Enhancing H11 Protein-Induced Immune Protection Against Haemonchus contortus in Goats: A Nano-Adjuvant Formulation Strategy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
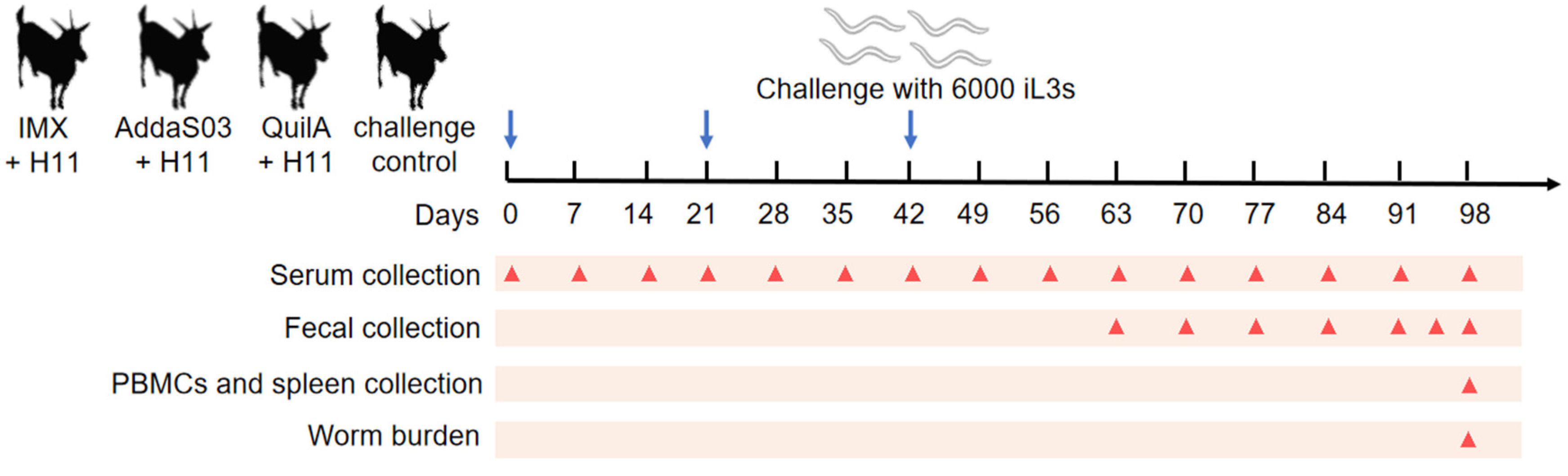
2.1. Experimental Animals
2.2. Parasites
2.3. Preparation of Antigens and Nanoparticles
2.4. Vaccination Trials
2.5. Parasitology
2.6. Detection of Serum IgG Antibody
2.7. Isolation of Goat PBMCs and Spleen Cells
2.8. Detection of the Transcription Levels of Cytokine-Encoding Genes
2.9. Statistical Analysis
3. Results
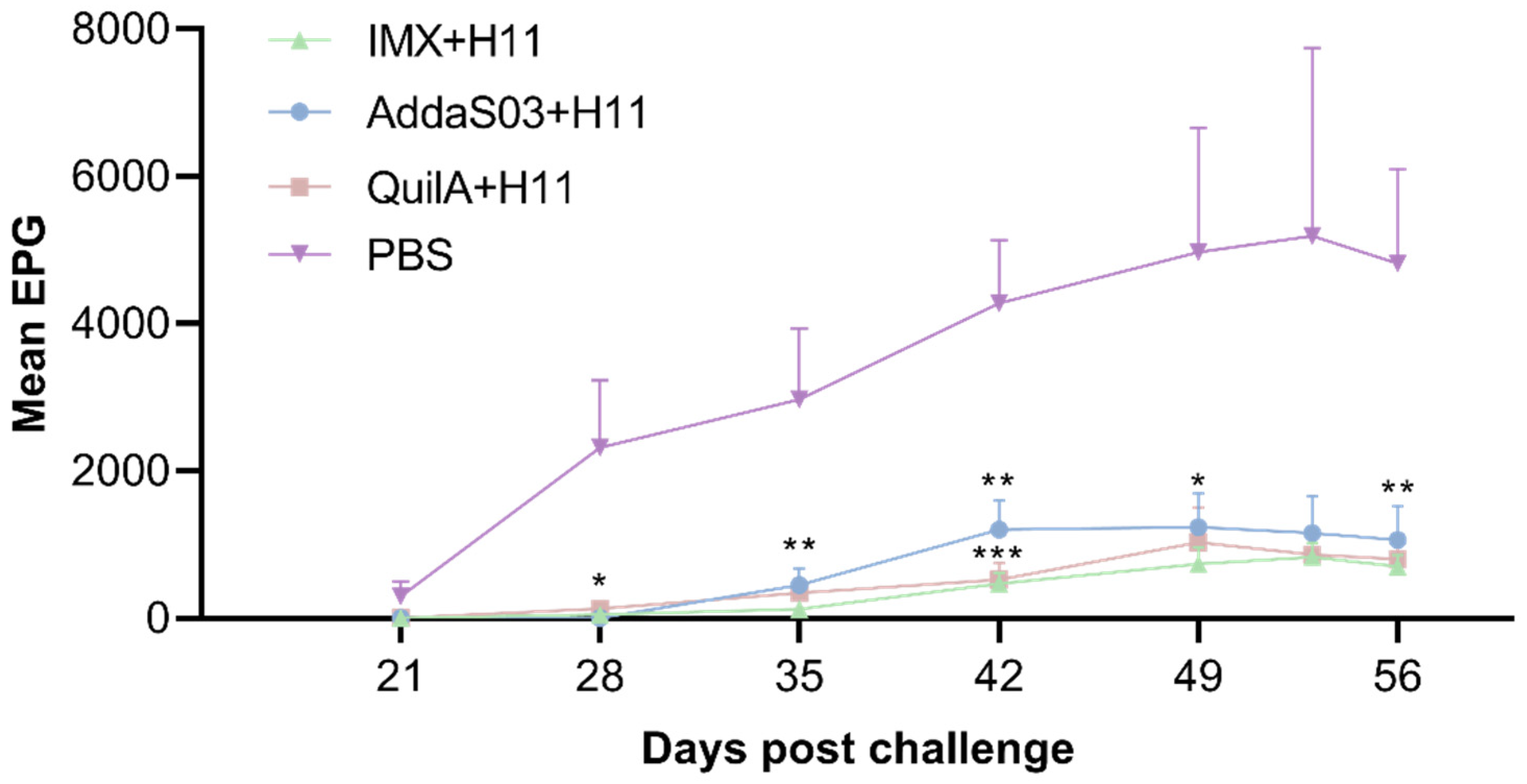
3.1. Fecal Egg Count (FEC)
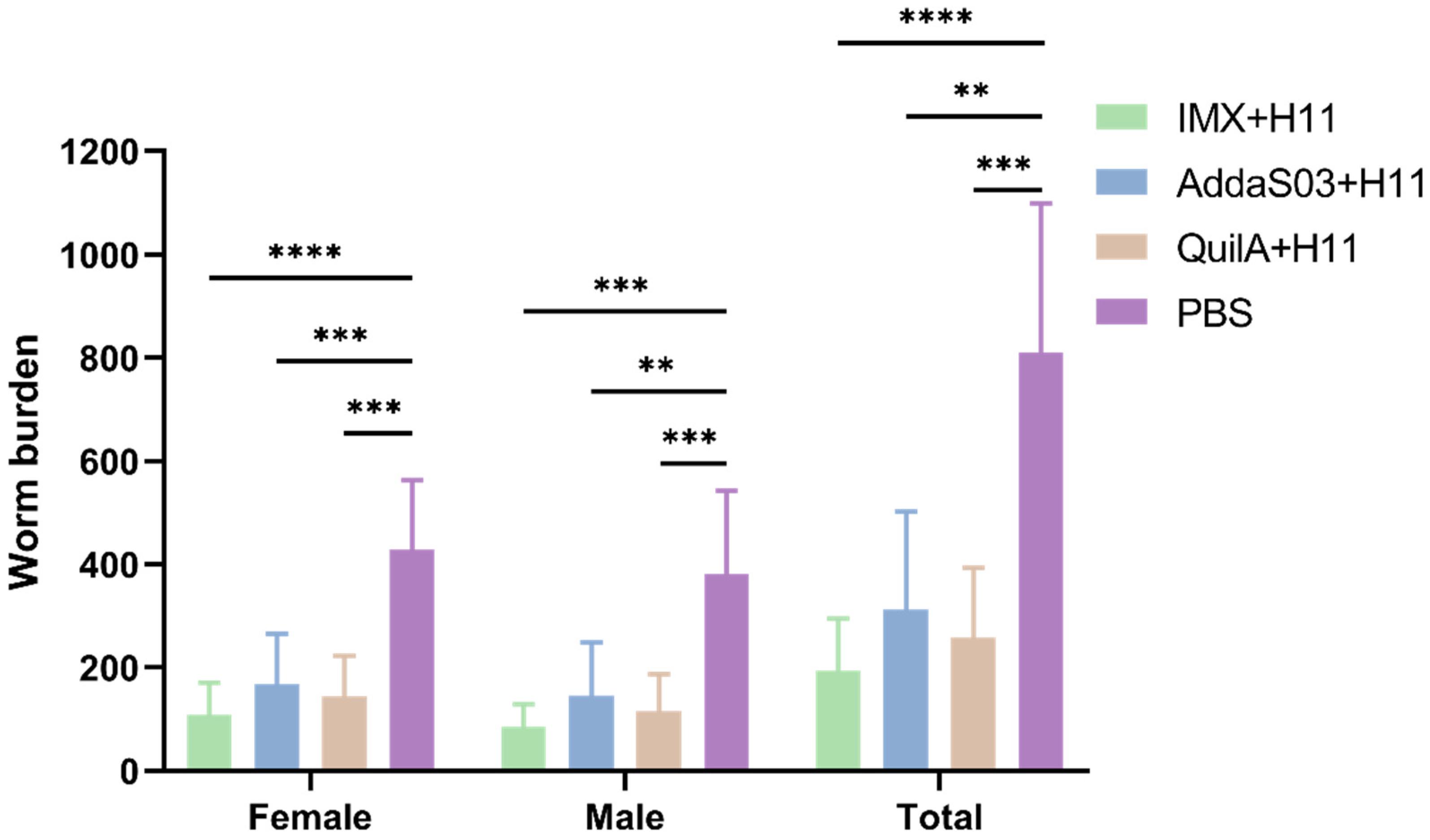
3.2. Worm Burdens
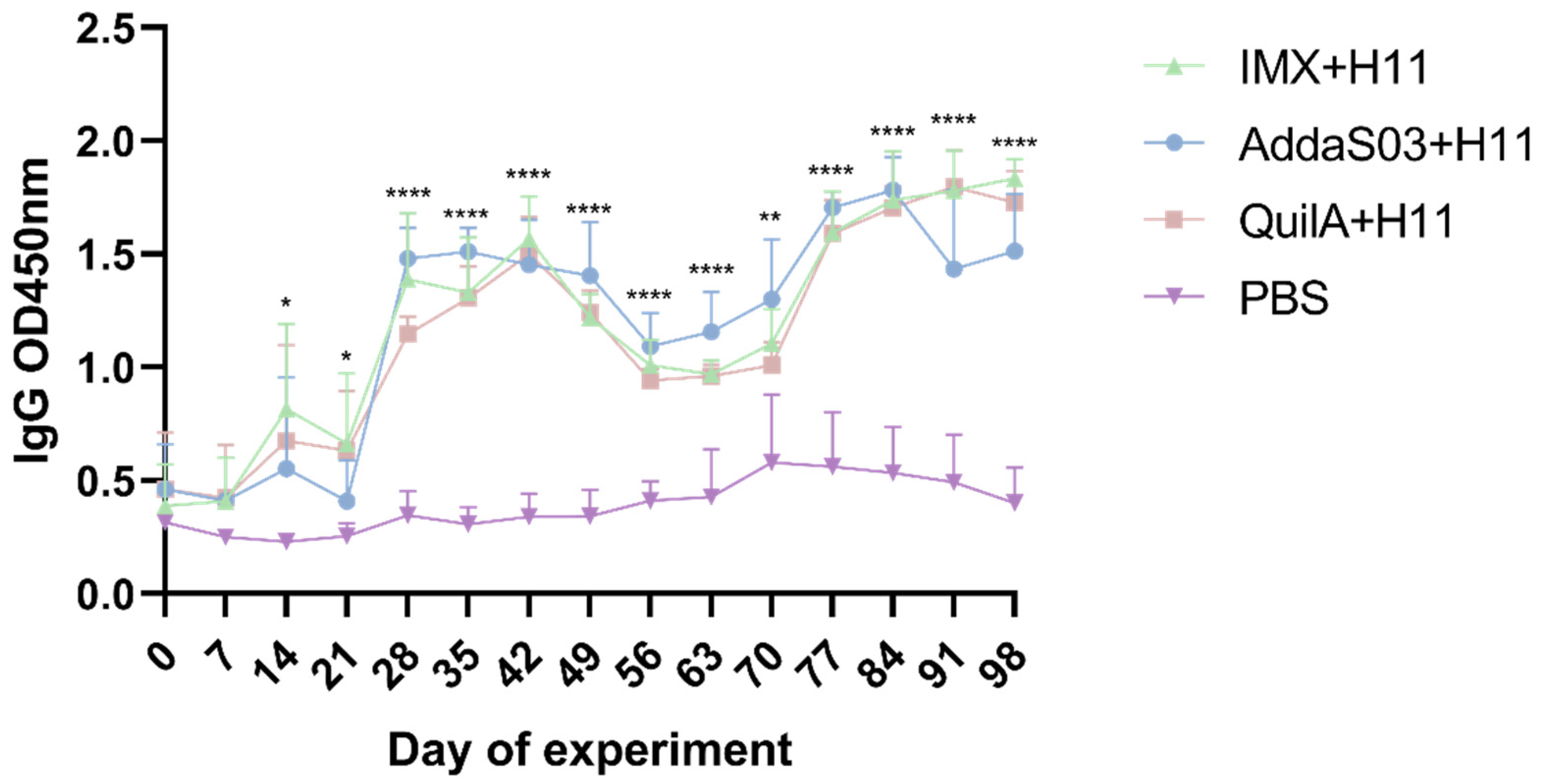
3.3. Serum IgG Antibody Level
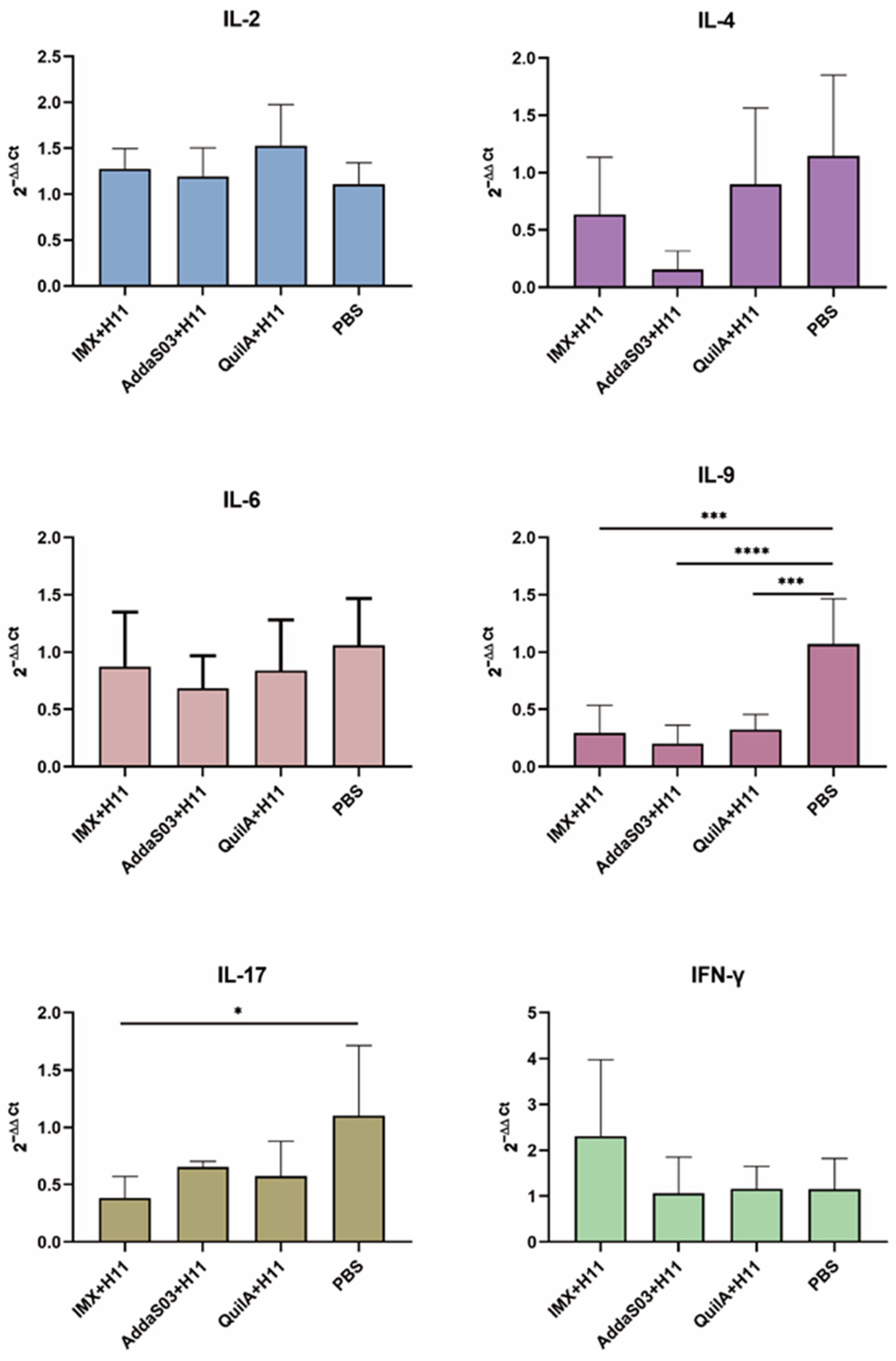
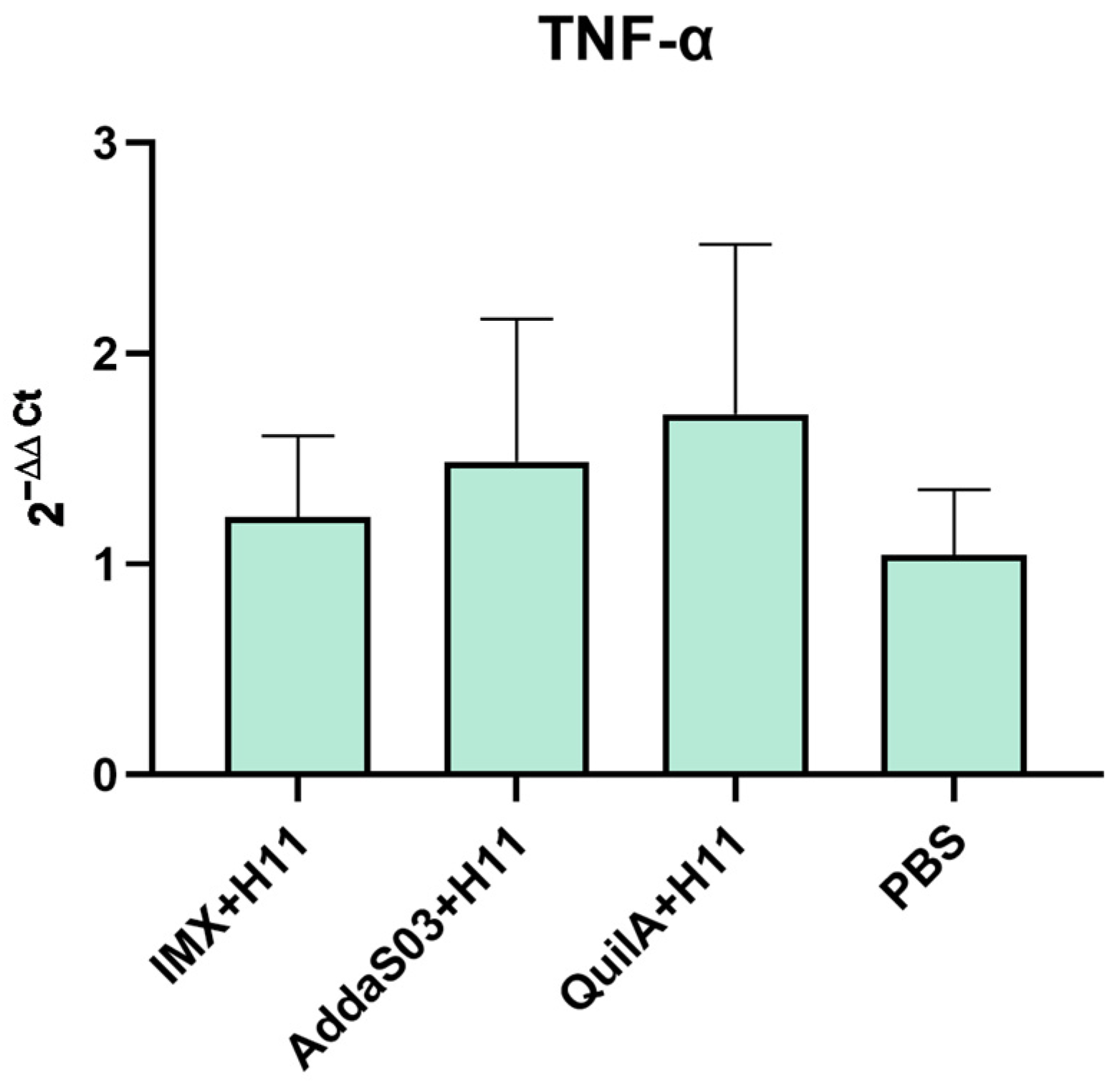
3.4. Transcription Level Changes of Cytokine-Encoding Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roeber, F.; Jex, A.R.; Gasser, R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance—An Australian perspective. Parasit. Vectors 2013, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef]
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv. Parasitol. 2016, 93, 95–143. [Google Scholar]
- Coop, R.L.; Holmes, P.H. Nutrition and parasite interaction. Int. J. Parasitol. 1996, 26, 951–962. [Google Scholar] [CrossRef]
- Bhagat, V.; Bhong, C.; Khillare, B.; Jadhav, N.; Narawade, M.; Khandekar, G.; Gaikwad, S.; Katkade, B.; Sharma, A.K.; Chigure, G. Prevalence status and detection of benzimidazole resistance using AS-PCR in Haemonchus contortus of goats from Marathwada region, Maharashtra, India. Vet. Parasitol. Reg. Stud. Rep. 2024, 55, 101119. [Google Scholar] [CrossRef]
- Cazajous, T.; Prevot, F.; Kerbiriou, A.; Milhes, M.; Grisez, C.; Tropee, A.; Godart, C.; Aragon, A.; Jacquiet, P. Multiple-resistance to ivermectin and benzimidazole of a Haemonchus contortus population in a sheep flock from mainland France, first report. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Munguía, B.; Teixeira, R.; Veroli, M.V.; Marín, M.; Domínguez, L. Molecular analysis of benzimidazole-resistance associated SNPs in Haemonchus contortus populations of Uruguay. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Aboelhadid, S.M.; Arafa, W.M.; El-Ashram, S.; Noaman, A.F.; Shokier, K.A.; Darwish, A.B.; Mahmoud, M.M.; Gadelhaq, S.M. Haemonchus contortus susceptibility and resistance to anthelmintics in naturally infected egyptian sheep. Acta Parasitol. 2021, 66, 329–335. [Google Scholar] [CrossRef]
- Broomfield, M.A.; Doyle, E.K.; Kahn, L.P.; Smith, W.D.; Walkden-Brown, S.W. A simplified Barbervax® vaccination regimen in lambs to evoke immunological protection to Haemonchus contortus. Vet. Parasitol. 2020, 287, 109243. [Google Scholar] [CrossRef]
- Kebeta, M.M.; Hine, B.C.; Walkden-Brown, S.W.; Kahn, L.P.; Doyle, E.K. Protective efficacy of Barbervax® in Merino weaner sheep trickle infected with five doses of Haemonchus contortus infective larvae. Vet. Parasitol. 2021, 292, 109386. [Google Scholar] [CrossRef]
- Teixeira, M.; Matos, A.; Albuquerque, F.; Bassetto, C.C.; Smith, W.D.; Monteiro, J.P. Strategic vaccination of hair sheep against Haemonchus contortus. Parasitol. Res. 2019, 118, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Bassetto, C.C.; Almeida, F.A.; Newlands, G.F.J.; Smith, W.D.; Amarante, A.F.T. Repeated vaccination against Haemonchus contortus provides continuous protection to young grazing sheep. Vet. Parasitol. 2020, 287, 109273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Suresh, M. Vaccine adjuvants to engage the cross-presentation pathway. Front. Immunol. 2022, 13, 940047. [Google Scholar] [CrossRef]
- Turley, J.L.; Lavelle, E.C. Resolving adjuvant mode of action to enhance vaccine efficacy. Curr. Opin. Immunol. 2022, 77, 102229. [Google Scholar] [CrossRef]
- Verma, S.K.; Mahajan, P.; Singh, N.K.; Gupta, A.; Aggarwal, R.; Rappuoli, R.; Johri, A.K. New-age vaccine adjuvants, their development, and future perspective. Front. Immunol. 2023, 14, 1043109. [Google Scholar] [CrossRef]
- Ben-Akiva, E.; Chapman, A.; Mao, T.; Irvine, D.J. Linking vaccine adjuvant mechanisms of action to function. Sci. Immunol. 2025, 10, eado5937. [Google Scholar] [CrossRef]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and vaccine development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Baljon, J.J.; Kwiatkowski, A.J.; Pagendarm, H.M.; Stone, P.T.; Kumar, A.; Bharti, V.; Schulman, J.A.; Becker, K.W.; Roth, E.W.; Christov, P.P.; et al. A cancer nanovaccine for co-delivery of peptide neoantigens and optimized combinations of STING and TLR4 agonists. ACS Nano 2024, 18, 6845–6862. [Google Scholar] [CrossRef]
- Lewis, J.S.; Zaveri, T.D.; Crooks, C.P., 2nd; Keselowsky, B.G. Microparticle surface modifications targeting dendritic cells for non-activating applications. Biomaterials 2012, 33, 7221–7232. [Google Scholar] [CrossRef]
- Ye, L.; Wang, T.; Wu, S.; Liu, H.; Liu, F.; Wang, C.; Hu, M. Effects of immune response on different nano-adjuvants combined with H11 antigen of Haemonchus contortus. Int. Immunopharmacol. 2024, 143, 113602. [Google Scholar] [CrossRef]
- Li, F.; Lok, J.B.; Gasser, R.B.; Korhonen, P.K.; Sandeman, M.R.; Shi, D.; Zhou, R.; Li, X.; Zhou, Y.; Zhao, J.; et al. Hc-daf-2 encodes an insulin-like receptor kinase in the barber’s pole worm, Haemonchus contortus, and restores partial dauer regulation. Int. J. Parasitol. 2014, 44, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Dicker, A.J.; Inglis, N.F.; Manson, E.D.; Subhadra, S.; Illangopathy, M.; Muthusamy, R.; Knox, D.P. Proteomic analysis of Mecistocirrus digitatus and Haemonchus contortus intestinal protein extracts and subsequent efficacy testing in a vaccine trial. PLoS Negl. Trop. Dis. 2014, 8, e2909. [Google Scholar] [CrossRef]
- Munn, E.A.; Smith, T.S.; Graham, M.; Tavernor, A.S.; Greenwood, C.A. The potential value of integral membrane proteins in the vaccination of lambs against Haemonchus contortus. Int. J. Parasitol. 1993, 23, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Wei, Y.; Feng, Z.; Gan, Y.; Liu, Z.; Liu, M.; Bai, F.; Shao, G. Protective efficacy of a live attenuated Mycoplasma hyopneumoniae vaccine with an ISCOM-matrix adjuvant in pigs. Vet. J. 2014, 199, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Pearse, M.J.; Drane, D. ISCOMATRIX adjuvant for antigen delivery. Adv. Drug Deliv. Rev. 2005, 57, 465–474. [Google Scholar] [CrossRef]
- Mines, J.J. Modifications of the McMaster worm egg counting method. Aust. Vet. J. 1977, 53, 342–343. [Google Scholar] [CrossRef]
- Kalyanasundaram, A.; Jawahar, S.; Ilangopathy, M.; Palavesam, A.; Raman, M. Comparative immunoprophylactic efficacy of Haemonchus contortus recombinant enolase (rHcENO) and Con A purified native glycoproteins in sheep. Exp. Parasitol. 2015, 154, 98–107. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, C.; Wang, S.; Song, X.; Xu, L.; Yan, R.; Hasson, I.A.; Li, X. Transcriptional and proteomic analysis reveal recombinant galectins of Haemonchus contortus down-regulated functions of goat PBMC and modulation of several signaling cascades in vitro. J. Proteom. 2014, 98, 123–137. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, L.; Hasan, M.W.; Lu, M.; Wang, W.; Yan, R.; Xu, L.; Song, X.; Li, X. Hepatocellular carcinoma-associated antigen 59 of Haemonchus contortus modulates the functions of PBMCs and the differentiation and maturation of monocyte-derived dendritic cells of goats in vitro. Parasit. Vectors 2019, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Lu, M.; Aleem, M.T.; Zhang, Y.; Wang, M.; Wen, Z.; Song, X.; Xu, L.; Li, X.; Yan, R. Identification of excretory and secretory proteins from Haemonchus contortus inducing a Th9 immune response in goats. Vet. Res. 2022, 53, 36. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, Y.; Wu, S.; Wang, Z.; Liu, F.; Wang, C.; Hu, M. Immunoprotection Efficacy of Con A-Purified Proteins against Haemonchus contortus in Goats. Vaccines 2022, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Blink, E.J.; Light, A.; Kallies, A.; Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J. Exp. Med. 2005, 201, 545–554. [Google Scholar] [CrossRef]
- Urdy, S.; Hanke, M.; Toledo, A.I.; Ratto, N.; Jacob, E.; Peyronnet, E.; Gourlet, J.B.; Chaves, S.S.; Thommes, E.; Coudeville, L.; et al. Multi-strain modeling of influenza vaccine effectiveness in older adults and its dependence on antigenic distance. Sci. Rep. 2024, 14, 27190. [Google Scholar] [CrossRef]
- Adduci, I.; Sajovitz, F.; Hinney, B.; Lichtmannsperger, K.; Joachim, A.; Wittek, T.; Yan, S. Haemonchosis in Sheep and Goats, Control Strategies and Development of Vaccines against Haemonchus contortus. Animals 2022, 12, 2339. [Google Scholar] [CrossRef]
- Carman, M.K.; Lakritz, J.; Cheng, T.Y.; Niehaus, A.J.; Lozier, J.W.; Marsh, A.E. Efficacy of a Haemonchus contortus vaccine under field conditions in young alpacas. Vet. Parasitol. 2024, 331, 110242. [Google Scholar] [CrossRef]
- Matos, A.F.I.M.d.; Nobre, C.O.R.; Monteiro, J.P.; Bevilaqua, C.M.L.; Smith, W.D.; Teixeira, M. Attempt to control Haemonchus contortus in dairy goats with Barbervax®, a vaccine derived from the nematode gut membrane glycoproteins. Small Rumin. Res. 2017, 151, 1–4. [Google Scholar] [CrossRef]
- Carson, A.; Reichel, R.; Bell, S.; Collins, R.; Smith, J.; Bartley, D. Haemonchus contortus: An overview. Vet. Rec. 2023, 192, 26–28. [Google Scholar] [CrossRef]
- Nisbet, A.J.; Meeusen, E.N.; González, J.F.; Piedrafita, D.M. Immunity to Haemonchus contortus and vaccine development. Adv. Parasitol. 2016, 93, 353–396. [Google Scholar]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: From basic science to medicine--40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Toscano, J.H.B.; Okino, C.H.; Dos Santos, I.B.; Giraldelo, L.A.; von Haehling, M.B.; Esteves, S.N.; de Souza Chagas, A.C. Innate immune responses associated with resistance against Haemonchus contortus in morada nova sheep. J. Immunol. Res. 2019, 2019, 3562672. [Google Scholar] [CrossRef] [PubMed]
- Lins, J.G.G.; Albuquerque, A.C.A.; Louvandini, H.; Amarante, A.F.T. Immunohistochemistry analyses of the abomasal mucosa show differences in cellular-mediated immune responses to Haemonchus contortus infection in resistant and susceptible young lambs. Dev. Comp. Immunol. 2024, 161, 105259. [Google Scholar] [CrossRef]
- Harty, J.T.; Badovinac, V.P. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 2008, 8, 107–119. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar]
- Raeber, M.E.; Zurbuchen, Y.; Impellizzieri, D.; Boyman, O. The role of cytokines in T-cell memory in health and disease. Immunol. Rev. 2018, 283, 176–193. [Google Scholar] [CrossRef]
- Williams, M.A.; Bevan, M.J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007, 25, 171–192. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Shim, Y.A.; Weliwitigoda, A.; Campbell, T.; Dosanjh, M.; Johnson, P. Splenic erythroid progenitors decrease TNF-α production by macrophages and reduce systemic inflammation in a mouse model of T cell-induced colitis. Eur. J. Immunol. 2021, 51, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Respatika, D.; Komori, S.; Washio, K.; Nishimura, T.; Kotani, T.; Murata, Y.; Okazawa, H.; Ohnishi, H.; Kaneko, Y.; et al. SIRPα+ dendritic cells regulate homeostasis of fibroblastic reticular cells via TNF receptor ligands in the adult spleen. Proc. Natl. Acad. Sci. USA 2017, 114, E10151–E10160. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liao, Y.; Willis, S.N.; Taubenheim, N.; Inouye, M.; Tarlinton, D.M.; Smyth, G.K.; Hodgkin, P.D.; Nutt, S.L.; Corcoran, L.M. Transcriptional profiling of mouse B cell terminal differentiation defines a signature for antibody-secreting plasma cells. Nat. Immunol. 2015, 16, 663–673. [Google Scholar] [CrossRef]
- Helms, T.; Boehm, B.O.; Asaad, R.J.; Trezza, R.P.; Lehmann, P.V.; Tary-Lehmann, M. Direct visualization of cytokine-producing recall antigen-specific CD4 memory T cells in healthy individuals and HIV patients. J. Immunol. 2000, 164, 3723–3732. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, J.; Liu, F.; Ye, L.; Liu, X.; Wang, C.; Hu, M. Identification and validation of protective glycoproteins in Haemonchus contortus H11. Front. Immunol. 2025, 16, 1521022. [Google Scholar] [CrossRef]
| Name of Cytokine (Reference No.) | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| GAPDH [30] | CCTGGAGAAACCTGCCAAGT | GCCAAATTCATTGTCGTACCA |
| IL-2 [31] | CAAACGGTGCACCTACTTCA | AGCTTGAGGTTCTCGGGATT |
| IL-4 [31] | GTACCAGCCACTTCGTCCAT | GCTGCTGAGATTCCTGTCAA |
| IL-6 [30] | CGTCGACAAAATCTCTGCAA | TTCCCTCAAACTCGTTCTGG |
| IL-9 [32] | GATGCGGCTGATTGTTT | CTCGTGCTCACTGTGGAGT |
| IL-17 [31] | TTGTAAAGGCAGGGGTCATC | GGTGGAGCGCTTGTGATAAT |
| TNF-α [30] | CAGGGCTCCAGAAGTTGCT | GGGCTACCGGCTTGTTATTT |
| IFN-γ [30] | TAAGGGTGGGCCTCTTTTCT | CATCCACCGGAATTTGAATC |
| Groups | Mean FEC | SD | Reduction (%) a |
|---|---|---|---|
| IMX + H11 | 2911.3 | 1551.6 | 88.3 ** |
| AddaS03 + H11 | 5119.8 | 4264.8 | 79.4 * |
| QuilA + H11 | 3688.3 | 3330.0 | 85.2 ** |
| Control | 24,846.0 | 19,946.8 | - |
| Groups | Total | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Reduction (%) a | Mean | SD | Reduction (%) a | Mean | SD | Reduction (%) a | |
| IMX + H11 | 195.7 | 101.7 | 75.8 **** | 109.5 | 60.9 | 74.5 **** | 86.2 | 43.3 | 77.4 *** |
| AddaS03 + H11 | 313.2 | 189.7 | 61.3 ** | 167.6 | 98.6 | 60.9 *** | 145.6 | 103.4 | 61.8 ** |
| QuilA + H11 | 259 | 135 | 68.0 *** | 143.7 | 79 | 66.5 *** | 115.3 | 71.6 | 69.8 *** |
| Control | 810.3 | 288.1 | - | 428.8 | 134.2 | - | 381.5 | 161 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Wu, S.; Liu, F.; Zhang, J.; Wan, J.; Wang, C.; Liu, H.; Hu, M. Enhancing H11 Protein-Induced Immune Protection Against Haemonchus contortus in Goats: A Nano-Adjuvant Formulation Strategy. Biology 2025, 14, 563. https://doi.org/10.3390/biology14050563
Ye L, Wu S, Liu F, Zhang J, Wan J, Wang C, Liu H, Hu M. Enhancing H11 Protein-Induced Immune Protection Against Haemonchus contortus in Goats: A Nano-Adjuvant Formulation Strategy. Biology. 2025; 14(5):563. https://doi.org/10.3390/biology14050563
Chicago/Turabian StyleYe, Lisha, Simin Wu, Fuqiang Liu, Juan Zhang, Jie Wan, Chunqun Wang, Hui Liu, and Min Hu. 2025. "Enhancing H11 Protein-Induced Immune Protection Against Haemonchus contortus in Goats: A Nano-Adjuvant Formulation Strategy" Biology 14, no. 5: 563. https://doi.org/10.3390/biology14050563
APA StyleYe, L., Wu, S., Liu, F., Zhang, J., Wan, J., Wang, C., Liu, H., & Hu, M. (2025). Enhancing H11 Protein-Induced Immune Protection Against Haemonchus contortus in Goats: A Nano-Adjuvant Formulation Strategy. Biology, 14(5), 563. https://doi.org/10.3390/biology14050563





