Resonance |
| O3FA | Omega-3 Fatty Acid |
| p-TsOH | p-toluenesulfonic acid |
| SD | Standard Deviation |
| TBME | tert-Butyl Methyl Ether |
| TEAC | Trolox equivalent antioxidant capacity |
| TG | Triglycerides |
| TLC | Thin layer chromatography |
| TPTZ | 2,4,6-Tris(2-pyridyl)-s-triazine |
| Trolox | (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid |
| VOO | Virgin Olive Oil |
References
- World Health Organization (WHO). Cardiovascular Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 14 March 2025).
- Lloyd-Jones, D.M.; Braun, L.T.; Ndumele, C.E.; Smith, S.C.; Sperling, L.S.; Virani, S.S.; Blumenthal, R.S. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2019, 73, 3153–3167. [Google Scholar] [CrossRef] [PubMed]
- Aberra, T.; Peterson, E.D.; Pagidipati, N.J.; Mulder, H.; Wojdyla, D.M.; Philip, S.; Granowitz, C.; Navar, A.M. The association between triglycerides and incident cardiovascular disease: What is “optimal”? J. Clin. Lipidol. 2020, 14, 438–447.e3. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Philip, S.; Reynolds, K.; Granowitz, C.B.; Fazio, S. Increased Cardiovascular Risk in Hypertriglyceridemic Patients With Statin-Controlled LDL Cholesterol. J. Clin. Endocrinol. Metab. 2018, 103, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Philip, S.; Reynolds, K.; Granowitz, C.B.; Fazio, S. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin-controlled LDL cholesterol. Diabetes Obes. Metab. 2019, 21, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. eClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Gabriel Steg, P.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Bhatt, D.L.; Kinninger, A.; Lakshmanan, S.; Muhlestein, J.B.; Le, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Roy, S.K.; et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: Final results of the EVAPORATE trial. Eur. Heart J. 2020, 41, 3925–3932. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; Mozaffarian, D.; et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin. Cardiol. 2018, 41, 1281–1288. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem Soc Rev 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Ismail, A.; Bannenberg, G.; Rice, H.; Schutt, E.; MacKay, D. Oxidation in EPA- and DHA-rich oils: An overview. Lipid Technol. 2016, 28, 55–59. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation and Atherosclerosis. Circulation 2017, 136, 1875–1877. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Lourenço, S.; Moldão Martins, M.; Alves, V. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of Phenols, Flavones, and Lignans in Virgin Olive Oils by Solid-Phase Extraction and High-Performance Liquid Chromatography with Diode Array Ultraviolet Detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- Brenes, M.; García, A.; García, P.; Rios, J.J.; Garrido, A. Phenolic Compounds in Spanish Olive Oils. J. Agric. Food Chem. 1999, 47, 3535–3540. [Google Scholar] [CrossRef]
- Milena, E.; Maurizio, M. Exploring the Cardiovascular Benefits of Extra Virgin Olive Oil: Insights into Mechanisms and Therapeutic Potential. Biomolecules 2025, 15, 284. [Google Scholar] [CrossRef]
- Noguera-Navarro, C.; Montoro-García, S.; Orenes-Piñero, E. Hydroxytyrosol: Its role in the prevention of cardiovascular diseases. Heliyon 2023, 9, e12963. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Kieserling, H.; Rohn, S.; Mocan, A. The Impact of Oleuropein, Hydroxytyrosol, and Tyrosol on Cardiometabolic Risk Factors: A Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2025, 65, 1–21. [Google Scholar] [CrossRef]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial Effects of Dietary Supplementation with Olive Oil, Oleic Acid, or Hydroxytyrosol in Metabolic Syndrome: Systematic Review and Meta-Analysis. Free Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef]
- Menezes, R.C.R.; Peres, K.K.; Costa-Valle, M.T.; Faccioli, L.S.; Dallegrave, E.; Garavaglia, J.; Dal Bosco, S.M. Oral Administration of Oleuropein and Olive Leaf Extract Has Cardioprotective Effects in Rodents: A Systematic Review. Rev. Port. Cardiol. 2022, 41, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Alcudia González, F. Method of Preparing Hydroxytyrosol Esters, Esters Thus Obtained and Use of Same. U.S. Patent US20050154058A1, 14 July 2005. [Google Scholar]
- Trujillo, M.; Mateos, R.; Collantes de Teran, L.; Espartero, J.L.; Cert, R.; Jover, M.; Alcudia, F.; Bautista, J.; Cert, A.; Parrado, J. Lipophilic Hydroxytyrosyl Esters. Antioxidant Activity in Lipid Matrices and Biological Systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Torres de Pinedo, A.; Peñalver, P.; Rondón, D.; Morales, J.C. Efficient lipase-catalyzed synthesis of new lipid antioxidants based on a catechol structure. Tetrahedron 2005, 61, 7654–7660. [Google Scholar] [CrossRef]
- Simeó, Y.; Sinisterra, J.V.; Alcántara, A.R. Regioselective enzymatic acylation of pharmacologically interesting nucleosides in 2-methyltetrahydrofuran, a greener substitute for THF. Green Chem. 2009, 11, 855–862. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Resende, D.; Monteiro, M.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Application of Hydroxytyrosol in the Functional Foods Field: From Ingredient to Dietary Supplements. Antioxidants 2020, 9, 1246. [Google Scholar] [CrossRef]
- Monteiro, M.; Silva, A.F.R.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Strategies to Broaden the Applications of Olive Biophenols Oleuropein and Hydroxytyrosol in Food Products. Antioxidants 2021, 10, 444. [Google Scholar] [CrossRef]
- Laszlo, J.A.; Cermak, S.C.; Evans, K.O.; Compton, D.L.; Evangelista, R.; Berhow, M.A. Medium-chain alkyl esters of tyrosol and hydroxytyrosol antioxidants by cuphea oil transesterification. Eur. J. Lipid Sci. Technol. 2013, 115, 363–371. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Siquet, C.; Orrù, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Bioorganic Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef]
- Floris, B.; Galloni, P.; Conte, V.; Sabuzi, F. Tailored Functionalization of Natural Phenols to Improve Biological Activity. Biomolecules 2021, 11, 1325. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Molecular mechanism of α-tocopherol action. Free. Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.L. An Update on Vitamin E, Tocopherol and Tocotrienol—Perspectives. Molecules 2010, 15, 2103–2113. [Google Scholar] [CrossRef]
- Mateos, R.; Domínguez, M.M.; Espartero, J.L.; Cert, A. Antioxidant Effect of Phenolic Compounds, α-Tocopherol, and Other Minor Components in Virgin Olive Oil. J. Agric. Food Chem. 2003, 51, 7170–7175. [Google Scholar] [CrossRef] [PubMed]
- Botterweck, A.A.M.; Verhagen, H.; Goldbohm, R.A.; Kleinjans, J.; van den Brandt, P.A. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands Cohort Study. Food Chem. Toxicol. 2000, 38, 599–605. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food, (ANS) Scientific Opinion on the re-evaluation of butylated hydroxytoluene BHT (E 321) as a food additive. EFSA J. 2012, 10, 2588.
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Yu, J.; Guo, X.; Tong, P.; Yin, F.; Liu, X.; Zhou, D. The potential of hydroxytyrosol fatty acid esters to enhance oral bioavailabilities of hydroxytyrosol and fatty acids: Continuous and slow-release ability in small intestine and blood. Food Chem. 2023, 422, 136246. [Google Scholar] [CrossRef]
- Torres de Pinedo, A.; Peñalver, P.; Pérez-Victoria, I.; Rondón, D.; Morales, J.C. Synthesis of new phenolic fatty acid esters and their evaluation as lipophilic antioxidants in an oil matrix. Food Chem. 2007, 105, 657–665. [Google Scholar] [CrossRef]
- Medina, I.; Lois, S.; Alcántara, D.; Lucas, R.; Morales, J.C. Effect of Lipophilization of Hydroxytyrosol on Its Antioxidant Activity in Fish Oils and Fish Oil-in-Water Emulsions. J. Agric. Food Chem. 2009, 57, 9773–9779. [Google Scholar] [CrossRef]
- Pazos, M.; Alonso, A.; Sánchez, I.; Medina, I. Hydroxytyrosol Prevents Oxidative Deterioration in Foodstuffs Rich in Fish Lipids. J. Agric. Food Chem. 2008, 56, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Crauste, C.; Rosell, M.; Durand, T.; Vercauteren, J. Omega-3 polyunsaturated lipophenols, how and why? Biochimie 2016, 120, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilized Epigallocatechin Gallate (EGCG) Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Barrow, C.J. Lipase-Produced Hydroxytyrosyl Eicosapentaenoate is an Excellent Antioxidant for the Stabilization of Omega-3 Bulk Oils, Emulsions and Microcapsules. Molecules 2018, 23, 275. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Bouaziz, M.; Lassoued, S.; Engasser, J.M.; Ghoul, M.; Sayadi, S. Hydroxytyrosol Acyl Esters: Biosynthesis and Activities. Appl. Biochem. Biotechnol. 2011, 163, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.Y.; Gong, T.; Zhao, P.T.; Niu, Y.J.; Hu, Y.Y.; Hu, C.Y.; Zhang, S.; Meng, Y.H. Hydroxytyrosyl oleate is a promising safe additive to inhibit the oxidation of olive oil. Food Control 2023, 153, 109895. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, Y.; Shahidi, F. Preparation and antioxidant activity of tyrosol and hydroxytyrosol esters. J. Funct. Foods 2017, 37, 66–73. [Google Scholar] [CrossRef]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing lipases for biocatalysis: A survey of chemical, physical and molecular biological approaches. J. Mol. Catal. B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Fjerbaek, L.; Christensen, K.V.; Norddahl, B. A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol. Bioeng. 2009, 102, 1298–1315. [Google Scholar] [CrossRef]
- Salta, F.N.; Kosma, I.S.; Lordan, R.; Vlachogianni, I.C.; Ladavos, A.K.; Zampounis, A. Olive phenolic compounds: A novel class of nutraceuticals and their impact on human health. Nutrients 2019, 11, 1325. [Google Scholar]
- Pereira-Caro, G.; Sarriá, B.; Madrona, A.; Espartero, J.L.; Escuderos, M.E.; Bravo, L.; Mateos, R. Digestive stability of hydroxytyrosol, hydroxytyrosyl acetate and alkyl hydroxytyrosyl ethers. Int. J. Food Sci. Nutr. 2012, 63, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, Y.; Li, Z.; Ma, C.; Lou, Z.; Yokoyama, W.; Wang, H. Lipase-catalyzed synthesis of acetylated EGCG and antioxidant properties of the acetylated derivatives. Food Res. Int. 2014, 56, 279–286. [Google Scholar] [CrossRef]
| Compound | FRAP TEAC (mM) | ABTS TEAC (mM) |
|---|---|---|
| Hydroxytyrosol (1, HT) | 1.33 ± 0.06 a | 1.47 ± 0.05 a |
| Hydroxytyrosol acetate (2, HT-Ac) | 1.28 ± 0.03 ab | 0.98 ± 0.03 b |
| Hydroxytyrosol eicosapentaenoate (3, HT-EPA) | 1.22 ± 0.06 b | 0.98 ± 0.05 b |
| α-Tocopherol (4, Alpha-T) | 0.64 ± 0.02 c | 0.79 ± 0.06 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Acosta, N.; Cert, R.; Jordán, M.; Goya, L.; Mateos, R.; Espartero, J.L. Hydroxytyrosyl Eicosapentaenoate as a Potential Antioxidant for Omega-3 Fatty Acids: Improved Synthesis and Comparative Evaluation with Other Natural Antioxidants. Biomolecules 2025, 15, 714. https://doi.org/10.3390/biom15050714
García-Acosta N, Cert R, Jordán M, Goya L, Mateos R, Espartero JL. Hydroxytyrosyl Eicosapentaenoate as a Potential Antioxidant for Omega-3 Fatty Acids: Improved Synthesis and Comparative Evaluation with Other Natural Antioxidants. Biomolecules. 2025; 15(5):714. https://doi.org/10.3390/biom15050714
Chicago/Turabian StyleGarcía-Acosta, Natalia, Rosa Cert, Marta Jordán, Luis Goya, Raquel Mateos, and Jose Luis Espartero. 2025. "Hydroxytyrosyl Eicosapentaenoate as a Potential Antioxidant for Omega-3 Fatty Acids: Improved Synthesis and Comparative Evaluation with Other Natural Antioxidants" Biomolecules 15, no. 5: 714. https://doi.org/10.3390/biom15050714
APA StyleGarcía-Acosta, N., Cert, R., Jordán, M., Goya, L., Mateos, R., & Espartero, J. L. (2025). Hydroxytyrosyl Eicosapentaenoate as a Potential Antioxidant for Omega-3 Fatty Acids: Improved Synthesis and Comparative Evaluation with Other Natural Antioxidants. Biomolecules, 15(5), 714. https://doi.org/10.3390/biom15050714






