Interplay Between Traditional and Scientific Knowledge: Phytoconstituents and Their Roles in Lung and Colorectal Cancer Signaling Pathways
Abstract
1. Introduction
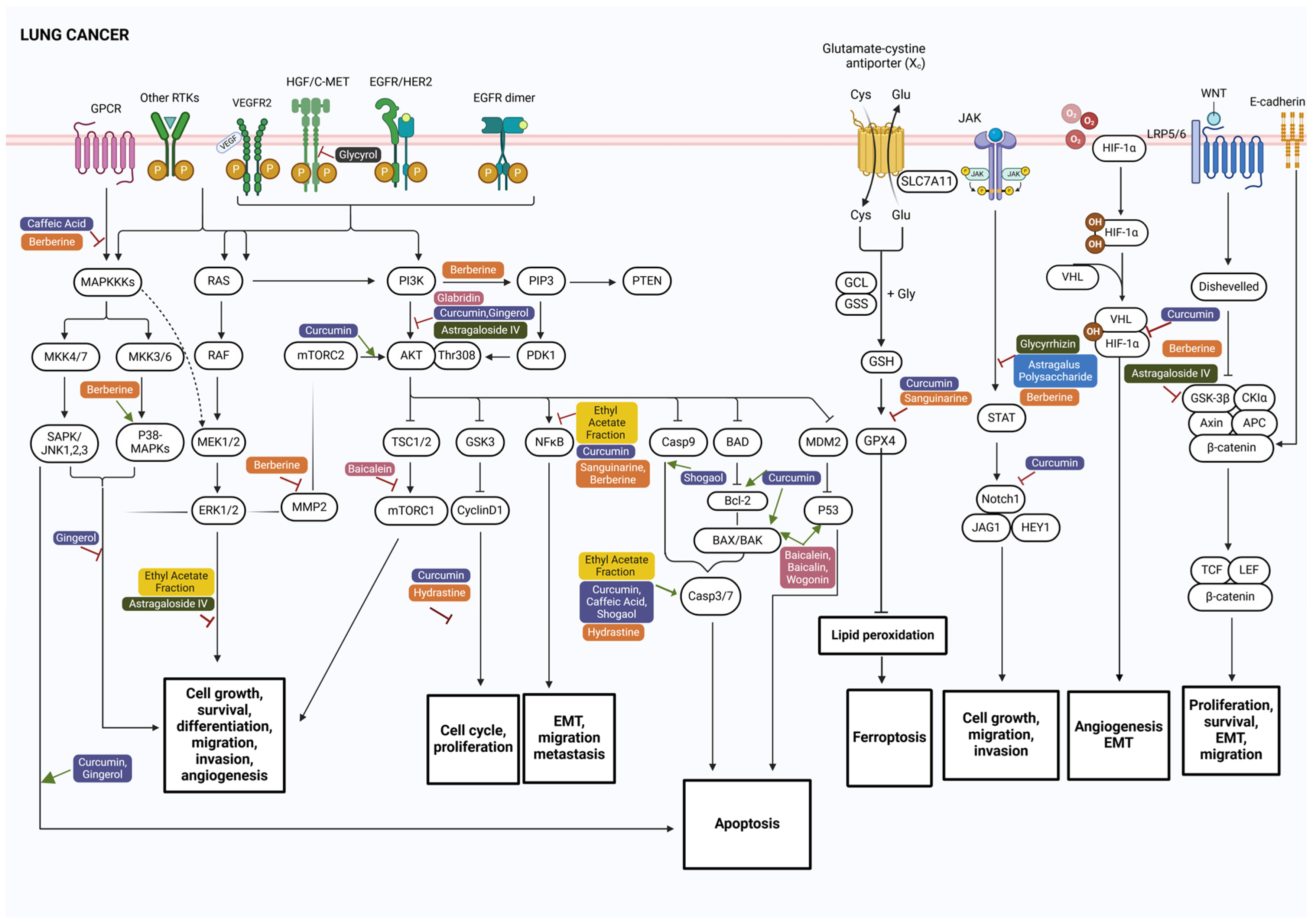
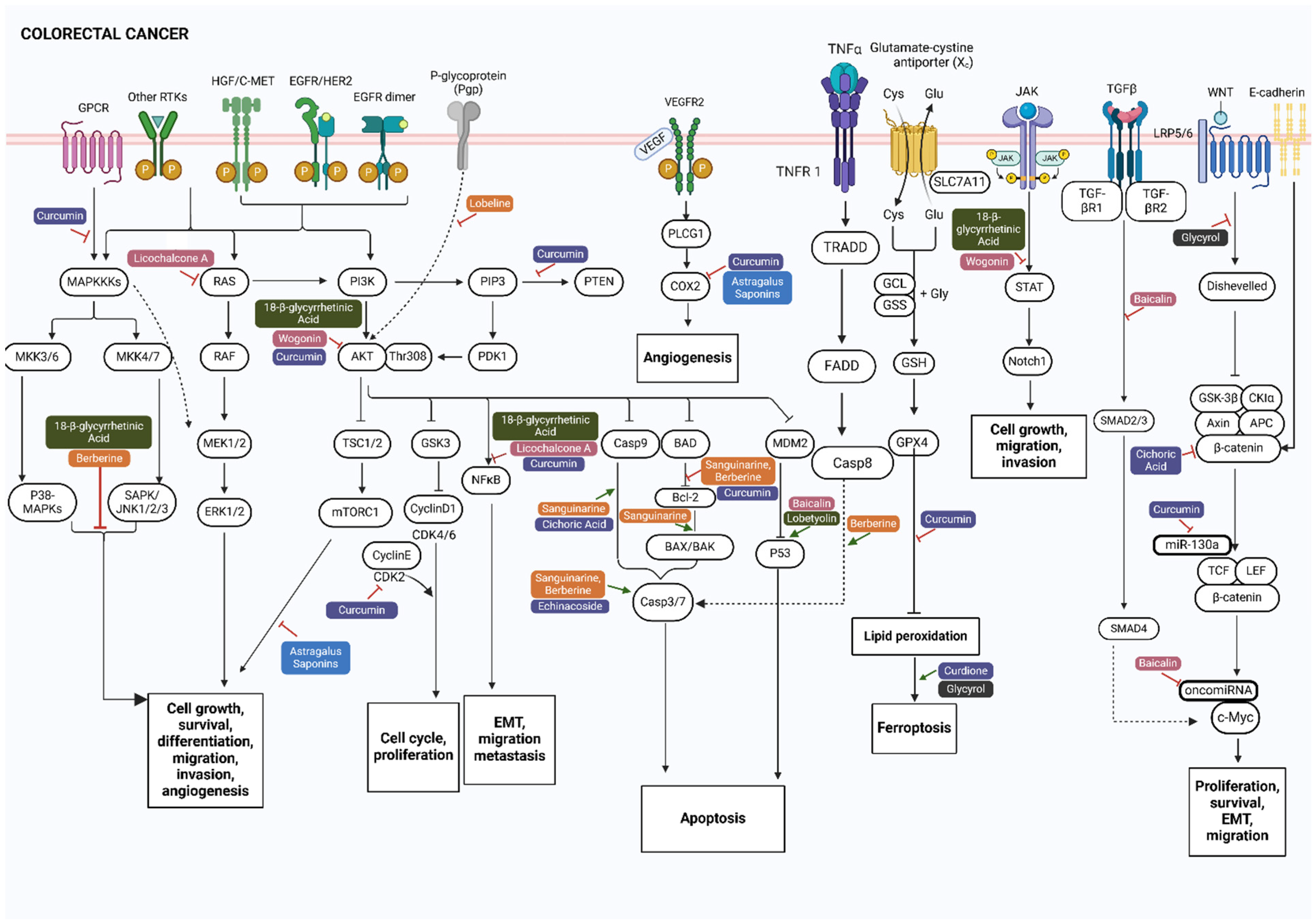
2. Key Signaling Pathways Involved in Lung and Colorectal Cancer
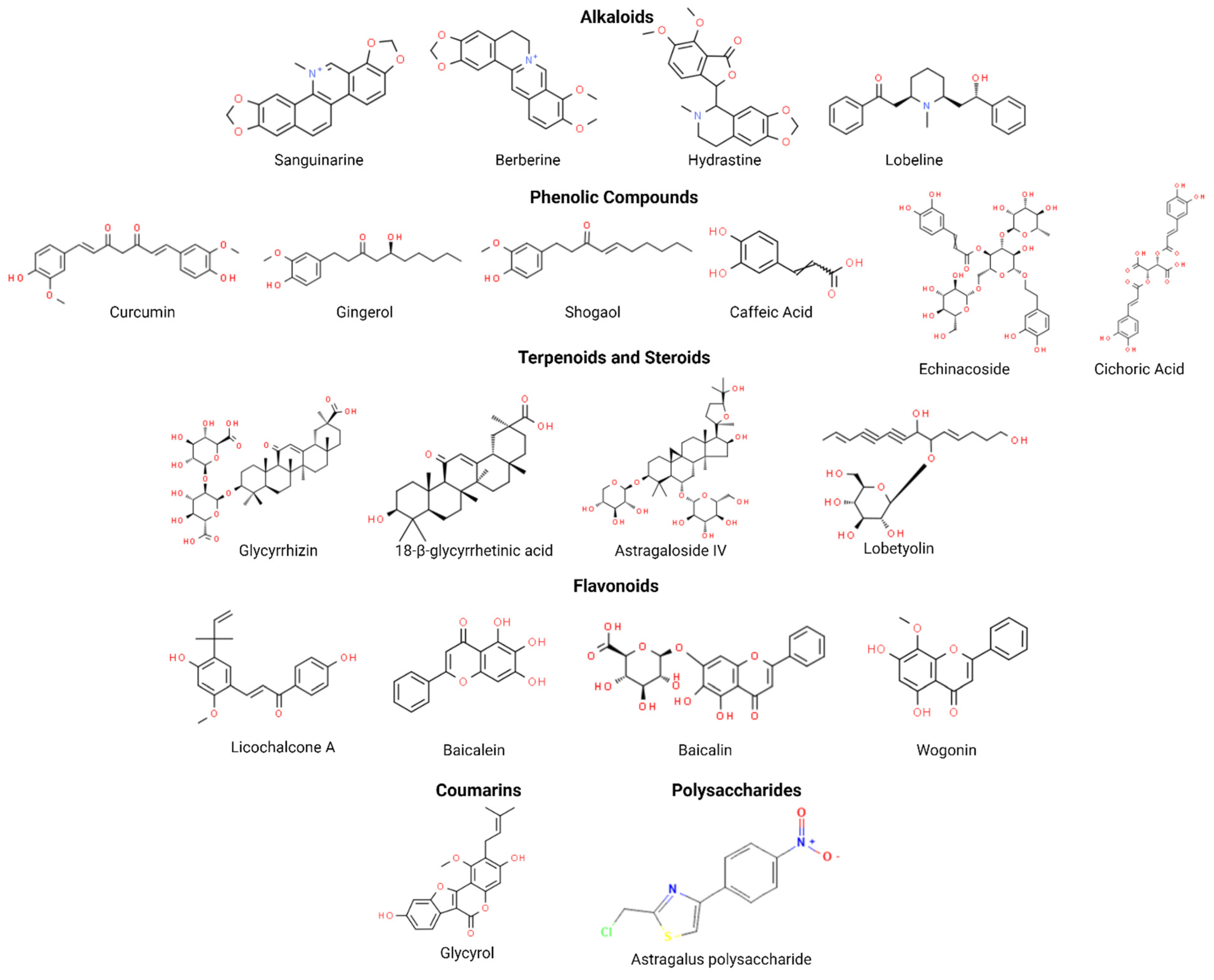
3. Anticancer Phytocompounds and Their Active Constituents
3.1. Alkaloids
3.2. Phenolic Compounds
3.3. Terpenoids and Steroids
3.4. Flavonoids
3.5. Coumarins
3.6. Polysaccharides
4. Current Progress of Clinical Trials of Phytoconstituents in Cancer Therapy
| Plant Species | Crude Extracts Tested | Isolated Compounds Tested | Anticancer Activity | Target (Protein/Pathway/miRNA) | Cancer Type | Reference |
|---|---|---|---|---|---|---|
| Curcuma longa | Not tested | Curcumin | Regulates angiogenesis, induces apoptosis, inhibits proliferation, suppresses cell division, activates autophagy | MAPK, p53, JAK/STAT pathways Wnt/β-catenin pathway (Notch, HIF-1 mRNA, VEGF and NF-κB) P13K/AKT signaling pathway (caspase 3 activity, miR-192-5p) P38 MAPK phosphorylation and ROS-DNA damage | Lung | [92,93,95,173,174,175] |
| Not tested | Curcumin Curdione | Regulates angiogenesis, inhibits proliferation, induces apoptosis, induces ferroptosis by activating autophagy (curcumin) Induces ferroptosis (curdione) | PPARy, Prp4B, NF-kB, E2F-1, CDK2, Bcl-2, HKII, COX-2, MAPK cell signaling pathway, Wnt/β-catenin pathway (miR-130a expression), PI3-K/PTEN/AKT pathway (EGFR), ↓ GPX4, FSP1 (curcumin) N6-methyladenosine pathway (curdione) | Colorectal | [58,94,98,176,177] | |
| Astragalus membranaceus | Ethyl acetate fraction of the root | Astragalus polysaccharide (APS)Astragaloside IV | Prevents the premetastatic niche (APS) Reduces proliferation, induces apoptosis (EAM) Reduces proliferation, survival, and metastasis and invasion (astragaloside IV) | S1PR1/STAT3 pathway (APS) Caspase 8 and 9, ERK pathway (EAM) PKC-α–ERK1/2–NF-κB pathway and AKT/GSK-3β/β-catenin signaling axis (astragaloside IV) | Lung | [125,126,127,160] |
| Powder of the whole root decoction Water extract Whole-plant extract | Total saponins isolated through the methanol extract | Reduces proliferation, induces cell cycle arrest, induces apoptosis, reduce migration (powder) Suppresses viability and proliferation, promotes apoptosis (water extract) Suppress angiogenesis (total saponins) Attenuates inflammation, oxidative stress and induces ferroptosis (whole-plant extract) | PI3K/AKT pathway, several mRNAs (specifically miR-590 expression) (powder) ERK1/2 signaling pathway (p-ERK1/2 and p-Akt expression) (water extract) mTOR and COX-2 signaling (VEGF) (total saponins) NF-κB activation and Nrf2 (whole plant extract) | Colorectal | [135,136,137,178] | |
| Glycyrrhiza glabra | Not tested | Glycyrrhizin Glabridin | Reduces tumor progression and the resistance and toxicity of cisplatin, reduces migration and invasion (glycyrrhizin) Reduces metastasis, invasion, and angiogenesis (glabridin) | TxA2 pathway and PCNA, JAK/STAT signaling pathway (HMGB1) (glycyrrhizin) FAK/Rho signaling pathway (glabridin) | Lung | [123,124,179] |
| Ethanol root extract | 18-β-glycyrrhetinic acid Licochalcone A Glycyrol | Reduces proliferation, invasion, and metastasis, induces apoptosis (18-β-glycyrrhetinic acid) Reduces proliferation, chemoprevention, induces apoptosis (root extract) Inhibits proliferation, induces apoptosis (licochalcone A) Inhibits proliferation and modulates ferroptosis (glycyrol) | PI3K and STAT3 signaling pathways (p-PI3K, p-AKT, p-STAT3, p-JNK, p-p38, and p-NF-κB p65) (18-β-glycyrrhetinic acid) HSP90 expression (root extract) NF-κB and Ras/Raf/MEK pathways (p65 and RAS) and programmed cell death ligand-1 (PD-L1) (licochalcone A) Wnt/β-catenin (glycyrol) | Colorectal | [120,135,139,157] | |
| Althaea officinalis | Aqueous root extract Aqueous flower extract | Not tested | Reduces cisplatin-induced cytotoxicity and cell proliferation (root extract) Reduces proliferation, anti-inflammatory activity, cytoprotective activity in red blood cells and antioxidant property (flower extract) | Reduce mRNA expression of iNOS (NOS2), IL-1β, TNF-α, IL-6 (flower extract) | Lung | [180,181] |
| Aqueous flower extract | Not tested | Reduces proliferation, anti-inflammatory activity, cytoprotective activity in red blood cells and antioxidant property | Reduces mRNA expression of iNOS (NOS2), IL-1β, TNF-α, IL-6 | Colorectal | [180] | |
| Echinacea purpurea | Dichloromethane root extract Intradermal injection Immunal forte tablets (dried extract of the plant’s juice) | Caffeic acid | Reduces viability, induces early apoptosis (root extract) Inhibits angiogenesis (injection) Stimulates metabolic activity of granulocytes (tablets) Regulates cell proliferation, migration, and apoptosis (caffeic acid) | ROS-induced caspase-dependent apoptosis (root extract) Increase CD16+ and CD56+ NK cells (tablets) MAPK pathway, inhibition of TMEM16A, calcium-activated chloride channel (caffeic acid) | Lung | [110,117,182,183] |
| Hexanic root extract 50% ethanol extract of flowers | Cichoric acid Echinacoside | Reduces viability (root extract) Reduce telomerase activity and induce apoptosis (flower extract and cichoric acid) Induces apoptosis, cell cycle arrest, and oxidative DNA damage (echinacoside) | DNA fragmentation, caspase 9 activation, PARP cleavage, and β-catenin downregulation (flower extract and cichoric acid) Increased active caspase 3, cleaved PARP, and G1/S-CDK blocker CDKN1B (p21) upregulation (echinacoside) | Colorectal | [112,115,116] | |
| Sanguinaria canadensis | Not tested | Sanguinarine | Facilitates ferroptosis and apoptosis, reduces proliferation, invasion, migration, metastasis | STUB1/GPX4-dependent ferroptosis (↑ Fe2+, ROS levels, and MDA, and ↓ GSH and GPX4) NF-κB pathway (↓ p-p65, TNF-α, IL-6, and CCL-2 expression) | Lung | [60,71] |
| Not tested | Sanguinarine | Induces apoptosis, inhibits proliferation and metabolism | ↑BAX, ↓Bcl-2 Activates caspase 3 and caspase 9 | Colorectal | [74,184] | |
| Codonopsislanceolata | Water extract of C. lanceolata polyacetylenes (CLP) | Not tested | Reduces proliferation and induces apoptosis | Ras/PI3K/AKT pathway (↓ Ras, PI3K, p-AKT, Bcl-2, cyclin D1, and CDK4 expression, and ↑ Bax, GSK-3β, clv-caspase 3, and clv-caspase 9 expression) | Lung | [185] |
| Codonopsispilosula | Not tested | Lobetyolin | Induces apoptosis, enhances the efficacy of chemotherapy (cisplatin) | ASCT2-mediated glutamine metabolism (p53) | Colorectal | [130,186] |
| Hydrastis canadensis | Not tested | Berberine, (-)-β-hydrastine | Inhibits metastasis and invasion (berberine) Reduces proliferation, migration, and invasion and induces apoptosis [(-)-β-hydrastine] | c-jun, c-fos, and NF-κB, ↓ MMP2, u-PA expression, TIMP-2 and PAI regulation (berberine) Mitochondrial apoptosis pathway (↓ cyclin D1/D3 and CDK2/4/6 expression) [(-)-β-hydrastine] | Lung | [72,73] |
| Liquid extract of root, leaf | Berberine | Induces apoptosis (berberine) Reduces viability (liquid extract) | ↑ROS, JNK/p38 MAPK pathway, and FasL, ↑ caspase 3 and caspase 8, PARP cleavage, and cytochrome C release, ↓ c-IAP1, Bcl-2, and Bcl-XL (berberine) ↓ P-gp function (liquid extract) | Colorectal | [75,187] | |
| Scutellaria baicalensis | Ethanolic root extract Water extract Qing-re-huo-xue decoction (QRHX) | Baicalein Baicalin Wogonin | Induce cell cycle arrest and apoptosis (root extract, baicalein, baicalin, and wogonin) Induces autophagy and cell cycle arrest (baicalein) Reduces metastasis and proliferation (water extract) Induces ferroptosis and apoptosis (QRHX) | ↑ p53 and BAX (root extract, baicalein, baicalin, and wogonin) MAP4K3/mTORC1/TFEB-dependent autophagy (baicalein) ↓ G1/S transition, cyclin D1, and MMP-2 (water extract) p53 and GSK-3β/Nrf2 (QRHX) | Lung | [149,150,151,188] |
| Not tested | Baicalin | Induces apoptosis (baicalin) Induces cell cycle arrest and apoptosis (baicalin) Induces cell cycle arrest, autophagy, and apoptosis (wogonin) | ↓ oncomiRNAs (miR-10a, miR-23a, miR-30c, miR-31, miR-151a, and miR-205) and c-Myc expression (baicalin) ↑ p53-independent apoptosis, ↓ TGF-β/Smad pathway (baicalin) ↓ STAT3 and PI3K/AKT (wogonin) | Colorectal | [144,145,147] | |
| Zingiber officinale | Phytocompounds extracted from ginger extract | 10-gingerol Gingerol 6-shogaol 6-gingerol | Induces apoptosis and inhibits metastasis (10-gingerol) Inhibits proliferation and invasion (gingerol) Induces cell death and reduces proliferation (6-shogaol) Inhibits growth (ginger extract) Induces ferroptosis (6-gingerol) | ↓ AKT and p38 MAPK (10-gingerol) ↓ AKT, p38 MAPK, and EGFR (gingerol) ↑ cytochrome C and caspase 3 and caspase 9 (6-shogaol) ↑ USP14 expression, modulates autophagy-dependent pathways (6-gingerol) | Lung | [99,100,104,105,189] |
| Leaf extract Phytocompounds extracted from ginger extract Bismuth oxide nanoparticles from Ginger root extract | Not tested | Reduces viability and induces apoptosis (leaf extract) Inhibit growth (extracted phytocompounds) Induce apoptosis (nanoparticles of ginger root extract) | ERK1/2 activation ↑ activating transcription factor 3 (ATF3) (leaf extract) PI3K/AKT/mTOR (nanoparticles of ginger root extract) | Colorectal | [105,106,107] | |
| Lobelia inflata | Not tested | Lobeline | Reverse P-glycoprotein (P-gp)-dependent multidrug resistance | P-glycoprotein (P-gp) | Colorectal | [76] |
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- World Health Organization. All Cancers Fact Sheet. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 1 June 2024).
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer development, progression, and therapy: An epigenetic overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead phytochemicals for anticancer drug development. Front. Plant Sci. 2016, 7, 1667. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5099879/ (accessed on 1 June 2024). [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer, J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Rebecca, L.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer, J. Clin. 2021, 3, 209–249. [Google Scholar] [CrossRef]
- Alam, M.; Hasan, G.M.; Eldin, S.M.; Adnan, M.; Riaz, M.B.; Islam, A.; Khan, I.; Hassan, M.I. Investigating regulated signaling pathways in therapeutic targeting of non-small cell lung carcinoma. Biomed. Pharmacother. 2023, 161, 114452. [Google Scholar] [CrossRef]
- Cooper, W.A.; Lam DC, L.; O’Toole, S.A.; Minna, J.D. Molecular biology of lung cancer. J. Thorac. Dis. 2013, 5, S479–S490. [Google Scholar]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Seyed Forootan, F.; Nasr Esfahani, M.H.; Ghaedi, K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 97. [Google Scholar] [CrossRef]
- White, B.D.; Chien, A.J.; Dawson, D.W. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology 2012, 142, 219–232. [Google Scholar] [CrossRef]
- Suzuki, H.; Watkins, D.N.; Jair, K.W.; Schuebel, K.E.; Markowitz, S.D.; Chen, W.D.; Pretlow, T.P.; Yang, B.; Akiyama, Y.; Van Engeland, M.; et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 2004, 36, 417–422. [Google Scholar] [CrossRef]
- Xu, S.; He, Y.; Lin, L.; Chen, P.; Chen, M.; Zhang, S. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, J.; Dumenil, T.; Bettington, M.; Pearson, S.-A.; Bond, C.; Fennell, L.; Liu, C.; McKeone, D.; Rosty, C.; Brown, I.; et al. The role of APC in WNT pathway activation in serrated neoplasia. Mod. Pathol. 2018, 31, 495–504. [Google Scholar] [CrossRef] [PubMed]
- De’ Angelis, G.L.; Bottarelli, L.; Azzoni, C.; De’ Angelis, N.; Leandro, G.; Di Mario, F.; Gaiani, F.; Negri, F. Microsatellite instability in colorectal cancer. Acta Biomed. 2018, 89, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, H. The Role of proinflammatory pathways in the pathogenesis of colitis-associated colorectal cancer. Mediat. Inflamm. 2017, 2017, 5126048. [Google Scholar] [CrossRef]
- Zhou, G. Tobacco, air pollution, environmental carcinogenesis, and thoughts on conquering strategies of lung cancer. Cancer Biol. Med. 2019, 16, 700–713. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, S. Hypoxia inducible factor-1α/vascular endothelial growth factor signaling activation correlates with response to radiotherapy and its inhibition reduces hypoxia-induced angiogenesis in lung cancer. J. Cell. Biochem. 2018, 119, 7707–7718. [Google Scholar] [CrossRef]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? a proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef]
- Heng HH, Q.; Bremer, S.W.; Stevens, J.B.; Ye, K.J.; Guo, L.; Ye, C.J. Genetic and epigenetic heterogeneity in cancer: A genome-centric perspective. J. Cell. Physiol. 2009, 220, 538–547. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Senosain, M.-F.; Massion, P.P. Intratumor heterogeneity in early lung adenocarcinoma. Front. Oncol. 2020, 10, 349. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Lehto, R.H. Psychosocial challenges for patients with advanced lung cancer: Interventions to improve well-being. Lung Cancer Targets Ther. 2017, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, J. Preventive effect and safety of chinese herbal medicine mouthwash in chemotherapy-induced oral mucositis. Medicine 2020, 99, e23387. [Google Scholar] [CrossRef] [PubMed]
- Growther, L. Anticancer activity of Annona muricata leaf extracts and screening for bioactive phytochemicals. Int. J. Pharm. Biol. Sci. 2018, 8, 475–481. [Google Scholar]
- Chopade, V.; Phatak, A.; Upaganlawar, A.; Tankar, A. Green tea (Camellia sinensis): Chemistry, traditional, medicinal uses and its pharmacological activities—A review. Pharmacogn. Rev. 2008, 2, 157–162. [Google Scholar]
- Wang, Y.; Ren, N.; Rankin, G.O.; Li, B.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Anti-proliferative effect and cell cycle arrest induced by saponins extracted from tea (Camellia sinensis) flower in human ovarian cancer cells. J. Funct. Foods 2017, 37, 310–321. [Google Scholar] [CrossRef]
- Imtiaz, I.; Schloss, J.; Bugarcic, A. Traditional and contemporary herbal medicines in management of cancer: A scoping review. J. Ayurveda Integr. Med. 2024, 15, 100904. [Google Scholar] [CrossRef]
- Tatsuno, S.; Yokosuka, A.; Hatsuma, F.; Mashiko, Y.; Mimaki, Y. Pregnane glycosides from the bark of Marsdenia cundurango and their cytotoxic activity. J. Nat. Med. 2019, 73, 93–103. [Google Scholar] [CrossRef]
- Brandon-Warner, E.; Eheim, A.L.; Foureau, D.M.; Walling, T.L.; Schrum, L.W.; McKillop, I.H. Silibinin (Milk thistle) potentiates ethanol-dependent hepatocellular carcinoma progression in male mice. Cancer Lett. 2012, 326, 88–95. [Google Scholar] [CrossRef]
- Asiimwe, J.B.; Nagendrappa, P.B.; Atukunda, E.C.; Kamatenesi, M.M.; Nambozi, G.; Tolo, C.U.; Sarki, A. Prevalence of the Use of Herbal Medicines Among Patients With Cancer: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2021, 2021, 9963038. [Google Scholar] [CrossRef]
- Safarzadeh, E.; Sandoghchian Shotorbani, S.; Baradaran, B. Herbal Medicine as Inducers of Apoptosis in Cancer Treatment. Adv. Pharm. Bull. 2014, 4 (Suppl. 1), 421–427. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Sadia, S.; Pan, K.; Ullah, I.; Mussarat, S.; Sun, F.; Song, D. A Systematic Review on Ethnomedicines of Anti-Cancer Plants. Phytother. Res. 2017, 31, 202–264. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, I.; Bugarcic, A.; Schloss, J.; Steel, A.; Redmond, R.; Browne, J.; Geldard, C. Naturopathic herbal management of lung, colorectal and skin cancer: International practitioner survey. In Proceedings of the Integrative Medicine and Health 2nd World Congress 2024, Rome, Italy, 20–23 September 2023. [Google Scholar] [CrossRef]
- Reissig, T.M.; Sara, L.; Ting, S.; Reis, H.; Metzenmacher, M.; Eberhardt, W.E.E.; Zaun, G.; Herold, T.; Aigner, C.; Darwiche, K.; et al. ERK phosphorylation as a marker of RAS activity and its prognostic value in non-small cell lung cancer. Lung Cancer 2020, 149, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Bawari, S.; Burcher, J.T.; Sinha, D.; Tewari, D.; Bishayee, A. Targeting cell signaling pathways in lung cancer by bioactive phytocompounds. Cancers 2023, 15, 3980. [Google Scholar] [CrossRef]
- Iksen; Pothongsrisit, S.; Pongrakhananon, V. Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: An update regarding potential drugs and natural products. Molecules 2021, 26, 4100. [Google Scholar] [CrossRef]
- Ahmad, R.; Singh, J.K.; Wunnava, A.; Al-Obeed, O.; Abdulla, M.; Srivastava, S.K. Emerging trends in colorectal cancer: Dysregulated signaling pathways (Review). Int. J. Mol. Med. 2021, 47, 14. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, W.R.; Park, E.G.; Lee, D.H.; Kim, J.M.; Shin, H.J.; Jeong, H.S.; Roh, H.Y.; Kim, H.S. Exploring the key signaling pathways and ncrnas in colorectal cancer. Int. J. Mol. Sci. 2024, 25, 4548. [Google Scholar] [CrossRef]
- García-Aranda, M.; Redondo, M. Targeting receptor kinases in colorectal cancer. Cancers 2019, 11, 433. [Google Scholar] [CrossRef]
- Palma, S.; Zwenger, A.; Croce, M.; Abba, M.; Lacunza, E. From molecular biology to clinical trials. Towards a personalized colorectal cancer therapy. Clin. Color. Cancer 2015, 15, 104–115. [Google Scholar] [CrossRef]
- Serafino, A.; Moroni, N.; Zonfrillo, M.; Andreola, F.; Mercuri, L.; Nicotera, G.; Nunziata, J.; Ricci, R.; Antinori, A.; Rasi, G.; et al. WNT-pathway components as predictive markers useful for diagnosis, prevention and therapy in inflammatory bowel disease and sporadic colorectal cancer. Oncotarget 2014, 5, 978–992. [Google Scholar] [CrossRef]
- Yuan, S.; Tao, F.; Zhang, X.; Zhang, Y.; Sun, X.; Wu, D. Role of Wnt/β-Catenin signaling in the chemoresistance modulation of colorectal cancer. BioMed Res. Int. 2020, 2020, 9390878. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Gan, W.J. Wnt/β-Catenin signaling pathway in the development and progression of colorectal cancer. Cancer Manag. Res. 2023, 15, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Park, J.-H.; Rhyu, S.Y.; Oh, S.-T.; Kang, W.-K.; Kim, H.-N. Wnt3a expression is associated with MMP-9 expression in primary tumor and metastatic site in recurrent or stage IV colorectal cancer. BMC Cancer 2014, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Guo, H.; Song, Y.; Zhao, X.; Shi, Y.; Lu, Y.; Hu, S.; Nie, Y.; Fan, D.; Wu, K. Loss of vinculin and membrane-bound β-catenin promotes metastasis and predicts poor prognosis in colorectal cancer. Mol. Cancer 2014, 13, 263. [Google Scholar] [CrossRef]
- Network, T.; Bainbridge, M.; Chang, K.; Dinh, H.; Drummond, J.; Fowler, G. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330. [Google Scholar] [CrossRef]
- Siena, S.; Sartore-Bianchi, A.; Nicolantonio, F.D.; Balfour, J.A.; Bardelli, A. Biomarkers predicting clinical outcome of epidermal growth factor receptor–targeted therapy in metastatic colorectal cancer. J. Natl. Cancer Inst. 2009, 101, 1308–1324. [Google Scholar] [CrossRef]
- Pennel, K.; Hatthakarnkul, P.; Wood, C.; Lian, G.-Y.; Al-Badran, S.; Quinn, J.; Legrini, A.; Inthagard, J.; Alexander, P.; Wyk, H.; et al. JAK/STAT3 represents a therapeutic target for colorectal cancer patients with stromal-rich tumors. J. Exp. Clin. Cancer Res. CR 2024, 43, 64. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Zhang, Y.; Fan, Z. Mechanism and application of ferroptosis in colorectal cancer. Biomed. Pharmacother. 2023, 158, 114102. [Google Scholar] [CrossRef]
- Song, Y.Q.; Yan, X.D.; Wang, Y.; Wang, Z.Z.; Mao, X.L.; Ye, L.P.; Li, S.W. Role of ferroptosis in colorectal cancer. World J. Gastrointest. Oncol. 2023, 15, 225–239. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Ganapathy, A.; Ezekiel, U.R. Phytochemical modulation of miRNAs in colorectal cancer. Medicines 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Wang, M.; Sun, N.X.; Zhu, C.; Lin, Y.M.; Cui, L.; Liu, F.; Zhu, W. Sulforaphane suppresses carcinogenesis of colorectal cancer through the ERK/Nrf2-UDP glucuronosyltransferase 1A metabolic axis activation. Oncol. Rep. 2020, 43, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.S.; Kruyt FA, E.; Cheah, S.C. Understanding lung carcinogenesis from a morphostatic perspective: Prevention and therapeutic potential of phytochemicals for targeting cancer stem cells. Int. J. Mol. Sci. 2021, 22, 5697. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. 2015, 21, 9262. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, R.; Chen, G.; Zhu, X.; Wu, W.; Chen, Z.; Jiang, C.; Lin, Z.; Ma, L.; Yu, H. Berberine synergises with ferroptosis inducer sensitizing NSCLC to ferroptosis in p53-dependent SLC7A11-GPX4 pathway. Biomed. Pharmacother. 2024, 176, 116832. [Google Scholar] [CrossRef]
- Miyazaki, K.; Xu, C.; Shimada, M.; Goel, A. Curcumin and Andrographis exhibit anti-tumor effects in colorectal cancer via activation of ferroptosis and dual suppression of glutathione peroxidase-4 and ferroptosis suppressor protein-1. Pharmaceuticals 2023, 16, 383. [Google Scholar] [CrossRef]
- Sun, K.; Zhi, Y.; Ren, W.; Li, S.; Zhou, X.; Gao, L.; Zhi, K. The mitochondrial regulation in ferroptosis signaling pathway and its potential strategies for cancer. Biomed. Pharmacother. 2023, 169, 115892. [Google Scholar] [CrossRef]
- Xu, R.; Wu, J.; Luo, Y.; Wang, Y.; Tian, J.; Teng, W.; Zhang, B.; Fang, Z.; Li, Y. Sanguinarine represses the growth and metastasis of non-small cell lung cancer by facilitating ferroptosis. Curr. Pharm. Des. 2022, 28, 760–768. [Google Scholar] [CrossRef]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef]
- Armstrong, L.; Machado, C.D.; Dell’Avanzi, G.G.; Spindola, T.; Raman, V.; Busch, J.; Maier, J.; da Silva NC, B.; Holandino, C.; Oliveira, A.P.; et al. Investigations on the morpho-anatomy and histochemistry of Aconitum napellus L. Microsc. Res. Tech. 2023, 87, 534–545. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Ding, K.; Chen, X.N.; Han, T. Study on optimization of extraction method of total alkaloids from zizyphi spinosi semen. In Proceedings of the 2016 International Forum on Mechanical, Control and Automation (IFMCA 2016), Shenzhen, China, 30–31 December 2016; Atlantis Press: Amsterdam, The Netherlands, 2017; pp. 846–854. [Google Scholar]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants used against cancer—An extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Savigni, D. Evaluation of the Anticancer Potential of Plants Used as Traditional Medicines by Aboriginal People. Doctoral Thesis, The University of Western Australia, Perth, Australia, 2010. Available online: https://research-repository.uwa.edu.au/files/3236021/Savigni_Donna_2010.pdf (accessed on 1 February 2025).
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, Y.; Fang, Z.; Teng, W.; Yu, Y.; Tian, J.; Guo, P.; Xu, R.; Wu, J.; Li, Y. Mechanism of sanguinarine in inhibiting macrophages to promote metastasis and proliferation of lung cancer via modulating the exosomes in A549 cells. OncoTargets Ther. 2020, 13, 8989–9003. [Google Scholar] [CrossRef]
- Peng, P.L.; Hsieh, Y.S.; Wang, C.J.; Hsu, J.L.; Chou, F.P. Inhibitory effect of berberine on the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Toxicol. Appl. Pharmacol. 2006, 214, 8–15. [Google Scholar] [CrossRef]
- Guo, B.; Li, X.; Song, S.; Chen, M.; Cheng, M.; Zhao, D.; Li, F. (-)-β-hydrastine suppresses the proliferation and invasion of human lung adenocarcinoma cells by inhibiting PAK4 kinase activity. Oncol. Rep. 2016, 35, 2246–2256. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, W.-K.; Jeong, M.H.; Yoon, T.R.; Kim, H.K. Sanguinarine induces apoptosis of HT-29 human colon cancer cells via the regulation of Bax/Bcl-2 Ratio and caspase-9-dependent pathway. Int. J. Toxicol. 2012, 31, 70–77. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Hsieh, Y.-S.; Kuo, H.-C.; Teng, C.-Y.; Huang, H.-I.; Wang, C.-J.; Yang, S.-F.; Liou, Y.-S.; Kuo, W.-H. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch. Toxicol. 2007, 81, 719–728. [Google Scholar] [CrossRef]
- Ma, Y.; Wink, M. Lobeline, a piperidine alkaloid from Lobelia can reverse P-gp dependent multidrug resistance in tumor cells. Phytomedicine 2008, 15, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Crooks, P.A.; Zheng, G.; Vartak, A.P.; Culver, J.P.; Zheng, F.; Horton, D.B.; Dwoskin, L.P. Design, synthesis and interaction at the vesicular monoamine transporter-2 of lobeline analogs: Potential pharmacotherapies for the treatment of psychostimulant abuse. Curr. Top. Med. Chem. 2011, 11, 1103–1127. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.U.; Lloyd, C.G. Drugs and Medicines of North America; 1884–1887. Available online: https://www.henriettes-herb.com/eclectic/dmna/index.html (accessed on 1 March 2025).
- Evans, W.C.; Evans, D. Alkaloids. In Trease and Evans’ Pharmacognosy, 16th ed.; Evans, W.C., Evans, D., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 353–415. [Google Scholar] [CrossRef]
- Folquitto, D.G.; Swiech, J.N.; Pereira, C.B.; Bobek, V.B.; Possagno GC, H.; Farago, P.V.; Miguel, M.D.; Duarte, J.L.; Miguel, O.G. Biological activity, phytochemistry and traditional uses of genus Lobelia (Campanulaceae): A systematic review. Fitoterapia 2019, 134, 23–38. [Google Scholar] [CrossRef]
- National Herbalists Association of Australia. Reforms to the Regulatory Framework for Complementary Medicines: Assessment Pathways Submission. (p. 7). Australia. 2017. Available online: https://www.tga.gov.au/sites/default/files/submissions-received-reforms-regulatory-framework-complementary-medicines-nhaa.pdf (accessed on 1 June 2024).
- Yeshi, K.; Turpin, G.; Jamtsho, T.; Wangchuk, P. Indigenous uses, phytochemical analysis, and anti-inflammatory properties of australian tropical medicinal plants. Molecules 2022, 27, 3849. [Google Scholar] [CrossRef] [PubMed]
- Boericke, W. Excerpt from Boericke’s Materia Medica: The Tinctures. 1901. Available online: https://www.henriettes-herb.com/eclectic/boericke/index.html (accessed on 1 June 2024).
- Mandal, S.K.; Maji, A.K.; Mishra, S.K.; Ishfaq, P.M.; Devkota, H.P.; Silva, A.S.; Das, N. Goldenseal (Hydrastis canadensis L.) and its active constituents: A critical review of their efficacy and toxicological issues. Pharmacol. Res. 2020, 160, 105085. [Google Scholar] [CrossRef]
- Culpeper, N. The complete herbal to which is now added, upwards of one hundred additional herbs ... To which are now first annexed 1847, the English physician enlarged, and Key to [Galen’s Method of] physic ... forming a complete family dispensatory and natural system of physic ... to which is also added ... receipts selected from the author’s Last legacy. To his wife. Available online: https://archive.org/details/b22011791/page/172/mode/2up?q=cancer (accessed on 1 June 2024).
- Madaus, G. Textbook of Biological Remedies. 1938. Available online: https://www.henriettes-herb.com/eclectic/madaus/index.html (accessed on 1 June 2024).
- Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Liu, L. Sanguinaria canadensis: Traditional medicine, phytochemical composition, biological activities and current uses. Int. J. Mol. Sci. 2016, 17, 1414. [Google Scholar] [CrossRef] [PubMed]
- Salmore, A.K.; Hunter, M.D. Environmental and genotypic influences on isoquinoline alkaloid content in Sanguinaria canadensis. J. Chem. Ecol. 2001, 27, 1729–1747. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–271. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Bhatia, M.; Bhalerao, M.; Cruz-Martins, N.; Kumar, D. Curcumin and cancer biology: Focusing regulatory effects in different signalling pathways. Phytother. Res. 2021, 35, 4913–4929. [Google Scholar] [CrossRef]
- Li, X.; Ma, S.; Yang, P.; Sun, B.; Zhang, Y.; Sun, Y.; Hao, M.; Mou, R.; Jia, Y. Anticancer effects of curcumin on nude mice bearing lung cancer A549 cell subsets SP and NSP cells. Oncol. Lett. 2018, 16, 6756–6762. [Google Scholar] [CrossRef]
- Mishra, A.P.; Swetanshu Singh, P.; Yadav, S.; Nigam, M.; Seidel, V.; Rodrigues, C.F. Role of the dietary phytochemical curcumin in targeting cancer cell signalling pathways. Plants 2023, 12, 1782. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Shen, R.; Tao, J.; Huang, L.; Shi, H.; Chen, H.; Wang, Y.; Wang, T. Curcumin suppresses the colon cancer proliferation by inhibiting Wnt/β-Catenin pathways via miR-130a [Original Research]. Front. Pharmacol. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Elgar, K. Curcumin: A review of clinical use and efficacy. Nutr. Med. J. 2022, 1, 10–31. [Google Scholar]
- Schloss, J. Chapter 21—Cancer—Advanced, I. In Advanced Clinical Naturopathic Medicine; Hechtman, L., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020; pp. 823–837. [Google Scholar]
- Sultana, S.; Munir, N.; Mahmood, Z.; Riaz, M.; Akram, M.; Rebezov, M.; Kuderinova, N.; Moldabayeva, Z.; Shariati, M.A.; Rauf, A.; et al. Molecular targets for the management of cancer using curcuma longa linn. phytoconstituents: A review. Biomed. Pharmacother. 2021, 135, 111078. [Google Scholar] [CrossRef]
- de Lima, R.M.T.; Dos Reis, A.C.; de Menezes, A.A.P.M.; Santos, J.V.D.O.; Filho, J.W.G.D.O.; Ferreira, J.R.D.O.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Funakoshi, H.; Momo, K.; Kashima, A.; Ida, H.; Miyata, Y.; Sagara, H.; Sasaki, T. Liver injury by the traditional chinese medicine hanshirento, zenshikunshito, and ninjin’yoeito in a patient with lung cancer: Probable causality assessed by the updated roussel uclaf causality assessment method. Integr. Cancer Ther. 2021, 20, 15347354211004734. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, K.I.; Choi, C.W.; Kim, J.Y.; Lee, J.H. Long-term progression-free survival in a patient with advanced non-small-cell lung cancer treated with low-dose gefitinib and traditional herbal medicine: A case report. Medicine 2021, 100, e24292. [Google Scholar] [CrossRef]
- Rasmussen, P. Ginger--Zingiber officinale Roscoe, Zingiberaceae. J. Prim. Health Care 2011, 3, 235–236. [Google Scholar] [CrossRef]
- Joo, J.-H.; Hong, S.-S.; Cho, Y.-R.; Seo, D.-W. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of Akt and p38MAPK activity. Oncol. Rep. 2016, 35, 779–784. [Google Scholar] [CrossRef]
- Warin, R.F.; Chen, H.; Soroka, D.N.; Zhu, Y.; Sang, S. Induction of lung cancer cell apoptosis through a p53 pathway by [6]-shogaol and its cysteine-conjugated metabolite M2. J. Agric. Food Chem. 2014, 62, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Hong, J.; Wu, H.; Liu, J.; Yang, C.S.; Pan, M.-H.; Badmaev, V.; Ho, C.-T. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J. Agric. Food Chem. 2009, 57, 10645–10650. [Google Scholar] [CrossRef] [PubMed]
- Park, G.H.; Park, J.H.; Song, H.M.; Eo, H.J.; Kim, M.K.; Lee, J.W.; Lee, M.H.; Cho, K.-H.; Lee, J.R.; Cho, H.J.; et al. Anti-cancer activity of Ginger (Zingiber officinale) leaf through the expression of activating transcription factor 3 in human colorectal cancer cells. BMC Complement. Altern. Med. 2014, 14, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Cheng, Z.; Wang, H. Regulation of the PI3K/AKT/mTOR signaling pathway with synthesized bismuth oxide nanoparticles from Ginger (Zingiber officinale) extract: Mitigating the proliferation of colorectal cancer cells. Arab. J. Chem. 2022, 15, 103607. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef]
- Nicoll, R.; Henein, M.Y. Ginger (Zingiber officinale Roscoe): A hot remedy for cardiovascular disease? Int. J. Cardiol. 2009, 131, 408–409. [Google Scholar] [CrossRef]
- Bai, X.; Li, S.; Liu, X.; An, H.; Kang, X.; Guo, S. Caffeic acid, an active ingredient in coffee, combines with dox for multitarget combination therapy of lung cancer. J. Agric. Food Chem. 2022, 70, 8326–8337. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Chen, J.; Tan, H.-Y.; Wang, N.; Chen, Z.; Feng, Y. Scutellaria baicalensis and cancer treatment: Recent progress and perspectives in biomedical and clinical studies. Am. J. Chin. Med. 2018, 46, 25–54. [Google Scholar] [CrossRef]
- Dong, L.; Yu, D.; Wu, N.; Wang, H.; Niu, J.; Wang, Y.; Zou, Z. Echinacoside induces apoptosis in human sw480 colorectal cancer cells by induction of oxidative DNA damages. Int. J. Mol. Sci. 2015, 16, 14655–14668. [Google Scholar] [CrossRef]
- Lin, S.-D.; Sung, J.-M.; Chen, C.-L. Effect of drying and storage conditions on caffeic acid derivatives and total phenolics of Echinacea Purpurea grown in Taiwan. Food Chem. 2011, 125, 226–231. [Google Scholar] [CrossRef]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Chiu, C.C.; Chen, J.Y.F.; Chan, K.C.; Lin, S.D. Cytotoxic Effects of Echinacea purpurea Flower Extracts and Cichoric Acid on Human Colon Cancer Cells Through Induction of Apoptosis. J. Ethnopharmacol. 2012, 143, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Chicca, A.; Adinolfi, B.; Martinotti, E.; Fogli, S.; Breschi, M.C.; Pellati, F.; Benvenuti, S.; Nieri, P. Cytotoxic effects of Echinacea root hexanic extracts on human cancer cell lines. J. Ethnopharmacol. 2007, 110, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Hosami, F.; Manayi, A.; Salimi, V.; Khodakhah, F.; Nourbakhsh, M.; Nakstad, B.; Tavakoli-Yaraki, M. The pro-apoptosis effects of Echinacea purpurea and Cannabis sativa extracts in human lung cancer cells through caspase-dependent pathway. BMC Complement. Med. Ther. 2021, 21, 37. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Mohammadi-Cheraghabadi, M.; Hazrati, S. Chapter 5 Terpenoids, steroids, and phenolic compounds of medicinal plants. In Phytochemicals in Medicinal Plants; Charu, A., Dakeshwar Kumar, V., Jeenat, A., Pramod Kumar, M., Eds.; De Gruyter: Berlin, Germany, 2023; pp. 105–130. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ara, I.; Mondal MS, A.; Kabir, Y. Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon 2021, 7, e07240. [Google Scholar] [CrossRef]
- Kaur, P.; Robin; Makanjuola, V.O.; Arora, R.; Singh, B.; Arora, S. Immunopotentiating Significance of Conventionally Used Plant Adaptogens as Modulators in Biochemical and Molecular Signalling Pathways in Cell-Mediated Processes. Biomed. Pharmacother. 2017, 95, 1815–1829. [Google Scholar] [CrossRef]
- Yoon, S.S.; Kim, E.H.; Lee, J.Y.; Yoon, S.W. Prolonged Progression-Free Survival in a Patient With Malignant Pleural Mesothelioma Following Korean Herbal Medicine Treatment Alone: A Case Report. Integr. Cancer Ther. 2020. [Google Scholar] [CrossRef]
- Deng, Q.-P.; Wang, M.-J.; Zeng, X.; Chen, G.G.; Huang, R.-Y. Effects of glycyrrhizin in a mouse model of lung adenocarcinoma. Cell. Physiol. Biochem. 2017, 41, 1383–1392. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Chen, Y.; Liu, X.; Wang, J.; Qin, X.; Yuan, D.; Yu, T.; Chen, G.; Mi, Y.; et al. Glycyrrhizin suppresses the growth of human NSCLC cell line HCC827 by downregulating HMGB1 level. BioMed Res. Int. 2018, 2018, 6916797. [Google Scholar] [CrossRef]
- Cheng, X.; Gu, J.; Zhang, M.; Yuan, J.; Zhao, B.; Jiang, J.; Jia, X. Astragaloside IV inhibits migration and invasion in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB pathway. Int. Immunopharmacol. 2014, 23, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Feng, C.; Chu, S.-J.; Zhang, R.; Lu, Y.; Zhu, J.; Zhang, J. Toosendanin inhibits growth and induces apoptosis in colorectal cancer cells through suppression of AKT/GSK-3β/β-catenin pathway. Int. J. Oncol. 2015, 47, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, S.H. Induction of Apoptosis by ethyl acetate fraction of Astragalus membranaceus in human non-small cell lung cancer cells: Apoptosis induction by Astragalus membranaceus. J. Pharmacopunct. 2018, 21, 268–276. [Google Scholar] [CrossRef]
- Xing, N.; Du, Q.; Guo, S.; Xiang, G.; Zhang, Y.; Meng, X.; Wang, S. Ferroptosis in Lung Cancer: A Novel Pathway Regulating Cell Death and a Promising Target for Drug Therapy. Cell Death Discov. 2023. [Google Scholar] [CrossRef]
- Wang, S.-S.; Zhang, T.; Wang, L.; Dong, S.; Wang, D.-H.; Li, B.; Cao, X.-Y. The Dynamic Changes in the Main Substances in Codonopsis pilosula Root Provide Insights into the Carbon Flux Between Primary and Secondary Metabolism During Different Growth Stages. Metabolites 2023, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Z.; Yang, L.; Wu, X.; Zhu, J.; Liu, L.; Liu, Y. Lobetyolin suppressed lung cancer in a mouse model by inhibiting epithelial-mesenchymal transition. Eur. J. Histochem. 2022, 66, 3423. [Google Scholar] [CrossRef]
- He, W.; Tao, W.; Zhang, F.; Jie, Q.; He, Y.; Zhu, W.; Tan, J.; Shen, W.; Li, L.; Yang, Y.; et al. Lobetyolin induces apoptosis of colon cancer cells by inhibiting glutamine metabolism. J. Cell. Mol. Med. 2020, 24, 3359–3369. [Google Scholar] [CrossRef]
- Wang, G.; Hiramoto, K.; Ma, N.; Yoshikawa, N.; Ohnishi, S.; Murata, M.; Kawanishi, S. Glycyrrhizin attenuates carcinogenesis by inhibiting the inflammatory response in a murine model of colorectal cancer. Int. J. Mol. Sci. 2021, 22, 2609. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.; Qiu, R.; Chen, Z.; Chen, Z.; Chen, W. 18 β-glycyrrhetinic acid exhibits potent antitumor effects against colorectal cancer via inhibition of cell proliferation and migration. Int. J. Oncol. 2017, 51, 615–624. [Google Scholar] [CrossRef]
- Nourazarian, S.M.; Nourazarian, A.; Majidinia, M.; Roshaniasl, E. Effect of root extracts of medicinal herb glycyrrhiza glabra on HSP90 gene expression and apoptosis in the HT-29 colon cancer cell line. Asian Pac. J. Cancer Prev. 2015, 16, 8563–8566. [Google Scholar] [CrossRef]
- Law, P.-C.; Auyeung, K.K.; Chan, L.-Y.; Ko, J.K. Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells. BMC Complement. Altern. Med. 2012, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Yang, C.H.; Chen, C.H.; Chen, C.H.; Hsu, S.L.; Lee, M.H.; Lee, H.C.; Su, L.J. An in vivo molecular response analysis of colorectal cancer treated with Astragalus membranaceus extract. Oncol. Rep. 2016, 35, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhai, W.; Tan, D.; Chen, H.; Zhang, G.; Tan, X.; Zheng, Y.; Gao, W.; Wei, Y.; Wu, J.; et al. Uncovering the effects and molecular mechanism of Astragalus membranaceus (Fisch.) Bunge and its bioactive ingredients formononetin and calycosin against colon cancer: An integrated approach based on network pharmacology analysis coupled with experimental validation and molecular docking [Original Research]. Front. Pharmacol. 2023, 14, 1111912. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Y.; Li, M.; Zhang, Z.; Wang, J.; Ri, M.; Jin, C.; Xu, G.; Piao, L.; Jin, H.; et al. Licochalcone A inhibits proliferation and promotes apoptosis of colon cancer cell by targeting programmed cell death-ligand 1 via the NF-κB and Ras/Raf/MEK pathways. J. Ethnopharmacol. 2021, 273, 113989. [Google Scholar] [CrossRef]
- Huang, H.-C.; Tsai, L.-L.; Tsai, J.-P.; Hsieh, S.-C.; Yang, S.-F.; Hsueh, J.-T.; Hsieh, Y.-H. Licochalcone A inhibits the migration and invasion of human lung cancer cells via inactivation of the Akt signaling pathway with downregulation of MMP-1/-3 expression. Tumor Biol. 2014, 35, 12139–12149. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, T.; Zhang, W.; Zhou, L.; Yu, B.; Wang, W.; Yang, Z.; Liu, Z.; Zou, P.; Liang, G. Licochalcone A inhibits the proliferation of human lung cancer cell lines A549 and H460 by inducing G2/M Cell cycle arrest and ER stress. Int. J. Mol. Sci. 2017, 18, 1761. [Google Scholar] [CrossRef]
- Cathcart, M.-C.; Useckaite, Z.; Drakeford, C.; Semik, V.; Lysaght, J.; Gately, K.; O’Byrne, K.J.; Pidgeon, G.P. Anti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivo. BMC Cancer 2016, 16, 707. [Google Scholar] [CrossRef]
- Sui, X.; Han, X.; Chen, P.; Wu, Q.; Feng, J.; Duan, T.; Chen, X.; Pan, T.; Yan, L.; Jin, T.; et al. Baicalin induces apoptosis and suppresses the cell cycle progression of lung cancer cells through downregulating Akt/mTOR signaling pathway [Original Research]. Front. Mol. Biosci. 2021, 7, 602282. [Google Scholar] [CrossRef]
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018, 8, 14477. [Google Scholar] [CrossRef]
- Yang, B.; Bai, H.; Sa, Y.; Zhu, P.; Liu, P. Inhibiting EMT, stemness and cell cycle involved in baicalin-induced growth inhibition and apoptosis in colorectal cancer cells. J. Cancer 2020, 11, 2303–2317. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jin, G.; Hu, Y.; Zhao, Z.; Nan, F.; Hu, X.; Hu, Y.; Han, Q. Wogonin restrains the malignant progression of lung cancer through modulating MMP1 and PI3K/AKT signaling pathway. Proteins Pept. Lett. 2023, 30, 25–34. [Google Scholar] [CrossRef]
- Tan, H.; Li, X.; Yang, W.-H.; Kang, Y. A flavone, Wogonin from Scutellaria baicalensis inhibits the proliferation of human colorectal cancer cells by inducing of autophagy, apoptosis and G2/M cell cycle arrest via modulating the PI3K/AKT and STAT3 signalling pathways. J. Buon 2019, 24, 1143–1149. [Google Scholar] [PubMed]
- Yingrui, W.; Zheng, L.; Guoyan, L.; Hongjie, W. Research progress of active ingredients of Scutellaria baicalensis in the treatment of type 2 diabetes and its complications. Biomed. Pharmacother. 2022, 148, 112690. [Google Scholar] [CrossRef]
- Gao, J.; Morgan, W.A.; Sanchez-Medina, A.; Corcoran, O. The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells. Toxicol. Appl. Pharmacol. 2011, 254, 221–228. [Google Scholar] [CrossRef]
- Park, K.-I.; Park, H.; Kang, S.-Y.; Nagappan, A.; Lee, D.H.; Kim, J.; Han, D.-Y.; Kim, G.S. Korean scutellaria baicalensis water extract inhibits cell cycle G1/S transition by suppressing cyclin d1 expression and matrix-metalloproteinase-2 activity in human lung cancer cells. J. Ethnopharmacol. 2011, 133, 634–641. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Ji, L.; Cui, W.; Cui, J.; Tang, Z.; Sun, N.; Zhang, G.; Guo, M.; Liu, B.; et al. Inhibition of non-small cell lung cancer by ferroptosis and apoptosis induction through P53 and GSK-3β/Nrf2 signal pathways using qingrehuoxue formula. J. Cancer 2023, 14, 336–349. [Google Scholar] [CrossRef]
- Hool, R.L. Health from British Wild Herbs. 1918. Available online: https://www.henriettes-herb.com/eclectic/hool/index.html (accessed on 1 June 2024).
- Ye, F.; Jiang, S.; Volshonok, H.; Wu, J.; Zhang, D.Y. Molecular mechanism of anti-prostate cancer activity of scutellaria baicalensis extract. Nutr. Cancer 2007, 57, 100–110. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, S.; Yang, S.; Lin, X.; He, X.; Wei, X.; Song, Q.; Li, R.; Fu, C.; Zhang, J.; et al. An “essential herbal medicine”—Licorice: A review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2020, 249, 112439. [Google Scholar] [CrossRef]
- Lu, S.; Yin, S.; Zhao, C.; Fan, L.; Hu, H. Synergistic anti-colon cancer effect of glycyrol and butyrate is associated with the enhanced activation of caspase-3 and structural features of glycyrol. Food Chem. Toxicol. 2020, 136, 110952. [Google Scholar] [CrossRef]
- Zhao, S.; Lu, S.; Fan, L.; Hu, H. Glycyrol alone or in combination with gefitinib is effective against gefitinib-resistant HCC827GR lung cancer cells. Appl. Sci. 2021, 11, 10526. [Google Scholar] [CrossRef]
- Wang, S.; Qin, C.; Shen, X.; Bi, C.; Wu, Y.; Jiang, Y.; Liu, Y.; Li, Y.; He, D.; Yang, Z. Three New compounds, licopyranol a–c, together with eighteen known compounds isolated from glycyrrhiza glabra l. and their antitumor activities. Metabolites 2022, 12, 896. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents-A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the pharmacological action of astragalus polysaccharide [Review]. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Y.-J.; Liu, Z.-H.; Chen, Y.-W.; Liang, Q.-K.; Li, Y.; Ming, H.-X. Inhibitory effect of Astragalus polysaccharide on premetastatic niche of lung cancer through the S1PR1-STAT3 signaling pathway. Evid.-Based Complement. Altern. Med. 2023, 2023, 4010797. [Google Scholar] [CrossRef]
- Zhi, Q.; Zhang, N.; Feng, G.-L.; Sun, W.-Y.; Zhao, Y.-Y.; Yang, S.-F. Study on the mechanism of astragalus polysaccharides regulating autophagy of colorectal cancer cells based on PI3K/Akt/mTOR signaling pathway. Front. Oncol. 2023, 14, 1334915. [Google Scholar] [CrossRef]
- Zhao, Z.; Qiu, Z.; Zhao, Y. Astragalus polysaccharides suppressed cisplatin-resistance of colorectal cancer TH-29/DDPcells via regulating miR-20a/TGFBR2 axis. Chin. J. Cancer Biother. 2019, 14, 417–425. [Google Scholar] [CrossRef]
- Jin, Z.; Huang, Z.; Zhang, F.; Gao, Y.; Guo, S.; Tao, X.; Lu, S.; Zhang, J.; Huang, J.; Zhai, Y.; et al. Targeting gastric cancer cell proliferation: Unraveling the therapeutic potential of oxidized matrine modulation of the TGF-β/Smad pathway. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Yu, M.; Li, L.; Du, G.; Xiao, W.; Yang, H. Involvement of miR-20a in promoting gastric cancer progression by targeting early growth response 2 (EGR2). Int. J. Mol. Sci. 2013, 14, 16226–16239. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.-B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, H.-W.; Li, J. Astragaloside IV: An effective drug for the treatment of cardiovascular diseases. Drug Des. Dev. Ther. 2020, 14, 3731–3746. [Google Scholar] [CrossRef] [PubMed]
- Xiaoli, Z.; Xinzhuang, Z.; Liuqing, D.; Shouchuan, W.; Baochang, C.; Qiu, D.; Jinjun, S.; Xiaolin, B.; Hongmei, W. Exploration of thoughts and methods in study on material base of traditional chinese medicinal herbs prescriptions. World Sci. Technol. 2009, 11, 488–492. [Google Scholar] [CrossRef]
- Esfahani, K.; Boodaghians, L.; Kasymjanova, G.; Agulnik, J.S.; Pepe, C.; Sakr, L.; Small, D.I.; Jagoe, T.R.; Cohen, V. A phase I open prospective cohort trial of curcumin plus tyrosine kinase inhibitors for EGFR-mutant advanced non-small cell lung cancer. J. Clin. Oncol. 2019, 37 (Suppl. 15), e20611. [Google Scholar] [CrossRef]
- Gbolahan, O.B.; O’Neil, B.H.; McRee, A.J.; Sanoff, H.K.; Fallon, J.K.; Smith, P.C.; Ivanova, A.; Moore, D.T.; Dumond, J.; Asher, G.N. A phase I evaluation of the effect of curcumin on dose-limiting toxicity and pharmacokinetics of irinotecan in participants with solid tumors. Clin. Transl. Sci. 2022, 15, 1304–1315. [Google Scholar] [CrossRef]
- Prizment, A.; Church, T.; Hatsukami, D.; Madoff, R.; Staley, C.; Straka, R.; Iwan, A.; Stromberg, J.; Wen, Y.-F.; Stibbe, C.; et al. Abstract A26: Pilot trial to examine the effect of ginger on the gut microbiome: The Minnesota Cancer Clinical Trials Network. Cancer Epidemiology, Biomark. Prev. 2020, 29, A26. [Google Scholar] [CrossRef]
- Zick, S.M.; Turgeon, D.K.; Ren, J.; Ruffin, M.T.; Wright, B.D.; Sen, A.; Djuric, Z.; Brenner, D.E. Pilot clinical study of the effects of ginger root extract on eicosanoids in colonic mucosa of subjects at increased risk for colorectal cancer. Mol. Carcinog. 2015, 54, 908–915. [Google Scholar] [CrossRef]
- Gu, Z.; Wei, G.; Zhu, L.; Zhu, L.; Hu, J.; Li, Q.; Cai, G.; Lu, H.; Liu, M.; Chen, C.; et al. Preventive efficacy and safety of yiqi-wenjing-fang granules on oxaliplatin-induced peripheral neuropathy: A protocol for a randomized, double-blind, placebo-controlled, multicenter trial. Evid. -Based Complement. Altern. Med. 2021, 2021, 5551568. [Google Scholar] [CrossRef]
- Jin, H.; Qiao, F.; Wang, Y.; Xu, Y.; Shang, Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol. Rep. 2015, 34, 2782–2789. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.; Shang, M.; Zou, W.; Zhang, M.; Wei, H.; Shao, H. Curcumin exerts cytotoxicity dependent on reactive oxygen species accumulation in non-small-cell lung cancer cells. Future Oncol. 2019, 15, 1243–1253. [Google Scholar] [CrossRef]
- Wu, M.F.; Huang, Y.H.; Chiu, L.Y.; Cherng, S.H.; Sheu, G.T.; Yang, T.Y. Curcumin Induces apoptosis of chemoresistant lung cancer cells via ROS-Regulated p38 MAPK phosphorylation. Int. J. Mol. Sci. 2022, 23, 8248. [Google Scholar] [CrossRef]
- Ruiz de Porras, V.; Layos, L.; Martínez-Balibrea, E. Curcumin: A therapeutic strategy for colorectal cancer? Semin. Cancer Biol. 2021, 73, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, Z.; Yang, H.; Yang, G.; Zhang, Q.; Yang, Q.; Zhu, Y.; Xu, W.; Wu, X. Curdione regulates ferroptosis in colorectal cancer via N6-Methyladenosine. Chin. Med. 2023, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus extract attenuates inflammation and oxidative stress in intestinal epithelial cells via NF-κB activation and Nrf2 response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef]
- Tsai, Y.-M.; Yang, C.-J.; Hsu, Y.-L.; Wu, L.-Y.; Tsai, Y.-C.; Hung, J.-Y.; Lien, C.-T.; Huang, M.-S.; Kuo, P.-L. Glabridin inhibits migration, invasion, and angiogenesis of human non–small cell lung cancer A549 cells by inhibiting the FAK/Rho signaling pathway. Integr. Cancer Ther. 2011, 10, 341–349. [Google Scholar] [CrossRef]
- Farhat, C.; Younes, H.; Alyamani, O.A.; Mrad, M.; Hourani, N.; Khalifeh, H.; El-Makhour, Y.; Dbaibo, G.; Hage-Sleiman, R. Chemical characterization and in vitro biological evaluation of aqueous extract of Althaea officinalis L. flower grown in Lebanon. J. Herb. Med. 2022, 34, 100575. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, F.; Zhang, L.; Li, C.; Zhang, R. Modulatory effect of Althaea officinalis L root extract on cisplatin-induced cytotoxicity and cell proliferation in A549 human lung cancer cell line. Trop. J. Pharm. Res. 2016, 15, 2647–2652. [Google Scholar] [CrossRef][Green Version]
- Bail, K.; Goss, J.; Draper, B.; Berry, H.; Karmel, R.; Gibson, D. The cost of hospital-acquired complications for older people with and without dementia; a retrospective cohort study. BMC Health Serv. Res. 2015, 15, 91. [Google Scholar] [CrossRef]
- Rogala, E.; Skopińska-Różewska, E.; Wasiutyński, A.; Siwicki, A.K.; Sommer, E.; Pastewka, K. Clinical immunology Echinacea purpurea diminishes neovascular reaction induced in mice skin by human cancer cells and stimulates non-specific cellular immunity in humans. J. Allergy Clin. Immunol. 2008, 33, 127–130. Available online: https://www.termedia.pl/Clinical-immunology-Echinacea-purpurea-diminishes-neovascular-reaction-induced-in-mice-skin-by-human-cancer-cells-and-stimulates-non-specific-cellular-immunity-in-humans,10,10368,1,1.html (accessed on 28 February 2025).
- Pica, F.; Balestrieri, E.; Serafino, A.; Sorrentino, R.; Gaziano, R.; Moroni, G.; Moroni, N.; Palmieri, G.; Mattei, M.; Garaci, E.; et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine in a rat syngeneic model of colorectal cancer. Anti-Cancer Drugs 2012, 23, 32–42. [Google Scholar] [CrossRef]
- Wang, M.C.; Wu, Y.F.; Yu, W.Y.; Yu, B.; Ying, H.Z. Polyacetylenes from codonopsis lanceolata root induced apoptosis of human lung adenocarcinoma cells and improved lung dysbiosis. Biomed. Res. Int. 2022, 2022, 7713355. [Google Scholar] [CrossRef]
- Van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.; Deng, N. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 2016, 35, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S. Anticancer Properties of Research Grade Hydrastis Canadensis (Goldenseal) and Characterization of Its Effect on the Mdr1-Encoded Phosphoglycoprotein Efflux Pump. Master’s Thesis, Clemson University, Clemson, SC, USA, 2009. [Google Scholar]
- Li, J.; Yan, L.; Luo, J.; Tong, L.; Gao, Y.; Feng, W.; Wang, F.; Cui, W.; Li, S.; Sun, Z. Baicalein suppresses growth of non-small cell lung carcinoma by targeting MAP4K3. Biomed. Pharmacother. 2021, 133, 110965. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Xia, C.; Sun, Z. The inhibitory effect of 6-gingerol on ubiquitin-specific peptidase 14 enhances autophagy-dependent ferroptosis and anti-tumor in vivo and in vitro [Original Research]. Frontiers in Pharmacology 2020, 11, 598555. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.A.; Saiman, M.Z.; Abdul Majid, N.; Karsani, S.A.; Yaacob, J.S. Berberine inhibits telomerase activity and induces cell cycle arrest and telomere erosion in colorectal cancer cell line, HCT 116. Molecules 2021, 26, 376. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting PI3K/Akt/mTOR pathway by different flavonoids: A cancer chemopreventive approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar] [CrossRef]
- Sun, L.-R.; Zhou, W.; Zhang, H.-M.; Guo, Q.-S.; Yang, W.; Li, B.-J.; Sun, Z.-H.; Gao, S.-H.; Cui, R.-J. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer [Review]. Front. Oncol. 2019, 9, 1153. [Google Scholar] [CrossRef]
- Bandara, N.; Chalamaiah, M. Bioactives from agricultural processing by-products. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 472–480. [Google Scholar] [CrossRef]
- Efimova, S.S.; Zakharova, A.A.; Ostroumova, O.S. Alkaloids modulate the functioning of ion channels produced by antimicrobial agents via an influence on the lipid host. Front. Cell Dev. Biol. 2020, 8, 537. [Google Scholar] [CrossRef]
- Rampogu, S.; Balasubramaniyam, T.; Lee, J.-H. Phytotherapeutic applications of alkaloids in treating breast cancer. Biomed. Pharmacother. 2022, 155, 113760. [Google Scholar] [CrossRef]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef]
- Mazurakova, A.; Koklesova, L.; Csizmár, S.H.; Samec, M.; Brockmueller, A.; Šudomová, M.; Biringer, K.; Kudela, E.; Pec, M.; Samuel, S.M.; et al. Significance of flavonoids targeting PI3K/Akt/HIF-1α signaling pathway in therapy-resistant cancer cells—A potential contribution to the predictive, preventive, and personalized medicine. J. Adv. Res. 2024, 55, 103–118. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Mersakova, S.; Strnadel, J.; Kajo, K.; Pec, M.; Zhai, K.; Smejkal, K.; Mirzaei, S.; et al. Flavonoids targeting HIF-1: Implications on cancer metabolism. Cancers 2021, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhuang, M.; Zhong, C.; Peng, J.; Wang, X.; Li, J.; Chen, Z.; Huang, Y. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1α signaling pathway. Oncol. Rep. 2015, 33, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, L.; Zhu, L.-T.; Wang, Y.; Pan, D.; Yao, J.; You, Q.-D.; Guo, Q.-L. Wogonin reverses hypoxia resistance of human colon cancer HCT116 cells via downregulation of HIF-1α and glycolysis, by inhibiting PI3K/Akt signaling pathway. Mol. Carcinog. 2014, 53, E107–E118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, X.; Chen, C.; Pei, Y.; Wang, Y. Baicalein suppresses rectal cancer progression through inhibition of cellular glycolysis and intracellular adenosine triphosphate level. Mater. Express 2023, 13, 800–805. [Google Scholar] [CrossRef]
- Endo, H.; Inoue, I.; Masunaka, K.; Tanaka, M.; Yano, M. Curcumin induces apoptosis in lung cancer cells by 14-3-3 protein-mediated activation of Bad. Bioscience, Biotechnol. Biochem. 2020, 84, 2440–2447. [Google Scholar] [CrossRef]
- Wu, S.-H.; Hang, L.-W.; Yang, J.-S.; Chen, H.-Y.; Lin, H.-Y.; Chiang, J.-H.; Lu, C.-C.; Yang, J.-L.; Lai, T.-Y.; Ko, Y.-C.; et al. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 Cells through ER stress and caspase cascade- and mitochondria-dependent pathways. Anticancer Res. 2010, 30, 2125. Available online: http://ar.iiarjournals.org/content/30/6/2125.abstract (accessed on 4 June 2024).
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Cunha, M.R.; Tavares, M.T.; Fernandes, T.B.; Parise-Filho, R. Peppers: A “hot” natural source for antitumor compounds. Molecules 2021, 26, 1521. [Google Scholar] [CrossRef]
- Islam, M.R.; Rauf, A.; Akash, S.; Trisha, S.I.; Nasim, A.H.; Akter, M.; Dhar, P.S.; Ogaly, H.A.; Hemeg, H.A.; Wilairatana, P.; et al. Targeted therapies of curcumin focus on its therapeutic benefits in cancers and human health: Molecular signaling pathway-based approaches and future perspectives. Biomed. Pharmacother. 2024, 170, 116034. [Google Scholar] [CrossRef]
- Meenakumari, R.; Suba, V.; Shakthi Paargavi, A.; Karthik, K. Spices in siddha traditional medicine. In Handbook of Spices in India: 75 Years of Research and Development; Ravindran, P.N., Sivaraman, K., Devasahayam, S., Babu, K.N., Eds.; Springer Nature: Singapore, 2024; pp. 4191–4216. [Google Scholar] [CrossRef]
- Zeng, X.; Cai, D.; Zeng, Q.; Chen, Z.; Zhong, G.; Zhuo, J.; Gan, H.; Huang, X.; Zhao, Z.; Yao, N.; et al. Selective reduction in the expression of ugts and sults, a novel mechanism by which piperine enhances the bioavailability of curcumin in rat. Biopharm. Drug Dispos. 2017, 38, 3–19. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and piperine-loaded emulsomes as combinational treatment approach enhance the anticancer activity of curcumin on HCT116 colorectal cancer model [Original Research]. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Lai, M.; Cavenagh, J. Long-term stabilisation of myeloma with curcumin. BMJ Case Rep. 2017, 2017, bcr2016218148. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Wang, Z.; Ma, L.; Peng, B.; Mao, K.; Li, C.; Su, M.; Zhou, C.; Peng, G. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget 2018, 9, 20089–20102. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Chen, X.; Zhong, D. Absorption and enterohepatic circulation of baicalin in rats. Life Sci. 2005, 78, 140–146. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, C.F.; Chen, L.; Anderson, S.; Lu, F.; Yuan, C.S. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int. J. Oncol. 2015, 47, 1749–1758. [Google Scholar] [CrossRef]
- Cui, Y.; Luo, Y.; Qian, Q.; Tian, J.; Fang, Z.; Wang, X.; Zeng, Y.; Wu, J.; Li, Y. Sanguinarine regulates tumor-associated macrophages to prevent lung cancer angiogenesis through the WNT/β-Catenin pathway. Front. Oncol. 2022, 12, 732860. [Google Scholar] [CrossRef]
- Cai, Y.; Xia, Q.; Luo, R.; Huang, P.; Sun, Y.; Shi, Y.; Jiang, W. Berberine inhibits the growth of human colorectal adenocarcinoma in vitro and in vivo. J. Nat. Med. 2014, 68, 53–62. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Tao, C.; Wang, L.; Chen, Z.; Li, X.; Zeng, Q.; Ma, M.; Zhang, R.; Wu, Z. Berberine chloride suppresses non-small cell lung cancer by deregulating Sin3A/TOP2B pathway in vitro and in vivo. Cancer Chemother. Pharmacol. 2020, 86, 151–161. [Google Scholar] [CrossRef]
- Ozawa, H.; Imaizumi, A.; Sumi, Y.; Hashimoto, T.; Kanai, M.; Makino, Y.; Tsuda, T.; Takahashi, N.; Kakeya, H. Curcumin β-d-glucuronide plays an important role to keep high levels of free-form curcumin in the blood. Biol. Pharm. Bull. 2017, 40, 1515–1524. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, T.; Liu, L.; Wu, Q.; Sun, L.; Li, L.; Wang, N.; Gong, C. Improving anti-tumor activity of curcumin by polymeric micelles in thermosensitive hydrogel system in colorectal peritoneal carcinomatosis model. J. Biomed. Nanotechnol. 2015, 11, 1173–1182. [Google Scholar] [CrossRef]
- Dance-Barnes, S.T.; Kock, N.D.; Moore, J.E.; Lin, E.Y.; Mosley, L.J.; D’Agostino, R.B., Jr.; McCoy, T.P.; Townsend, A.J.; Miller, M.S. Lung tumor promotion by curcumin. Carcinogenesis 2009, 30, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Peng, H.; Li, Y.; Xiong, J.; Xu, Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater. Sci. Eng. C 2013, 33, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Kumar, A.P.; Ghosh, R. Food-based natural products for cancer management: Is the whole greater than the sum of the parts? Semin Cancer Biol 2016, 40–41, 233–246. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10 (Suppl. 1), S4. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Peng, C.; Liu, Y. Regulation of ferroptosis by PI3K/Akt signaling pathway: A promising therapeutic axis in cancer [Review]. Front. Cell Dev. Biol. 2024, 12, 1372330. [Google Scholar] [CrossRef]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Ye, Q.; Raese, R.; Luo, D.; Cao, S.; Wan, Y.W.; Qian, Y.; Guo, N.L. MicroRNA, mRNA, and proteomics biomarkers and therapeutic targets for improving lung cancer treatment outcomes. Cancers 2023, 15, 2294. [Google Scholar] [CrossRef]
- Sell, M.C.; Ramlogan-Steel, C.A.; Steel, J.C.; Dhungel, B.P. MicroRNAs in cancer metastasis: Biological and therapeutic implications. Expert Rev. Mol. Med. 2023, 25, e14. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Zhou, S.; Mao, J.; Zhan, Z.; Duan, S. miRNA interplay: Mechanisms and therapeutic interventions in cancer. MedComm–Oncol. 2024, 3, e93. [Google Scholar] [CrossRef]
- Yu, S.; Lei, X.; Qu, C. MicroRNA Sensors Based on CRISPR/Cas12a technologies: Evolution from indirect to direct detection. Crit. Rev. Anal. Chem. 2024, 1–17. [Google Scholar] [CrossRef]
- Pagoni, M.; Cava, C.; Sideris, D.C.; Avgeris, M.; Zoumpourlis, V.; Michalopoulos, I.; Drakoulis, N. miRNA-Based technologies in cancer therapy. J. Pers. Med. 2023, 13, 1586. [Google Scholar] [CrossRef] [PubMed]
- Ellingwood, F. Ellingwood’s Therapeutist. 1908. Available online: https://www.henriettes-herb.com/eclectic/journals/elth/elth1908/index.html (accessed on 1 June 2024).
- Hage-Sleiman, R.; Mroueh, M.; Daher, C.F. Pharmacological evaluation of aqueous extract of Althaea officinalis flower grown in Lebanon. Pharm. Biol. 2011, 49, 327–333. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, M.; Tang, C.; Yue, Y.; Liu, X.; Zheng, Z.; Dong, H.; Liu, D. Dietary daidzein inhibits hepatitis C virus replication by decreasing microRNA-122 levels. Virus Res. 2021, 298, 198404. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lee, T.Y.; Cheng, Y.J.; Cho, D.Y.; Chen, J.Y. The dietary flavonol kaempferol inhibits epstein-barr virus reactivation in nasopharyngeal carcinoma cells. Molecules 2022, 27, 8158. [Google Scholar] [CrossRef]
- Manivannan, A.C.; Devaraju, V.; Velmurugan, P.; Sathiamoorthi, T.; Sivakumar, S.; Subbiah, S.K.; Ravi, A.V. Tumorigenesis and diagnostic practice applied in two oncogenic viruses: Epstein Barr virus and T-cell lymphotropic virus-1-Mini review. Biomed. Pharmacother. 2021, 142, 111974. [Google Scholar] [CrossRef]
- Kenney, A. Plants as Medicine. Reedbird. Information Adapted from Alfs, M. 300 Herbs: Their Indications & Contraindications: A Materia Medica & Repertory. Old Theology Book House. 2003. Available online: http://www.reedbird.com/plants-as-medicine.html (accessed on 18 December 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imtiaz, I.; Schloss, J.; Bugarcic, A. Interplay Between Traditional and Scientific Knowledge: Phytoconstituents and Their Roles in Lung and Colorectal Cancer Signaling Pathways. Biomolecules 2025, 15, 380. https://doi.org/10.3390/biom15030380
Imtiaz I, Schloss J, Bugarcic A. Interplay Between Traditional and Scientific Knowledge: Phytoconstituents and Their Roles in Lung and Colorectal Cancer Signaling Pathways. Biomolecules. 2025; 15(3):380. https://doi.org/10.3390/biom15030380
Chicago/Turabian StyleImtiaz, Ilma, Janet Schloss, and Andrea Bugarcic. 2025. "Interplay Between Traditional and Scientific Knowledge: Phytoconstituents and Their Roles in Lung and Colorectal Cancer Signaling Pathways" Biomolecules 15, no. 3: 380. https://doi.org/10.3390/biom15030380
APA StyleImtiaz, I., Schloss, J., & Bugarcic, A. (2025). Interplay Between Traditional and Scientific Knowledge: Phytoconstituents and Their Roles in Lung and Colorectal Cancer Signaling Pathways. Biomolecules, 15(3), 380. https://doi.org/10.3390/biom15030380








