OX40–OX40L Axis in Cutaneous T-Cell Lymphomas: Pathogenic, Prognostic, and Potential Therapeutic Perspectives
Abstract
1. Introduction
2. Interactions Between CD4+ T-Cells and Antigen-Presenting Cells in Cutaneous T-Cell Lymphomas and Physiological Conditions: Similarities and Differences
3. A Focus on Antigen-Presenting Cells in Cutaneous T-Cell Lymphomas
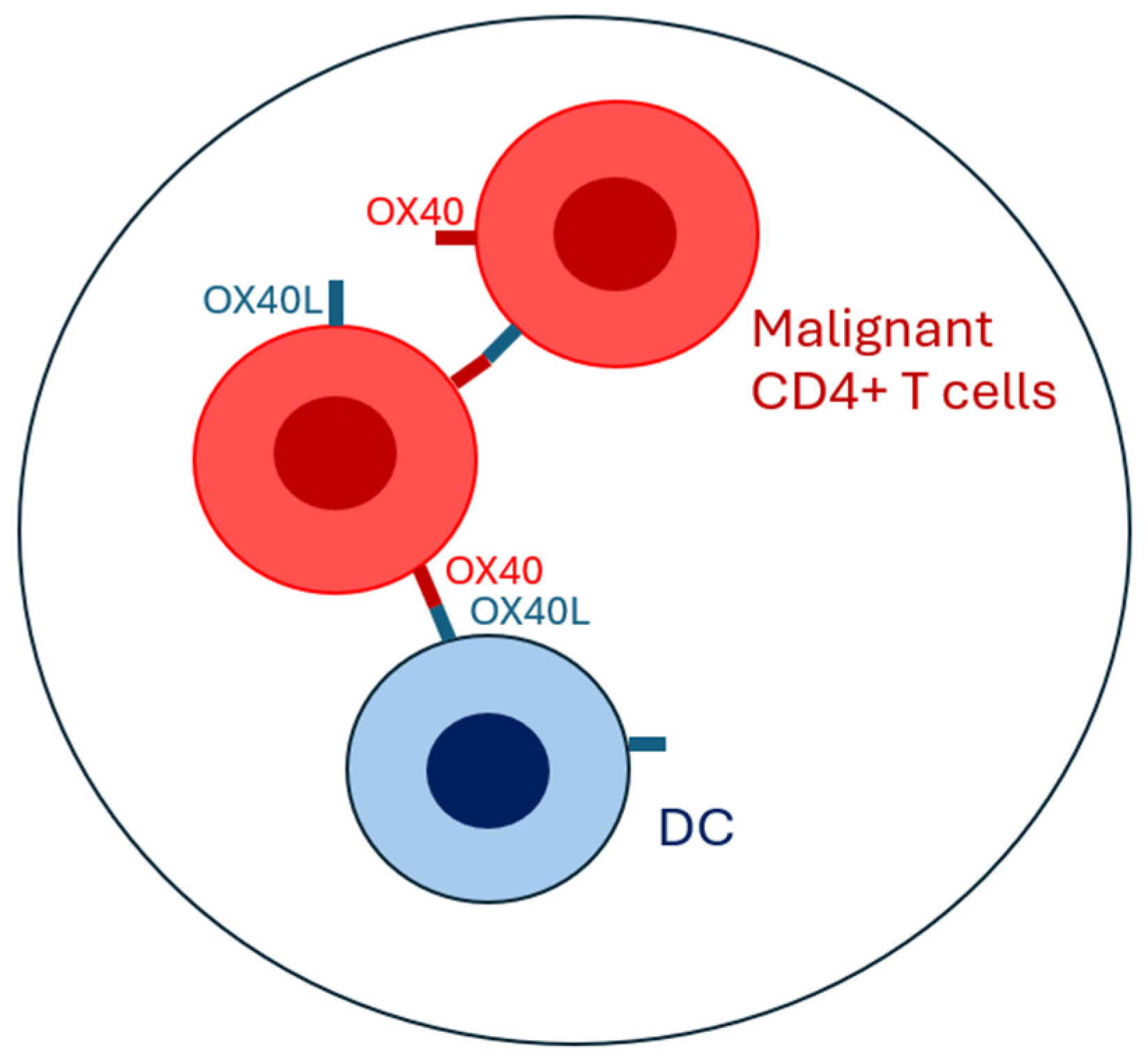
4. Role of the OX40–OX40L Axis in Cutaneous T-CELL Lymphoma and Benign Inflammatory Dermatoses
5. Future Perspectives and Therapeutic Opportunities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latzka, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Guenova, E.; Gniadecki, R.; Hodak, E.; Jonak, C.; Klemke, C.-D.; et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2023. Eur. J. Cancer 1990, 195, 113343. [Google Scholar] [CrossRef] [PubMed]
- Pileri, A.; Guglielmo, A.; Grandi, V.; Violetti, S.A.; Fanoni, D.; Fava, P.; Agostinelli, C.; Berti, E.; Quaglino, P.; Pimpinelli, N. The Microenvironment’s Role in Mycosis Fungoides and Sézary Syndrome: From Progression to Therapeutic Implications. Cells 2021, 10, 2780. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Whittaker, S.; Kim, Y.H.; Duvic, M.; Prince, H.M.; Lessin, S.R.; Wood, G.S.; Willemze, R.; Demierre, M.F.; Pimpinelli, N.; et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: A consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2598–2607. [Google Scholar]
- Olsen, E.A.; Whittaker, S.; Willemze, R.; Pinter-Brown, L.; Foss, F.; Geskin, L.; Schwartz, L.; Horwitz, S.; Guitart, J.; Zic, J.; et al. Primary cutaneous lymphoma: Recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood 2022, 140, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Fava, P.; Pileri, A.; Grandi, V.; Sanlorenzo, M.; Panasiti, V.; Guglielmo, A.; Alberti-Violetti, S.; Novelli, M.; Astrua, C.; et al. Phenotypical Markers, Molecular Mutations, and Immune Microenvironment as Targets for New Treatments in Patients with Mycosis Fungoides and/or Sézary Syndrome. J. Investig. Dermatol. 2021, 141, 484–495. [Google Scholar] [CrossRef]
- Gonzalez, B.R.; Zain, J.; Rosen, S.T.; Querfeld, C. Tumor microenvironment in mycosis fungoides and Sézary syndrome. Curr. Opin. Oncol. 2016, 28, 88–96. [Google Scholar] [CrossRef]
- Guglielmo, A.; Zengarini, C.; Agostinelli, C.; Motta, G.; Sabattini, E.; Pileri, A. The Role of Cytokines in Cutaneous T Cell Lymphoma: A Focus on the State of the Art and Possible Therapeutic Targets. Cells 2024, 13, 584. [Google Scholar] [CrossRef]
- Dobos, G.; Pohrt, A.; Ram-Wolff, C.; Lebbé, C.; Bouaziz, J.-D.; Battistella, M.; Bagot, M.; de Masson, A. Epidemiology of Cutaneous T-Cell Lymphomas: A Systematic Review and Meta-Analysis of 16,953 Patients. Cancers 2020, 12, 2921. [Google Scholar] [CrossRef]
- Ghazawi, F.M.; Netchiporouk, E.; Rahme, E.; Tsang, M.; Moreau, L.; Glassman, S.; Provost, N.; Gilbert, M.; Jean, S.-E.; Roshdy, O.; et al. Distribution and Clustering of Cutaneous T-Cell Lymphoma (CTCL) Cases in Canada During 1992 to 2010. J. Cutan. Med. Surg. 2018, 22, 154–165. [Google Scholar] [CrossRef]
- Campbell, J.J.; Clark, R.A.; Watanabe, R.; Kupper, T.S. Sézary syndrome and mycosis fungoides arise from distinct T-cell subsets: A biologic rationale for their distinct clinical behaviors. Blood 2010, 116, 767–771. [Google Scholar] [CrossRef]
- Ma, J.; Wei, K.; Zhang, H.; Tang, K.; Li, F.; Zhang, T.; Liu, J.; Xu, P.; Yu, Y.; Sun, W.; et al. Mechanisms by Which Dendritic Cells Present Tumor Microparticle Antigens to CD8+ T Cells. Cancer Immunol. Res. 2018, 6, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Caron, C.; Chan, Y.-Y.; Lee, C.K.; Xu, X.; Zhang, J.; Masubuchi, T.; Wu, C.; Bui, J.D.; Hui, E. cis-B7:CD28 interactions at invaginated synaptic membranes provide CD28 co-stimulation and promote CD8+ T cell function and anti-tumor immunity. Immunity 2023, 56, 1187–1203.e12. [Google Scholar] [CrossRef]
- McVey, J.C.; Beatty, G.L. Facts and Hopes of CD40 Agonists in Cancer Immunotherapy. Clin. Cancer Res. 2025, OF1–OF9. [Google Scholar] [CrossRef]
- David, A.; Crawford, F.; Garside, P.; Kappler, J.W.; Marrack, P.; MacLeod, M. Tolerance induction in memory CD4 T cells requires two rounds of antigen-specific activation. Proc. Natl. Acad. Sci. USA 2014, 111, 7735–7740. [Google Scholar] [CrossRef]
- Cribier, B.J. The myth of Pautrier’s microabscesses. J. Am. Acad. Dermatol. 2003, 48, 796–797, author reply 797. [Google Scholar] [CrossRef] [PubMed]
- Cerroni, L. Mycosis fungoides. In Skin Lymphoma; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 23–112. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119485933.ch3 (accessed on 25 March 2025).
- Goteri, G.; Filosa, A.; Mannello, B.; Stramazzotti, D.; Rupoli, S.; Leoni, P.; Fabris, G. Density of neoplastic lymphoid infiltrate, CD8+ T cells, and CD1a+ dendritic cells in mycosis fungoides. J. Clin. Pathol. 2003, 56, 453–458. Available online: https://www.proquest.com/docview/1781120774/abstract/FE1D8D6197EE4DF0PQ/1 (accessed on 2 March 2025). [CrossRef] [PubMed][Green Version]
- Tosca, A.D.; Varelzidis, A.G.; Economidou, J.; Stratigos, J.D. Mycosis fungoides: Evaluation of immunohistochemical criteria for the early diagnosis of the disease and differentiation between stages. J. Am. Acad. Dermatol. 1986, 15, 237–245. [Google Scholar] [CrossRef]
- Cerroni, L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin. Cutan. Med. Surg. 2018, 37, 2–10. Available online: https://pubmed.ncbi.nlm.nih.gov/29719014/ (accessed on 24 September 2024). [CrossRef]
- Pileri, A.; Agostinelli, C.; Sessa, M.; Quaglino, P.; Santucci, M.; Tomasini, C.; Grandi, V.; Fava, P.; Astrua, C.; Righi, S.; et al. Langerhans, plasmacytoid dendritic and myeloid-derived suppressor cell levels in mycosis fungoides vary according to the stage of the disease. Virchows Arch. 2017, 470, 575–582. [Google Scholar] [CrossRef]
- Der-Petrossian, M.; Valencak, J.; Jonak, C.; Klosner, G.; Dani, T.; Müllauer, L.; Pehamberger, H.; Knobler, R.; Trautinger, F. Dermal infiltrates of cutaneous T-cell lymphomas with epidermotropism but not other cutaneous lymphomas are abundant with langerin+ dendritic cells. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 922–927. [Google Scholar] [CrossRef]
- Luftl, M.; Feng, A.; Licha, E.; Schuler, G. Dendritic cells and apoptosis in mycosis fungoides. Br. J. Dermatol. 2002, 147, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Pimpinelli, N.; Santucci, M.; Romagnoli, P.; Giannotti, B. Dendritic cells in T- and B-cell proliferation in the skin. Dermatol. Clin. 1994, 12, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, P.; Obermoser, G.; Nguyen, V.; Fritsch, P.; Sepp, N.; Romani, N. Distribution and maturation of skin dendritic cell subsets in two forms of cutaneous T-cell lymphoma: Mycosis fungoides and Sézary syndrome. Acta Dermato-Venereol. 2012, 92, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Cioplea, M.; Caruntu, C.; Zurac, S.; Bastian, A.; Sticlaru, L.; Cioroianu, A.; Boda, D.; Jugulete, G.; Nichita, L.; Popp, C. Dendritic cell distribution in mycosis fungoides vs. inflammatory dermatosis and other T-cell skin lymphoma. Oncol. Lett. 2019, 17, 4055–4059. [Google Scholar] [CrossRef]
- Schlapbach, C.; Ochsenbein, A.; Kaelin, U.; Hassan, A.S.; Hunger, R.E.; Yawalkar, N. High numbers of DC-SIGN+ dendritic cells in lesional skin of cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2010, 62, 995–1004. [Google Scholar] [CrossRef]
- Berger, C.L.; Hanlon, D.; Kanada, D.; Dhodapkar, M.; Lombillo, V.; Wang, N.; Christensen, I.; Howe, G.; Crouch, J.; El-Fishawy, P.; et al. The growth of cutaneous T-cell lymphoma is stimulated by immature dendritic cells. Blood 2002, 99, 2929–2939. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Giordano, S.; Fava, P.; Pileri, A.; Guglielmo, A.; Tonella, L.; Sanlorenzo, M.; Ribero, S.; Fierro, M.T.; Quaglino, P. Immune Check Point Inhibitors in Primary Cutaneous T-Cell Lymphomas: Biologic Rationale, Clinical Results and Future Perspectives. Front. Oncol. 2021, 11, 733770. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, X.; Hwang, S.T.; Liu, J. The Role of Tumor Microenvironment in Mycosis Fungoides and Sézary Syndrome. Ann. Dermatol. 2021, 33, 487–496. [Google Scholar] [CrossRef]
- Papadavid, E.; Economidou, J.; Psarra, A.; Kapsimali, V.; Mantzana, V.; Antoniou, C.; Limas, K.; Stratigos, A.; Stavrianeas, N.; Avgerinou, G.; et al. The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sézary syndrome. Br. J. Dermatol. 2003, 148, 709–718. [Google Scholar] [CrossRef]
- Elbendary, A.; Parikh, K.; Elattar, I.; Truong, J.; Elston, D.M. Expression of T-bet and GATA-3 in early mycosis fungoides and spongiotic dermatitis. J. Am. Acad. Dermatol. 2016, 74, 1012–1014. [Google Scholar] [CrossRef]
- Fu, Y.; Lin, Q.; Zhang, Z.; Zhang, L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm. Sin. B 2020, 10, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Croft, M. Control of Immunity by the TNFR-Related Molecule OX40 (CD134). Annu. Rev. Immunol. 2010, 28, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, J.; Griffiths, J.; Tews, I.; Cragg, M.S. OX40: Structure and function—What questions remain? Mol. Immunol. 2017, 83, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J.L. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef]
- Hoshino, A.; Tanaka, Y.; Akiba, H.; Asakura, Y.; Mita, Y.; Sakurai, T.; Takaoka, A.; Nakaike, S.; Ishii, N.; Sugamura, K.; et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur. J. Immunol. 2003, 33, 861–869. [Google Scholar] [CrossRef]
- Sadrolashrafi, K.; Guo, L.; Kikuchi, R.; Hao, A.; Yamamoto, R.K.; Tolson, H.C.; Bilimoria, S.N.; Yee, D.K.; Armstrong, A.W. An OX-Tra’Ordinary Tale: The Role of OX40 and OX40L in Atopic Dermatitis. Cells 2024, 13, 587. [Google Scholar] [CrossRef]
- Nakae, S.; Saijo, S.; Horai, R.; Sudo, K.; Mori, S.; Iwakura, Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA 2003, 100, 5986–5990. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, W.; Hinrichs, D.; Wu, X.; Weinberg, A.; Hall, M.; Spencer, D.; Wegmann, K.; Rosenbaum, J.T. Activation of OX40 augments Th17 cytokine expression and antigen-specific uveitis. Am. J. Pathol. 2010, 177, 2912–2920. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, X.; Lan, P.; Li, J.; Dou, Y.; Chen, W.; Ishii, N.; Chen, S.; Xia, B.; Chen, K.; et al. OX40 Costimulation Inhibits Foxp3 Expression and Treg Induction via BATF3-Dependent and Independent Mechanisms. Cell Rep. 2018, 24, 607–618. [Google Scholar] [CrossRef]
- Tahiliani, V.; Hutchinson, T.E.; Abboud, G.; Croft, M.; Salek-Ardakani, S. OX40 Cooperates with ICOS To Amplify Follicular Th Cell Development and Germinal Center Reactions during Infection. J. Immunol. 2017, 198, 218–228. [Google Scholar] [CrossRef]
- Deng, Z.; Tian, Y.; Wang, J.; Xu, Y.; Liu, Z.; Xiao, Z.; Wang, Z.; Hu, M.; Liu, R.; Yang, P. Enhanced Antitumor Immunity Through T Cell Activation with Optimized Tandem Double-OX40L mRNAs. Int. J. Nanomed. 2025, 20, 3607–3621. [Google Scholar] [CrossRef] [PubMed]
- Kawana, Y.; Suga, H.; Kamijo, H.; Miyagaki, T.; Sugaya, M.; Sato, S. Roles of OX40 and OX40 Ligand in Mycosis Fungoides and Sézary Syndrome. Int. J. Mol. Sci. 2021, 22, 12576. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Fletcher, C.D.M.; Pulford, K.; Shahsafaei, A.; Dorfman, D.M. The T-Cell Activation Markers CD30 and OX40/CD134 Are Expressed in Nonoverlapping Subsets of Peripheral T-Cell Lymphoma. Blood 1999, 93, 3487–3493. [Google Scholar] [CrossRef]
- Kempf, W.; Pfaltz, K.; Vermeer, M.H.; Cozzio, A.; Ortiz-Romero, P.L.; Bagot, M.; Olsen, E.; Kim, Y.H.; Dummer, R.; Pimpinelli, N.; et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: Lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood 2011, 118, 4024–4035. [Google Scholar] [CrossRef]
- Gniadecki, R.; Rossen, K. Expression of T-cell activation marker CD134 (OX40) in lymphomatoid papulosis. Br. J. Dermatol. 2003, 148, 885–891. [Google Scholar] [CrossRef]
- Arestides, R.S.S.; He, H.; Westlake, R.M.; Chen, A.I.; Sharpe, A.H.; Perkins, D.L.; Finn, P.W. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur. J. Immunol. 2002, 32, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Croft, M.; Geng, B.; Rynkiewicz, N.; Lucchesi, D.; Peakman, M.; van Krinks, C.; Valdecantos, W.; Xing, H.; Weidinger, S. The role of OX40 ligand/OX40 axis signalling in atopic dermatitis. Br. J. Dermatol. 2024, 191, 488–496. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Shang, X.; Benson, J.; Merle Elloso, M.; Schantz, A.; Bracht, M.; Orlovsky, Y.; Sweet, R. Negative regulation of IL-17 production by OX40/OX40L interaction. Cell Immunol. 2008, 253, 31–37. [Google Scholar] [CrossRef]
- Remedios, K.A.; Zirak, B.; Sandoval, P.M.; Lowe, M.M.; Boda, D.; Henley, E.; Bhattrai, S.; Scharschmidt, T.C.; Liao, W.; Naik, H.B.; et al. The TNFRSF members CD27 and OX40 coordinately limit TH17 differentiation in regulatory T cells. Sci. Immunol. 2018, 3, eaau2042. [Google Scholar] [CrossRef]
- Guo, R.; Zhang , T.; Meng, X.; Lin, Z.; Lin, J.; Gong , Y.; Liu, X.; Yu, Y.; Zhao , G.; Ding , X. Lymphocyte mass cytometry identifies a CD3-CD4+ cell subset with a potential role in psoriasis. JCI Insight 2019, 4, e125306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilves, T.; Harvima, I. OX40 ligand and OX40 are increased in atopic dermatitis lesions but do not correlate with clinical severity. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e197–e205. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yang, Z.; Su, Q.; Fang, L.; Xiang, Q.; Tian, C.; Gao, Q.; Mao, C.; Huang, C.Z.; Zuo, H. Bivalent OX40 Aptamer and CpG as Dual Agonists for Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2025, 17, 7353–7362. [Google Scholar] [CrossRef]
- Case, K.B.; Allen, P.B. Advances in Novel Systemic Therapies for the Management of Cutaneous T Cell Lymphoma (CTCL). Curr. Hematol. Malig. Rep. 2025, 20, 5. [Google Scholar] [CrossRef]
- Bagot, M. Therapeutic advances for cutaneous T-cell lymphoma. Br. J. Dermatol. 2025, ljaf105. [Google Scholar] [CrossRef]
- He, B.; Zhao, R.; Zhang, B.; Pan, H.; Liu, J.; Huang, L.; Wei, Y.; Yang, D.; Liang, J.; Wang, M.; et al. Endothelial OX40 activation facilitates tumor cell escape from T cell surveillance through S1P/YAP-mediated angiogenesis. J. Clin. Investig. 2025, 135, e186291. [Google Scholar] [CrossRef]
- Imura, A.; Hori, T.; Imada, K.; Kawamata, S.; Tanaka, Y.; Imamura, S.; Uchiyama, T. OX40 Expressed on Fresh Leukemic Cells from Adult T-Cell Leukemia Patients Mediates Cell Adhesion to Vascular Endothelial Cells: Implication for the Possible Involvement of OX40 in Leukemic Cell Infiltration. Blood 1997, 89, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Y.; Hirsch, F.R.; Zhou, C. 163P—OX40/OX40L protein expression in non-small cell lung cancer and its role in clinical outcome and relationships with other immune biomarkers. Ann. Oncol. 2019, 30, v51. [Google Scholar] [CrossRef]
- Bell, R.B.; Leidner, R.S.; Crittenden, M.R.; Curti, B.D.; Feng, Z.; Montler, R.; Gough, M.J.; Fox, B.A.; Weinberg, A.D.; Urba, W.J. OX40 signaling in head and neck squamous cell carcinoma: Overcoming immunosuppression in the tumor microenvironment. Oral Oncol. 2016, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Chiappori, A.A.; Thompson, J.A.; Doi, T.; Hu-Lieskovan, S.; Eskens, F.A.L.M.; Ros, W.; Diab, A.; Spano, J.-P.; Rizvi, N.A.; et al. First-in-human study of an OX40 (ivuxolimab) and 4-1BB (utomilumab) agonistic antibody combination in patients with advanced solid tumors. J. Immunother. Cancer 2022, 10, e005471. [Google Scholar] [CrossRef]
- Han, M.G.; Wee, C.W.; Kang, M.H.; Kim, M.J.; Jeon, S.H.; Kim, I.A. Combination of OX40 Co-Stimulation, Radiotherapy, and PD-1 Inhibition in a Syngeneic Murine Triple-Negative Breast Cancer Model. Cancers 2022, 14, 2692. [Google Scholar] [CrossRef]
- Yan, L.H.; Liu, X.L.; Mo, S.S.; Zhang, D.; Mo, X.W.; Tang, W.Z. OX40 as a novel target for the reversal of immune escape in colorectal cancer. Am. J. Transl. Res. 2021, 13, 923–934. [Google Scholar] [PubMed]
- Thapa, B.; Kato, S.; Nishizaki, D.; Miyashita, H.; Lee, S.; Nesline, M.K.; Previs, R.A.; Conroy, J.M.; DePietro, P.; Pabla, S.; et al. OX40/OX40 ligand and its role in precision immune oncology. Cancer Metastasis Rev. 2024, 43, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
| OX40 Expression Compared to Healthy Skin | OX40L Expression Compared to Healthy Skin | Pathogenetic Mechanism | Prognostic Significance of OX40–OX40L Axis | |
|---|---|---|---|---|
| Cutaneous T-Cell Lymphoma | Increased | Increased | In early MF: increased Th1, cytokine expression.In advanced MF and SS: increased Th2 and Treg cytokine expression | Increased disease-specific mortality (Kawana et al. [43]) |
| Atopic dermatitis | Increased | Increased | In acute phase: increased Th2 cytokine expression.In chronic phase: increased Th1, Th17, Th22 cytokine expression | No correlation with AD severity (Ilves and Harvima [52]) |
| Psoriasis | Increased | Low | Increased Th17 cytokine expression | Not known (Guo et al. [51]) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guglielmo, A.; Borghi, A.; Zengarini, C.; Piraccini, B.M.; Corazza, M.; Pileri, A. OX40–OX40L Axis in Cutaneous T-Cell Lymphomas: Pathogenic, Prognostic, and Potential Therapeutic Perspectives. Biomolecules 2025, 15, 715. https://doi.org/10.3390/biom15050715
Guglielmo A, Borghi A, Zengarini C, Piraccini BM, Corazza M, Pileri A. OX40–OX40L Axis in Cutaneous T-Cell Lymphomas: Pathogenic, Prognostic, and Potential Therapeutic Perspectives. Biomolecules. 2025; 15(5):715. https://doi.org/10.3390/biom15050715
Chicago/Turabian StyleGuglielmo, Alba, Alessandro Borghi, Corrado Zengarini, Bianca Maria Piraccini, Monica Corazza, and Alessandro Pileri. 2025. "OX40–OX40L Axis in Cutaneous T-Cell Lymphomas: Pathogenic, Prognostic, and Potential Therapeutic Perspectives" Biomolecules 15, no. 5: 715. https://doi.org/10.3390/biom15050715
APA StyleGuglielmo, A., Borghi, A., Zengarini, C., Piraccini, B. M., Corazza, M., & Pileri, A. (2025). OX40–OX40L Axis in Cutaneous T-Cell Lymphomas: Pathogenic, Prognostic, and Potential Therapeutic Perspectives. Biomolecules, 15(5), 715. https://doi.org/10.3390/biom15050715








