Comparative Evaluation of Compression Testing Methods for Murine Lumbar Vertebral Bodies: Identifying Most Reliable and Reproducible Techniques for Assessing Compressive Strength
Abstract
1. Introduction
2. Materials and Methods
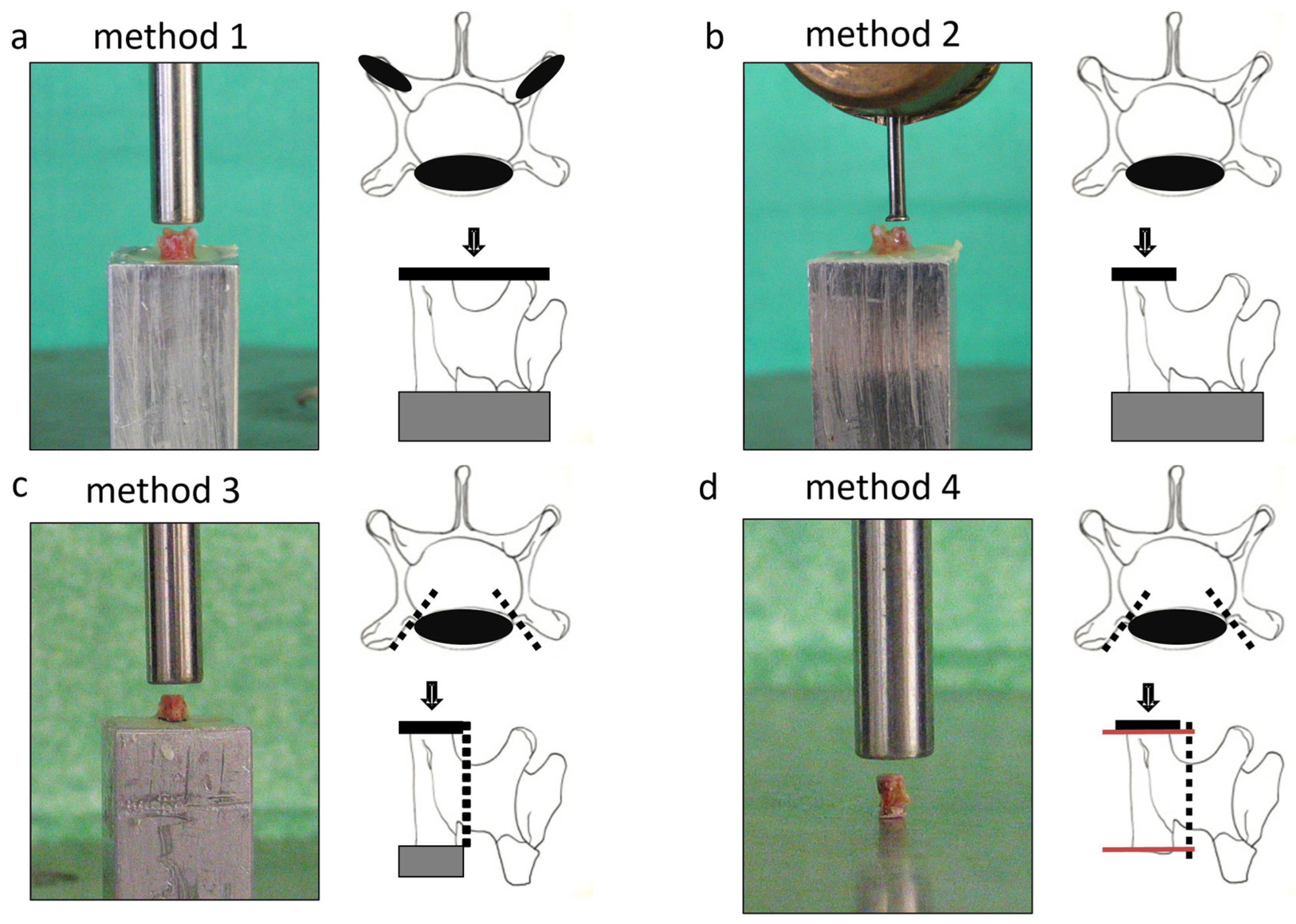
2.1. Specimen Preparation
- Method 1: The whole vertebral body was placed upright and fixated without bone manipulation using bone cement (Palacos R, Heraeus Kulzer GmbH, Germany) to provide a planar surface and unidirectional force application. The dorsal process was pressed into the cement until the caudal surface was horizontal.
- Method 2: The placement and fixation were the same as Method 1, but a smaller diameter compression tool was used, excluding the transversal process.
- Method 3: The transversal and dorsal processes were resected, and the remaining vertebral body was fixated into the cement.
- Method 4: Similar to Method 3, but the cranial and caudal surfaces were ground to create parallel surfaces, eliminating the need for resin embedding.
2.2. Determination of Dimensional Parameters of the Vertebral Bodies
2.3. Compression Testing
2.4. Statistical Analysis
2.5. Data Availability
3. Results
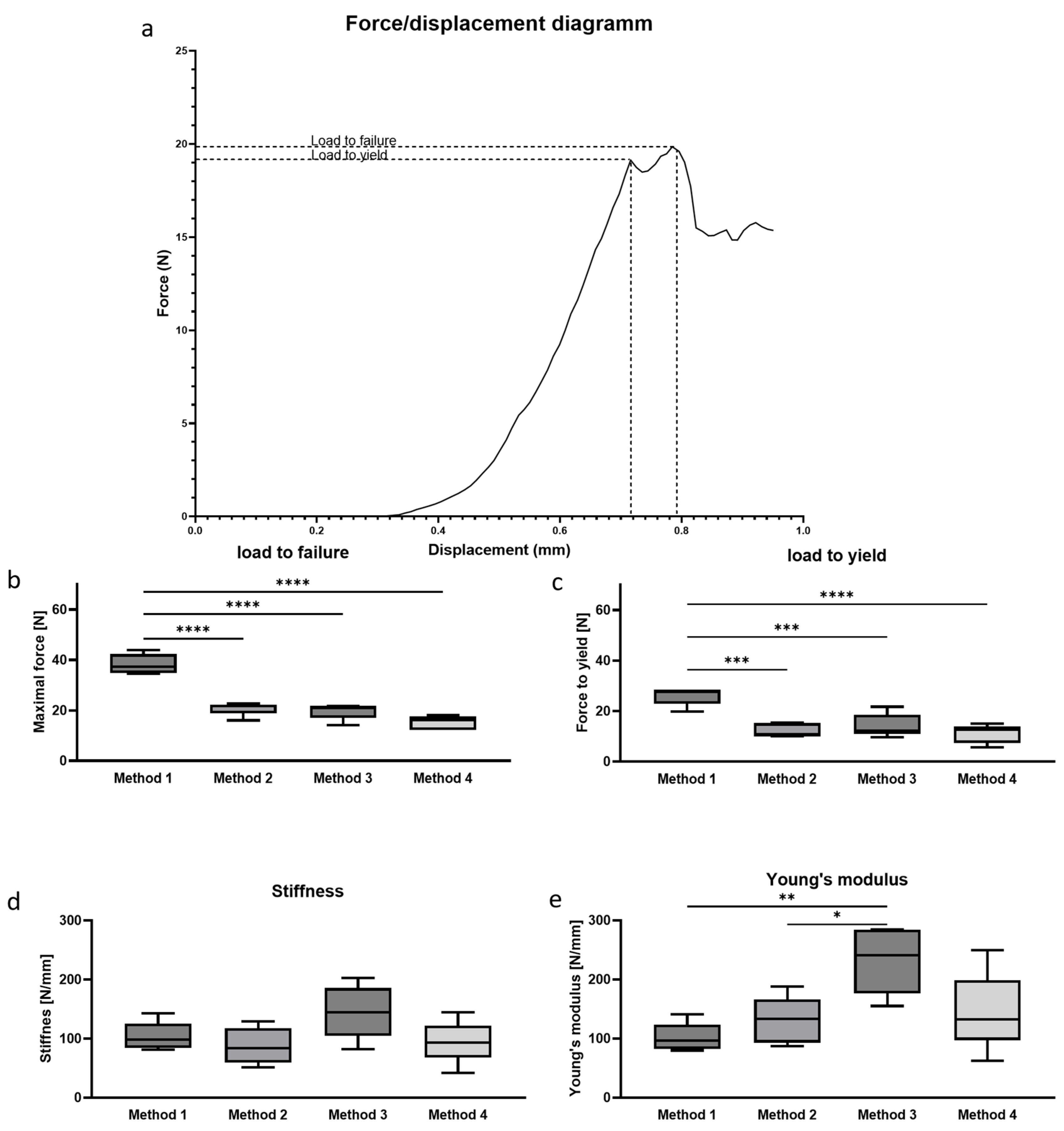
3.1. Determination of Absolute Biomechanical Properties
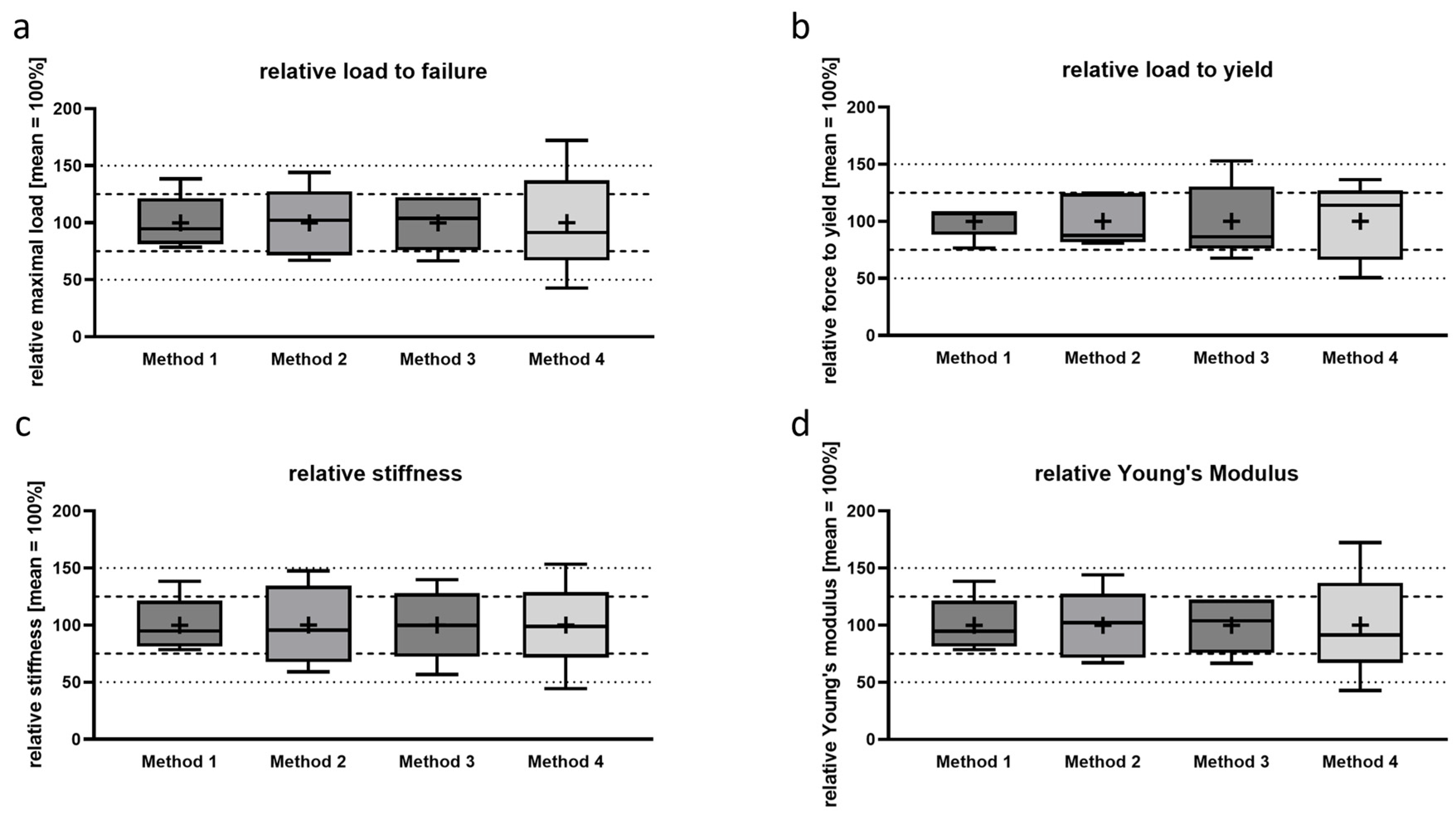
3.2. Determination of Relative Biomechanical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhter, M.P.; Cullen, D.M.; Gong, G.; Recker, R.R. Bone Biomechanical Properties in Prostaglandin Ep1 and Ep2 Knockout Mice. Bone 2001, 29, 121–125. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Chase, J.; Havey, R.M.; Voronov, L.; Sartori, M.; McEwen, H.A.; Beamer, W.G.; Patwardhan, A.G. Effects of Growth Hormone Transgene Expression on Vertebrae in a Mouse Model of Osteogenesis Imperfecta. Spine 2005, 30, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Brodt, M.D.; Uthgenannt, B.A. Morphological and Mechanical Properties of Caudal Vertebrae in the Samp6 Mouse Model of Senile Osteoporosis. Bone 2004, 35, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Hsieh, Y.F.; Muller, R.; Bouxsein, M.L.; Baylink, D.J.; Rosen, C.J.; Grynpas, M.D.; Donahue, L.R.; Beamer, W.G. Genetic Regulation of Cortical and Trabecular Bone Strength and Microstructure in Inbred Strains of Mice. J. Bone Miner. Res. 2000, 15, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Binder, N.B.; Niederreiter, B.; Hoffmann, O.; Stange, R.; Pap, T.; Stulnig, T.M.; Mack, M.; Erben, R.G.; Smolen, J.S.; Redlich, K. Estrogen-Dependent and C-C Chemokine Receptor-2-Dependent Pathways Determine Osteoclast Behavior in Osteoporosis. Nat. Med. 2009, 15, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Stange, R.; Kronenberg, D.; Timmen, M.; Everding, J.; Hidding, H.; Eckes, B.; Hansen, U.; Holtkamp, M.; Karst, U.; Pap, T.; et al. Age-Related Bone Deterioration is Diminished by Disrupted Collagen Sensing in Integrin Alpha2beta1 Deficient Mice. Bone 2013, 56, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mosekilde, L. Normal Vertebral Body Size and Compressive Strength: Relations to Age and to Vertebral and Iliac Trabecular Bone Compessive Strength. Bone 1986, 7, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Goetzen, N.; Kummer, T.; Schilling, A.; Amling, M.; Morlock, M.M. The Mouse as a Model Organism for Human Skeletal Diseases—A Biomechanical Study. In Proceedings of the XXth Congress of the International Society of Biomechanics, Cleveland, OH, USA, 4 August 2005; p. 784. [Google Scholar]
- Akhter, M.P.; Otero, J.K.; Iwaniec, U.T.; Cullen, D.M.; Haynatzki, G.R.; Recker, R.R. Differences in Vertebral Structure and Strength of Inbred Female Mouse Strains. J. Musculoskelet. Neuronal Interact. 2004, 4, 33–40. [Google Scholar] [PubMed]
- Bell, G.H.; Dunbar, O.; Beck, J.S.; Gibb, A. Variations in Strength of Vertebrae with Age and Their Relation to Osteoporosis. Calcif. Tissue Res. 1967, 1, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.J. The Anatomy of the Laboratory Mouse; Academic Press: London, UK, 1956. [Google Scholar]
- Tommasini, S.M.; Morgan, T.G.; van der Meulen, M.; Jepsen, K.J. Genetic Variation in Structure-Function Relationships for the Inbred Mouse Lumbar Vertebral Body. J. Bone Miner Res. 2005, 20, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.; Schumacher, U.; Schünke, M.; Voll, M.; Wesker, K.H. Prometheus Lernatlas Der Anatomie; Thieme: New York, NY, USA, 2005; Volume 1. [Google Scholar]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal Involution by Age-Associated Oxidative Stress and Its Acceleration by Loss of Sex Steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef] [PubMed]
- Steinke, B.; Patwardhan, A.G.; Havey, R.M.; King, D. Human Growth Hormone Transgene Expression Increases the Biomechanical Structural Properties of Mouse Vertebrae. Spine 1999, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Beaupied, H.; Lespessailles, E.; Benhamou, C.L. Evaluation of Macrostructural Bone Biomechanics. Joint Bone Spine 2007, 74, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Isaksson, P.; Ferguson, S.J.; Persson, C. Young’s Modulus of Trabecular Bone at the Tissue Level: A Review. Acta Biomater 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Milstrey, A.; Wieskoetter, B.; Hinze, D.; Grueneweller, N.; Stange, R.; Pap, T.; Raschke, M.; Garcia, P. Dose-Dependent Effect of Parathyroid Hormone on Fracture Healing and Bone Formation in Mice. J. Surg. Res. 2017, 220, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q. Resveratrol Enhances the Protective Effects of Calcium Supplements on Spinal Cord Injury-Induced Osteoporosis by Targeting the Sirt1/Foxo3a Pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Komrakova, M.; Lang, J.G.; Roch, P.J.; Hoffmann, D.B.; Böker, K.O.; Lehmann, W.; Schilling, A.F.; Sehmisch, S. Hyponatremia Does Not Cause Further Bone Loss in Estrogen-Deficient Rats. Osteologie 2024, 33, 118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kronenberg, D.; Wieskoetter, B.; Soeger, S.; Hidding, H.; Timmen, M.; Raschke, M.J.; Stange, R. Comparative Evaluation of Compression Testing Methods for Murine Lumbar Vertebral Bodies: Identifying Most Reliable and Reproducible Techniques for Assessing Compressive Strength. Bioengineering 2025, 12, 273. https://doi.org/10.3390/bioengineering12030273
Kronenberg D, Wieskoetter B, Soeger S, Hidding H, Timmen M, Raschke MJ, Stange R. Comparative Evaluation of Compression Testing Methods for Murine Lumbar Vertebral Bodies: Identifying Most Reliable and Reproducible Techniques for Assessing Compressive Strength. Bioengineering. 2025; 12(3):273. https://doi.org/10.3390/bioengineering12030273
Chicago/Turabian StyleKronenberg, Daniel, Britta Wieskoetter, Sarah Soeger, Heriburg Hidding, Melanie Timmen, Michael J. Raschke, and Richard Stange. 2025. "Comparative Evaluation of Compression Testing Methods for Murine Lumbar Vertebral Bodies: Identifying Most Reliable and Reproducible Techniques for Assessing Compressive Strength" Bioengineering 12, no. 3: 273. https://doi.org/10.3390/bioengineering12030273
APA StyleKronenberg, D., Wieskoetter, B., Soeger, S., Hidding, H., Timmen, M., Raschke, M. J., & Stange, R. (2025). Comparative Evaluation of Compression Testing Methods for Murine Lumbar Vertebral Bodies: Identifying Most Reliable and Reproducible Techniques for Assessing Compressive Strength. Bioengineering, 12(3), 273. https://doi.org/10.3390/bioengineering12030273








