Trace Element and Mineral Levels in Serum, Hair, and Urine of Obese Women in Relation to Body Composition, Blood Pressure, Lipid Profile, and Insulin Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Design
2.3. Bioimpedance
2.4. Anthropometric Measurements
2.5. Blood Pressure Measurement
2.6. Blood, Urine, and Hair Sampling
2.7. Biochemical Analysis
2.8. Hair, Serum, and Urine Sample Preparation
2.9. ICP-MS Analysis
2.10. Laboratory Quality Control
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. N. Am. 2016, 45, 571–579. [Google Scholar] [CrossRef] [Green Version]
- WHO. Available online: https://www.who.int/features/factfiles/obesity/en/ (accessed on 15 March 2021).
- García, O.P.; Long, K.Z.; Rosado, J.L. Impact of micronutrient deficiencies on obesity. Nutr. Rev. 2009, 67, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Shen, Y.; Fang, X.; Wang, Y.; Wang, F. Obesity and iron deficiency: A quantitative meta-analysis. Obes. Rev. 2015, 16, 1081–1093. [Google Scholar] [CrossRef]
- Sarrafzadegan, N.; Khosravi-Boroujeni, H.; Lotfizadeh, M.; Pourmogaddas, A.; Salehi-Abargouei, A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrients 2016, 32, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, K.; Xiang, W.; Zhang, Y.; Sun, K.; Jiang, X. The association between serum zinc level and overweight/obesity: A meta-analysis. Eur. J. Nutr. 2019, 58, 2971–2982. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Ajsuvakova, O.P.; Filippini, T.; Zhou, J.-C.; Lei, X.G.; Gatiatulina, E.R.; Michalke, B.; Skalnaya, M.G.; Vinceti, M.; Aschner, M.; et al. Selenium and Selenoproteins in Adipose Tissue Physiology and Obesity. Biomolecules 2020, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Li, X.; Xiang, W.; Jiang, X. The Relationship Between Serum Copper and Overweight/Obesity: A Meta-analysis. Biol. Trace Elem. Res. 2019, 194, 336–347. [Google Scholar] [CrossRef]
- Błażewicz, A.; Klatka, M.; Astel, A.; Partyka, M.; Kocjan, R. Differences in Trace Metal Concentrations (Co, Cu, Fe, Mn, Zn, Cd, and Ni) in Whole Blood, Plasma, and Urine of Obese and Nonobese Children. Biol. Trace Elem. Res. 2013, 155, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Yerlikaya, F.H.; Toker, A.; Arıbaş, A. Serum trace elements in obese women with or without diabetes. Indian J. Med. Res. 2013, 137, 339–345. [Google Scholar]
- Lee, Y.-A.; Kim, S.-H.; Kim, H.-N.; Song, S.-W. Are There Differences in Hair Mineral Concentrations Between Metabolically Healthy and Unhealthy Obese Adults? Biol. Trace Elem. Res. 2019, 193, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, Y.; Long, T.; Wang, R.; Li, Z.; Yu, C.; Wu, T.; He, M. Association between urinary metals levels and metabolic phenotypes in overweight and obese individuals. Chemosphere 2020, 254, 126763. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdański, P.; Pupek-Musialik, D.; Krejpcio, Z. Dietary Intake and Serum and Hair Concentrations of Minerals and their Relationship with Serum Lipids and Glucose Levels in Hypertensive and Obese Patients with Insulin Resistance. Biol. Trace Elem. Res. 2010, 139, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Nikonorov, A.A.; Skalnaya, M.G.; Tinkov, A.A.; Skalny, A.V. Mutual interaction between iron homeostasis and obesity pathogenesis. J. Trace Elem. Med. Biol. 2015, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Thethi, T.K.; Parsha, K.; Rajapurkar, M.; Mukhopadhyay, B.; Shah, S.; Yau, C.L.; Japa, S.; Fonseca, V. Urinary Catalytic Iron in Obesity. Clin. Chem. 2011, 57, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Liu, C.-N.; Wolf, R.M.; Ralle, M.; Dev, S.; Pierson, H.; Askin, F.; Steele, K.E.; Magnuson, T.H.; Schweitzer, M.A.; et al. Obesity is associated with copper elevation in serum and tissues. Metallomics 2019, 11, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Polyakova, V.S.; Nikonorov, A.A. Chronic administration of iron and copper potentiates adipogenic effect of high fat diet in Wistar rats. BioMetals 2013, 26, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Skalnaya, M.G.; Skalny, A.V.; Tinkov, A.A. Serum copper, zinc, and iron levels, and markers of carbohydrate metabolism in postmenopausal women with prediabetes and type 2 diabetes mellitus. J. Trace Elem. Med. Biol. 2017, 43, 46–51. [Google Scholar] [CrossRef]
- Mokhberi, V.; Bagheri, B.; Akbari, N.; Tabiban, S.; Habibi, V. Serum level of copper in patients with coronary artery disease. Niger. Med. J. 2015, 56, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Burkhead, J.L.; Lutsenko, S. The role of copper as a modifier of lipid metabolism. In Lipid Metabolism; Baez, R.V., Ed.; IntechOpen: London, UK, 2013; pp. 39–60. [Google Scholar]
- Darroudi, S.; Saberi-Karimian, M.; Tayefi, M.; Tayefi, B.; Khashyarmanesh, Z.; Fereydouni, N.; Haghighi, H.M.; Mahmoudi, A.A.; Kharazmi-Khorassani, J.; Gonoodi, K.; et al. Asso-ciation between hypertension in healthy participants and zinc and copper status: A population-based study. Biol. Trace Elem. Res. 2019, 190, 38–44. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, H.; Li, S.; Zhang, D. The association of serum zinc and copper with hypertension: A meta-analysis. J. Trace Elem. Med. Biol. 2019, 53, 41–48. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A Review of Dietary Selenium Intake and Selenium Status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Rothman, K.J. Selenium exposure and the risk of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Epidemiol. 2018, 33, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, K.; Lei, X.G. Selenium and diabetes—Evidence from animal studies. Free. Radic. Biol. Med. 2013, 65, 1548–1556. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-N.; Song, S.-W. Concentrations of Chromium, Selenium, and Copper in the Hair of Viscerally Obese Adults are Associated with Insulin Resistance. Biol. Trace Elem. Res. 2014, 158, 152–157. [Google Scholar] [CrossRef]
- Stupin, A.; Cosic, A.; Novak, S.; Vesel, M.; Jukic, I.; Popovic, B.; Karalic, K.; Loncaric, Z.; Drenjancevic, I. Reduced Dietary Selenium Impairs Vascular Function by Increasing Oxidative Stress in Sprague-Dawley Rat Aortas. Int. J. Environ. Res. Public Health 2017, 14, 591. [Google Scholar] [CrossRef] [Green Version]
- Tinkov, A.A.; Skalnaya, M.G.; Ajsuvakova, O.P.; Serebryansky, E.P.; Chao, J.C.-J.; Aschner, M.; Skalny, A.V. Selenium, Zinc, Chromium, and Vanadium Levels in Serum, Hair, and Urine Samples of Obese Adults Assessed by Inductively Coupled Plasma Mass Spectrometry. Biol. Trace Elem. Res. 2021, 199, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Król, E.; Bogdański, P.; Suliburska, J.; Krejpcio, Z. The Relationship between Dietary, Serum and Hair Levels of Minerals (Fe, Zn, Cu) and Glucose Metabolism Indices in Obese Type 2 Diabetic Patients. Biol. Trace Elem. Res. 2019, 189, 34–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Khorsandi, H.; Nikpayam, O.; Yousefi, R.; Parandoosh, M.; Hosseinzadeh, N.; Saidpour, A.; Ghorbani, A. Zinc supplementation improves body weight management, inflammatory biomarkers and insulin resistance in individuals with obesity: A ran-domized, placebo-controlled, double-blind trial. Diabetol. Metab. Syndr. 2019, 11, 101. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.R.; Mistry, M.; Cheriyan, A.M.; Williams, J.M.; Naraine, M.K.; Ellis, C.L.; Mallick, R.; Mistry, A.C.; Gooch, J.L.; Ko, B.; et al. Zinc deficiency induces hypertension by promoting renal Na+ reabsorption. Am. J. Physiol. Renal. Physiol. 2019, 316, F646–F653. [Google Scholar] [CrossRef] [PubMed]
- Treviño, S.; Diaz, A. Vanadium and insulin: Partners in metabolic regulation. J. Inorg. Biochem. 2020, 208, 111094. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Popova, E.V.; Polyakova, V.S.; Kwan, O.V.; Skalny, A.V.; Nikonorov, A.A. Adipose tissue chromium and vanadium disbalance in high-fat fed Wistar rats. J. Trace Elem. Med. Biol. 2015, 29, 176–181. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Kim, S.M. Vanadium-protein complex inhibits human adipocyte differentiation through the activation of β-catenin and LKB1/AMPK signaling pathway. PLoS ONE 2020, 15, e0239547. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Youn, C.-K.; Hyun, J.W.; You, H.J. The Anti-obesity Effect of Natural Vanadium-Containing Jeju Ground Water. Biol. Trace Elem. Res. 2012, 151, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Sagua, R.; Reyes, M.; Lavandero, S.; Cifuentes, M. Calcium in Obesity and Related Diseases: The Calcium-Sensing Receptor as a Novel Mediator. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Academic Press: Cambridge, MA, USA, 2017; pp. 35–44. [Google Scholar]
- Li, P.; Fan, C.; Lu, Y.; Qi, K. Effects of calcium supplementation on body weight: A meta-analysis. Am. J. Clin. Nutr. 2016, 104, 1263–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Ye, J.; Zhu, X.; Wang, L.; Gao, P.; Shu, G.; Jiang, Q.; Wang, S. Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms. Int. J. Mol. Sci. 2019, 20, 3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Choudhuri, D. Dietary calcium regulates the insulin sensitivity by altering the adipokine secretion in high fat diet induced obese rats. Life Sci. 2020, 250, 117560. [Google Scholar] [CrossRef]
- Cruz, K.J.; De Oliveira, A.R.; De Freitas, S.T.; Henriques, G.S.; Marreiro, D.D.N. Hypomagnesemia in Obese Subjects: Evidence of Systematic Review and Meta-analysis. Curr. Nutr. Food Sci. 2020, 16, 1044–1051. [Google Scholar] [CrossRef]
- Huerta, M.G.; Roemmich, J.N.; Kington, M.L.; Bovbjerg, V.E.; Weltman, A.L.; Holmes, V.F.; Patrie, J.T.; Rogol, A.D.; Nadler, J.L. Magnesium Deficiency Is Associated with Insulin Resistance in Obese Children. Diabetes Care 2005, 28, 1175–1181. [Google Scholar] [CrossRef] [Green Version]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Gutiérrez, A.; Sánchez-Pimienta, T.G.; Carriquiry, A.; Da Costa, T.H.M.; Ariza, A.C. Higher dietary magnesium intake is associated with lower body mass index, waist circumference and serum glucose in Mexican adults. Nutr. J. 2018, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Rodriguez-Moran, M. Serum magnesium in the metabolically-obese normal-weight and healthy-obese subjects. Eur. J. Intern. Med. 2013, 24, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Schutten, J.C.; Joosten, M.M.; De Borst, M.H.; Bakker, S.J. Magnesium and Blood Pressure: A Physiology-Based Approach. Adv. Chronic Kidney Dis. 2018, 25, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Mikulewicz, M. Biomarkers of Trace Element Status. In Recent Advances in Trace Elements; Chojnacka, K., Saeid, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 457–467. [Google Scholar]
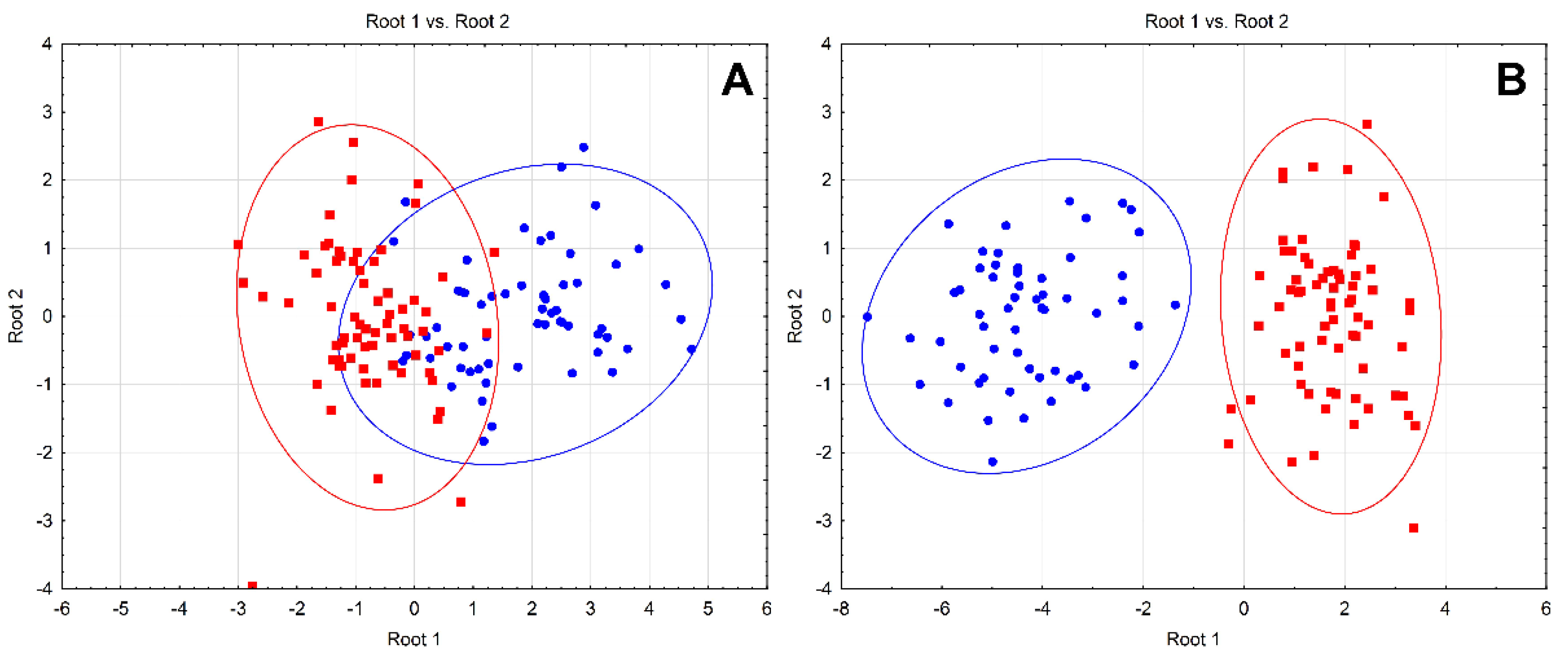
| Parameter | Control (n = 40) | Obese (n = 40) | p Value |
|---|---|---|---|
| Age, y.o. | 52.5 ± 11.8 | 51.4 ± 12.2 | 0.603 |
| Height, cm | 162.2 ± 6.6 | 163.6 ± 6.9 | 0.209 |
| Body mass, kg | 59.9 ± 6.2 | 97.4 ± 16.8 | <0.001 * |
| BMI, kg/m2 | 22.7 ± 1.4 | 36.4 ± 5 | <0.001 * |
| BF, % | 30.4 ±5.4 | 46.3 ±5.4 | <0.001 * |
| SBP, mmHg | 128.4 ± 18.5 | 142.8 ± 18.6 | <0.001 * |
| DBP, mmHg | 76.3 ± 11.5 | 85.9 ± 10.7 | <0.001 * |
| HR, bpm | 77.7 ± 13.8 | 80.2 ± 11.9 | 0.381 |
| TC, mg/dL | 213.3 ± 43.4 | 184 ± 44.2 | <0.001 * |
| HDL-C, mg/dL | 77.7 ± 18.5 | 46 ± 17.7 | <0.001 * |
| LDL-C, mg/dL | 115.2 ± 40.6 | 120.8 ± 45.6 | 0.379 |
| TG, mg/dL | 110.3 ± 54.3 | 143 ± 69.3 | 0.003 * |
| FPG, mg/dL | 90.3 ± 14.9 | 88.4 ± 25.9 | 0.622 |
| Insulin, µU/mL | 11.4 ± 8.5 | 16 ± 11.4 | 0.012 * |
| HOMA-IR | 2.5 ± 2 | 3.7 ± 3.4 | 0.023 * |
| Element | Control (n = 40) | Obese (n = 40) | p Value |
|---|---|---|---|
| Serum | |||
| Ca, µg/mL | 101.8 (97.9–106.6) | 74.2 (70.7–78.6) | <0.001 * |
| Cu, µg/mL | 1.136 (0.938–1.315) | 1.170 (1.07–1.290) | 0.018 * |
| Fe, µg/mL | 1.243 (0.923–1.505) | 0.910 (0.736–1.150) | 0.001 * |
| Mg, µg/mL | 22.7 (21.3–23.3) | 19.5 (17.9–20.7) | <0.001 * |
| Se, µg/mL | 0.106 (0.096–0.116) | 0.067 (0.061–0.078) | <0.001 * |
| V, ng/mL | 5.316 (4.412–5.766) | 3.1 (2.7–3.2) | <0.001 * |
| Zn, µg/mL | 0.88 (0.805–0.947) | 0.814 (0.762–0.87) | 0.001 * |
| Hair | |||
| Ca, µg/g | 1406.3 (773.6–2755.9) | 1738 (650–2929) | 0.699 |
| Cu, µg/g | 14.456 (10.705–25.87) | 13.5 (12.03–18.57) | 0.936 |
| Fe, µg/g | 14.381 (9.183–24.417) | 9.3 (6.75–13.58) | 0.008 * |
| Mg, µg/g | 152.3 (67.3–265.5) | 89.7 (53.4–154) | 0.017 * |
| Se, µg/g | 0.391 (0.331–0.461) | 0.332 (0.278–0.42) | 0.074 |
| V, µg/g | 0.014 (0.01–0.034) | 0.007 (0.004–0.011) | <0.001 * |
| Zn, µg/g | 198.7 (160.3–227.5) | 160 (132–188) | 0.062 |
| Urine | |||
| Ca, µg/mL | 97.7 (53.5–154.5) | 96.2 (46.3–157) | 0.932 |
| Cu, µg/mL | 0.011 (0.008–0.017) | 0.011 (0.008–0.016) | 0.808 |
| Fe, µg/mL | 0.022 (0.015–0.041) | 0.046 (0.018–0.213) | 0.012 * |
| Mg, µg/mL | 77.7 (50.7–128.8) | 67.7 (47.7–98.3) | 0.142 |
| Se, µg/mL | 0.026 (0.015–0.041) | 0.018 (0.01–0.026) | 0.016 * |
| V, ng/mL | 0.068 (0.041–0.157) | 0.040 (0.005–0.09) | 0.006 * |
| Zn, µg/mL | 0.284 (0.183–0.465) | 0.282 (0.177–0.431) | 0.400 |
| Element | BMI, kg/m2 | BF, % | SBP, mmHg | DBP, mmHg | TC, mg/dL | HDL-C, mg/dL | TG, mg/dL | insulin, µU/mL | HOMA-IR |
| Serum Ca | −0.776 * | −0.748* | −0.343 * | −0.369 * | 0.339 * | 0.605 * | −0.245 * | −0.174 * | −0.151 |
| Serum Cu | 0.293 * | 0.245* | 0.156 | 0.195 * | −0.045 | −0.153 | 0.097 | 0.364 * | 0.302 * |
| Serum Fe | −0.193 * | −0.260* | −0.167 | −0.138 | 0.141 | 0.191 * | −0.027 | −0.174 * | −0.143 |
| Serum Mg | −0.592 * | −0.560* | −0.321 * | −0.323 * | 0.205 * | 0.477 * | −0.269 * | −0.214 * | −0.186 * |
| Serum Se | −0.686 * | −0.610* | −0.183 * | −0.248 * | 0.376 * | 0.569 * | −0.210 * | −0.181 * | −0.146 |
| Serum V | −0.544 * | −0.553* | −0.219 * | −0.264 * | 0.272 * | 0.445 * | −0.177 * | −0.097 | −0.089 |
| Serum Zn | −0.261 * | −0.218* | −0.250 * | −0.206 * | 0.250 * | 0.222 * | −0.219 * | −0.274 * | −0.224 * |
| Hair Mg | −0.228 * | −0.204* | −0.208 * | −0.151 | 0.075 | 0.160 | −0.115 | −0.098 | −0.084 |
| Hair Se | −0.130 | −0.103 | −0.031 | −0.022 | 0.141 | 0.015 | 0.109 | −0.019 | −0.058 |
| Hair V | −0.235 * | −0.194* | −0.027 | −0.065 | 0.059 | 0.204 * | −0.084 | 0.166 | 0.146 |
| Urine Mg | −0.132 | −0.120 | −0.144 | −0.142 | 0.080 | 0.157 | −0.062 | −0.212 * | −0.215 * |
| Urine Se | −0.239 * | −0.274* | −0.106 | −0.126 | 0.223 * | 0.239 * | 0.030 | −0.185 * | −0.157 |
| Parameter | Model 1 | Model 2 (Serum) | Model 3 (Hair) | Model 4 (Urine) | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| FPG, mg/dL | −0.026 | 0.824 | −0.009 | 0.924 | −0.090 | 0.473 | 0.003 | 0.984 |
| TC, mg/dL | −0.149 | 0.090 | 0.012 | 0.869 | −0.132 | 0.163 | −0.090 | 0.376 |
| HDL-C mg/dL | −0.529 | <0.001 * | −0.164 | 0.029 * | −0.476 | <0.001 * | −0.540 | <0.001 * |
| LDL-C, mg/dL | 0.040 | 0.631 | −0.052 | 0.446 | 0.040 | 0.658 | 0.053 | 0.562 |
| HOMA-IR | 0.323 | 0.279 | 0.097 | 0.692 | 0.384 | 0.217 | 0.348 | 0.274 |
| TG, mg/dL | 0.071 | 0.380 | 0.096 | 0.139 | 0.087 | 0.290 | 0.047 | 0.613 |
| Insulin, µU/mL | −0.222 | 0.411 | 0.003 | 0.989 | −0.241 | 0.395 | −0.242 | 0.406 |
| Age, y.o. | −0.033 | 0.638 | 0.011 | 0.841 | −0.011 | 0.884 | −0.032 | 0.688 |
| SBP, mmHg | 0.212 | 0.024 * | 0.155 | 0.041 * | 0.170 | 0.083 | 0.250 | 0.016 * |
| DBP, mmHg | 0.159 | 0.085 | 0.021 | 0.773 | 0.159 | 0.090 | 0.135 | 0.186 |
| HR, bpm | 0.014 | 0.849 | 0.055 | 0.338 | 0.016 | 0.830 | 0.019 | 0.818 |
| Ca, µg/mL | - | - | −0.391 | <0.001 * | −0.001 | 0.996 | 0.015 | 0.880 |
| Cu, µg/mL | - | - | 0.179 | 0.001 * | 0.011 | 0.867 | 0.035 | 0.689 |
| Fe, µg/mL | - | - | 0.037 | 0.481 | −0.062 | 0.357 | 0.030 | 0.701 |
| Mg, µg/mL | - | - | −0.021 | 0.777 | −0.093 | 0.296 | −0.211 | 0.083 |
| Se, µg/mL | - | - | −0.230 | 0.009 * | −0.127 | 0.075 | 0.065 | 0.412 |
| V, ng/mL | - | - | −0.103 | 0.129 | −0.111 | 0.091 | 0.168 | 0.147 |
| Zn, µg/mL | - | - | −0.198 | 0.001 * | −0.002 | 0.974 | 0.035 | 0.755 |
| Multiple R | 0.755 | 0.871 | 0.777 | 0.761 | ||||
| Multiple R2 | 0.570 | 0.759 | 0.603 | 0.579 | ||||
| Adjusted R2 | 0.530 | 0.718 | 0.536 | 0.500 | ||||
| p for a model | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinkov, A.A.; Bogdański, P.; Skrypnik, D.; Skrypnik, K.; Skalny, A.V.; Aaseth, J.; Skalnaya, M.G.; Suliburska, J. Trace Element and Mineral Levels in Serum, Hair, and Urine of Obese Women in Relation to Body Composition, Blood Pressure, Lipid Profile, and Insulin Resistance. Biomolecules 2021, 11, 689. https://doi.org/10.3390/biom11050689
Tinkov AA, Bogdański P, Skrypnik D, Skrypnik K, Skalny AV, Aaseth J, Skalnaya MG, Suliburska J. Trace Element and Mineral Levels in Serum, Hair, and Urine of Obese Women in Relation to Body Composition, Blood Pressure, Lipid Profile, and Insulin Resistance. Biomolecules. 2021; 11(5):689. https://doi.org/10.3390/biom11050689
Chicago/Turabian StyleTinkov, Alexey A., Paweł Bogdański, Damian Skrypnik, Katarzyna Skrypnik, Anatoly V. Skalny, Jan Aaseth, Margarita G. Skalnaya, and Joanna Suliburska. 2021. "Trace Element and Mineral Levels in Serum, Hair, and Urine of Obese Women in Relation to Body Composition, Blood Pressure, Lipid Profile, and Insulin Resistance" Biomolecules 11, no. 5: 689. https://doi.org/10.3390/biom11050689






