Cholesterol Redistribution in Pancreatic β-Cells: A Flexible Path to Regulate Insulin Secretion
Abstract
1. Introduction
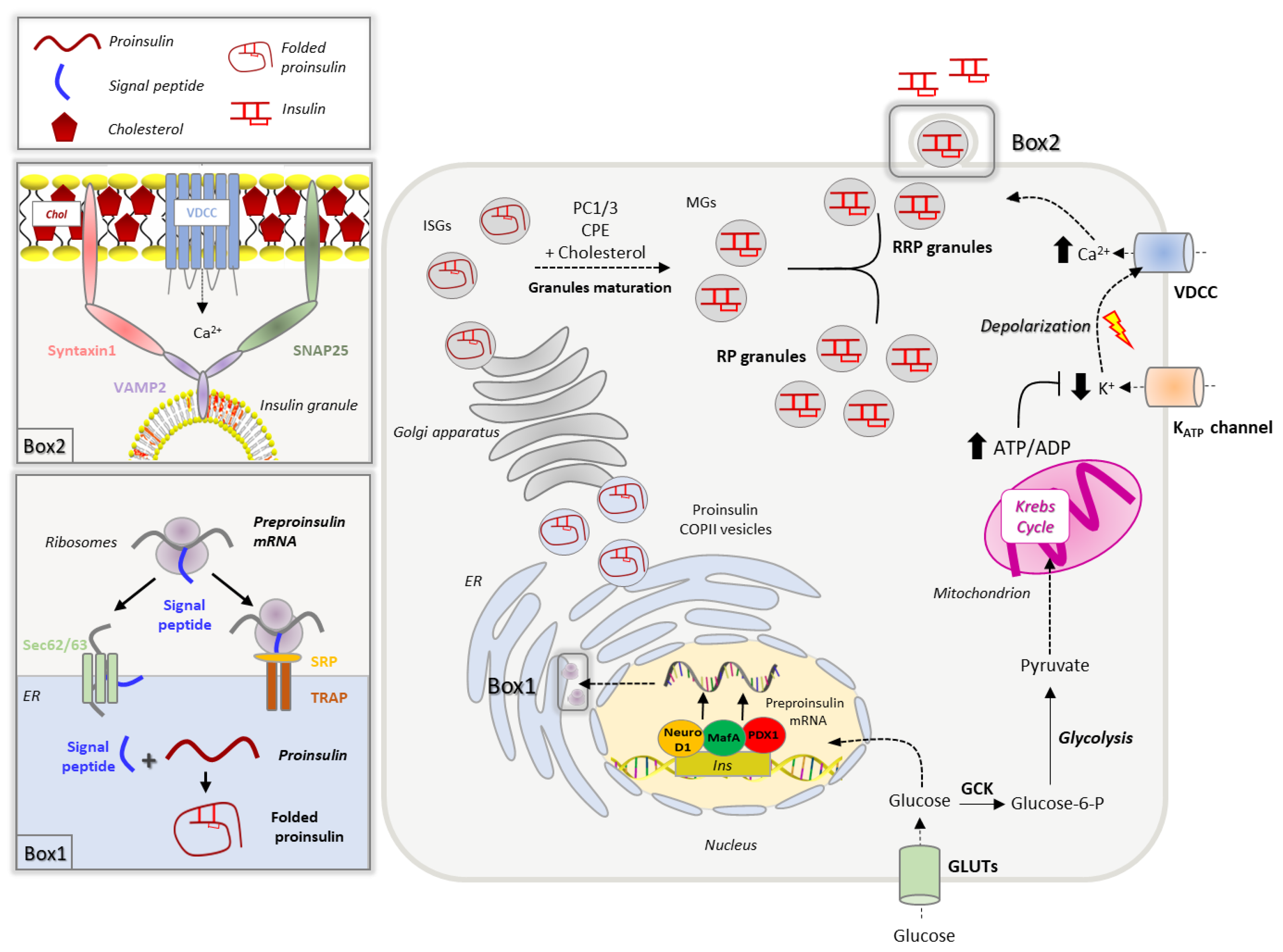
2. Insulin Biosynthesis, Sorting and Secretion
2.1. Insulin Biogenesis, Processing and Storage into Secretory Granules
2.2. Insulin Secretion
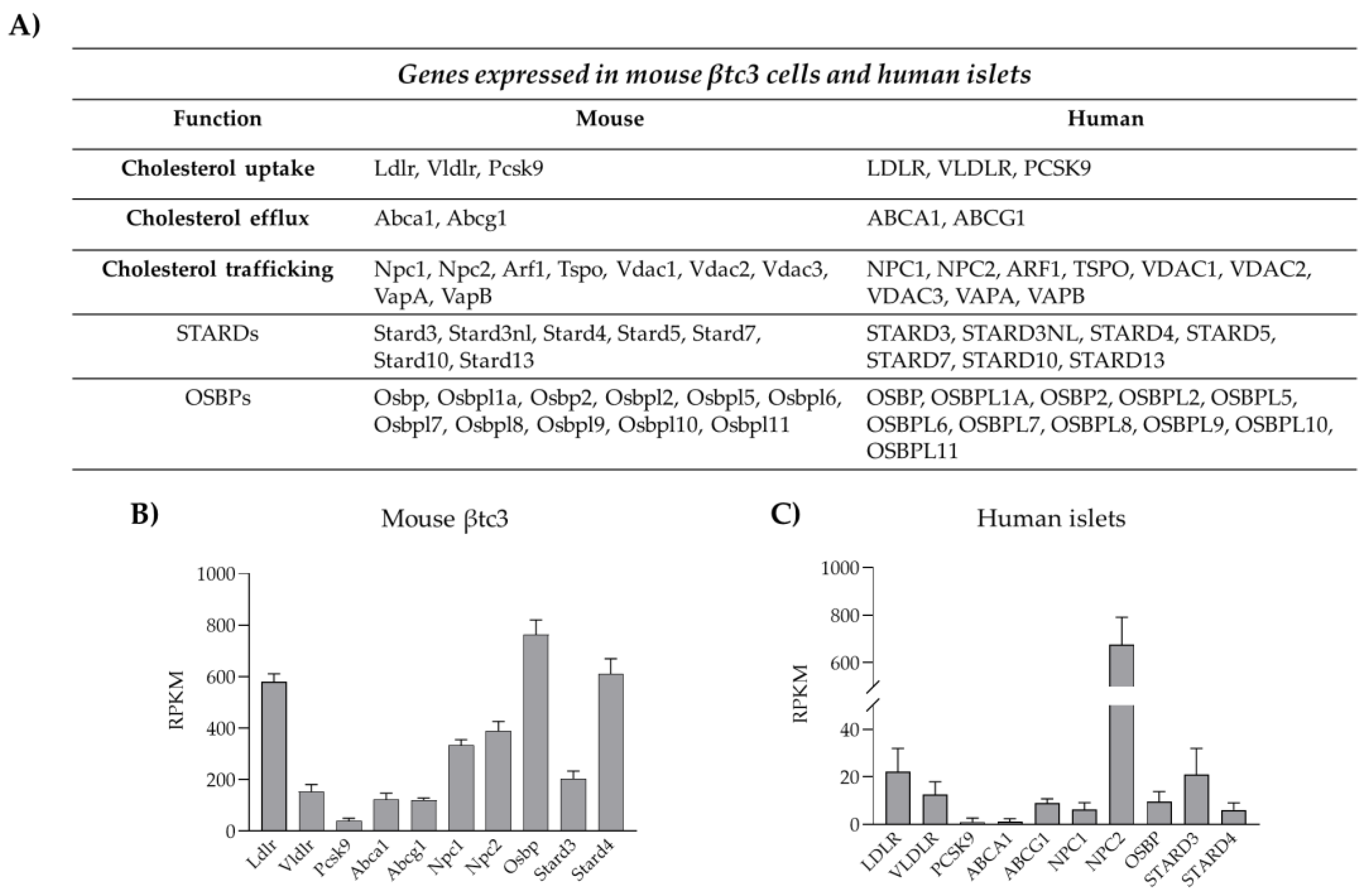
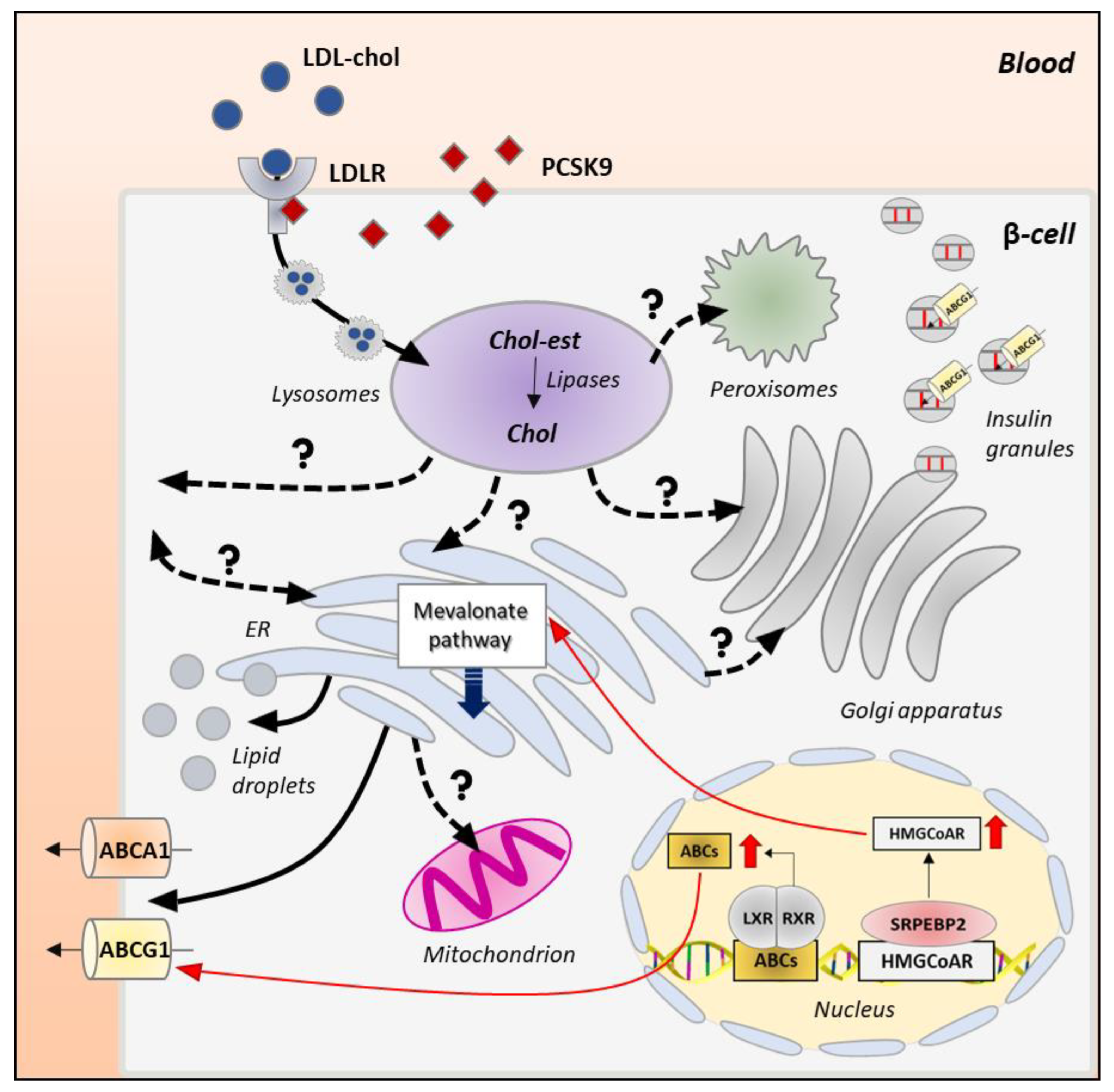
3. Cholesterol Biosynthesis, Homeostasis and Turnover
3.1. Cholesterol Biosynthesis
3.2. Cholesterol Uptake
3.3. Cholesterol Esterification and Efflux
4. Cholesterol Distribution and Trafficking
4.1. Mechanisms of Intracellular Cholesterol Trafficking
4.2. Intracellular Itinerary of Cholesterol
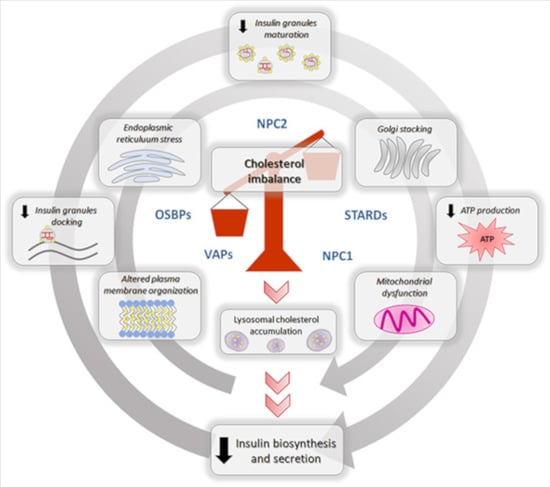
5. Effects of Cholesterol Imbalance on the Insulin Biosynthetic and Secretive Pathway
5.1. Impaired Cholesterol Regulation Affects β-Cell ER Homeostasis
5.2. Cholesterol Imbalance, Insulin Biogenesis and Granules Formation
5.3. Dysregulated Cholesterol Homeostasis and Insulin Secretion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, H.; Morral, N.; McEvoy, R.; Meseck, M.; Thung, S.N.; Woo, S.L. Hepatic Insulin Expression Improves Glycemic Control in Type 1 Diabetic Rats. Diabetes Res. Clin. Pract. 2001, 52, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Csajbók, É.A.; Tamás, G. Cerebral Cortex: A Target and Source of Insulin? Diabetologia 2016, 59, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.M.; Rorsman, P. Diabetes Mellitus and the β Cell: The Last Ten Years. Cell 2012, 148, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.H.; Braun, M.; Mahdi, T.; Andersson, S.A.; Travers, M.E.; Shigeto, M.; Zhang, E.; Almgren, P.; Ladenvall, C.; Axelsson, A.S.; et al. Reduced Insulin Exocytosis in Human Pancreatic β-Cells with Gene Variants Linked to Type 2 Diabetes. Diabetes 2012, 61, 1726–1733. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Borén, J. New Insights into the Pathophysiology of Dyslipidemia in Type 2 Diabetes. Atherosclerosis 2015, 239, 483–495. [Google Scholar] [CrossRef]
- Gregg, E.W.; Gu, Q.; Cheng, Y.J.; Venkat Narayan, K.M.; Cowie, C.C. Mortality Trends in Men and Women with Diabetes, 1971 to 2000. Ann. Intern. Med. 2007, 147, 149. [Google Scholar] [CrossRef]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol Metabolism, Pancreatic β-Cell Function and Diabetes. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef]
- Dirkx, R., Jr.; Solimena, M. Cholesterol-Enriched Membrane Rafts and Insulin Secretion: Membrane Rafts and Insulin Secretion. J. Diabetes Investig. 2012, 3, 339–346. [Google Scholar] [CrossRef]
- Hao, M.; Head, W.S.; Gunawardana, S.C.; Hasty, A.H.; Piston, D.W. Direct Effect of Cholesterol on Insulin Secretion. Diabetes 2007, 56, 2328–2338. [Google Scholar] [CrossRef]
- Bogan, J.S.; Xu, Y.; Hao, M. Cholesterol Accumulation Increases Insulin Granule Size and Impairs Membrane Trafficking: Cholesterol in Insulin Granule Regulation. Traffic 2012, 13, 1466–1480. [Google Scholar] [CrossRef]
- Fryirs, M.; Barter, P.J.; Rye, K.-A. Cholesterol Metabolism and Pancreatic β-Cell Function. Curr. Opin. Lipidol. 2009, 20, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kruit, J.K.; Wijesekara, N.; Fox, J.E.M.; Dai, X.-Q.; Brunham, L.R.; Searle, G.J.; Morgan, G.P.; Costin, A.J.; Tang, R.; Bhattacharjee, A.; et al. Islet Cholesterol Accumulation Due to Loss of ABCA1 Leads to Impaired Exocytosis of Insulin Granules. Diabetes 2011, 60, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Kruit, J.K.; Brunham, L.R.; Verchere, C.B.; Hayden, M.R. HDL and LDL Cholesterol Significantly Influence β-Cell Function in Type 2 Diabetes Mellitus. Curr. Opin. Lipidol. 2010, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kojima, I.; Medina, J.; Nakagawa, Y. Role of the Glucose-Sensing Receptor in Insulin Secretion. Diabetes Obes. Metab. 2017, 19, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Renström, E. Insulin Granule Dynamics in Pancreatic Beta Cells. Diabetologia 2003, 46, 1029–1045. [Google Scholar] [CrossRef]
- Rutter, G.A. Insulin Secretion: Feed-Forward Control of Insulin Biosynthesis? Curr. Biol. 1999, 9, R443–R445. [Google Scholar] [CrossRef]
- Dumonteil, E.; Philippe, J. Insulin Gene: Organisation, Expression and Regulation. Diabetes Metab. 1996, 22, 164–173. [Google Scholar]
- Inada, A.; Seino, Y. The regulation of insulin gene transcription by transcription factors. Nihon Rinsho 2002, 60 (Suppl. S7), 159–164. [Google Scholar]
- Melloul, D.; Cerasi, E. Transcription of the Insulin Gene: Towards Defining the Glucose-Sensitive Cis-Element and Trans-Acting Factors. Diabetologia 1994, 37 (Suppl. S2), S3–S10. [Google Scholar] [CrossRef]
- Someya, Y.; Ishida, H.; Seino, Y. Insulin gene: Gene organization and its regulatory mechanism of transcription. Nihon Rinsho 1997, 55, 73–78. [Google Scholar] [PubMed]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Han, S.; Shioda, S.; Hirai, M.; Nishizawa, M.; Handa, H. MafA Is a Glucose-Regulated and Pancreatic Beta-Cell-Specific Transcriptional Activator for the Insulin Gene. J. Biol. Chem. 2002, 277, 49903–49910. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Zhao, L.; Artner, I.; Jarrett, H.W.; Friedman, D.; Means, A.; Stein, R. Members of the Large Maf Transcription Family Regulate Insulin Gene Transcription in Islet Beta Cells. Mol. Cell Biol. 2003, 23, 6049–6062. [Google Scholar] [CrossRef] [PubMed]
- Raum, J.C.; Gerrish, K.; Artner, I.; Henderson, E.; Guo, M.; Sussel, L.; Schisler, J.C.; Newgard, C.B.; Stein, R. FoxA2, Nkx2.2, and PDX-1 Regulate Islet β-Cell-Specific MafA Expression through Conserved Sequences Located between Base Pairs −8118 and −7750 Upstream from the Transcription Start Site. Mol. Cell Biol. 2006, 26, 5735–5743. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Aramata, S.; Yasuda, K.; Kataoka, K. MafA Stability in Pancreatic β Cells Is Regulated by Glucose and Is Dependent on Its Constitutive Phosphorylation at Multiple Sites by Glycogen Synthase Kinase 3. Mol. Cell Biol. 2007, 27, 6593–6605. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Y.; Xu, X.; Li, X.; Alam, M.; Arunagiri, A.; Haataja, L.; Ding, L.; Wang, S.; Itkin-Ansari, P.; et al. Normal and Defective Pathways in Biogenesis and Maintenance of the Insulin Storage Pool. J. Clin. Investig. 2021, 131, e142240. [Google Scholar] [CrossRef]
- Wang, S.Y.; Halban, P.A.; Rowe, J.W. Effects of Aging on Insulin Synthesis and Secretion. Differential Effects on Preproinsulin Messenger RNA Levels, Proinsulin Biosynthesis, and Secretion of Newly Made and Preformed Insulin in the Rat. J. Clin. Investig. 1988, 81, 176–184. [Google Scholar] [CrossRef]
- Akopian, D.; Shen, K.; Zhang, X.; Shan, S. Signal Recognition Particle: An Essential Protein-Targeting Machine. Annu. Rev. Biochem. 2013, 82, 693–721. [Google Scholar] [CrossRef]
- Egea, P.F.; Stroud, R.M.; Walter, P. Targeting Proteins to Membranes: Structure of the Signal Recognition Particle. Curr. Opin. Struct. Biol. 2005, 15, 213–220. [Google Scholar] [CrossRef]
- Guo, H.; Sun, J.; Li, X.; Xiong, Y.; Wang, H.; Shu, H.; Zhu, R.; Liu, Q.; Huang, Y.; Madley, R.; et al. Positive Charge in the N-Region of the Signal Peptide Contributes to Efficient Post-Translational Translocation of Small Secretory Preproteins. J. Biol. Chem. 2018, 293, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Weiss, M.A.; Arunagiri, A.; Yong, J.; Rege, N.; Sun, J.; Haataja, L.; Kaufman, R.J.; Arvan, P. Biosynthesis, Structure, and Folding of the Insulin Precursor Protein. Diabetes Obes. Metab. 2018, 20 (Suppl. S2), 28–50. [Google Scholar] [CrossRef] [PubMed]
- Fons, R.D.; Bogert, B.A.; Hegde, R.S. Substrate-Specific Function of the Translocon-Associated Protein Complex during Translocation across the ER Membrane. J. Cell Biol. 2003, 160, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Lakkaraju, A.K.K.; Thankappan, R.; Mary, C.; Garrison, J.L.; Taunton, J.; Strub, K. Efficient Secretion of Small Proteins in Mammalian Cells Relies on Sec62-Dependent Posttranslational Translocation. Mol. Biol. Cell 2012, 23, 2712–2722. [Google Scholar] [CrossRef] [PubMed]
- Bachar-Wikstrom, E.; Wikstrom, J.D.; Ariav, Y.; Tirosh, B.; Kaiser, N.; Cerasi, E.; Leibowitz, G. Stimulation of Autophagy Improves Endoplasmic Reticulum Stress–Induced Diabetes. Diabetes 2013, 62, 1227–1237. [Google Scholar] [CrossRef]
- Cunningham, C.N.; Williams, J.M.; Knupp, J.; Arunagiri, A.; Arvan, P.; Tsai, B. Cells Deploy a Two-Pronged Strategy to Rectify Misfolded Proinsulin Aggregates. Mol. Cell 2019, 75, 442–456.e4. [Google Scholar] [CrossRef]
- Kadowaki, H.; Nagai, A.; Maruyama, T.; Takami, Y.; Satrimafitrah, P.; Kato, H.; Honda, A.; Hatta, T.; Natsume, T.; Sato, T.; et al. Pre-Emptive Quality Control Protects the ER from Protein Overload via the Proximity of ERAD Components and SRP. Cell Rep. 2015, 13, 944–956. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Prins, J.B.; McGuckin, M.A. Oxidative and Endoplasmic Reticulum Stress in β-Cell Dysfunction in Diabetes. J. Mol. Endocrinol. 2016, 56, R33–R54. [Google Scholar] [CrossRef]
- Mori, K. Tripartite Management of Unfolded Proteins in the Endoplasmic Reticulum. Cell 2000, 101, 451–454. [Google Scholar] [CrossRef]
- Harding, H.P.; Zeng, H.; Zhang, Y.; Jungries, R.; Chung, P.; Plesken, H.; Sabatini, D.D.; Ron, D. Diabetes Mellitus and Exocrine Pancreatic Dysfunction in Perk−/− Mice Reveals a Role for Translational Control in Secretory Cell Survival. Mol. Cell 2001, 7, 1153–1163. [Google Scholar] [CrossRef]
- Scheuner, D.; Song, B.; McEwen, E.; Liu, C.; Laybutt, R.; Gillespie, P.; Saunders, T.; Bonner-Weir, S.; Kaufman, R.J. Translational Control Is Required for the Unfolded Protein Response and In Vivo Glucose Homeostasis. Mol. Cell 2001, 7, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Teske, B.F.; Wek, S.A.; Bunpo, P.; Cundiff, J.K.; McClintick, J.N.; Anthony, T.G.; Wek, R.C. The EIF2 Kinase PERK and the Integrated Stress Response Facilitate Activation of ATF6 during Endoplasmic Reticulum Stress. MBoC 2011, 22, 4390–4405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kaufman, R.J. The Unfolded Protein Response: A Stress Signaling Pathway Critical for Health and Disease. Neurology 2006, 66, S102–S109. [Google Scholar] [CrossRef] [PubMed]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian Transcription Factor ATF6 Is Synthesized as a Transmembrane Protein and Activated by Proteolysis in Response to Endoplasmic Reticulum Stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef]
- Yang, H.; Niemeijer, M.; van de Water, B.; Beltman, J.B. ATF6 Is a Critical Determinant of CHOP Dynamics during the Unfolded Protein Response. iScience 2020, 23, 100860. [Google Scholar] [CrossRef]
- Yoshida, H.; Okada, T.; Haze, K.; Yanagi, H.; Yura, T.; Negishi, M.; Mori, K. ATF6 Activated by Proteolysis Binds in the Presence of NF-Y (CBF) Directly to the Cis -Acting Element Responsible for the Mammalian Unfolded Protein Response. Mol. Cell Biol. 2000, 20, 6755–6767. [Google Scholar] [CrossRef]
- Korennykh, A.V.; Egea, P.F.; Korostelev, A.A.; Finer-Moore, J.; Zhang, C.; Shokat, K.M.; Stroud, R.M.; Walter, P. The Unfolded Protein Response Signals through High-Order Assembly of Ire1. Nature 2009, 457, 687–693. [Google Scholar] [CrossRef]
- Arunagiri, A.; Haataja, L.; Pottekat, A.; Pamenan, F.; Kim, S.; Zeltser, L.M.; Paton, A.W.; Paton, J.C.; Tsai, B.; Itkin-Ansari, P.; et al. Proinsulin Misfolding Is an Early Event in the Progression to Type 2 Diabetes. eLife 2019, 8, e44532. [Google Scholar] [CrossRef]
- Sun, J.; Cui, J.; He, Q.; Chen, Z.; Arvan, P.; Liu, M. Proinsulin Misfolding and Endoplasmic Reticulum Stress during the Development and Progression of Diabetes. Mol. Asp. Med. 2015, 42, 105–118. [Google Scholar] [CrossRef]
- Miyake, M.; Sobajima, M.; Kurahashi, K.; Shigenaga, A.; Denda, M.; Otaka, A.; Saio, T.; Sakane, N.; Kosako, H.; Oyadomari, S. Identification of an Endoplasmic Reticulum Proteostasis Modulator That Enhances Insulin Production in Pancreatic β Cells. Cell Chem. Biol. 2022, 29, 996–1009.e9. [Google Scholar] [CrossRef]
- Hutton, J.C. Insulin Secretory Granule Biogenesis and the Proinsulin-Processing Endopeptidases. Diabetologia 1994, 37 (Suppl. S2), S48–S56. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Lockridge, A.; Alejandro, E.U. EIF4G1 and Carboxypeptidase E Axis Dysregulation in O-GlcNAc Transferase–Deficient Pancreatic β-Cells Contributes to Hyperproinsulinemia in Mice. J. Biol. Chem. 2019, 294, 13040–13050. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, A.; Asadi, A.; Kieffer, T.J. Revisiting Proinsulin Processing: Evidence That Human β-Cells Process Proinsulin With Prohormone Convertase (PC) 1/3 but Not PC2. Diabetes 2020, 69, 1451–1462. [Google Scholar] [CrossRef]
- Arvan, P.; Castle, D. Sorting and Storage during Secretory Granule Biogenesis: Looking Backward and Looking Forward. Biochem. J. 1998, 332 Pt 3, 593–610. [Google Scholar] [CrossRef]
- Traub, L.M.; Kornfeld, S. The Trans-Golgi Network: A Late Secretory Sorting Station. Curr. Opin. Cell Biol. 1997, 9, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.; Montesano, R.; Meda, P.; Malaisse-Lagae, F.; Brown, D.; Perrelet, A.; Vassalli, P. Heterogeneous Distribution of Filipin--Cholesterol Complexes across the Cisternae of the Golgi Apparatus. Proc. Natl. Acad. Sci. USA 1981, 78, 293–297. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Westhead, E.W. Lipid Composition and Orientation in Secretory Vesicles. Ann. N. Y. Acad. Sci. 1987, 493, 92–100. [Google Scholar] [CrossRef]
- Dodson, G.; Steiner, D. The Role of Assembly in Insulin’s Biosynthesis. Curr. Opin. Struct. Biol. 1998, 8, 189–194. [Google Scholar] [CrossRef]
- Olofsson, C.S.; Göpel, S.O.; Barg, S.; Galvanovskis, J.; Ma, X.; Salehi, A.; Rorsman, P.; Eliasson, L. Fast Insulin Secretion Reflects Exocytosis of Docked Granules in Mouse Pancreatic B-Cells. Pflug. Arch. 2002, 444, 43–51. [Google Scholar] [CrossRef]
- Henquin, J.C. Triggering and Amplifying Pathways of Regulation of Insulin Secretion by Glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef]
- Henquin, J.-C.; Nenquin, M.; Stiernet, P.; Ahren, B. In Vivo and in Vitro Glucose-Induced Biphasic Insulin Secretion in the Mouse: Pattern and Role of Cytoplasmic Ca2+ and Amplification Signals in Beta-Cells. Diabetes 2006, 55, 441–451. [Google Scholar] [CrossRef] [PubMed]
- De Vos, A.; Heimberg, H.; Quartier, E.; Huypens, P.; Bouwens, L.; Pipeleers, D.; Schuit, F. Human and Rat Beta Cells Differ in Glucose Transporter but Not in Glucokinase Gene Expression. J. Clin. Investig. 1995, 96, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, L.J.; van de Bunt, M.; Braun, M.; Frayn, K.N.; Clark, A.; Gloyn, A.L. GLUT2 (SLC2A2) Is Not the Principal Glucose Transporter in Human Pancreatic Beta Cells: Implications for Understanding Genetic Association Signals at This Locus. Mol. Genet. Metab. 2011, 104, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Sugawara, K.; Yokoi, N.; Takahashi, H. β-Cell Signalling and Insulin Secretagogues: A Path for Improved Diabetes Therapy. Diabetes Obes. Metab. 2017, 19, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ammälä, C.; Eliasson, L.; Bokvist, K.; Larsson, O.; Ashcroft, F.M.; Rorsman, P. Exocytosis Elicited by Action Potentials and Voltage-Clamp Calcium Currents in Individual Mouse Pancreatic B-Cells. J. Physiol. 1993, 472, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Wollheim, C.B.; Sharp, G.W. Regulation of Insulin Release by Calcium. Physiol. Rev. 1981, 61, 914–973. [Google Scholar] [CrossRef]
- Bruns, D.; Jahn, R. Molecular Determinants of Exocytosis. Pflug. Arch. 2002, 443, 333–338. [Google Scholar] [CrossRef]
- Xia, F.; Gao, X.; Kwan, E.; Lam, P.P.L.; Chan, L.; Sy, K.; Sheu, L.; Wheeler, M.B.; Gaisano, H.Y.; Tsushima, R.G. Disruption of Pancreatic β-Cell Lipid Rafts Modifies Kv2.1 Channel Gating and Insulin Exocytosis. J. Biol. Chem. 2004, 279, 24685–24691. [Google Scholar] [CrossRef]
- Dolai, S.; Xie, L.; Zhu, D.; Liang, T.; Qin, T.; Xie, H.; Kang, Y.; Chapman, E.R.; Gaisano, H.Y. Synaptotagmin-7 Functions to Replenish Insulin Granules for Exocytosis in Human Islet β-Cells. Diabetes 2016, 65, 1962–1976. [Google Scholar] [CrossRef]
- Huang, C.; Walker, E.M.; Dadi, P.K.; Hu, R.; Xu, Y.; Zhang, W.; Sanavia, T.; Mun, J.; Liu, J.; Nair, G.G.; et al. Synaptotagmin 4 Regulates Pancreatic β Cell Maturation by Modulating the Ca2+ Sensitivity of Insulin Secretion Vesicles. Dev. Cell 2018, 45, 347–361.e5. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Matschinsky, F.M.; Madiraju, S.R.M. Metabolic Signaling in Fuel-Induced Insulin Secretion. Cell Metab. 2013, 18, 162–185. [Google Scholar] [CrossRef] [PubMed]
- Di Cairano, E.S.; Moretti, S.; Marciani, P.; Sacchi, V.F.; Castagna, M.; Davalli, A.; Folli, F.; Perego, C. Neurotransmitters and Neuropeptides: New Players in the Control of Islet of Langerhans’ Cell Mass and Function. J. Cell Physiol. 2016, 231, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Churchward, M.A.; Coorssen, J.R. Cholesterol, Regulated Exocytosis and the Physiological Fusion Machine. Biochem. J. 2009, 423, 1–14. [Google Scholar] [CrossRef]
- Segerstolpe, Å.; Palasantza, A.; Eliasson, P.; Andersson, E.-M.; Andréasson, A.-C.; Sun, X.; Picelli, S.; Sabirsh, A.; Clausen, M.; Bjursell, M.K.; et al. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab. 2016, 24, 593–607. [Google Scholar] [CrossRef]
- Shao, W.; Espenshade, P.J. Lipids. Cholesterol Synthesis and Regulation. In Encyclopedia of Biological Chemistry III; Elsevier: Amsterdam, The Netherlands, 2021; pp. 732–738. ISBN 978-0-12-822040-5. [Google Scholar]
- Ishikawa, M.; Iwasaki, Y.; Yatoh, S.; Kato, T.; Kumadaki, S.; Inoue, N.; Yamamoto, T.; Matsuzaka, T.; Nakagawa, Y.; Yahagi, N.; et al. Cholesterol Accumulation and Diabetes in Pancreatic β-Cell-Specific SREBP-2 Transgenic Mice: A New Model for Lipotoxicity. J. Lipid Res. 2008, 49, 2524–2534. [Google Scholar] [CrossRef]
- Grupping, A.Y.; Cnop, M.; Van Schravendijk, C.F.H.; Hannaert, J.C.; Van Berkel, T.J.C.; Pipeleers, D.G. Low Density Lipoprotein Binding and Uptake by Human and Rat Islet β Cells. Endocrinology 1997, 138, 4064–4068. [Google Scholar] [CrossRef]
- Fujino, T.; Asaba, H.; Kang, M.-J.; Ikeda, Y.; Sone, H.; Takada, S.; Kim, D.-H.; Ioka, R.X.; Ono, M.; Tomoyori, H.; et al. Low-Density Lipoprotein Receptor-Related Protein 5 (LRP5) Is Essential for Normal Cholesterol Metabolism and Glucose-Induced Insulin Secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 229–234. [Google Scholar] [CrossRef]
- d Oliveira, R.B.; Carvalho, C.P.d.F.; Polo, C.C.; Dorighello, G.d.G.; Boschero, A.C.; d Oliveira, H.C.F.; Collares-Buzato, C.B. Impaired Compensatory Beta-Cell Function and Growth in Response to High-Fat Diet in LDL Receptor Knockout Mice. Int. J. Exp. Pathol. 2014, 95, 296–308. [Google Scholar] [CrossRef]
- Ye, R.; Gordillo, R.; Shao, M.; Onodera, T.; Chen, Z.; Chen, S.; Lin, X.; SoRelle, J.A.; Li, X.; Tang, M.; et al. Intracellular Lipid Metabolism Impairs β Cell Compensation during Diet-Induced Obesity. J. Clin. Investig. 2018, 128, 1178–1189. [Google Scholar] [CrossRef]
- Norata, G.D.; Tibolla, G.; Catapano, A.L. Targeting PCSK9 for Hypercholesterolemia. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.; Mayer, G.; Zaid, A.; Rousselet, E.; Nassoury, N.; Poirier, S.; Essalmani, R.; Prat, A. The Activation and Physiological Functions of the Proprotein Convertases. Int. J. Biochem. Cell Biol. 2008, 40, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Si-Tayeb, K.; Le May, C. Role of PCSK9 beyond Liver Involvement. Curr. Opin. Lipidol. 2015, 26, 155–161. [Google Scholar] [CrossRef]
- Da Dalt, L.; Ruscica, M.; Bonacina, F.; Balzarotti, G.; Dhyani, A.; Di Cairano, E.; Baragetti, A.; Arnaboldi, L.; De Metrio, S.; Pellegatta, F.; et al. PCSK9 Deficiency Reduces Insulin Secretion and Promotes Glucose Intolerance: The Role of the Low-Density Lipoprotein Receptor. Eur. Heart J. 2019, 40, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Marku, A.; Da Dalt, L.; Galli, A.; Dule, N.; Corsetto, P.; Rizzo, A.M.; Moregola, A.; Uboldi, P.; Bonacina, F.; Marciani, P.; et al. Pancreatic PCSK9 Controls the Organization of the β-Cell Secretory Pathway via LDLR-Cholesterol Axis. Metabolism 2022, 136, 155291. [Google Scholar] [CrossRef]
- Mbikay, M.; Sirois, F.; Mayne, J.; Wang, G.-S.; Chen, A.; Dewpura, T.; Prat, A.; Seidah, N.G.; Chretien, M.; Scott, F.W. PCSK9-Deficient Mice Exhibit Impaired Glucose Tolerance and Pancreatic Islet Abnormalities. FEBS Lett. 2010, 584, 701–706. [Google Scholar] [CrossRef]
- Ramin-Mangata, S.; Thedrez, A.; Nativel, B.; Diotel, N.; Blanchard, V.; Wargny, M.; Aguesse, A.; Billon-Crossouard, S.; Vindis, C.; Le May, C.; et al. Effects of Proprotein Convertase Subtilisin Kexin Type 9 Modulation in Human Pancreatic Beta Cells Function. Atherosclerosis 2021, 326, 47–55. [Google Scholar] [CrossRef]
- Tong, X.; Liu, S.; Stein, R.; Imai, Y. Lipid Droplets’ Role in the Regulation of β-Cell Function and β-Cell Demise in Type 2 Diabetes. Endocrinology 2022, 163, bqac007. [Google Scholar] [CrossRef]
- Neumann, J.; Rose-Sperling, D.; Hellmich, U.A. Diverse Relations between ABC Transporters and Lipids: An Overview. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2017, 1859, 605–618. [Google Scholar] [CrossRef]
- Oram, J. Tangier Disease and ABCA1. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2000, 1529, 321–330. [Google Scholar] [CrossRef]
- Singaraja, R.R.; Brunham, L.R.; Visscher, H.; Kastelein, J.J.P.; Hayden, M.R. Efflux and Atherosclerosis: The Clinical and Biochemical Impact of Variations in the ABCA1 Gene. ATVB 2003, 23, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Sturek, J.M.; Castle, J.D.; Trace, A.P.; Page, L.C.; Castle, A.M.; Evans-Molina, C.; Parks, J.S.; Mirmira, R.G.; Hedrick, C.C. An Intracellular Role for ABCG1-Mediated Cholesterol Transport in the Regulated Secretory Pathway of Mouse Pancreatic β Cells. J. Clin. Investig. 2010, 120, 2575–2589. [Google Scholar] [CrossRef] [PubMed]
- Brunham, L.R.; Kruit, J.K.; Pape, T.D.; Timmins, J.M.; Reuwer, A.Q.; Vasanji, Z.; Marsh, B.J.; Rodrigues, B.; Johnson, J.D.; Parks, J.S.; et al. β-Cell ABCA1 Influences Insulin Secretion, Glucose Homeostasis and Response to Thiazolidinedione Treatment. Nat. Med. 2007, 13, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.T.; Tarling, E.J.; Bojanic, D.D.; Edwards, P.A.; Baldán, Á. Emerging New Paradigms for ABCG Transporters. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2009, 1791, 584–593. [Google Scholar] [CrossRef]
- Gerin, I.; Dolinsky, V.W.; Shackman, J.G.; Kennedy, R.T.; Chiang, S.-H.; Burant, C.F.; Steffensen, K.R.; Gustafsson, J.-Å.; MacDougald, O.A. LXRβ Is Required for Adipocyte Growth, Glucose Homeostasis, and β Cell Function. J. Biol. Chem. 2005, 280, 23024–23031. [Google Scholar] [CrossRef]
- Lange, Y.; Swaisgood, M.H.; Ramos, B.V.; Steck, T.L. Plasma Membranes Contain Half the Phospholipid and 90% of the Cholesterol and Sphingomyelin in Cultured Human Fibroblasts. J. Biol. Chem. 1989, 264, 3786–3793. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, L.; Yang, H.; Song, B.-L. Routes and Mechanisms of Post-Endosomal Cholesterol Trafficking: A Story That Never Ends. Traffic 2017, 18, 209–217. [Google Scholar] [CrossRef]
- Vance, J.E. Cellular Itinerary of LDL Cholesterol. Proc. Natl. Acad. Sci. USA 2022, 119, e2122584119. [Google Scholar] [CrossRef]
- Ikonen, E. Cellular Cholesterol Trafficking and Compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Ikonen, E. Mechanisms of Cellular Cholesterol Compartmentalization: Recent Insights. Curr. Opin. Cell Biol. 2018, 53, 77–83. [Google Scholar] [CrossRef]
- Arenas, F.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Intracellular Cholesterol Trafficking and Impact in Neurodegeneration. Front. Mol. Neurosci. 2017, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Thorsell, A.-G.; Lee, W.H.; Persson, C.; Siponen, M.I.; Nilsson, M.; Busam, R.D.; Kotenyova, T.; Schüler, H.; Lehtiö, L. Comparative Structural Analysis of Lipid Binding START Domains. PLoS ONE 2011, 6, e19521. [Google Scholar] [CrossRef]
- Clark, B.J. The Mammalian START Domain Protein Family in Lipid Transport in Health and Disease. J. Endocrinol. 2012, 212, 257–275. [Google Scholar] [CrossRef]
- Soccio, R.E.; Breslow, J.L. StAR-Related Lipid Transfer (START) Proteins: Mediators of Intracellular Lipid Metabolism. J. Biol. Chem. 2003, 278, 22183–22186. [Google Scholar] [CrossRef] [PubMed]
- Beh, C.T.; Rine, J. A Role for Yeast Oxysterol-Binding Protein Homologs in Endocytosis and in the Maintenance of Intracellular Sterol-Lipid Distribution. J. Cell Sci. 2004, 117, 2983–2996. [Google Scholar] [CrossRef] [PubMed]
- Lehto, M.; Laitinen, S.; Chinetti, G.; Johansson, M.; Ehnholm, C.; Staels, B.; Ikonen, E.; Olkkonen, V.M. The OSBP-Related Protein Family in Humans. J. Lipid. Res. 2001, 42, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, V. OSBP-Related Proteins: Liganding by Glycerophospholipids Opens New Insight into Their Function. Molecules 2013, 18, 13666–13679. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Olkkonen, V.M. OSBP-Related Proteins: Lipid Sensors or Transporters? Future Lipidol. 2007, 2, 85–94. [Google Scholar] [CrossRef]
- Lev, S. Non-Vesicular Lipid Transport by Lipid-Transfer Proteins and Beyond. Nat. Rev. Mol. Cell. Biol. 2010, 11, 739–750. [Google Scholar] [CrossRef]
- Carrat, G.R.; Hu, M.; Nguyen-Tu, M.-S.; Chabosseau, P.; Gaulton, K.J.; van de Bunt, M.; Siddiq, A.; Falchi, M.; Thurner, M.; Canouil, M.; et al. Decreased STARD10 Expression Is Associated with Defective Insulin Secretion in Humans and Mice. Am. J. Hum. Genet. 2017, 100, 238–256. [Google Scholar] [CrossRef]
- Carrat, G.R.; Haythorne, E.; Tomas, A.; Haataja, L.; Müller, A.; Arvan, P.; Piunti, A.; Cheng, K.; Huang, M.; Pullen, T.J.; et al. The Type 2 Diabetes Gene Product STARD10 Is a Phosphoinositide-Binding Protein That Controls Insulin Secretory Granule Biogenesis. Mol. Metab. 2020, 40, 101015. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Harris, M.T.; Kreutzberger, A.J.B.; Inouye, C.M.; Doyle, C.A.; Castle, A.M.; Arvan, P.; Castle, J.D. Control of Insulin Granule Formation and Function by the ABC Transporters ABCG1 and ABCA1 and by Oxysterol Binding Protein OSBP. Mol. Biol. Cell 2018, 29, 1238–1257. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Inter-Organelle Membrane Contact Sites: Implications for Lipid Metabolism. Biol. Direct. 2020, 15, 24. [Google Scholar] [CrossRef]
- Scorrano, L.; De Matteis, M.A.; Emr, S.; Giordano, F.; Hajnóczky, G.; Kornmann, B.; Lackner, L.L.; Levine, T.P.; Pellegrini, L.; Reinisch, K.; et al. Coming Together to Define Membrane Contact Sites. Nat. Commun. 2019, 10, 1287. [Google Scholar] [CrossRef]
- Vance, J.E. Historical Perspective: Phosphatidylserine and Phosphatidylethanolamine from the 1800s to the Present. J. Lipid Res. 2018, 59, 923–944. [Google Scholar] [CrossRef] [PubMed]
- Maffioli, E.; Galli, A.; Nonnis, S.; Marku, A.; Negri, A.; Piazzoni, C.; Milani, P.; Lenardi, C.; Perego, C.; Tedeschi, G. Proteomic Analysis Reveals a Mitochondrial Remodeling of ΒTC3 Cells in Response to Nanotopography. Front. Cell Dev. Biol. 2020, 8, 508. [Google Scholar] [CrossRef]
- Rutter, G.A.; Pinton, P. Mitochondria-Associated Endoplasmic Reticulum Membranes in Insulin Signaling. Diabetes 2014, 63, 3163–3165. [Google Scholar] [CrossRef]
- Thivolet, C.; Vial, G.; Cassel, R.; Rieusset, J.; Madec, A.-M. Reduction of Endoplasmic Reticulum- Mitochondria Interactions in Beta Cells from Patients with Type 2 Diabetes. PLoS ONE 2017, 12, e0182027. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, R.; Zhang, C.; He, S.; Su, Z. Mitochondria-Associated Endoplasmic Reticulum Membranes in the Pathogenesis of Type 2 Diabetes Mellitus. Front. Cell Dev. Biol. 2020, 8, 571554. [Google Scholar] [CrossRef]
- Rieusset, J. The Role of Endoplasmic Reticulum-Mitochondria Contact Sites in the Control of Glucose Homeostasis: An Update. Cell Death. Dis. 2018, 9, 388. [Google Scholar] [CrossRef]
- Vig, S.; Lambooij, J.M.; Zaldumbide, A.; Guigas, B. Endoplasmic Reticulum-Mitochondria Crosstalk and Beta-Cell Destruction in Type 1 Diabetes. Front. Immunol. 2021, 12, 669492. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and Regulation of Cholesterol Homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Navarro-Lérida, I.; Sánchez-Alvarez, M.; Bosch, M.; Calvo, C.; López, J.A.; Calvo, E.; Ferguson, C.; Giacomello, M.; Serafini, A.; et al. Interplay between Hepatic Mitochondria-Associated Membranes, Lipid Metabolism and Caveolin-1 in Mice. Sci. Rep. 2016, 6, 27351. [Google Scholar] [CrossRef] [PubMed]
- Haddad, D.; Al Madhoun, A.; Nizam, R.; Al-Mulla, F. Role of Caveolin-1 in Diabetes and Its Complications. Oxidative Med. Cell. Longev. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Nevins, A.K.; Thurmond, D.C. Caveolin-1 Functions as a Novel Cdc42 Guanine Nucleotide Dissociation Inhibitor in Pancreatic β-Cells. J. Biol. Chem. 2006, 281, 18961–18972. [Google Scholar] [CrossRef]
- Cohen, A.W.; Razani, B.; Wang, X.B.; Combs, T.P.; Williams, T.M.; Scherer, P.E.; Lisanti, M.P. Caveolin-1-Deficient Mice Show Insulin Resistance and Defective Insulin Receptor Protein Expression in Adipose Tissue. Am. J. Physiol. -Cell Physiol. 2003, 285, C222–C235. [Google Scholar] [CrossRef]
- Kepner, E.M.; Yoder, S.M.; Oh, E.; Kalwat, M.A.; Wang, Z.; Quilliam, L.A.; Thurmond, D.C. Cool-1/ΒPIX Functions as a Guanine Nucleotide Exchange Factor in the Cycling of Cdc42 to Regulate Insulin Secretion. Am. J. Physiol. -Endocrinol. Metab. 2011, 301, E1072–E1080. [Google Scholar] [CrossRef]
- Prasad, M.; Kaur, J.; Pawlak, K.J.; Bose, M.; Whittal, R.M.; Bose, H.S. Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Regulates Steroidogenic Activity via Steroidogenic Acute Regulatory Protein (StAR)-Voltage-Dependent Anion Channel 2 (VDAC2) Interaction. J. Biol. Chem. 2015, 290, 2604–2616. [Google Scholar] [CrossRef]
- Perera, R.M.; Zoncu, R. The Lysosome as a Regulatory Hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. A Receptor-Mediated Pathway for Cholesterol Homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Chang, C.C.Y.; Ohgami, N.; Yamauchi, Y. Cholesterol Sensing, Trafficking, and Esterification. Annu. Rev. Cell Dev. Biol. 2006, 22, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Sugii, S.; Reid, P.C.; Ohgami, N.; Du, H.; Chang, T.-Y. Distinct Endosomal Compartments in Early Trafficking of Low Density Lipoprotein-Derived Cholesterol. J. Biol. Chem. 2003, 278, 27180–27189. [Google Scholar] [CrossRef] [PubMed]
- Lamri, A.; Pigeyre, M.; Garver, W.S.; Meyre, D. The Extending Spectrum of NPC1-Related Human Disorders: From Niemann–Pick C1 Disease to Obesity. Endocr. Rev. 2018, 39, 192–220. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.P.; Chen, F.W.; Ioannou, Y.A. Transmembrane Molecular Pump Activity of Niemann-Pick C1 Protein. Science 2000, 290, 2295–2298. [Google Scholar] [CrossRef]
- Infante, R.E.; Wang, M.L.; Radhakrishnan, A.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. NPC2 Facilitates Bidirectional Transfer of Cholesterol between NPC1 and Lipid Bilayers, a Step in Cholesterol Egress from Lysosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 15287–15292. [Google Scholar] [CrossRef]
- Kwon, H.J.; Abi-Mosleh, L.; Wang, M.L.; Deisenhofer, J.; Goldstein, J.L.; Brown, M.S.; Infante, R.E. Structure of N-Terminal Domain of NPC1 Reveals Distinct Subdomains for Binding and Transfer of Cholesterol. Cell 2009, 137, 1213–1224. [Google Scholar] [CrossRef]
- Xu, S.; Benoff, B.; Liou, H.-L.; Lobel, P.; Stock, A.M. Structural Basis of Sterol Binding by NPC2, a Lysosomal Protein Deficient in Niemann-Pick Type C2 Disease. J. Biol. Chem. 2007, 282, 23525–23531. [Google Scholar] [CrossRef]
- Garver, W.S.; Jelinek, D.; Oyarzo, J.N.; Flynn, J.; Zuckerman, M.; Krishnan, K.; Chung, B.H.; Heidenreich, R.A. Characterization of Liver Disease and Lipid Metabolism in the Niemann-Pick C1 Mouse. J. Cell Biochem. 2007, 101, 498–516. [Google Scholar] [CrossRef]
- Castellano, B.M.; Thelen, A.M.; Moldavski, O.; Feltes, M.; van der Welle, R.E.N.; Mydock-McGrane, L.; Jiang, X.; van Eijkeren, R.J.; Davis, O.B.; Louie, S.M.; et al. Lysosomal Cholesterol Activates MTORC1 via an SLC38A9-Niemann-Pick C1 Signaling Complex. Science 2017, 355, 1306–1311. [Google Scholar] [CrossRef]
- Eid, W.; Dauner, K.; Courtney, K.C.; Gagnon, A.; Parks, R.J.; Sorisky, A.; Zha, X. MTORC1 Activates SREBP-2 by Suppressing Cholesterol Trafficking to Lysosomes in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2017, 114, 7999–8004. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The MTOR-Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.; Millat, G. Niemann-Pick Disease Type C. Clin. Genet. 2003, 64, 269–281. [Google Scholar] [CrossRef]
- Rocha, N.; Kuijl, C.; van der Kant, R.; Janssen, L.; Houben, D.; Janssen, H.; Zwart, W.; Neefjes, J. Cholesterol Sensor ORP1L Contacts the ER Protein VAP to Control Rab7-RILP-P150 Glued and Late Endosome Positioning. J. Cell Biol. 2009, 185, 1209–1225. [Google Scholar] [CrossRef]
- Tsujishita, Y.; Hurley, J.H. Structure and Lipid Transport Mechanism of a StAR-Related Domain. Nat. Struct. Biol. 2000, 7, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Trinh, M.N.; Brown, M.S.; Goldstein, J.L.; Han, J.; Vale, G.; McDonald, J.G.; Seemann, J.; Mendell, J.T.; Lu, F. Last Step in the Path of LDL Cholesterol from Lysosome to Plasma Membrane to ER Is Governed by Phosphatidylserine. Proc. Natl. Acad. Sci. USA 2020, 117, 18521–18529. [Google Scholar] [CrossRef]
- Garbarino, J.; Pan, M.; Chin, H.F.; Lund, F.W.; Maxfield, F.R.; Breslow, J.L. STARD4 Knockdown in HepG2 Cells Disrupts Cholesterol Trafficking Associated with the Plasma Membrane, ER, and ERC. J. Lipid Res. 2012, 53, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Agudo, D.; Ren, S.; Wong, E.; Marques, D.; Redford, K.; Gil, G.; Hylemon, P.; Pandak, W.M. Intracellular Cholesterol Transporter StarD4 Binds Free Cholesterol and Increases Cholesteryl Ester Formation. J. Lipid. Res. 2008, 49, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.T.; Zeng, Q.; Hong, W. VAMP4 Cycles from the Cell Surface to the Trans-Golgi Network via Sorting and Recycling Endosomes. J. Cell Sci. 2007, 120, 1028–1041. [Google Scholar] [CrossRef]
- Urano, Y.; Watanabe, H.; Murphy, S.R.; Shibuya, Y.; Geng, Y.; Peden, A.A.; Chang, C.C.Y.; Chang, T.Y. Transport of LDL-Derived Cholesterol from the NPC1 Compartment to the ER Involves the Trans-Golgi Network and the SNARE Protein Complex. Proc. Natl. Acad. Sci. USA 2008, 105, 16513–16518. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, P.; Dwyer, N.K.; Christenson, L.K.; Fujimoto, T.; Martinez, F.; Comly, M.; Hanover, J.A.; Blanchette-Mackie, E.J.; Strauss, J.F. MLN64 Mediates Mobilization of Lysosomal Cholesterol to Steroidogenic Mitochondria. J. Biol. Chem. 2002, 277, 33300–33310. [Google Scholar] [CrossRef]
- Chu, B.-B.; Liao, Y.-C.; Qi, W.; Xie, C.; Du, X.; Wang, J.; Yang, H.; Miao, H.-H.; Li, B.-L.; Song, B.-L. Cholesterol Transport through Lysosome-Peroxisome Membrane Contacts. Cell 2021, 184, 289. [Google Scholar] [CrossRef]
- Igoillo-Esteve, M.; Marselli, L.; Cunha, D.A.; Ladrière, L.; Ortis, F.; Grieco, F.A.; Dotta, F.; Weir, G.C.; Marchetti, P.; Eizirik, D.L.; et al. Palmitate Induces a Pro-Inflammatory Response in Human Pancreatic Islets That Mimics CCL2 Expression by Beta Cells in Type 2 Diabetes. Diabetologia 2010, 53, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Boslem, E.; Weir, J.M.; MacIntosh, G.; Sue, N.; Cantley, J.; Meikle, P.J.; Biden, T.J. Alteration of Endoplasmic Reticulum Lipid Rafts Contributes to Lipotoxicity in Pancreatic β-Cells. J. Biol. Chem. 2013, 288, 26569–26582. [Google Scholar] [CrossRef] [PubMed]
- Roomp, K.; Kristinsson, H.; Schvartz, D.; Ubhayasekera, K.; Sargsyan, E.; Manukyan, L.; Chowdhury, A.; Manell, H.; Satagopam, V.; Groebe, K.; et al. Combined Lipidomic and Proteomic Analysis of Isolated Human Islets Exposed to Palmitate Reveals Time-Dependent Changes in Insulin Secretion and Lipid Metabolism. PLoS ONE 2017, 12, e0176391. [Google Scholar] [CrossRef]
- Barlow, J.; Jensen, V.H.; Jastroch, M.; Affourtit, C. Palmitate-Induced Impairment of Glucose-Stimulated Insulin Secretion Precedes Mitochondrial Dysfunction in Mouse Pancreatic Islets. Biochem. J. 2016, 473, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-J.; Wu, J.-H.; Sun, S.-Y.; Zhou, J.-Q. The Endoplasmic Reticulum Stress/Autophagy Pathway Is Involved in Cholesterol-Induced Pancreatic β-Cell Injury. Sci. Rep. 2017, 7, 44746. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Hou, F.; Liu, Z.; Cao, X.; Seo, H.; Gao, B. Cholesterol Induces Pancreatic β Cell Apoptosis through Oxidative Stress Pathway. Cell Stress Chaperones 2011, 16, 539–548. [Google Scholar] [CrossRef]
- Cnop, M.; Hannaert, J.C.; Grupping, A.Y.; Pipeleers, D.G. Low Density Lipoprotein Can Cause Death of Islet Beta-Cells by Its Cellular Uptake and Oxidative Modification. Endocrinology 2002, 143, 3449–3453. [Google Scholar] [CrossRef]
- Plaisance, V.; Brajkovic, S.; Tenenbaum, M.; Favre, D.; Ezanno, H.; Bonnefond, A.; Bonner, C.; Gmyr, V.; Kerr-Conte, J.; Gauthier, B.R.; et al. Endoplasmic Reticulum Stress Links Oxidative Stress to Impaired Pancreatic Beta-Cell Function Caused by Human Oxidized LDL. PLoS ONE 2016, 11, e0163046. [Google Scholar] [CrossRef]
- Li, Y.; Ge, M.; Ciani, L.; Kuriakose, G.; Westover, E.J.; Dura, M.; Covey, D.F.; Freed, J.H.; Maxfield, F.R.; Lytton, J.; et al. Enrichment of Endoplasmic Reticulum with Cholesterol Inhibits Sarcoplasmic-Endoplasmic Reticulum Calcium ATPase-2b Activity in Parallel with Increased Order of Membrane Lipids: Implications for Depletion of Endoplasmic Reticulum Calcium Stores and Apoptosis in Cholesterol-Loaded Macrophages. J. Biol. Chem. 2004, 279, 37030–37039. [Google Scholar] [CrossRef]
- Iida, H.; Kono, T.; Lee, C.-C.; Krishnan, P.; Arvin, M.C.; Weaver, S.A.; Jarvela, T.S.; Bone, R.N.; Tong, X.; Arvan, P.; et al. SERCA2 Regulates Proinsulin Processing and Processing Enzyme Maturation in the Pancreatic β Cell. Physiology 2022. [CrossRef]
- Lee, A.K.; Yeung-Yam-Wah, V.; Tse, F.W.; Tse, A. Cholesterol Elevation Impairs Glucose-Stimulated Ca2+ Signaling in Mouse Pancreatic β-Cells. Endocrinology 2011, 152, 3351–3361. [Google Scholar] [CrossRef] [PubMed]
- Andrali, S.S.; Sampley, M.L.; Vanderford, N.L.; Özcan, S. Glucose Regulation of Insulin Gene Expression in Pancreatic β-Cells. Biochem. J. 2008, 415, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Abdulkarim, B.; Bottu, G.; Cunha, D.A.; Igoillo-Esteve, M.; Masini, M.; Turatsinze, J.-V.; Griebel, T.; Villate, O.; Santin, I.; et al. RNA Sequencing Identifies Dysregulation of the Human Pancreatic Islet Transcriptome by the Saturated Fatty Acid Palmitate. Diabetes 2014, 63, 1978–1993. [Google Scholar] [CrossRef] [PubMed]
- Haataja, L.; Snapp, E.; Wright, J.; Liu, M.; Hardy, A.B.; Wheeler, M.B.; Markwardt, M.L.; Rizzo, M.A.; Arvan, P. Proinsulin Intermolecular Interactions during Secretory Trafficking in Pancreatic β Cells. J. Biol. Chem. 2013, 288, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Ramanadham, S.; Ma, Z.; Arita, H.; Zhang, S.; Turk, J. Type IB Secretory Phospholipase A2 Is Contained in Insulin Secretory Granules of Pancreatic Islet β-Cells and Is Co-Secreted with Insulin from Glucose-Stimulated Islets. Biochim. Et Biophys. Acta (BBA)—Lipids Lipid Metab. 1998, 1390, 301–312. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Hosaka, M.; Moriguchi, T.; Zhang, S.; Suda, M.; Yokota-Hashimoto, H.; Shinozuka, K.; Takeuchi, T. Cholesterol Biosynthesis Pathway Intermediates and Inhibitors Regulate Glucose-Stimulated Insulin Secretion and Secretory Granule Formation in Pancreatic β-Cells. Endocrinology 2010, 151, 4705–4716. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing Mitochondrial Dysfunction in Cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Stiles, L.; Shirihai, O.S. Mitochondrial Dynamics and Morphology in Beta-Cells. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 725–738. [Google Scholar] [CrossRef]
- Gerhold, J.M.; Cansiz-Arda, Ş.; Lõhmus, M.; Engberg, O.; Reyes, A.; van Rennes, H.; Sanz, A.; Holt, I.J.; Cooper, H.M.; Spelbrink, J.N. Human Mitochondrial DNA-Protein Complexes Attach to a Cholesterol-Rich Membrane Structure. Sci. Rep. 2015, 5, 15292. [Google Scholar] [CrossRef]
- Kruit, J.K.; Kremer, P.H.C.; Dai, L.; Tang, R.; Ruddle, P.; de Haan, W.; Brunham, L.R.; Verchere, C.B.; Hayden, M.R. Cholesterol Efflux via ATP-Binding Cassette Transporter A1 (ABCA1) and Cholesterol Uptake via the LDL Receptor Influences Cholesterol-Induced Impairment of Beta Cell Function in Mice. Diabetologia 2010, 53, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Efanov, A.M.; Sewing, S.; Bokvist, K.; Gromada, J. Liver X Receptor Activation Stimulates Insulin Secretion via Modulation of Glucose and Lipid Metabolism in Pancreatic Beta-Cells. Diabetes 2004, 53 (Suppl. S3), S75–S78. [Google Scholar] [CrossRef]
- Bonfleur, M.L.; Vanzela, E.C.; Ribeiro, R.A.; de Gabriel Dorighello, G.; de França Carvalho, C.P.; Collares-Buzato, C.B.; Carneiro, E.M.; Boschero, A.C.; de Oliveira, H.C.F. Primary Hypercholesterolaemia Impairs Glucose Homeostasis and Insulin Secretion in Low-Density Lipoprotein Receptor Knockout Mice Independently of High-Fat Diet and Obesity. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2010, 1801, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ohara-Imaizumi, M.; Nishiwaki, C.; Nakamichi, Y.; Kikuta, T.; Nagai, S.; Nagamatsu, S. Correlation of Syntaxin-1 and SNAP-25 Clusters with Docking and Fusion of Insulin Granules Analysed by Total Internal Reflection Fluorescence Microscopy. Diabetologia 2004, 47, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Vikman, J.; Jimenez-Feltström, J.; Nyman, P.; Eliasson, L. Insulin Secretion Is Highly Sensitive to Desorption of Plasma Membrane Cholesterol. FASEB J. 2009, 23, 58–67. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, A.; Arunagiri, A.; Dule, N.; Castagna, M.; Marciani, P.; Perego, C. Cholesterol Redistribution in Pancreatic β-Cells: A Flexible Path to Regulate Insulin Secretion. Biomolecules 2023, 13, 224. https://doi.org/10.3390/biom13020224
Galli A, Arunagiri A, Dule N, Castagna M, Marciani P, Perego C. Cholesterol Redistribution in Pancreatic β-Cells: A Flexible Path to Regulate Insulin Secretion. Biomolecules. 2023; 13(2):224. https://doi.org/10.3390/biom13020224
Chicago/Turabian StyleGalli, Alessandra, Anoop Arunagiri, Nevia Dule, Michela Castagna, Paola Marciani, and Carla Perego. 2023. "Cholesterol Redistribution in Pancreatic β-Cells: A Flexible Path to Regulate Insulin Secretion" Biomolecules 13, no. 2: 224. https://doi.org/10.3390/biom13020224
APA StyleGalli, A., Arunagiri, A., Dule, N., Castagna, M., Marciani, P., & Perego, C. (2023). Cholesterol Redistribution in Pancreatic β-Cells: A Flexible Path to Regulate Insulin Secretion. Biomolecules, 13(2), 224. https://doi.org/10.3390/biom13020224