The Pharmacological Activity of Camellia sinensis (L.) Kuntze on Metabolic and Endocrine Disorders: A Systematic Review
Abstract
:1. Introduction
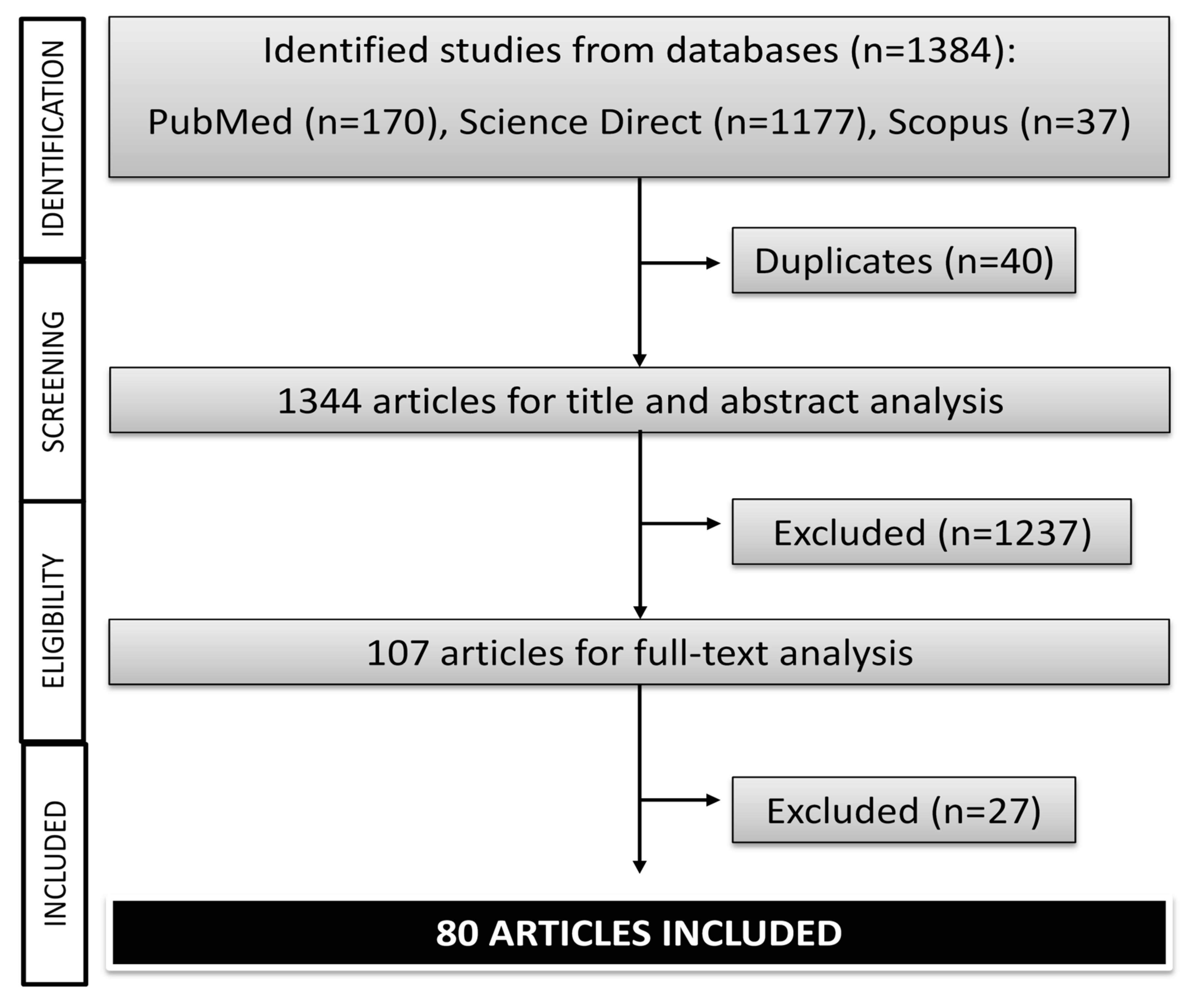
2. Method
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
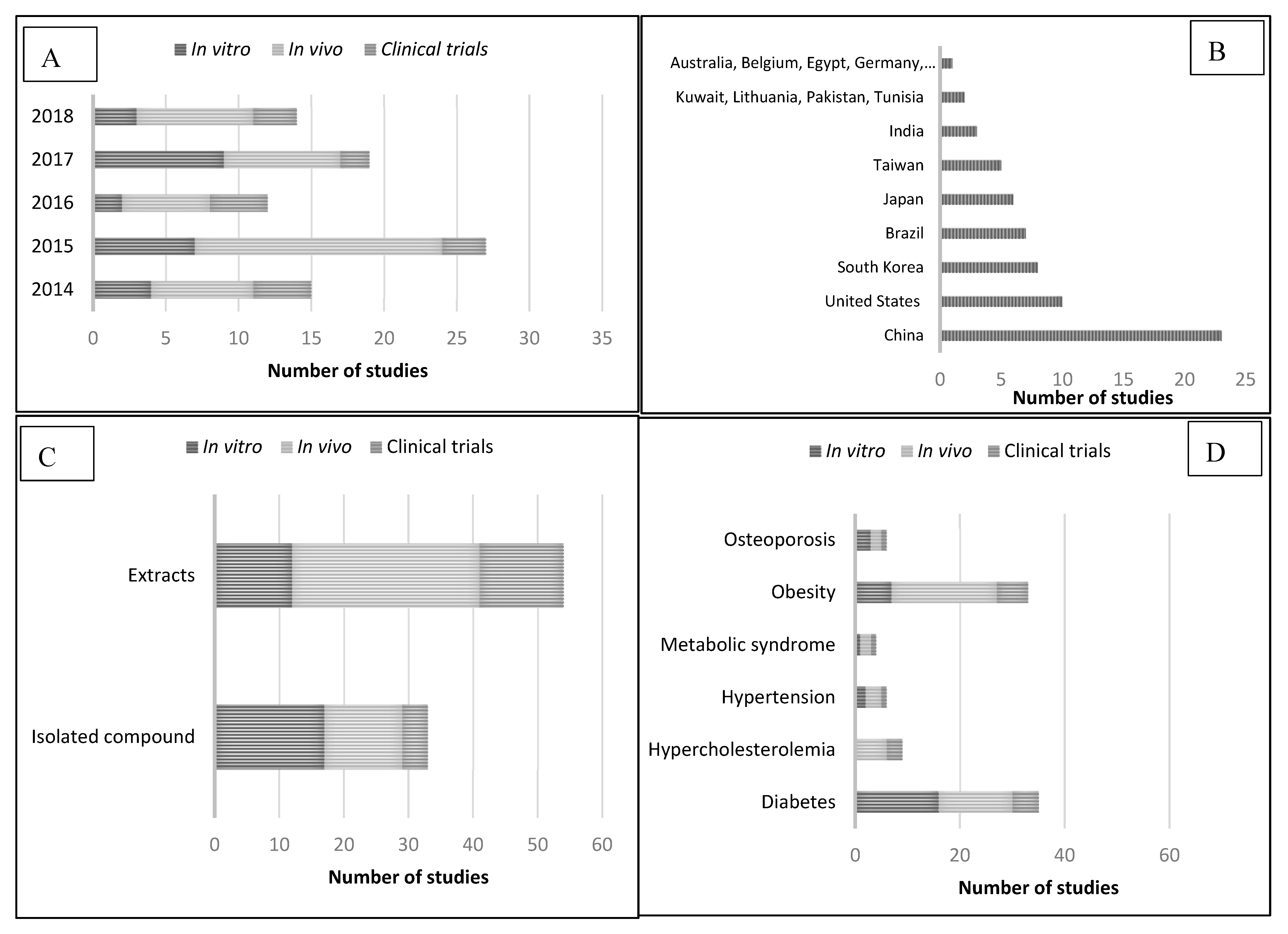
3. Pharmacological Activity. Description of the Data
3.1. Camellia sinensis and Diabetes
3.2. Camellia sinensis and Hypercholesterolemia
3.3. Camellia sinensis and Hypertension
3.4. Camellia sinensis and Metabolic Syndrome
3.5. Camellia sinensis and Obesity
3.6. Camellia sinensis and Osteoporosis
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Disease | Extract/Isolated Compound | Experimental Model | Treatments | Major Findings | References |
|---|---|---|---|---|---|
| Diabetes | Amelliaone A | α-Glucosidase model | - | α-Glucosidase inhibition: IC50 = 10.2 µg/mL | [40] |
| Arabinogalactan | Rat islet tumor RIN-5F cells | 50 or 200 μg/mL, 2 h | ↑ Insulin secretion | [26] | |
| Black and green teas | Mouse 3T3-L1 preadipocytes | 10 µg/mL, 24 h | ↑ SOD, CAT, and GPx activities ↓ Protein glycation ↓ α-Amylase and α-glucosidase activities | [42] | |
| Black tea aqueous extract | α-Glucosidase model α-Amylase model Caco-2 cells | - | ↓ α-Glucosidase activity No effect on GLUT2 and SGLT1 uptake | [33] | |
| Black, green, and dark tea extracts | Human liver HepG2 cancer cells | - | ↓ α-Glucosidase, aldose reductase, advanced glycation end-products ↑ Glucose uptake (dark tea extracts) | [109] | |
| Epicatechin gallate | α-Amylase model α-Glucosidase models | - | ↓ α-Amylase activity (IC50 = 45.30 μg/mL) ↓ α-Glucosidase activity (IC50 = 4.03 μg/mL) | [39] | |
| Epigallocatechin gallate | Mouse 3T3-L1 adipocytes | 20 μM, 2 h | ↓ IGF-I and IGF-II stimulation | [27] | |
| Epigallocatechin-3-O-gallate | Rat skeletal muscle L6 cells | 0, 20, 40, 50, and 60 μM, 48 h | ↓ α-Glucosidase activity (IC50 = 19.5 μM) ↑ Glucose uptake Promotion GLUT4 translocation to plasma | [15] | |
| Flavanols | α-Glucosidase model | - | ↓ Sucrase activity and maltase activity (EGCG IC50 = 32.5 and 1.3 μM, respectively) | [14] | |
| Flavone and flavone glycosides | α-Glucosidase model α-Amylase model | - | ↓ α-Glucosidase activity (kaempferol monoglycoside IC50 = 40.02 μM) ↓ α-Amylase activity (kaempferol diglycoside IC50 = 0.09 μM) | [41] | |
| Green tea polyphenols Green, black, and oolong tea extracts | α-Glucosidase model | - | ↓ α-Glucosidase activity (green tea polyphenols IC50 = 2.33 µg/mL, green tea IC50 = 2.82 µg/mL, black tea IC50 = 2.25 µg/mL, and oolong tea IC50 = 1.38 µg/mL) | [31] | |
| Diabetes (continued) | Green, oolong, and black water and pomace tea extracts | Rat intestinal α-glucosidase activity | - | ↓ α-glucosidase activity (tea water extract IC50 = 2040 µg/mL and tea pomace extract IC50 = 1950 µg/mL) | [32] |
| Non-catechin flavonoids | Human liver HepG2 cancer cells | Insulin (5 µM) Insulin + TNF-α (30 ng/mL) Insulin + TNF-α + NCF (2000 ppm) Insulin + TNF-α + NCF (1000 ppm) Insulin + TNF-α + NCF (500 ppm) 2, 4, and 6 h | ↑ TNF-α induced insulin resistance ↓ Glucose levels | [29] | |
| Pu-erh tea polysaccharides | α-Glucosidase model α-Amylase model | - | ↓ α-glucosidase activity No α-amylase inhibition | [37] | |
| Qingzhuan dark tea | α-Glucosidase model | IC50 2270 µg/mL for ethyl acetate fraction | ↓ α-Glucosidase activity (ethyl acetate fraction, EGCG, ECG) | [110] | |
| Tea polysaccharides | α-Glycosidase model | - | ↑ α-Glycosidase inhibitory activities (polysaccharides with 5 years aging) | [38] | |
| Hypertension | Black tea aqueous extracts Thearubigin, theaflavin, catechin, epicatechin, epigallocatechin gallate, gallic acid, caffeine | Angiotensin converting enzyme model | Aqueous tea extract (15 µg/mL) Isolated compounds (37 µM) | ↑ ACE inhibitory activity (Thearubigin, theaflavin, catechin) | [56] |
| Black tea extract Theaflavin-3,3’-digallate | Endothelial cells from rat thoracic artery | Black tea (0.3–5 μg/mL), 30 min TF3 (0.03–0.5 μg/mL), 30 min | Endothelium dependent relaxations restored ↓ ROS production | [57] | |
| Metabolic Syndrome | Green tea extract | Mouse 3T3-L1 preadipocytes | Green tea extract (0.2%–0.5%, w/v), 2 days | ↓ Adipogenesis induced lipid accumulation ↓ C/EBPα and PPARγ expression | [64] |
| Obesity | Black tea theaflavins | Pancreatic lipase model | - | Pancreatic lipase inhibition Theaflavin-3,3′-digallate IC50 = 1.9 μM Theaflavin-3′-gallate IC50 = 4.2 μM Theaflavin-3-gallate IC50 = 3.0 μM Theaflavin IC50 > 10 μM | [81] |
| Ethanol tea extracts | Porcine pancreatic lipase type II | 5 mg/mL ethanol | Antilipase activity (IC50 = 500 µg/mL) | [80] | |
| Flavanols | Lipase model | - | ↓ Lipase activity ECG (IC50 = 16.0 μM) CG (IC50 = 13.6 μM) Epiafzelechin-3-O-gallate (IC50 = 19.8 μM) EGCG (IC50 = 13.3 μM ) | [14] | |
| Gallocatechin gallate Epigallocatechin gallate | Mouse 3T3-L1 preadipocytes | Gallates 0–20 μg/mL | Anti-adipogenic activity ↓ Intracellular lipid droplets (GCG, EGCG) ↓ PPAR γ, SREBP-1c and C/EBP α adipogenic transcription factors (GCG, EGCG) ↓ ROS levels (GCG) ↓ NF-κB activation (GCG) ↓ IL-6 production (GCG) | [73] | |
| Green tea catechins | Mouse 3T3-L1 preadipocytes | Green tea catechins with/without norepinephrine (0.1 or 1 μM) for 6 or 24 h | ↑ Lipolysis via PKA-dependent pathway | [71] | |
| Green tea polyphenols Epigallocatechin-3-gallate | Mouse 3T3-L1 preadipocytes | Green tea polyphenols (1, 10, and 100 μg/mL) EGCG (6.8 μg/mL) | ↓ Triglyceride accumulation ↓ Adipogenic factor C/EBPα, SREBP-1c, and PPARγ expression | [72] | |
| Traditional Korean Chungtaejeon | Mouse 3T3-L1 preadipocytes | Traditional Korean Chungtaejeon (250 μg/mL) | ↓ Lipid accumulation ↓ PPARγ expression ↓ Adipocyte lipid-binding protein | [92] | |
| Osteoporosis | Green tea extract | Mouse macrophage RAW 264.7 cells treated with RANKL (50 ng/mL) | 25, 50, or 100 μg/mL for 48 h | ↓ mRNA expression osteoclast-associated genes ↓ NFATc1, c-Fos, c-src and cath-K protein levels | [103] |
| Gallocatechin gallate Epigallocatechin-3-gallate | Mouse macrophage RAW 264.7 cells | 10 μM, 20 min | ↓ RANKL-induced osteoclast differentiation ↓ F-actin ring formation ↓ Osteoclastogenesis-related marker genes and proteins expression, especially gallocatechin gallate | [104] | |
| Flavones | Rat osteoblastic cells C2C12 mouse myoblast cell line | From 3.125 to 50 μg/mL, 48 h | ↑ Alkaline phosphatase activity (epicatechin) ↑ Hydroxyproline content (epicatechin) ↑ Area of mineralized bone nodules (epiafzelechin) | [106] |
Appendix B
| Disease | Extract/Isolated Compound | Experimental Model | Treatments | Major Findings | References |
|---|---|---|---|---|---|
| Diabetes | Black tea aqueous extract | GK rats | Group 1: black tea 31.3, 62.5, and 250 mg/kg Group 2: acarbose 0.1, 0.3, and 3.0 mg/kg Group 3: acarbose 0.3 mg/kg + black tea 31.3 mg/kg | ↓ Plasma glucose levels | [33] |
| Black tea aqueous extract | Alloxan-induced diabetic rats | Group 1: control Group 2: alloxan Group 3: black tea extract (1 mL/100 g body w/d for 10 days before alloxan injection and 35 days after alloxan injection) Group 4: black tea extract (35 days) Group 5: diabetic insulin group (twice a day/subcutaneous injection of three units of insulin) | ↑ Plasma antioxidant potential ↓ Lipid peroxidation levels ↑ GSH levels | [22] | |
| Epigallocatechin-3-gallate | C57BL/6J mice | Group 1: low fat diet Group 2: high fat diet Group 3: high fat diet + EGCG (25 mg/kg) Group 4: high fat diet + EGCG (75 mg/kg) | ↓ Plasma glucose ↓ Insulin level ↓ Advanced glycation end products | [11] | |
| Epigallocatechin-3-gallate | Wistar rats streptozotocin-nicotinamide-induced diabetic rats | Group 1: control Group 2: EGCG (2 mg/kg body wt) Group 3: diabetic control group Group 4: diabetic control group + EGCG 1 month | ↓ Glucose, glycosylated hemoglobin, HOMA-IR and lipid profile level ↑ Insulin levels ↑ GSH levels and SOD and CAT activities | [23] | |
| Green tea decoctions Epigallocatechin gallate Epigallocatechin | Wistar rats | Group 1: water Group 2: green tea decoctions Group 3: EGCG, EGC | ↓ SGLT-1 activity ↑ GLUT2 activity ↑ Glucose tolerance | [12] | |
| Green tea ethanol extracts | Sprague-Dawley rats | Group 1: hyperglycemic rats Group 2: hyperglycemic rats + tea extract 10% Group 3: hyperglycemic rats + tea extract 5% 8 weeks | ↓ Serum glucose | [16] | |
| Green tea extract | Nematode Caenorhabditis elegans | 0.1%, 48 h | ↓ Glucose induced damage | [24] | |
| Diabetes (continued) | Green tea extract | Rat model High sodium diet | Group 1: high sodium diet Group 2: high sodium diet + 2 g green tea extract in kg diet Group 3: high sodium diet + 4 g green tea extract in kg diet 6 weeks | ↓ Insulin level and homeostatic model assessment | [13] |
| Green tea polysaccharides | Kunming mice | Group 1: high fat diabetic control Group 2: rosiglitazone Groups 3, 4, and 5: green Tea polysaccharides (200, 400, and 800 mg/kgb.w. per day) 4 weeks | ↓ Insulin resistance PI3K/Akt signal pathway | [10] | |
| Pu-erh tea and green tea | BALB/c mice | Group 1: glucose (2000 mg/kg) Group 2: glucose (2000 mg/kg) + pu-erh tea (800 mg/kg) Group 3: glucose (2000 mg/kg) + green tea (800 mg/kg) Group 4: glucose (2000 mg/kg) + EGCG (240 mg/kg) Group 5: glucose (2000 mg/kg) + EGCG (240 mg/kg) + caffeine (80 mg/kg) Group 6: caffeine (80 mg/kg) | ↓ Blood glucose levels | [111] | |
| Pu-erh tea polysaccharides (TPS) | ICR mice | Group 1: control Group 2: acarbose (5 mg kg−1) Group 3: TPS (1 mg kg−1) Group 4: TPS (5 mg kg−1) | ↓ Blood glucose levels | [37] | |
| Pu-erh tea extract | C57BL/6J mice | Group 1: normal chow diet Group 2: high fat diet Group 3: normal chow diet + pu-erh tea extract (5 mg/mL, 17 weeks) Group 4: high fat diet + pu-erh tea extract (5 mg/ml, 17 weeks) | ↓ Gluconeogenesis related genes expression | [9] | |
| Tea polypeptides from green tea | High fat diet/streptozocin induced (30 mg/kg bw) diabetic mice | 1000 mg/kg bw/day, p.o., 5 weeks | ↓ Total urinary protein, creatinine, and urine nitrogen | [28] | |
| Tea water extract and tea pomace extract of green and black tea | Sprague-Dawley rats | Group 1: sucrose Group 2: tea extracts (0.5 g/kg body wt) | ↓ Glucose level | [32] | |
| Hypercholes-terolemia | Chungtaejeon aqueous extracts | Wistar rats high fat atherogenic diet (HFAD) | Group 1: normal basal diet Group 2: HFAD Group 3: 100 mg/kg day tea extract + HFAD Group 4: 200 mg/kg day tea extract + HFAD Group 5: 400 mg/kg day tea extract + HFAD | ↓ LDL cholesterol ↓ Total serum cholesterol ↓ Hepatic cholesterol | [50] |
| Epigallocatechin-gallate | Wistar rats Fluoride-induced oxidative stress mediated cardiotoxicity | Group 1: normal saline Group 2: EGCG (40 mg/kg BW/day) Group 3: sodium fluoride (25 mg/kg body weight/day, 4 weeks) Group 4: EGCG (40 mg/kg BW/day) + sodium fluoride (25 mg/kg body weight/day, 4 weeks) | ↓ Lipid peroxidative markers ↓ Plasma total cholesterol ↓ Triglycerides ↓ Phospholipids ↓ LDL cholesterol ↑ HDL cholesterol | [48] | |
| Green tea ethanol extracts | Sprague-Dawley rats | Group 1: hypercholesterolemic rats Group 2: hypercholesterolemic rats + diet containing green tea extracts 5% Group 3: hypercholesterolemic rats + diet containing tea powder 10% 8 weeks | ↓ LDL ↓ Triglycerides ↓ Cholesterol | [16] | |
| Green tea extracts | Rat model High sodium diet | Group 1: high sodium diet Group 2: high sodium diet + 2 g green tea extract in kg diet Group 3: high sodium diet + 4 g green tea extract in kg diet 6 weeks | ↓ Total cholesterol, LDL, cholesterol serum concentrations | [13] | |
| Green tea polysaccharides | Kunming mice | Group 1: high fat diabetic control Group 2: rosiglitazone Groups 3, 4, and 5: green tea polysaccharides (200, 400, and 800 mg/kgb.w. per day) 4 weeks | ↓ Total cholesterol ↓ LDL cholesterol | [47] | |
| Tea flavonols (“Sofu” green tea leaves and “Yabukita” tea leaves) | Mice model High cholesterol diet induced | Group 1: high cholesterol diet Group 2: high cholesterol diet + water Group 3: high cholesterol diet + “Sofu” green tea Group 4: high cholesterol diet + “Yabukita” tea | ↓ Plasma oxidized LDL level | [59] | |
| Hypertension | Black tea extract | Sprague-Dawley rats Angiotensin II induced | Group 1: control Group 2: angiotensin II (50 ng/kg/min) Group 3: angiotensin II + black tea extract (15 mg/kg/day, 14 days) | ↑ Endothelium-dependent relaxations ↓ Endoplasmic reticulum stress markers levels ↓ ROS production | [57] |
| Green tea from three cultivars “Yabukita”, “Sofu” and “Sunrouge” | Hypertensive rats High salt diet | Group 1: high salt water Group 2: high salt water + Yabukita Group 3: high salt water + Sofu Group 4: high salt water + Sunrouge | ↓ Urinary NO metabolite ↑ Soluble guanyilate cyclase expression (Yabukita and Sofu) | [59] | |
| Metabolic Syndrome | Green tea aqueous extract | Olanzapine induced Wistar rats | Group 1: control Group 2: olanzapine (5 mg/kg/day) Groups 3, 4, and 5: green tea aqueous extract (25, 50, and 100 mg/kg/day) + olanzapine Groups 6, 7, and 8: green tea aqueous extract (25, 50, and 100 mg/kg/day) | ↓ Body weight gain ↓ Average food and water intake Improved changes in lipid profile ↓ Hyperleptinemia and hypertension | [67] |
| Yellow tea | C57BL/6 male mice High fat diet | Group 1: low fat diet Group 2: high fat diet Group 3: high fat diet + 2.5% yellow tea Group 4: high fat diet + 0.5% yellow tea 12 weeks | ↓ Body weight, liver weight, and adipose tissue weight ↓ Serum glucose, TC, TG, LDL-C, and HDL-C ↓ Glucose intolerance and insulin resistance | [68] | |
| Obesity | Black tea and green tea decoctions | Male Wistar rats | Group 1: high fat diet Group 2: green tea decoction Group 3: black tea decoction 10 weeks | ↑ Fecal triglycerides excretion ↓ Liver triglycerides ↓ Plasma triglycerides ↓ Body weight ↓ Glucose | [76] |
| Decaffeinated green tea extract rich in EGCG | Male Swiss mice | Group 1: control diet + water (0.1 mL/day) Group 2: control diet + EGCG (50 mg/kg/day) Group 3: hyperlipidic diet + water Group 4: hyperlipidic diet + EGCG 16 weeks | ↓ Body weight ↓ Insulin level ↓ Liver fat accumulation ↑ Glucose uptake | [90] | |
| Obesity (continued) | Decaffeinated polyphenol extracts (green tea, black tea, and oolong tea) | C57BL/6J mice High fat/high sucrose | Group 1: low fat/high sucrose diet Group 2: high fat/high sucrose diet Group 3: high fat/high sucrose diet + green tea polyphenols Group 4: high fat/high sucrose diet + black tea polyphenols Group 5: high fat/high sucrose diet + oolong tea polyphenols | ↓ Body weight ↓ Total visceral fat volume ↓ Liver lipid weight ↓ Food intake (green tea polyphenols) | [74] |
| Decaffeinated green tea extract rich in EGCG | Swiss mice High fat diet | Group 1: control diet Group 2: high fat diet Group 3: control diet + placebo Group 4: high fat diet + placebo Group 5: control diet + EGCG Group 6: high fat diet + EGCG 8 weeks | ↓ Body weights ↓ Serum triglyceride levels ↓ Adipocyte area | [91] | |
| Epigallocatechin 3-gallate | C57BL/6J mice | Group 1: low fat diet (negative control) Group 2: high fat diet (positive control) Group 3: high fat diet + EGCG (25 mg/kg) Group 4: high fat diet + EGCG (75 mg/kg) | ↓Body weight ↓ Liver and kidney weight | [11] | |
| Epigallocatechin-3-gallate | C57BL/6 mice | Group 1: high fat diet Group 2: high fat diet + EGCG (20 mg/kg) | ↓ Body weight ↓ Fat infiltration in liver tissue ↑ Serum lipid profiles | [93] | |
| Fermented green tea extract | C57BL/6 mice | Group 1: normal diet Group 2: high fat diet Group 3: high fat diet + fermented green tea extract | ↓ Body weight gain ↓ Fat mass ↓ Glucose intolerance ↓ Fatty liver symptoms | [84] | |
| Green tea | C57BL/6J mice | Group 1: normal diet Group 2: high fat (60% energy as fat) Group 3: high fat + 0.25% (w/w) Green tea 12 weeks | ↓ Body weight gain ↑ Energy expenditure ↓ Adiposity | [85] | |
| Obesity (continued) | Green tea | C57BL/6J mice | Group 1: normal diet Group 2: high fat diet Group 3: high fat diet + 0.25% (w/w) green tea extract | ↑ Lysophospholipids levels | [87] |
| Green tea decoctions Epigallocatechin gallate Epigallocatechin | Wistar rats | Group 1: normal diet Group 2: high fat diet Group 3: high fat diet + green tea decoctions | ↓ Body weight ↓ Triglycerides ↓ Cholesterol | [12] | |
| Green tea extract | C57BL/6J mice | Group 1: green tea extract (77 mg/g) Group 2: voluntary exercise Group 3: green tea extract + voluntary exercise | ↑ Adipose tissue conversion into brown fat like adipose | [83] | |
| Green tea extracts | C57BL/6 mice | Group 1: control diet Group 2: high fat diet Group 3: high fat diet + 0.5% polyphenolic green tea extracts 8 weeks | ↓ Adiposity ↓ High diet inflammation ↓ Adipocyte size ↓ Lipid droplet size | [86] | |
| Green tea extract | Sprague–Dawley rats | Group 1: normal diet control Group 2: high fat diet control Group 3: orlistat control (50 mg/kg/d + high fat diet) Group 4: green tea extract (100 mg/kg/d + high fat diet) 50 days | ↓ Body weight ↓ White adipose tissue fat | [89] | |
| Oolong tea water extract | C57BL/6J mice | Group 1: normal diet Group 2: high fat diet Group 3/4/5: oolong tea (different storage years) 6 weeks | ↓ Body weight ↓ Fat accumulation ↓ Triglyceride levels ↓ LDL cholesterol ↑ HDL cholesterol level | [75] | |
| Polyphenol-rich green tea extract | C57BL/6 mice | Group 1: fed a standard diet + gavage with water Group 2: standard diet + gavage with 500 mg/kg GT Group 3: HFD + gavage with water Group 4: HFD+ gavage with GT 16 weeks | ↓ Body weight ↓ Body adiposity ↓ Inflammation ↑ Insulin sensitivity | [88] | |
| Obesity (continued) | Polysaccharides, polyphenols and caffeine from green tea | Sprague-Dawley rats High fat rats | Groups control, polysaccharides, polyphenols, and caffeine at two doses (low and high) | ↓ Body weight and fat accumulation ↑ Antioxidant levels ↓ Leptin levels ↓ Fatty acids absorption | [112] |
| Pu-erh tea extract | C57BL/6J mice | Group 1: normal chow diet Group 2: high fat diet Group 3: normal diet + tea extract (5 mg/mL, 17 weeks) Group 4: high fat diet + tea extract (5 mg/mL, 17 weeks) | ↓ Obesity ↓ Hepatic steatosis and liver inflammation ↓ Liver injury | [9] | |
| Teasaponin | High fat diet C57BL/6 male mice | High fat diet (8 weeks) + oral teasaponin (0.5%) with high diet (6 weeks) | ↓ Neuroinflammation ↑ Brain derived neurotrophic factor ↑ Glucose tolerance ↓ Body weight gain | [82] | |
| Traditional Korean Chungtaejeon | C57BL6J-Lep ob/ob mice | Traditional Korean Chungtaejeon (200 or 400 mg/kg body weight, 10 weeks) | ↓ Body weight gain ↓ Fat mass ↓ Food efficacy ratio ↓ Levels of plasma triglyceride and total cholesterol | [92] | |
| Water extract of white tea, yellow tea, oolong tea, green tea, white tea, and raw pu-erh tea | High fat diet induced obese mice | Group 1: untreated Group 2: atorvastatin-treated (oral daily at 10 mg/kg body weight) Group 3: green tea Group 4: yellow tea Group 5: black tea Group 6: white tea Group 7: raw pu-erh tea Group 8: oolong tea Teas: daily oral 1000 mg/kg body weight for 9 weeks | ↓ Body weight ↓ White fat accumulation ↑ Energy expenditure and fatty acid oxidation (white, yellow, and oolong teas) ↓ Fatty acid synthesis (green, white, and raw pu-erh teas) Best tea: white tea | [77] | |
| Osteoporosis | Green tea aqueous extract | Ovariectomized female rats | GTE (60, 120, and 370 mg/kg, 13 weeks) | ↑ Bone mass ↓ Trabecular bone loss | [103] |
| Green tea polyphenols | Sprague-Dawley | Group 1: high fat diet Group 2: caloric restricted diet Group 3: high fat diet + 0.5% green tea polyphenols Group 4: caloric restricted diet + 0.5% green tea polyphenols | ↑ Femoral mass and strength ↑ Trabecular thickness and number ↑ Cortical thickness of tibia ↓ Trabecular separation ↓ Formation rate and eroded surface at proximal tibia ↓ Insulin-like growth factor-I and leptin | [108] |
Appendix C
| Study (Author, Year, Country) | Study Design | Sample Size | Population | Type of Plant | Intervention | Duration of Treatment | Results |
|---|---|---|---|---|---|---|---|
| DIABETES | |||||||
| Alves Ferreira et al., 2017 [43] Brazil | Randomized, double-blind, placebo-controlled study | 120 | Women (20–45 years) abnormal glucose values | Green tea capsules | Group 1: control (cellulose) Group 2: green tea (1 g) Group 3: metformin (1 g) Group 4: green tea (1 g) + metformin (1 g) | 12 weeks | Improving glycemic and lipid profile ↓ Fasting glucose ↓ Total cholesterol and LDL |
| Lasaite et al., 2014 [113] Lithuania | Randomized double-blind placebo-controlled study | 56 | Patients (37–78 years) with diabetes mellitus type II and diabetic retinopathy, nephropathy or neuropathy | Green tea extract | Group 1: placebo Group 2: Gingko biloba dry extract Group 3: green tea extract For extracts: one capsule twice a day (9 months) and one capsule three times a day (9 months) | 18 months | No statistically significant differences in HbA1c level, antioxidant state, and psychological data |
| Mahmoud et al., 2016 [44] Kuwait | Randomly assigned | 34 | Male and female type 2 diabetics | Black tea infusions | Group 1: three cups black tea daily (600 mL) Group 2: one cup black tea daily (200 mL) | 12 weeks | ↓ HbA1c levels ↑ Regulatory T cells ↓ Pro-inflammatory |
| Spadiene et al., 2014 [45] Lithuania | Randomized, double-blind, placebo-controlled study | 45 | Patients (35-80 years) with diabetes mellitus type II and diabetic retinopathy, nephropathy or neuropathy | Green tea extract | Group 1: green tea extract Group 2: placebo | 9–18 months | ↓ Lipid peroxidation |
| Vaz et al., 2018 [46] Brazil | Randomized, double-blind, placebo-controlled study | 60 | Patients with diabetes | Green tea extract | Group 1: green tea extract (two capsules/day, containing 560 mg of polyphenols/each) Group 2: cellulose (two capsules/day) | 20 weeks | No effect on total antioxidant capacity, glycemic control markers, and renal function ↑ SOD activity |
| HYPERCHOLESTEROLEMIA | |||||||
| Imbe et al., 2016 [53] Japan | Randomized, double-blind, placebo-controlled trial | 155 | Healthy volunteers High LDL cholesterol levels Aged 20–80 years | “Benifuuki” green tea | Group 1: “Benifuuki” Group 2: “Yabukita” Group 3: barley infusion drinker | 12 weeks | ↓ LDL cholesterol levels ↓ Lectin-like oxidized LDL receptor-1 containing LAB level |
| Orem et al., 2017 [51] Canada | Randomized, double-blind, placebo-controlled study | 125 | Subjects 25–60 years hypercholesterolemia | Black tea | Group 1: placebo Group 2: instant black tea Group 3: functional black tea | 4 weeks | Functional black tea: ↓ Total cholesterol ↓ LDL ↓ Oxidative stress index ↑ Total antioxidant status |
| Troup et al., 2015 [52] United States | Randomized, double-blind, crossover trial | 57 | 45–65 years, hypercholesterolemia | Black tea | Group 1: controlled low flavonoid diet plus five cups per day of black tea Group 2: Placebo | 4 weeks | ↓ LDL/HDL ratio ↓ Total cholesterol |
| HYPERTENSION | |||||||
| Alkerwi et al., 2015 [60] Luxembourg | National cross-sectional stratified sample | 1352 | 18–69 years | Tea | Group 1: nonconsumers Group 2: ≤ 3-dL/d consumers (tea/coffee) Group 3: > 3-dL/d consumers (tea/coffee) | - | ↓ Systolic BP and pulse pressure |
| METABOLIC SYNDROME | |||||||
| Yang et al., 2014 [64] China | - | 134 | Metabolic syndrome | Green tea extract | Group 1: green tea extract (500 mg). Two capsules/time/day Group 2: control (water) | 45 days | ↑ Adiponectin serum concentrations ↓ Visfatin levels |
| OBESITY | |||||||
| Chen et al., 2016 [94] Taiwan | Randomized, double-blind trial | 102 | Women BMI ≥ 27 kg/m2 Waist circumference ≥ 80 cm | EGCG | Group 1: placebo Group 2: high dose green tea | 12 weeks | ↓ Weight ↓ Waist circumference ↓ Total cholesterol and LDL plasma levels |
| Dostal et al., 2016 [96] USA | Randomized, double-blind, placebo-controlled clinical trial | 937 | Postmenopausal women aged 50–70 with high breast density and overweight/obese | Green tea extract | Group 1: placebo Group 2: EGCG (843 mg), four capsules daily | 12 months | No ↓ adiposity No improvements in BMI ↓ Tissue fat and gynoid fat |
| Huang et al., 2018 [95] Taiwan | Randomized, double-blind, crossover, placebo-controlled | 90 | Women (18 - 65 years) BMI ≥ 27 kg/m2 LDL-C ≥ 130 mg/dL | Green tea extract | Group 1: placebo Group 2: one capsule 30 min after meal, three times a day, green tea extract | 6 weeks | ↑ Leptin ↓ LDL |
| Janssens et al., 2015 [98] The Netherlands | Randomized, placebo-controlled, single-blind design | 60 | Caucasian men and women with body mass index from 18 kg/m², age: 18–50 | Green tea extract | Group 1: placebo Group 2: green tea (capsules > 0.06 g EGCG and 0.03–0.05 g caffeine) | 12 weeks | No effect on fecal energy content, fecal fat content, resting energy expenditure, respiratory quotient, and body composition |
| Mielgo-Ayuso et al., 2014 [97] Spain | Randomized, double-blind, parallel design | 83 | Obese (30 kg/m2. BMI, 40 kg/m2) premenopausal women | EGCG | Group 1: placebo (lactose) Group 2: EGCG (300 mg/d) | 12 weeks | No changes in body weight No changes in adiposity |
| Nicoletti et al., 2019 [99] Brazil | Longitudinal interventional study | 11 | Women (18–60 years) (BMI) > 40 kg/m2 | EGCG | Group 1: eutrophic women Group 2: decaffeinated green tea capsules with 450.7 mg of EGCG, two capsules/day | 8 weeks | ↑ RICTOR ↑ HIF1-α expression |
| OSTEOPOROSIS | |||||||
| Amorim et al., 2018 [114] Brazil | Double-blind, randomized, controlled clinical trial | 35 | ≥ 18 years old Diabetes for more than 5 years. | Green tea extract | Group 1: cellulose Group 2: 1120 mg of green tea extract contains 560 mg of polyphenols/day | 10 and 20 weeks | ↑ Bone mineral content |
References
- Bansal, A.; Henao-Mejia, J.; Simmons, R.A. Immune system: An emerging player in mediating effects of endocrine disruptors on metabolic health. Endocrinology 2017, 159, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.H.; Patnaik, S.K. Incidence of endocrine disorders in Indian adult male population. Indian J. Endocr. Metab. 2017, 21, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, M.; BiBi, J.; Kamboh, A.A.; Suheryani, I.; Kakar, I.; Fazlani, S.A.; Noreldin, A.E. Pharmacological values and therapeutic properties of black tea (Camellia sinensis): A comprehensive overview. Biomed. Pharm. 2018, 100, 521–531. [Google Scholar] [CrossRef]
- Tang, G.Y.; Zhao, C.N.; Xu, X.Y.; Gan, R.Y.; Cao, S.Y.; Liu, Q.; Shang, A.; Mao, Q.Q.; Li, H.B. Phytochemical Composition and Antioxidant Capacity of 30 Chinese Teas. Antioxidants (Basel). 2019, 8, 180. [Google Scholar] [CrossRef] [Green Version]
- Konieczynski, P.; Viapiana, A.; Wesolowski, M. Comparison of infusions from black and green teas (Camellia sinensis L. Kuntze) and yerva-mate (Ilex paraguariensis A. St.-Hil.) based on the content of essential elements, secondary metabolites, and antioxidant activity. Food Anal. Methods 2017, 10, 3063–3070. [Google Scholar] [CrossRef] [Green Version]
- Valduga, A.T.; Gonçalves, I.L.; Magri, E.; Finzer, J.R.D. Chemistry, pharmacology and new trends in traditional functional and medicinal beverages. Food Res. Int. 2019, 120, 478–503. [Google Scholar] [CrossRef]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [CrossRef]
- Cai, X.; Fang, C.; Hayashi, S.; Hao, S.; Zhao, M.; Tsutsui, H.; Nishiguchi, S.; Sheng, J. Pu-erh tea extract ameliorates high-fat diet-induced nonalcoholic steatohepatitis and insulin resistance by modulating hepatic IL-6/STAT3 signaling in mice. J. Gastroenterol. 2016, 51, 819–829. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Wang, J.; Wang, X.; Hu, B.; Lv, F. Involvement of the PI3K/Akt signal pathway in the hypoglycemic effects of tea polysaccharides on diabetic mice. Int. J. Biol. Macromol. 2015, 81, 967–974. [Google Scholar] [CrossRef]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharm. 2017, 87, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, C.; Ducroc, R.; Hamdaoui, M.H.; Dhaouadi, K.; Abaidi, H.; Cluzeaud, F.; Bado, A. Green tea decoction improves glucose tolerance and reduces weight gain of rats fed normal and high-fat diet. J. Nutr. Biochem. 2014, 25, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Kujawska-Luczak, M.; Szulinska, M.; Kregielska-Narozna, M.; Skrypnik, D.; Suliburska, J.; Skrypnik, K.; Regula, J.; Bogdanski, P. Beneficial dose-independent influence of Camellia sinensis supplementation on lipid profile, glycemia, and insulin resistance in a NaCl-induced hypertensive rat model. J. Physiol. Pharm. 2018, 69, 275–282. [Google Scholar]
- Wang, X.; Liu, Q.; Zhu, H.; Wang, H.; Kang, J.; Shen, Z.; Chen, R. Flavanols from the Camellia sinensis var. Assamica and their hypoglycemic and hypolipidemic activities. Acta Pharm. Sin. B 2017, 7, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, W.; Chen, Z.; Guo, Q.; Wang, C.; Santhanam, R.K.; Chen, H. Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells. Int. J. Biol. Macromol. 2019, 125, 605–611. [Google Scholar] [CrossRef]
- Yousaf, S.; Butt, M.S.; Suleria, H.A.; Iqbal, M.J. The role of green tea extract and powder in mitigating metabolic syndromes with special reference to hyperglycemia and hypercholesterolemia. Food Funct. 2014, 5, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Fan, W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. Metab. 2017, 6, 8–16. [Google Scholar] [CrossRef]
- International Diabetes Federation. Available online: www.idf.org (accessed on 1 December 2019).
- Thomas, N.J.; Jones, S.E.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; Hattersley, A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: A cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018, 6, 122–129. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Szmuilowicz, E.D.; Josefson, J.L.; Metzger, B.E. Gestational diabetes mellitus. Endocrinol. Metab. Clin. 2019, 48, 479–493. [Google Scholar] [CrossRef]
- Kumar, D.; Rizvi, S.I. Black tea extract improves anti-oxidant profile in experimental diabetic rats. Arch. Physiol. Biochem. 2015, 121, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.I.; El-Sawi, M.R.; El-Missiry, M.A.; Abukhalil, M.H. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharm. 2017, 94, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Deusing, D.J.; Winter, S.; Kler, A.; Kriesl, E.; Bonnländer, B.; Wenzel, U.; Fitzenberger, E. A catechin-enriched green tea extract prevents glucose-induced survival reduction in Caenorhabditis elegans through sir-2.1 and uba-1 dependent hormesis. Fitoterapia 2015, 102, 163–170. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Bhandari, K.; Chakravorty, N.; Mukherjee, R.; Gundamaraju, R.; Singla, R.K.; Katakam, P.; Adiki, S.K.; Ghosh, B.; Mitra, A. Computational pharmacokinetics and in vitro-in vivo correlation of anti-diabetic synergistic phyto-composite blend. World J. Diabetes 2015, 6, 1179–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Shi, S.; Bao, B.; Li, X.; Wang, S. Structure characterisation of an arabinogalactam from green tea and its anti-diabetic effect. Carbohydr. Polym. 2015, 124, 98–108. [Google Scholar] [CrossRef]
- Ku, H.C.; Tsuei, Y.W.; Kao, C.C.; Weng, J.T.; Shih, L.J.; Chang, H.H.; Kao, Y.H. Green tea (−)-epigallocatechin gallate suppresses IGF-I and IGF-II stimulation of 3T3-L1 adipocyte glucose uptake via the glucose transporter 4, but not glucose transporter 1 pathway. Gen. Comp. Endocr. 2014, 199, 46–55. [Google Scholar] [CrossRef]
- Deng, X.; Sun, L.; Lai, X.; Xiang, L.; Li, Q.; Zhang, W.; Zhang, L.; Sun, S. Tea polypeptide ameliorates diabetic nephropathy through RAGE and NF-κB signaling pathway in type 2 diabetes mice. J. Agr. Food Chem. 2018, 66, 11957–11967. [Google Scholar] [CrossRef]
- Chen, F.C.; Shen, K.P.; Ke, L.Y.; Lin, H.L.; Wu, C.C.; Shaw, S.Y. Flavonoids from Camellia sinensis (L.) O. Kuntze seed ameliorates TNF-α induced insulin resistance in HepG2 cells. Saudi Pharm. J. 2019, 27, 507–516. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharm. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Kong, F. Evaluation of the in vitro α-glucosidase inhibitory activity of green tea polyphenols and different tea types. J. Sci. Food Agric. 2016, 96, 777–782. [Google Scholar] [CrossRef]
- Oh, J.; Jo, S.H.; Kim, J.S.; Ha, K.S.; Lee, J.Y.; Choi, H.Y.; Yu, S.Y.; Kwon, Y.I.; Kim, Y.C. Selected tea and tea pomace extracts inhibit intestinal α-glucosidase activity in vitro and postprandial hyperglycemia in vivo. Int. J. Mol. Sci. 2015, 16, 8811–8825. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Igarashi, M.; Yamada, S.; Takahashi, N.; Watanabe, K. Inhibitory effect of black tea and its combination with acarbose on small intestinal α-glucosidase activity. J. Ethnopharmacol. 2015, 161, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Weerawatanakorna, M.; Hung, W.-L.; Pan, M.-H.; Li., S.; Li, D.; Wan, X.; Ho, C.-T. Chemistry and health beneficial effects of oolong tea and theasinensins. FSHW 2015, 4, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans, No. 51. In Coffee, Tea, Mate, Methylxanthines and Methylglyoxal; IARC Working Group on the Evaluation of Carcinogenic Risk to Humans: Lyon, France, 1991. [Google Scholar]
- Deng, Y.T.; Lin-Shiau, S.Y.; Shyur, L.F.; Lin, J.K. Pu-erh tea polysaccharides decrease blood sugar by inhibition of α-glucosidase activity in vitro and in mice. Food Funct. 2015, 6, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y. Physicochemical characterization of pu-erh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554. [Google Scholar] [CrossRef]
- Wu, X.; Hu, M.; Hu, X.; Ding, H.; Gong, D.; Zhang, G. Inhibitory mechanism of epicatechin gallate on α-amylase and α-glucosidase and its combinational effect with acarbose or epigallocatechin gallate. J. Mol. Liq. 2019, 290, 111202. [Google Scholar] [CrossRef]
- Zhou, H.; Li, H.-M.; Du, Y.-M.; Yan, R.-A.; Fu, L. C-geranylated flavanones from Ying De black tea and their antioxidant and α-glucosidase inhibition activities. Food Chem. 2017, 235, 227–333. [Google Scholar] [CrossRef]
- Hua, F.; Zhou, P.; Wu, H.Y.; Chu, G.X.; Xie, Z.W.; Bao, G.H. Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu’an GuaPian tea: Molecular docking and interaction mechanism. Food Funct. 2018, 9, 4173–4183. [Google Scholar] [CrossRef]
- Ramlagan, P.; Rondeau, P.; Planesse, C.; Neergheen-Bhujun, V.S.; Bourdon, E.; Bahorun, T. Comparative suppressing effects of black and green teas on the formation of advanced glycation end products (AGEs) and AGE-induced oxidative stress. Food Funct. 2017, 8, 4194–4209. [Google Scholar] [CrossRef]
- Alves Ferreira, M.; Oliveira Gomes, A.P.; Guimarães de Moraes, A.P.; Ferreira Stringhini, M.L.; Mota, J.F.; Siqueira Guedes Coelho, A.; Borges Botelho, P. Green tea extract outperforms metformin in lipid profile and glycaemic control in overweight women: A double-blind, placebo-controlled, randomized trial. Clin Nutr. 2017, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Al-Ozairi, E.; Haines, D.; Novotny, L.; Dashti, A.; Ibrahim, B.; Abdel-Hamid, M. Effect of Diabetea tea™ consumption on inflammatory cytokines and metabolic biomarkers in type 2 diabetes patients. J. Ethnopharmacol. 2016, 194, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Spadiene, A.; Savickiene, N.; Ivanauskas, L.; Jakstas, V.; Skesters, A.; Silova, A.; Rodovicius, H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J. Food Drug Anal. 2014, 22, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, S.R.; de Amorim, L.M.N.; de Nascimento, P.V.F.; Veloso, V.S.P.; Nogueira, M.S.; Castro, I.A.; Botelho, P.B. Effects of green tea extract on oxidative stress and renal function in diabetic individuals: A randomized, double-blinded, controlled trial. J. Funct. Foods 2018, 46, 195–201. [Google Scholar] [CrossRef]
- Li, S.B.; Li, Y.F.; Mao, Z.F.; Hu, H.H.; Ouyang, S.H.; Wu, Y.P.; Tsoi, B.; Gong, P.; Kurihara, H.; He, R.R. Differing chemical compositions of three teas may explain their different effects on acute blood pressure in spontaneously hypertensive rats. J. Sci. Food Agric. 2015, 95, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Miltonprabu, S.; Thangapandiyan, S. Epigallocatechin gallate potentially attenuates Fluoride induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. J. Trace. Elem. Med. Biol. 2015, 29, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Monobe, M.; Ema, K.; Matsunaga, A.; Maeda-Yamamoto, M.; Horie, H. Effects of flavonol-rich green tea cultivar (Camellia sinensis L.) on plasma oxidized LDL levels in hypercholesterolemic mice. Biosci. Biotechnol. Biochem. 2016, 80, 360–362. [Google Scholar] [CrossRef] [Green Version]
- Paudel, K.R.; Lee, U.W.; Kim, D.W. Chungtaejeon, a Korean fermented tea, prevents the risk of atherosclerosis in rats fed a high-fat atherogenic diet. J. Integr. Med. 2016, 14, 134–142. [Google Scholar] [CrossRef]
- Orem, A.; Alasalvar, C.; Kural, B.V.; Yaman, S.; Orem, C.; Karadag, A.; Zawistowski, J. Cardio-protective effects of phytosterol-enriched functional black tea in mild hypercholesterolemia subjects. J. Funct. Foods 2017, 31, 311–319. [Google Scholar] [CrossRef]
- Troup, R.; Hayes, J.H.; Raatz, S.K.; Thyagarajan, B.; Khaliq, W.; Jacobs, D.R., Jr.; Gross, M. Effect of black tea intake on blood cholesterol concentrations in individuals with mild hypercholesterolemia: A diet-controlled randomized trial. J. Acad. Nutr. Diet 2015, 115, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Imbe, H.; Sano, H.; Miyawaki, M.; Fujisawa, R.; Miyasato, M.; Nakatsuji, F.; Tachibana, H. “Benifuuki” green tea, containing O-methylated EGCG, reduces serum low-density lipoprotein cholesterol and lectin-like oxidized low-density lipoprotein receptor-1 ligands containing apolipoprotein B: A double-blind, placebo-controlled randomized trial. J. Funct. Foods 2016, 25, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Gumprecht, J.; Domek, M.; Lip, G.Y.; Shantsila, A. Invited review: Hypertension and atrial fibrillation: Epidemiology, pathophysiology, and implications for management. J. Hum. Hypertens. 2019, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pragle, A. Screening for endocrine hypertension. Clin. Rev. 2019, 29, 5e–7e. [Google Scholar]
- Ray, S.; Dutta, M.; Chaudhury, K.; De, B. GC–MS based metabolite profiling and angiotensin I-converting enzyme inhibitory property of black tea extracts. Rev. Bras. Farm. 2017, 27, 580–586. [Google Scholar] [CrossRef]
- Ghaffari, S.; Roshanravan, N. The role of nutraceuticals in prevention and treatment of hypertension: An updated review of the literature. Food Res. Int. 2020, 128, 108749. [Google Scholar] [CrossRef]
- San Cheang, W.; Yuen Ngai, C.; Yen Tam, Y.; Yu Tian, X.; Tak Wong, W.; Zhang, Y.; Wai Lau, C.; Chen, Z.Y.; Bian, Z.X.; Huang, Y.; et al. Black tea protects against hypertension-associated endothelial dysfunction through alleviation of endoplasmic reticulum stress. Sci. Rep. 2015, 15, 10340. [Google Scholar] [CrossRef]
- Nomura, S.; Monobe, M.; Ema, K.; Maeda-Yamamoto, M.; Nesumi, A. comparison of the effects of three tea cultivars (Camellia sinensis L.) on nitric oxide production and aortic soluble guanylate cyclase expression in high-salt diet-fed spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2017, 63, 306–314. [Google Scholar] [CrossRef]
- Alkerwi, A.; Sauvageot, N.; Crichton, G.E.; Elias, M.F. Tea, but not coffee consumption, is associated with components of arterial pressure. The observation of cardiovascular risk factors study in Luxembourg. Nutr. Res. 2015, 35, 557–565. [Google Scholar] [CrossRef]
- Dichi, I.; Simão, A.N.; Vannucchi, H.; Curi, R.; Calder, P.C. Metabolic syndrome: Epidemiology, pathophysiology, and nutrition intervention. J. Nutr. Metab. 2012, 2012, 584541. [Google Scholar] [CrossRef] [Green Version]
- Fornari, E.; Maffeis, C. Treatment of metabolic syndrome in children. Front. Endocrinol. 2019, 10, 702. [Google Scholar] [CrossRef] [Green Version]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Yin, L.; Li, T.; Chen, Z. Green tea extracts reduce adipogenesis by decreasing expression of transcription factors C/EBPα and PPARγ. Int. J. Clin. Exp. Med. 2014, 7, 4906–4914. [Google Scholar] [PubMed]
- Gracious, B.L.; Meyer, A.E. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry 2005, 2, 36–42. [Google Scholar] [PubMed]
- Kirk, S.L.; Glazebrook, J.; Grayson, B.; Neill, J.C.; Reynolds, G.P. Olanzapine-induced weight gain in the rat: Role of 5-HT2C and histamine H1 receptors. Psychopharmacology 2009, 207, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Lookian, F.; Hosseinzadeh, H. Protective effects of green tea on olanzapine-induced-metabolic syndrome in rats. Biomed. Pharm. 2017, 92, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chu, J.; Wang, M.; Chen, L.; Zhang, L.; Xie, Z.; Zhang, J.; Ho, C.T.; Li, D.; Wan, X. Large yellow tea Attenuates macrophage-related chronic inflammation and metabolic syndrome in high-fat diet treated mice. J. Agr. Food Chem. 2018, 66, 3823–3832. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K. Health effects of overweight and obesity in 195 countries over 25 years. N. Eng. J. Med. 2017, 377, 13–27. [Google Scholar]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 228–298. [Google Scholar] [CrossRef]
- Chen, S.; Osaki, N.; Shimotoyodome, A. Green tea catechins enhance norepinephrine-induced lipolysis via a protein kinase A-dependent pathway in adipocytes. Biochem. Bioph. Res. Commun. 2015, 461, 1–7. [Google Scholar] [CrossRef]
- Lao, W.; Tan, Y.; Jin, X.; Xiao, L.; Kim, J.J.; Qu, X. Comparison of cytotoxicity and the anti-adipogenic effect of green tea polyphenols with epigallocatechin-3-gallate in 3T3-L1 preadipocytes. Am. J. Chin. Med. 2015, 43, 1177–1190. [Google Scholar] [CrossRef]
- Li, K.K.; Peng, J.M.; Zhu, W.; Cheng, B.H.; Li, C.M. Gallocatechin gallate (GCG) inhibits 3T3-L1 differentiation and lipopolysaccharide induced inflammation through MAPK and NF-κB signaling. J. Funct. Foods 2017, 30, 159–167. [Google Scholar] [CrossRef]
- Heber, D.; Zhang, Y.; Yang, J.; Ma, J.E.; Henning, S.M.; Li, Z. Green tea, black tea, and oolong tea polyphenols reduce visceral fat and inflammation in mice fed high-fat, high-sucrose obesogenic diets. Nutr. J. 2014, 144, 1385–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, E.; Duan, X.; Xiang, L.; Ren, J.; Lai, X.; Li, Q.; Sun, L.; Sun, S. Aged oolong tea reduces high-fat diet-induced fat accumulation and dyslipidemia by regulating the AMPK/ACC signaling pathway. Nutrients 2018, 10, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdaoui, M.H.; Snoussi, C.; Dhaouadi, K.; Fattouch, S.; Ducroc, R.; Le Gall, M.; Bado, A. Tea decoctions prevent body weight gain in rats fed high-fat diet; black tea being more efficient than green tea. J. Nutr. Intermed. Metab. 2016, 6, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Guo, Y.; Sun, L.; Lai, X.; Li, Q.; Zhang, W.; Xiang, L.; Sun, S.; Cao, F. Six types of tea reduce high-fat-diet-induced fat accumulation in mice by increasing lipid metabolism and suppressing inflammation. Food Funct. 2019, 10, 2061–2074. [Google Scholar] [CrossRef]
- Ray, I.; Mahata, S.K.; De, R.K. Obesity: An immunometabolic perspective. Front. Endocrinol. 2016, 7, 157. [Google Scholar] [CrossRef] [Green Version]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Jamous, R.M.; Abu-Zaitoun, S.Y.; Akkawi, R.J.; Ali-Shtayeh, M.S. Antiobesity and antioxidant potentials of selected palestinian medicinal plants. Evid. Based Complement. Altern. Med. 2018, 13, 8426752. [Google Scholar] [CrossRef]
- Glisan, S.L.; Grove, K.A.; Yennawar, N.H.; Lambert, J.D. Inhibition of pancreatic lipase by black tea theaflavins: Comparative enzymology and in silico modeling studies. Food Chem. 2017, 216, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Huang, X.F.; Zhang, P.; Newell, K.A.; Wang, H.; Zheng, K.; Yu, Y. Dietary teasaponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci. Rep. 2017, 7, 12203. [Google Scholar] [CrossRef]
- Sae-Tan, S.; Rogers, C.J.; Lambert, J.D. Decaffeinated green tea and voluntary exercise induce gene Changes related to beige adipocyte formation in high fat-fed obese mice. J. Funct. Foods 2015, 14, 210–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, D.B.; Jeong, H.W.; Cho, D.; Lee, B.J.; Lee, J.H.; Choi, J.Y.; Bae, I.H.; Lee, S.J. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J. Med. Food 2015, 18, 549–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.Y.; Kim, Y.J.; Ryu, R.; Cho, S.J.; Kwon, E.Y.; Choi, M.S. Effect of green tea extract on systemic metabolic homeostasis in diet-induced obese mice determined via RNA-Seq transcriptome profiles. Nutrients 2016, 8, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyrinck, A.M.; Bindels, L.B.; Geurts, L.; Van Hul, M.; Cani, P.D.; Ashfaq, U.A. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J. Nutr. Biochem. 2017, 49, 15–21. [Google Scholar] [CrossRef]
- Nam, M.; Choi, M.S.; Choi, J.Y.; Kim, N.; Kim, M.S.; Jung, S.; Kim, J.; Ryu, D.H.; Hwang, G.S. Effect of green tea on hepatic lipid metabolism in mice fed a high-fat diet. J. Nutr. Biochem. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Otton, R.; Bolin, A.P.; Ferreira, L.T.; Marinovic, M.P.; Rocha, A.L.S.; Mori, M.A. Polyphenol-rich green tea extract improves adipose tissue metabolism by down-regulating miR-335 expression and mitigating insulin resistance and inflammation. J. Nutr. Biochem. 2018, 57, 170–179. [Google Scholar] [CrossRef]
- Chaudhary, N.; Bhardwaj, J.; Seo, H.J.; Kim, M.Y.; Shin, T.S.; Kim, J.D. Camellia sinensis fruit peel extract inhibits angiogenesis and ameliorates obesity induced by high-fat diet in rats. J. Funct. Foods 2014, 7, 479–486. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Carvalho-Silva, M.; Gomes, L.M.; Okuda, M.H.; Santana, A.A.; Streck, E.L.; Oyama, L.M. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. J. Nutr. Biochem. 2015, 26, 1348–1356. [Google Scholar] [CrossRef] [Green Version]
- Santana, A.; Santamarina, A.; Souza, G.; Mennitti, L.; Okuda, M.; Venancio, D.; Seelaender, M.; do Nascimento, C.O.; Ribeiro, E.; Lira, F.; et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate improves insulin resistance and metabolic profiles in normolipidic diet—But not high-fat diet-fed mice. J. Nutr. Biochem. 2015, 26, 893–902. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, D.W.; Rhyu, D.Y. Korean Chungtaejeon tea extract attenuates body weight gain in C57BL/6J-Lep ob/ob mice and regulates adipogenesis and lipolysis in 3T3-L1 adipocytes. J. Integr. Med. 2017, 15, 56–63. [Google Scholar] [CrossRef]
- Byun, J.K.; Yoon, B.Y.; Jhun, J.Y.; Oh, H.J.; Kim, E.K.; Min, J.K.; Cho, M.L. Epigallocatechin-3-gallate ameliorates both obesity and autoinflammatory arthritis aggravated by obesity by altering the balance among CD4+ T-cell subsets. Immunol. Lett. 2014, 157, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Liu, C.Y.; Wang, L.Y.; Huang, C.J.; Hsu, C.H. Effects of green tea extract on overweight and obese women with high levels of low density-lipoprotein-cholesterol (LDL-C): A randomised, double-blind, and cross-over placebo-controlled clinical trial. BMC Complement. Altern. Med. 2018, 18, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Arikawa, A.; Espejo, L.; Kurzer, M.S. Long-term supplementation of green tea extract does not modify adiposity or bone mineral density in a randomized trial of overweight and obese postmenopausal women. J. Nutr. 2016, 46, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Mielgo-Ayuso, J.; Barrenechea, L.; Alcorta, P.; Larrarte, E.; Margareto, J.; Labayen, I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. Nutr. J. 2015, 145, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, C.F.; Delfino, H.B.P.; Pinhel, M.; Noronha, N.Y.; Pinhanelli, V.C.; Quinhoneiro, D.C.G.; de Oliveira, B.A.P.; Marchini, J.S.; Nonino, C.B. Impact of green tea epigallocatechin-3-gallate on HIF1-α and mTORC2 expression in obese women: Anti-cancer and anti-obesity effects? Nutr. Hosp. 2019, 36, 315–320. [Google Scholar]
- Al Anouti, F.; Taha, Z.; Shamim, S.; Khalaf, K.; Al Kaabi, L.; Alsafar, H. An insight into the paradigms of osteoporosis: From genetics to biomechanics. Bone 2019, 11, 100216. [Google Scholar] [CrossRef]
- Ralston, S.H.; de Crombrugghe, B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006, 20, 2492–2506. [Google Scholar] [CrossRef] [Green Version]
- Kalu, D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Min. 1991, 15, 175–191. [Google Scholar] [CrossRef]
- Wu, X.; Xie, C.Q.; Zhu, Q.Q.; Wang, M.Y.; Sun, B.; Huang, Y.P.; Shen, C.; An, M.F.; Zhao, Y.L.; Wang, X.J.; et al. Green tea (Camellia sinensis) aqueous extract alleviates postmenopausal osteoporosis in ovariectomized rats and prevents RANKL-induced osteoclastogenesis in vitro. Food Nutr. Res. 2018, 62, 1478–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Liu, T.; Li, J.; Xu, J.; Chen, F.; Hu, L.; Sheng, J. Oxidation derivative of (-)-epigallocatechin-3-gallate (EGCG) inhibits RANKL-induced osteoclastogenesis by suppressing RANK signaling pathways in RAW 264.7 cells. Biomed. Pharm. 2019, 118, 109237. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Min. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tian, J.; Cai, K.; Wu, X.; Wang, Y.; Zheng, Y.; Su, Y.; Cui, L. Promoting osteoblast differentiation by the flavanes from Huangshan Maofeng tea is linked to a reduction of oxidative stress. Phytomedicine 2014, 21, 217–224. [Google Scholar] [CrossRef]
- Xu, H.; Yin, D.; Liu, T.; Chen, F.; Chen, Y.; Wang, X.; Sheng, J. Tea polysaccharide inhibits RANKL-induced osteoclastogenesis in raw264. 7 cells and ameliorates ovariectomy-induced osteoporosis in rats. Biomed. Pharm. 2018, 102, 539–548. [Google Scholar] [CrossRef]
- Shen, C.L.; Han, J.; Wang, S.; Chung, E.; Chyu, M.C.; Cao, J.J. Green tea supplementation benefits body composition and improves bone properties in obese female rats fed with high-fat diet and caloric restricted diet. Nutr. Res. 2015, 35, 1095–1105. [Google Scholar] [CrossRef]
- Wu, Y.T.; Du, W.H.; Shi, L.; Liang, Q.; Zou, X.Q. Vasculoprotective effects of water extracts of black, green and dark tea in vitro. Nat. Prod. Commun. 2017, 12, 387–390. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yu, Z.; Zhu, H.; Zhang, W.; Chen, Y. In vitro α-glucosidase inhibitory activity of isolated fractions from water extract of Qingzhuan dark tea. BMC Complement. Altern. Med. 2016, 16, 378. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.Y.; Wang, X.J.; Huang, Y.W.; Hao, S.M.; Sheng, J. Caffeine is responsible for the blood glucose-lowering effects of green tea and Pu-erh tea extracts in BALB/c mice. Chin. J. Nat. Med. 2015, 13, 595–601. [Google Scholar]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 297–304. [Google Scholar] [CrossRef]
- Lasaite, L.; Spadiene, A.; Savickiene, N.; Skesters, A.; Silova, A. The effect of Ginkgo biloba and Camellia sinensis extracts on psychological state and glycemic control in patients with type 2 diabetes mellitus. Nat. Prod. Commun. 2014, 9, 1345–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Amorim, L.M.N.; Vaz, S.R.; Cesário, G.; Coelho, A.S.G.; Botelho, P.B. Effect of green tea extract on bone mass and body composition in individuals with diabetes. J. Funct. Foods. 2018, 40, 589–594. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.; González-Burgos, E.; Iglesias, I.; Lozano, R.; Gómez-Serranillos, M.P. The Pharmacological Activity of Camellia sinensis (L.) Kuntze on Metabolic and Endocrine Disorders: A Systematic Review. Biomolecules 2020, 10, 603. https://doi.org/10.3390/biom10040603
Sánchez M, González-Burgos E, Iglesias I, Lozano R, Gómez-Serranillos MP. The Pharmacological Activity of Camellia sinensis (L.) Kuntze on Metabolic and Endocrine Disorders: A Systematic Review. Biomolecules. 2020; 10(4):603. https://doi.org/10.3390/biom10040603
Chicago/Turabian StyleSánchez, Marta, Elena González-Burgos, Irene Iglesias, Rafael Lozano, and M. Pilar Gómez-Serranillos. 2020. "The Pharmacological Activity of Camellia sinensis (L.) Kuntze on Metabolic and Endocrine Disorders: A Systematic Review" Biomolecules 10, no. 4: 603. https://doi.org/10.3390/biom10040603





