A Mechanistic Insight into the Anti-Staphylococcal Mode of Action of (+)-Usnic Acid and Its Synergy with Norfloxacin Against Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Culture Conditions, Chemicals, and Enzymes
2.2. Determination of Minimum Inhibitory Concentrations (MICs)
2.3. In Vitro Synergy of UA with Different Antibiotics
2.4. Growth Kinetics Study of MRSA 2071
2.5. Scanning Electron Microscopy (SEM)
2.6. Cytoplasmic Leakage Assay
2.7. Ethidium Bromide (EtBr) Efflux Assay
2.8. Relative Expression of Efflux Pump Genes
2.9. Proteome Profiling for Identification of Differentially Expressed Protein by Nano-LC-ESI-QTOF-MS/MS
2.10. Validation of Identified Protein Through RT-qPCR
2.11. Effect of Thiourea on Survival of MRSA 2071
2.12. Determining In Vivo Efficacy Using Swiss Albino Mice Model
2.13. In Vivo Toxicity Assessment of UA
2.14. Statistical Analysis
3. Results
3.1. The Anti-Staphylococcal Activity of UA and Its Synergy with Antibiotics
3.2. Growth Kinetics Study
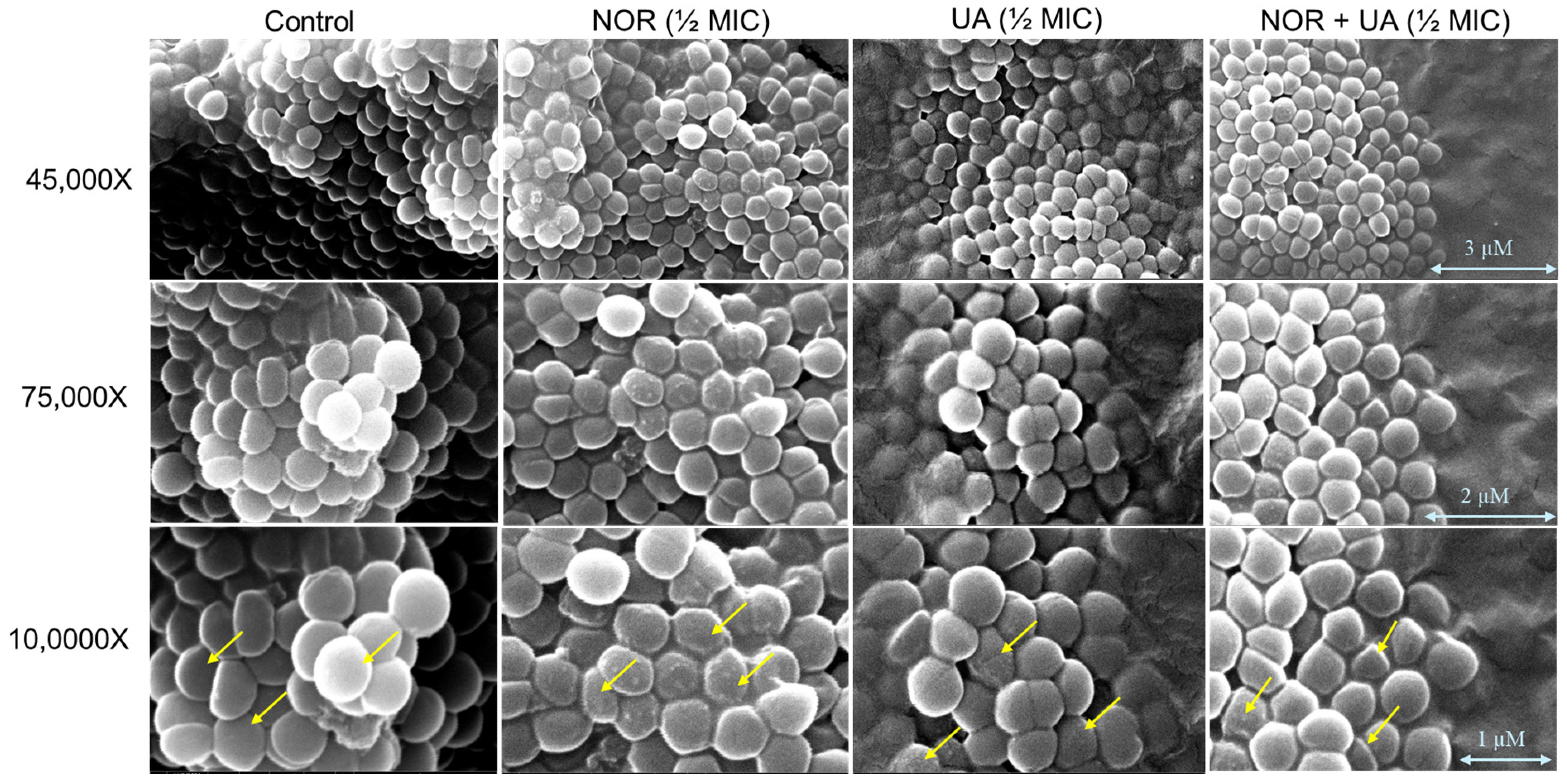
3.3. Conducting a Cell Morphology Study by Scanning Electron Microscopy
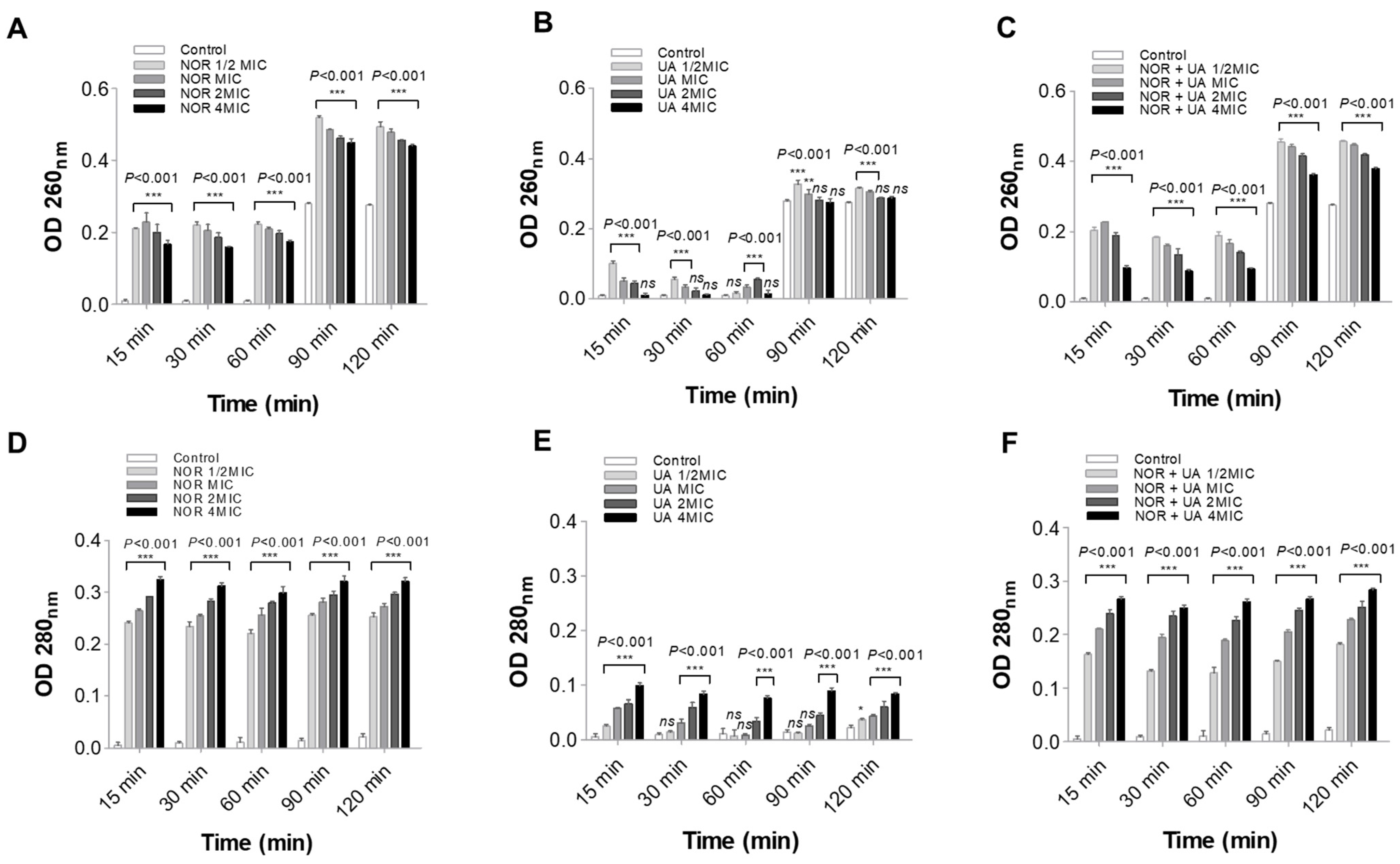
3.4. Cell Leakage Assay
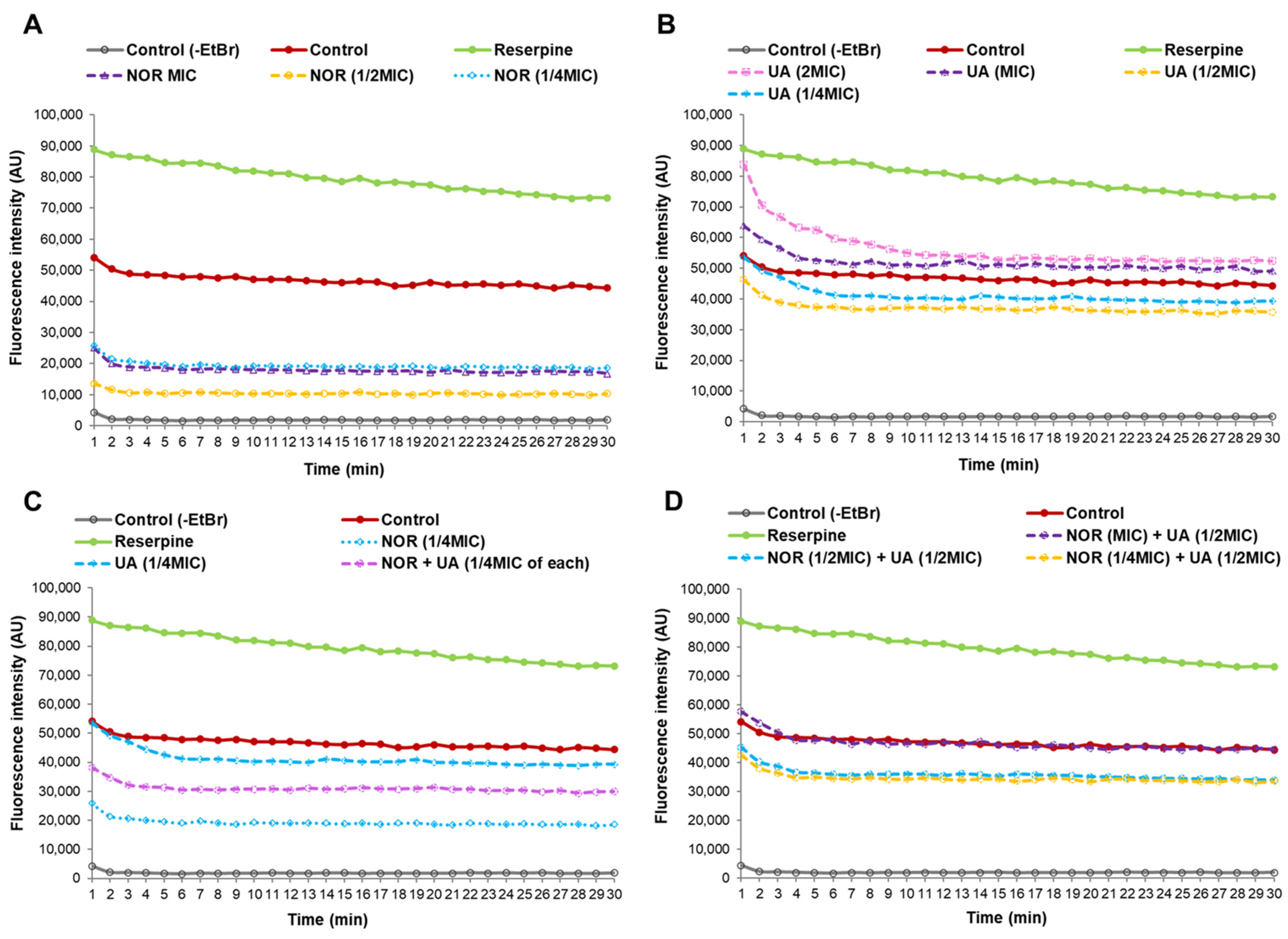
3.5. Efflux Pump Assay
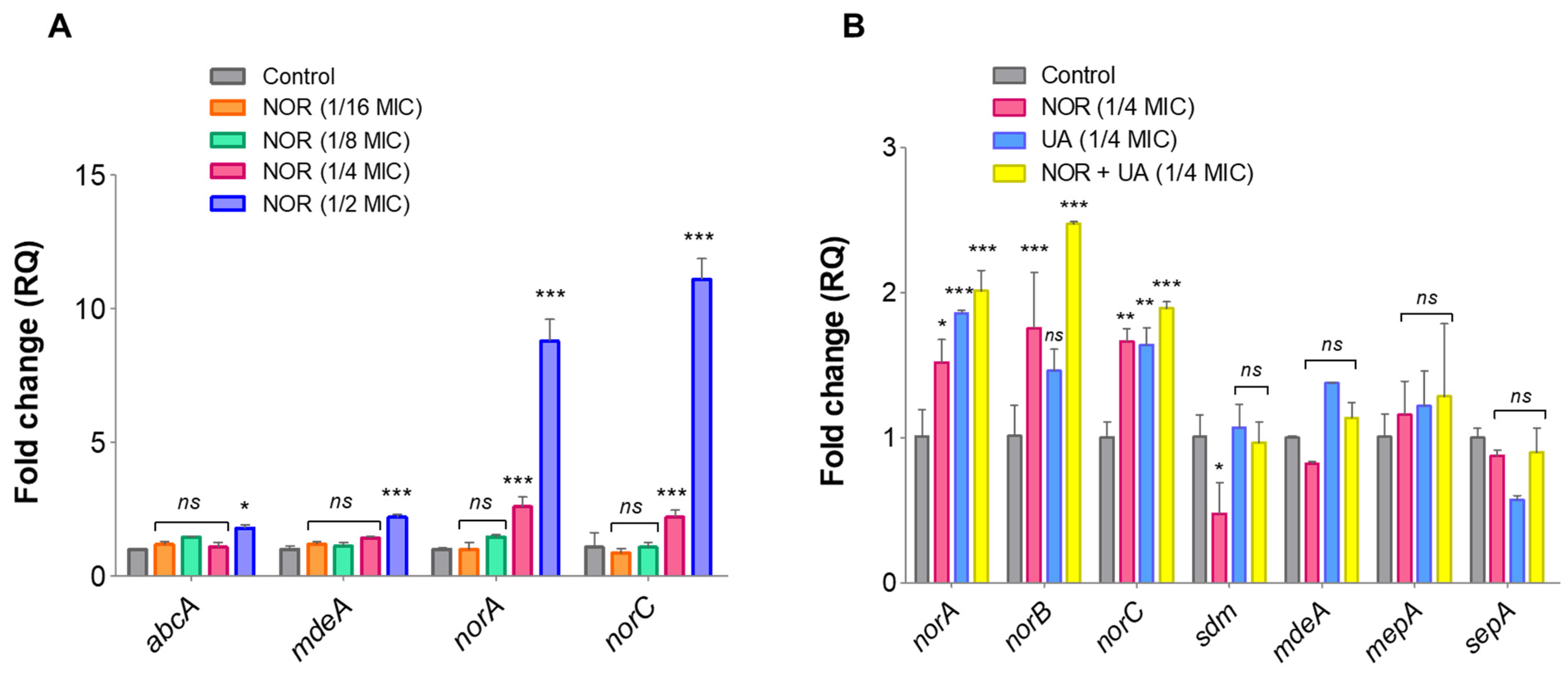
3.6. Expression Analysis of Efflux Pump Genes and Drug Transporters Through RT-qPCR
3.7. Analysis of Differentially Expressed Proteins by Nano-LC-ESI-QTOF-MS/MS
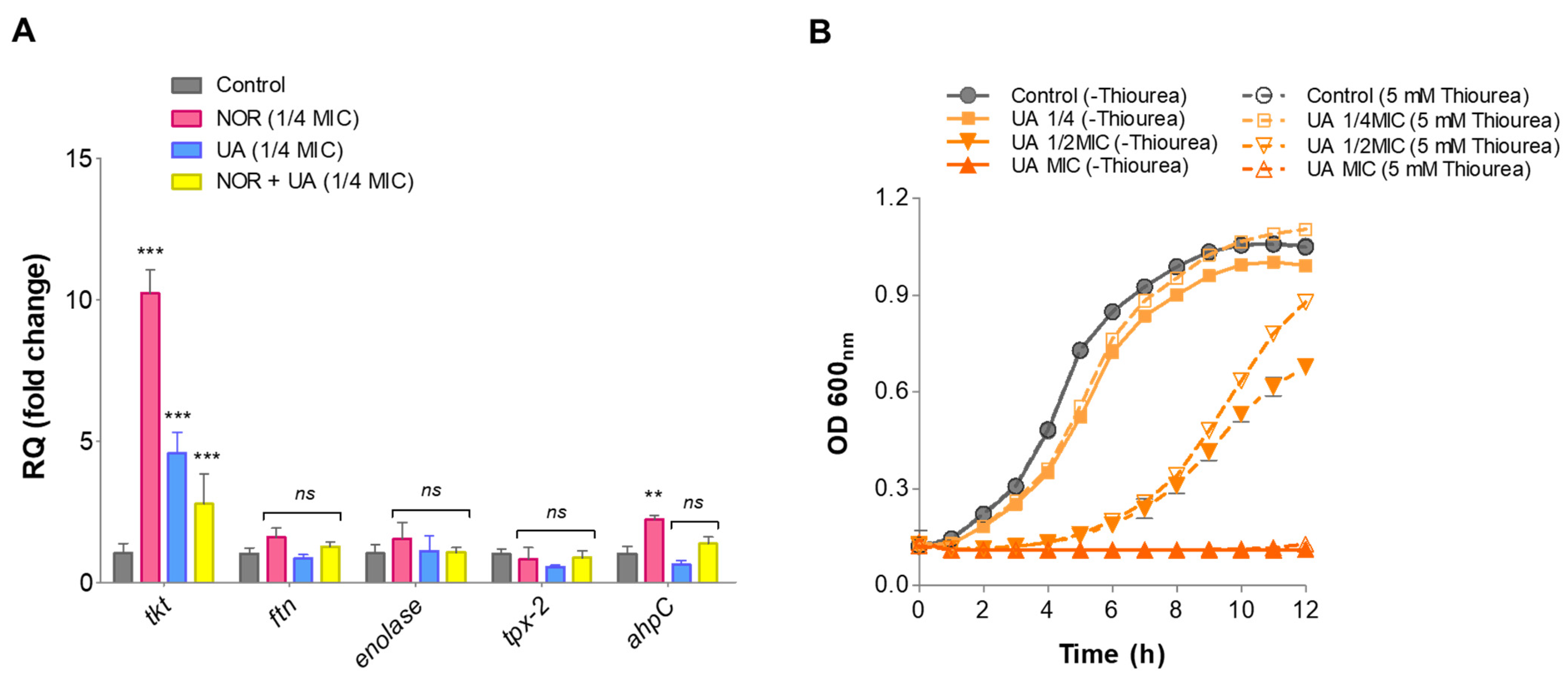
3.8. Gene Expression Analysis of Identified Protein Through RT-qPCR
3.9. Thiourea Rescued Growth Inhibition by UA
3.10. In Vivo Anti-Staphylococcal Efficacy and Toxicity Assessment
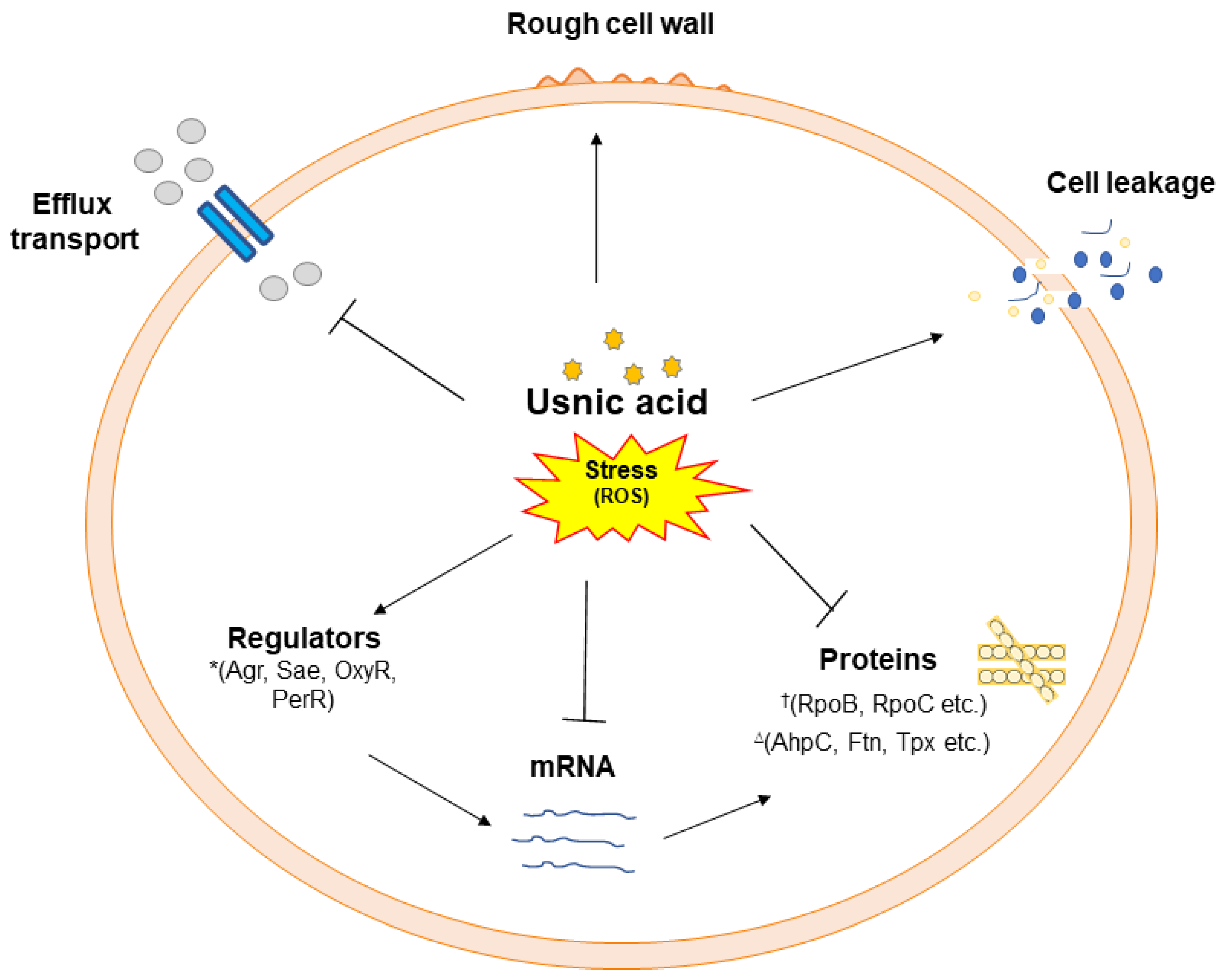
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Rincón, S.; Panesso, D.; Díaz, L.; Carvajal, L.P.; Reyes, J.; Munita, J.M.; Arias, C.A. Resistencia a antibióticos de última línea en cocos Gram positivos: La era posterior a la vancomicina [Resistance to “last resort” antibiotics in Gram-positive cocci: The post-vancomycin era]. Biomed. Rev. Inst. Nac. Salud 2014, 34 (Suppl. 1), 191–208. [Google Scholar] [CrossRef]
- Coates, A.R.M.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation, and resistance reduction. Expert Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug Efflux Pumps in Staphylococcus aureus: An Update. Open Microbiol. J. 2013, 7, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Dashtbani-Roozbehani, A.; Melissa, H.B. Efflux Pump Mediated Antimicrobial Resistance by Staphylococci in Health-Related Environments: Challenges and the Quest for Inhibition. Antibiotics 2021, 10, 1502. [Google Scholar] [CrossRef]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Dieu, A.; Mambu, L.; Champavier, Y.; Chaleix, V.; Sol, V.; Gloaguen, V. Antibacterial activity of the lichens Usnea florida and Flavoparmelia caperata (Parmeliaceae). Nat. Prod. Res. 2020, 34, 3358–3362. [Google Scholar] [CrossRef]
- Galanty, A.; Paśko, P.; Podolak, I. Enantioselective activity of UA: A comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548. [Google Scholar] [CrossRef]
- Francolini, I.; Norris, P.; Piozzi, A.; Donelli, G.; Stoodley, P. UA, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob. Agents Chemother. 2004, 48, 4360–4365. [Google Scholar] [CrossRef]
- Maciąg-Dorszyńska, M.; Węgrzyn, G.; Guzow-Krzemińska, B. Antibacterial activity of lichen secondary metabolite UA is primarily caused by inhibition of RNA and DNA synthesis. FEMS Microbiol. Lett. 2014, 353, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Nithyanand, P.; Shafreen, R.M.B.; Muthamil, S.; Pandian, S.K. UA inhibits biofilm formation and virulent morphological traits of Candida albicans. Microbiol. Res. 2015, 179, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, B.; Kumar, S.; Darokar, M.P. UA: A Potential Natural Anti-Staphylococcal Agent. Ann. Clin. Pathol. 2022, 9, 1156. [Google Scholar]
- Gupta, V.K.; Verma, S.; Gupta, S.; Singh, A.; Pal, A.; Srivastava, S.K.; Srivastava, P.K.; Singh, S.C.; Darokar, M.P. Membrane-damaging potential of natural L-(-)-UA in Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and its derivatives: Part 1. Activity against unicellular organisms. Russ. J. Bioorg. Chem. 2016, 42, 115–132. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Dyrkheeva, N.S.; Luzina, O.A.; Filimonov, A.S.; Mozhaitsev, E.S.; Malakhova, A.A.; Medvedev, S.P.; Zakian, S.M.; Salakhutdinov, N.F.; Lavrik, O.I. Usnic Acid Derivatives Inhibit DNA Repair Enzymes Tyrosyl-DNA Phosphodiesterases 1 and 2 and Act as Potential Anticancer Agents. Genes 2023, 14, 1931. [Google Scholar] [CrossRef]
- Lauterwein, M.; Oethinger, M.; Belsner, K.; Peters, T.; Marre, R. In vitro activities of the lichen secondary metabolites vulpinic acid, (+)- and (−)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob. Agents Chemother. 1995, 39, 2541–2543. [Google Scholar] [CrossRef]
- Lage, T.C.A.; Maciel, T.M.S.; Mota, Y.C.C.; Sisto, F.; Sabino, J.R.; Santos, J.C.C.; Figueiredo, I.M.; Masia, C.; de Fátima, Â.; Fernandes, S.A.; et al. In vitro inhibition of Helicobacter pylori and interaction studies of lichen natural products with jack bean urease. New J. Chem. 2018, 42, 5356–5366. [Google Scholar] [CrossRef]
- Sinha, S.; Gupta, V.K.; Kumar, P.; Kumar, R.; Joshi, R.; Pal, A.; Darokar, M.P. UA modifies MRSA drug resistance through down-regulation of proteins involved in peptidoglycan and fatty acid biosynthesis. FEBS Open Bio 2019, 9, 2025–2040. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Khailova, L.S.; Rokitskaya, T.I.; Nosikova, E.S.; Nazarov, P.A.; Luzina, O.A.; Salakhutdinov, N.F.; Kotova, E.A. Mechanism of action of an old antibiotic revisited: Role of calcium ions in protonophoric activity of usnic acid. Biochim. Biophys. Acta. (BBA) Bioenerg. 2019, 1860, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Diţu, L.M.; Oprea, E.; Liţescu, S.; Bucur, M.; Măruţescu, L.; Enache, G.; Saviuc, C.; Burlibaşa, M.; Trăistaru, T.; et al. In vitro study of the inhibitory activity of usnic acid on dental plaque biofilm. Roum. Arch. Microbiol. Immunol. 2009, 68, 215–222. [Google Scholar] [PubMed]
- Marzhoseyni, Z.; Rashki, S.; Khaledi, A. Antibacterial, antibiofilm, and antioxidant impacts of usnic acid against vancomycin-resistant enterococcus. Polym. Bull. 2024, 81, 10337–10350. [Google Scholar] [CrossRef]
- Pompilio, A.; Riviello, A.; Crocetta, V.; Di Giuseppe, F.; Pomponio, S.; Sulpizio, M.; Di Ilio, C.; Angelucci, S.; Barone, L.; Di Giulio, A.; et al. Evaluation of antibacterial and antibiofilm mechanisms by usnic acid against methicillin-resistant Staphylococcus aureus. Future Microbiol. 2016, 11, 1315–1338. [Google Scholar] [CrossRef]
- Segatore, B.; Bellio, P.; Setacci, D.; Brisdelli, F.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Amicosante, G.; Perilli, M.; Celenza, G. In vitro interaction of usnic acid in combination with antimicrobial agents against methicillin-resistant Staphylococcus aureus clinical isolates determined by FICI and ΔE model methods. Phytomedicine 2012, 19, 341–347. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Y.; Huang, Z.; Huang, Y.; Kong, J.; Sun, Y.; Cao, J.; Zhou, T. Restoring Colistin Sensitivity and Combating Biofilm Formation: Synergistic Effects of Colistin and Usnic Acid against Colistin-Resistant Enterobacteriaceae. ACS Infect. Dis. 2023, 9, 2457–2470. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Annam, S.S.P. Antimycobacterial activity of acetone extract and isolated metabolites from folklore medicinal lichen Usnea laevis Nyl. against drug-sensitive and multidrug-resistant tuberculosis strains. J. Ethnopharmacol. 2022, 282, 114641. [Google Scholar] [CrossRef] [PubMed]
- da Costa Júnior, S.D.; da Silva, W.R.C.; da Silva, A.M.C.M.; Maciel, M.A.V.; Cavalcanti, I.M.F. Synergistic Effect between Usnic Acid and Polymyxin B against Resistant Clinical Isolates of Pseudomonas aeruginosa. Evid.-Based Complement. Altern. Med. 2020, 2020, 9852145. [Google Scholar] [CrossRef]
- Victor, K.; Boris, L.; Athina, G.; Anthi, P.; Marija, S.; Marina, K.; Oliver, R.; Marina, S. Design, synthesis and antimicrobial activity of usnic acid derivatives. MedChemComm 2018, 9, 870–882. [Google Scholar] [CrossRef]
- Qi, W.; Lu, C.; Huang, H.; Zhang, W.; Song, S.; Liu, B. (+)-Usnic Acid Induces ROS-dependent Apoptosis via Inhibition of Mitochondria Respiratory Chain Complexes and Nrf2 Expression in Lung Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 876. [Google Scholar] [CrossRef]
- Wang, H.; Xuan, M.; Huang, C.; Wang, C. Advances in Research on Bioactivity, Toxicity, Metabolism, and Pharmacokinetics of Usnic Acid In Vitro and In Vivo. Molecules 2022, 27, 7469. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Popiół, J.; Paczkowska-Walendowska, M.; Studzińska-Sroka, E.; Paśko, P.; Cielecka-Piontek, J.; Pękala, E.; Podolak, I. (+)-Usnic Acid as a Promising Candidate for a Safe and Stable Topical Photoprotective Agent. Molecules 2021, 26, 5224. [Google Scholar] [CrossRef] [PubMed]
- Croce, N.; Pitaro, M.; Gallo, V.; Antonini, G. Toxicity of Usnic Acid: A Narrative Review. J. Toxicol. 2022, 2022, 8244340. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, B.; Kumar, S.; Darokar, M.P. Glabridin averts biofilms formation in methicillin-resistant Staphylococcus aureus by modulation of the surfaceome. Front. Microbiol. 2020, 11, 1779. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI Supplement M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Miranda-Novales, G.; Leaños-Miranda, B.E.; Vilchis-Pérez, M.; Solórzano-Santos, F. In vitro activity effects of combinations of cephalothin, dicloxacillin, imipenem, vancomycin and amikacin against methicillin-resistant Staphylococcus spp. strains. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 25. [Google Scholar] [CrossRef][Green Version]
- Aelenei, P.; Rimbu, C.M.; Horhogea, C.E.; Lobiuc, A.; Neagu, A.N.; Dunca, S.I.; Motrescu, I.; Dimitriu, G.; Aprotosoaie, A.C.; Miron, A. Prenylated phenolics as promising candidates for combination antibacterial therapy: Morusin and kuwanon G. Saudi Pharm. J. 2020, 28, 1172–1181. [Google Scholar] [CrossRef]
- Viveiros, M.; Rodrigues, L.; Martins, M.; Couto, I.; Spengler, G.; Martins, A.; Amaral, L. Evaluation of efflux activity of bacteria by a semi-automated fluorometric system. Methods Mol. Biol. 2010, 642, 159–172. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, A.K.; Mishra, M.N.; Shukla, M.; Singh, P.K.; Tripathi, A.K. RpoH2 sigma factor controls the photooxidative stress response in a non-photosynthetic rhizobacterium, Azospirillum brasilense Sp7. Microbiology 2012, 158, 2891–2902. [Google Scholar] [CrossRef]
- Liu, H.; Sadygov, R.G.; Yates, J.R. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 2004, 76, 4193–4201. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Song, X.; Liu, T.; Wang, L.; Liu, L.; Li, X.; Wu, X. Antibacterial Effects and Mechanism of Mandarin (Citrus reticulata L.) Essential Oil against Staphylococcus aureus. Molecules 2020, 25, 4956. [Google Scholar] [CrossRef] [PubMed]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Su, H.L.; Chou, C.C.; Hung, D.J.; Lin, S.H.; Pao, I.C.; Lin, J.H.; Huang, F.L.; Dong, R.X.; Lin, J.J. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials 2009, 30, 5979–5987. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef]
- Wong, F.; Stokes, J.M.; Cervantes, B.; Penkov, S.; Friedrichs, J.; Renner, L.D.; Collins, J.J. Cytoplasmic condensation induced by membrane damage is associated with antibiotic lethality. Nat. Commun. 2021, 12, 2321. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; Fluit, A.C.; Luckefahr, M.; Engler, B.; Hofmann, B.; Verhoef, J.; Heinz, H.P.; Hadding, U.; Jones, M.E. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of S. aureus. J. Antimicrob. Chemother. 1998, 42, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; McCusker, M.P.; Viveiros, M.; Couto, I.; Fanning, S.; Pagès, J.M.; Amaral, L. A Simple Method for Assessment of MDR Bacteria for Over-Expressed Efflux Pumps. Open Microbiol. J. 2013, 7, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Pramyothin, P.; Janthasoot, W.; Pongnimitprasert, N.; Phrukudom, S.; Ruangrungsi, N. Hepatotoxic effect of (+)usnic acid from Usnea siamensis Wainio in rats, isolated rat hepatocytes and isolated rat liver mitochondria. J. Ethnopharmacol. 2004, 90, 381–387. [Google Scholar] [CrossRef]
- Abo-Khatwa, A.N.; al-Robai, A.A.; al-Jawhari, D.A. Lichen acids as uncouplers of oxidative phosphorylation of mouse-liver mitochondria. Nat. Toxins 1996, 4, 96–102. [Google Scholar] [CrossRef]
- Sonko, B.J.; Schmitt, T.C.; Guo, L.; Shi, Q.; Boros, L.G.; Leakey, J.E.; Beger, R.D. Assessment of usnic acid toxicity in rat primary hepatocytes using 13C isotopomer distribution analysis of lactate, glutamate and glucose. Food Chem. Toxicol. 2011, 49, 2968–2974. [Google Scholar] [CrossRef]
- Han, D.; Matsumaru, K.; Rettori, D.; Kaplowitz, N. Usnic acid-induced necrosis of cultured mouse hepatocytes: Inhibition of mitochondrial function and oxidative stress. Biochem. Pharmacol. 2004, 67, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Viveiros, M.; Rosato, A.E. Impact of efflux in the development of multidrug resistance phenotypes in Staphylococcus aureus. BMC Microbiol. 2015, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Onodera, Y.; Lee, J.C.; Hooper, D.C. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. J. Bacteriol. 2008, 190, 7123–7129. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Touati, D. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 2000, 373, 1–6. [Google Scholar] [CrossRef]
- Smith, J.L. The physiological role of ferritin-like compounds in bacteria. Crit. Rev. Microbiol. 2004, 30, 173–185. [Google Scholar] [CrossRef]
- Panchal, V.V.; Griffiths, C.; Mosaei, H.; Bilyk, B.; Sutton, J.A.F.; Carnell, O.T.; Hornby, D.P.; Green, J.; Hobbs, J.K.; Kelley, W.L.; et al. Evolving MRSA: High-level β-lactam resistance in Staphylococcus aureus is associated with RNA Polymerase alterations and fine tuning of gene expression. PLoS Pathog. 2020, 16, e1008672. [Google Scholar] [CrossRef]
- De Vos, M.; Müller, B.; Borrell, S.; Black, P.A.; van Helden, P.D.; Warren, R.M.; Gagneux, S.; Victor, T.C. Putative compensatory mutations in the rpoC gene of rifampin-resistant Mycobacterium tuberculosis are associated with ongoing transmission. Antimicrob. Agents Chemother. 2013, 57, 827–832. [Google Scholar] [CrossRef]
- Laurent, J.M.; Vogel, C.; Kwon, T.; Craig, S.A.; Boutz, D.R.; Huse, H.K.; Nozue, K.; Walia, H.; Whiteley, M.; Ronald, P.C.; et al. Protein abundances are more conserved than mRNA abundances across diverse taxa. Proteomics 2010, 10, 4209–4212. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Qing, T.; Ren, Z.; Yu, D.; Couch, L.; Ning, B.; Mei, N.; Shi, L.; Tolleson, W.H.; et al. Activation of the Nrf2 signaling pathway in usnic acid-induced toxicity in HepG2 cells. Arch. Toxicol. 2017, 91, 1293–1307. [Google Scholar] [CrossRef]
- Shi, Q.; Greenhaw, J.; Salminen, W.F. Inhibition of cytochrome P450s enhances (+)-usnic acid cytotoxicity in primary cultured rat hepatocytes. J. Appl. Toxicol. 2014, 34, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, X.; Lu, X.; Fan, X.; Wang, Y. Proteomic study on usnic-acid-induced hepatotoxicity in rats. J. Agric. Food Chem. 2012, 60, 7312–7317. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Q.; Tian, Y.; Xiao, S.; Jin, T.; Fan, X. A metabonomic characterization of (+)-usnic acid-induced liver injury by gas chromatography-mass spectrometry-based metabolic profiling of the plasma and liver in rat. Int. J. Toxicol. 2011, 30, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos, N.P.; Nascimento, S.C.; Wanderley, M.S.; Pontes-Filho, N.T.; da Silva, J.F.; de Castro, C.M.; Pereira, E.C.; da Silva, N.H.; Honda, N.K.; Santos-Magalhães, N.S. Nanoencapsulation of usnic acid: An attempt to improve antitumour activity and reduce hepatotoxicity. Eur. J. Pharm. Biopharm. 2006, 64, 154–160. [Google Scholar] [CrossRef] [PubMed]

| Clinical Isolates | MIC of Antibiotics Alone (µg/mL) | MIC of Antibiotics in Presence of Usnic Acid (µg/mL) | |||||||||||||||||
| UA | OXA | FOX | CFZ | NOR | CIP | TET | ERY | STR | VAN | OXA | FOX | CFZ | NOR | CIP | TET | ERY | STR | VAN | |
| MTCC 96 | 3.9 ± 0.0 | 0.39 ± 0.11 | 1.56 ± 0.67 | 1.56 ± 0.67 | 0.39 ± 0.45 | 0.78 ± 0.33 | 0.78 ± 0.11 | 0.78 ± 0.33 | 1.56 ± 0.67 | 0.78 ± 0.33 | |||||||||
| MRSA 2071 | 7.8 ± 0.0 | 1000 ± 288.6 | 500 ± 144.3 | 500 ± 144.3 | 500 ± 0.00 | 500 ± 144.3 | 25 ± 7.2 | 1000 ± 288.6 | 1000 ± 288.6 | 6.25 ± 1.8 | 250 ± 72.1 | 250 ± 72.1 | 250 ± 72.1 | 125 ± 0 | 125 ± 36.0 | 12.5 ± 7.2 | 250 ± 72.1 | 250 ± 0 | 0.78 ± 0.22 |
| MRSA 4627 | 3.9 ± 2.2 | 1000 ± 288.6 | 500 ± 144.3 | 500 ± 144.3 | 500 ± 0.00 | 500 ± 144.3 | 50 ± 7.2 | 1000 ± 288.6 | 1000 ± 288.6 | 3.12 ± 5.4 | 250 ± 72.1 | 250 ± 72.1 | 250 ± 72.1 | 125 ± 0 | 125 ± 36.0 | 25 ± 7.2 | 500 ± 144.3 | 500 ± 144.3 | 0.78 ± 0.22 |
| MRSA 1745 | 7.8 ± 0.0 | 1000 ± 288.6 | 500 ± 144.3 | 500 ± 144.3 | 500 ± 144.3 | 500 ± 144.3 | 50 ± 7.2 | 1000 ± 288.6 | 1000 ± 288.6 | 6.25 ± 1.8 | 250 ± 72.1 | 250 ± 72.1 | 250 ± 72.1 | 125 ± 36.0 | 125 ± 36.0 | 25 ± 7.2 | 500 ± 144.3 | 500 ± 144.3 | 0.78 ± 1.3 |
| MRSA 4423 | 7.8 ± 0.0 | 1000 ± 288.6 | 500 ± 144.3 | 500 ± 144.3 | 500 ± 144.3 | 500 ± 144.3 | 25 ± 7.2 | 1000 ± 288.6 | 1000 ± 288.6 | 3.12 ± 5.4 | 250 ± 72.1 | 250 ± 72.1 | 250 ± 72.1 | 125 ± 36.0 | 125 ± 72.1 | 12.5 ± 3.6 | 250 ± 0 | 250 ± 72.1 | 0.78 ± 1.3 |
| Clinical Isolates | OXA | FOX | CFZ | NOR | CIP | TET | ERY | STR | VAN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR (OXA/UA) | FICI/ Interaction | FR (FOX/UA) | FICI/ Interaction | FR (CFZ/UA) | FICI/ Interaction | FR (NOR/UA) | FICI/ Interaction | FR (CIP/UA) | FICI/ Interaction | FR (TET/UA) | FICI/ Interaction | FR (ERY/UA) | FICI/ Interaction | FR (STR/UA) | FICI/ Interaction | FR (VAN/UA) | FICI/ Interaction | |
| MRSA 2071 | 04/04 | 0.5/ Synergy | 04/02 | 0.75/ Additive | 04/02 | 0.75/ Additive | 04/04 | 0.5/ Synergy | 04/04 | 0.5/ Synergy | 02/02 | 01/ Additive | 04/04 | 0.5/ Synergy | 04/04 | 0.5/ Synergy | 08/08 | 0.24/ Synergy |
| MRSA 4627 | 04/04 | 0.5/ Synergy | 04/02 | 0.75/ Additive | 04/02 | 0.75/ Additive | 04/04 | 0.5/ Synergy | 04/04 | 0.5/ Synergy | 02/02 | 01/ Additive | 02/04 | 0.75/ Additive | 02/04 | 0.75/ Additive | 04/08 | 0.37/ Synergy |
| MRSA 1745 | 04/02 | 0.75/ Additive | 02/04 | 0.75/ Additive | 02/04 | 0.75/ Additive | 04/04 | 0.5/ Synergy | 04/02 | 0.75/ Additive | 02/04 | 0.75/ Additive | 02/04 | 0.75/ Additive | 02/04 | 0.75/ Additive | 08/08 | 0.25/ Synergy |
| MRSA 4423 | 04/04 | 0.5/ Synergy | 02/04 | 0.75/ Additive | 02/04 | 0.75/ Additive | 04/04 | 0.5/ Synergy | 04/02 | 0.75/ Additive | 02/04 | 0.75/ Additive | 04/04 | 0.5/ Synergy | 04/04 | 0.5/ Synergy | 04/08 | 0.37/ Synergy |
| Gene Name | Primer Sequence (5′-3′) |
|---|---|
| Primers of antibiotic efflux and drug resistance genes | |
| abcA | F-TGCGTCGCCACTTAGATAAC R-GTGGCGACGCAAAATTGGAT |
| mdeA | F-GTTTATGCGATTCGAATGGTTGGT R-AATTAATGCAGCTGTTCCGATAGA |
| norA | F-GGCGGTATATTTGGGGCACT R-TGTCGAGTTCAATCCGCCTG |
| norB | F-ATGGAAAAGCCGTCAAGAGA R-AACCAATGATTGTGCAAATAGC |
| norC | F-ATGAATGAAACGTATCGCGG R-GTCTGCACCAAAACTTTGTTGTAAA |
| sdm | F-AAGCGGTCCAATGATACTCG R-CGCGTGATACAAGGTTTTGG |
| mepA | F-TGCTGCTGCTCTGTTCTTTA R-GCGAAGTTTCCATAATGTGC |
| sepA | F-CCATGATGACCCAAAAATCGA R-GGCGCGACTTTTCATTTG |
| Primers used for RT-qPCR to validate genes identified through proteome analysis | |
| enolase | F-GGTGCTACAACGTTCAAAGAATCA R-TTCAAATTTAGGAGCGAAACCACC |
| tkt | F-GGTGCTACAACGTTCAAAGAATCA R-TTCAAATTTAGGAGCGAAACCACC |
| ahpC | F-GGTGCTACAACGTTCAAAGAATCA R-TTCAAATTTAGGAGCGAAACCACC |
| tpx-2 | F-ATCAAGACGGAACTGTCATTACAAAT R-CTTCTGTGGTACAAGTAGGTGTATTATCTCT |
| ftn | F-AATCGTACGAAGGATTTGCAAAC R-CTTTTGTCCATGGAAACGTTCTT |
| S.N. | Control | NOR | UA | NOR + UA | Protein Name | Protein MW | pI | Database Accession # | %AA Coverage | MS/MS Search Score | Fold Change | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # Spectra | # Spectra | # Spectra | # Spectra | (Da) | NOR | UA | NOR + UA | ||||||
| Total Intensity | Total Intensity | Total Intensity | Total Intensity | ||||||||||
| 1 | 31 | 30 | 14 | 30 | formate acetyltransferase | 85,316.8 | 5.31 | AIA26805.1 | 45.2 | 496.38 | −1.03 | −2.2 | −1.03 |
| 2.94 × 107 | 1.56 × 107 | 1.96 × 106 | 2.19 × 107 | ||||||||||
| 2 | 759 | 662 | 363 | 692 | elongation factor Tu | 43,159.9 | 4.74 | AIA27105.1 | 59.6 | 370.22 | −1.14 | −2.09 | −1.09 |
| 6.76 × 108 | 5.13 × 108 | 2.64 × 108 | 7.39 × 108 | ||||||||||
| 3 | 9 | 5 | 8 | 13 | elongation factor Tu, partial | 8757.6 | 4.96 | KMR26954.1 | 38.2 | 32.81 | −1.8 | −1.12 | +1.44 |
| 3.81 × 107 | 3.60 × 106 | 1.29 × 107 | 1.22 × 107 | ||||||||||
| 4 | 90 | 79 | 66 | 96 | elongation factor G | 76,926.4 | 4.8 | OWU45296.1 | 40.4 | 341.22 | −1.13 | −1.36 | +1.06 |
| 1.31 × 108 | 5.03 × 107 | 3.35 × 107 | 1.00 × 108 | ||||||||||
| 5 | 42 | 28 | 22 | 28 | DNA-directed RNA polymerase subunit beta’ | 135,976.6 | 6.53 | AKK57709.1 | 23.8 | 320.81 | −1.5 | −1.90 | −1.5 |
| 3.20 × 107 | 6.59 × 106 | 5.42 × 106 | 1.46 × 107 | ||||||||||
| 6 | 31 | 35 | 22 | 38 | transketolase | 72,222.5 | 4.97 | KMS30787.1 | 46.5 | 303.16 | +1.12 | −1.59 | +1.22 |
| 2.74 × 107 | 1.02 × 107 | 9.59 × 106 | 3.25 × 107 | ||||||||||
| 7 | 17 | 12 | 3 | 12 | DNA-directed RNA polymerase subunit beta | 133,587.5 | 4.91 | ALK38466.1 | 25.5 | 285.11 | −1.41 | −5.6 | −1.41 |
| 3.64 × 106 | 3.43 × 105 | 2.45 × 105 | 1.39 × 106 | ||||||||||
| 8 | 50 | 55 | 35 | 60 | 1-pyrroline-5-carboxylate dehydrogenase | 57,037.7 | 4.98 | AIA29027.1 | 32.2 | 276.79 | +1.1 | −1.42 | +1.2 |
| 7.91 × 107 | 4.40 × 107 | 2.81 × 107 | 8.67 × 107 | ||||||||||
| 9 | 39 | 41 | 32 | 52 | aconitate hydratase | 99,196.1 | 4.83 | AIA27839.1 | 25.5 | 272.58 | +1.05 | −1.21 | +1.3 |
| 3.00 × 107 | 1.52 × 107 | 1.04 × 107 | 3.06 × 107 | ||||||||||
| 10 | 31 | 37 | 20 | 26 | molecular chaperone DnaK | 66,417.2 | 4.65 | AIA28120.1 | 36 | 268.51 | +1.19 | −1.55 | −1.19 |
| 5.18 × 107 | 2.82 × 107 | 1.06 × 107 | 3.23 × 107 | ||||||||||
| 11 | 21 | 27 | 15 | 34 | malate/quinone oxidoreductase | 56,183.1 | 6.12 | KMS00114.1 | 40.9 | 268.46 | +1.28 | −1.4 | +1.61 |
| 3.87 × 107 | 1.61 × 107 | 5.47 × 106 | 3.81 × 107 | ||||||||||
| 12 | 32 | 52 | 33 | 47 | succinyl-CoA synthetase subunit beta | 42,283.7 | 4.91 | AIA27731.1 | 40.2 | 263.16 | +1.62 | +1.03 | +1.46 |
| 4.67 × 107 | 4.51 × 107 | 2.16 × 107 | 5.46 × 107 | ||||||||||
| 13 | 12 | 6 | 2 | 5 | carbamoyl phosphate synthase large subunit | 117,669.9 | 4.87 | KMR30550.1 | 20.3 | 262.05 | −2.0 | −6.0 | −2.4 |
| 4.99 × 106 | 1.08 × 106 | 6.11 × 105 | 1.81 × 106 | ||||||||||
| 14 | 33 | 31 | 28 | 36 | ATP F0F1 synthase subunit beta | 51,399.4 | 4.68 | AIA28597.1 | 43.1 | 259.03 | −1.06 | −1.17 | 1.09 |
| 2.06 × 107 | 6.86 × 106 | 6.68 × 106 | 1.09 × 107 | ||||||||||
| 15 | 26 | 20 | 14 | 20 | pyruvate kinase | 63,329.4 | 5.24 | AIA28225.1 | 35.2 | 258.15 | −1.3 | −1.85 | −1.3 |
| 1.29 × 107 | 5.26 × 106 | 2.53 × 106 | 8.53 × 106 | ||||||||||
| 16 | 28 | 35 | 26 | 22 | branched-chain alpha-keto acid dehydrogenase subunit E2 | 46,454.6 | 4.9 | AHZ98881.1 | 50.4 | 256.66 | 1.25 | −1.07 | −1.27 |
| 4.98 × 107 | 3.75 × 107 | 2.30 × 107 | 3.28 × 107 | ||||||||||
| 17 | 43 | 36 | 33 | 44 | fructose-bisphosphate aldolase | 33,027.9 | 5.01 | QBC22365.1 | 56 | 254.66 | −1.19 | −1.30 | 1.02 |
| 5.40 × 107 | 1.84 × 107 | 1.66 × 107 | 3.74 × 107 | ||||||||||
| 18 | 41 | 42 | 54 | 48 | 2-oxoisovalerate dehydrogenase | 35,303.5 | 4.62 | KMR38463.1 | 43.3 | 239.53 | +1.04 | +1.31 | +1.17 |
| 6.31 × 107 | 3.28 × 107 | 3.58 × 107 | 4.01 × 107 | ||||||||||
| 19 | 37 | 28 | 20 | 29 | glutamine synthetase | 51,125 | 5.08 | AIA27794.1 | 44.3 | 239.26 | −1.32 | −1.85 | −1.27 |
| 3.93 × 107 | 1.92 × 107 | 5.97 × 106 | 2.24 × 107 | ||||||||||
| 20 | 47 | 28 | 21 | 35 | enolase | 47,173.1 | 4.55 | AIA27354.1 | 39.6 | 235.87 | −1.67 | −2.23 | −1.34 |
| 5.65 × 107 | 2.94 × 107 | 1.02 × 107 | 4.32 × 107 | ||||||||||
| Parameters | Control | UA (2 mg/kg Body Weight) | NOR (10 mg/kg Body Weight) | NOR + UA (10 + 2 mg/kg Body Weight) |
|---|---|---|---|---|
| Change in body weight (gm) | 7.45 ± 1.06 | 4.63 ± 1.42 | 0.82 ± 1.36 | 0.88 ± 2.08 |
| Relative organ weight (gm); (a) spleen and (b) liver | 0.24 ± 0.37 0.40 ± 0.33 | 0.45 ± 0.44 0.56 ± 0.39 | 0.21 ± 0.04 0.60 ± 0.12 | 0.12 ± 0.02 0.29 ± 0.05 |
| RBC count (million/mm3) (normal range: 4.7 to 6.1 million cells/mcL) | 4.89 ± 0.332 | 5.34 ± 1.078 | 5.39 ± 1.015 | 5.91 ± 0.391 |
| WBC count (thousand/mm3) (normal range: 4.5 to 11.0 × 109/L) | 6.46 ± 0.55 | 6.44 ± 0.57 | 10.45 ± 2.58 | 6.86 ± 0.31 |
| Hemoglobin (g/dL) (normal range for males: 13.8 to 17.2 g/dL; females: 12.1 to 15.1 g/dL) | 11.8 ± 0.99 | 12.3 ± 1.13 | 12.4 ± 1.27 | 12.4 ± 1.20 |
| SGPT (U/L) (normal range: 49 U/L) | 17.101 ± 0.006 | 9.11 ± 0.004 | 16.43 ± 0.002 | 16.29 ± 0.009 |
| SGOT (U/L) (normal range: 46 U/L) | 41.29 ± 0.005 | 33.155 ± 0.016 | 34.9 ± 0.0002 | 34.9 ± 0.0047 |
| Serum creatinine (mg/dL) (normal range for males: 0.6–1.1 mg/dL; females: 0.5–0.8 mg/dL) | 0.797 ± 0.004 | 0.730 ± 0.007 | 0.784 ± 0.001 | 0.919 ± 0.004 |
| Serum ALKP (U/L) (normal range for females: 64–306 U/L; males: 80–306 U/L; children: 180–1200 U/L) | 220 ± 0.059 | 90.5 ± 0.039 | 220 ± 0.018 | 176.61 ± 0.003 |
| Serum total cholesterol (mg/dL) (normal range: 150–220 mg/dL) | 140.97 ± 0.127 | 116.58 ± 0.089 | 130.08 ± 0.049 | 114.14 ± 0.012 |
| Serum bilirubin (mg/dL) (normal range: 1.2 mg/dL) | 12.103 ± 7.105 | 0.102 ± 0.723 | 0.184 ± 0.024 | 0.276 ± 0.046 |
| Serum triglycerides (mg/dL) (normal range for males: 60–165 mg/dL; females: 40–140 mg/dL) | 69.59 ± 0.034 | 84.58 ± 0.013 * | 65.91 ± 0.027 | 86.22 ± 0.040 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangwar, B.; Kumar, S.; Kumar, P.; Pal, A.; Darokar, M.P. A Mechanistic Insight into the Anti-Staphylococcal Mode of Action of (+)-Usnic Acid and Its Synergy with Norfloxacin Against Methicillin-Resistant Staphylococcus aureus. Biomolecules 2025, 15, 750. https://doi.org/10.3390/biom15060750
Gangwar B, Kumar S, Kumar P, Pal A, Darokar MP. A Mechanistic Insight into the Anti-Staphylococcal Mode of Action of (+)-Usnic Acid and Its Synergy with Norfloxacin Against Methicillin-Resistant Staphylococcus aureus. Biomolecules. 2025; 15(6):750. https://doi.org/10.3390/biom15060750
Chicago/Turabian StyleGangwar, Bhavana, Santosh Kumar, Parmanand Kumar, Anirban Pal, and Mahendra P. Darokar. 2025. "A Mechanistic Insight into the Anti-Staphylococcal Mode of Action of (+)-Usnic Acid and Its Synergy with Norfloxacin Against Methicillin-Resistant Staphylococcus aureus" Biomolecules 15, no. 6: 750. https://doi.org/10.3390/biom15060750
APA StyleGangwar, B., Kumar, S., Kumar, P., Pal, A., & Darokar, M. P. (2025). A Mechanistic Insight into the Anti-Staphylococcal Mode of Action of (+)-Usnic Acid and Its Synergy with Norfloxacin Against Methicillin-Resistant Staphylococcus aureus. Biomolecules, 15(6), 750. https://doi.org/10.3390/biom15060750








