Non-Typhoidal Salmonella Infection in Children: Influence of Antibiotic Therapy on Postconvalescent Excretion and Clinical Course—A Systematic Review
Abstract
:1. Introduction
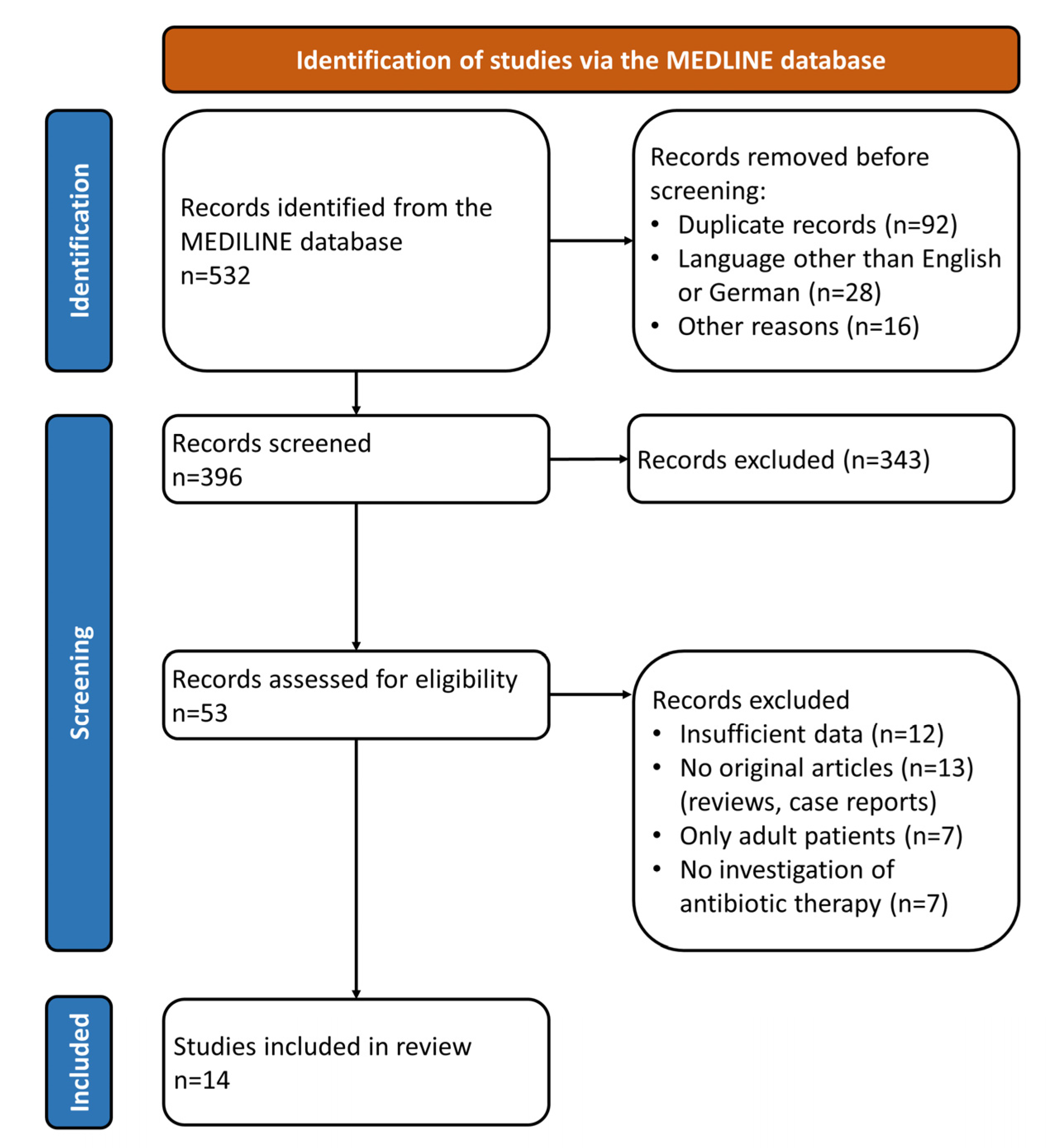
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stanaway, J.D. The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef] [Green Version]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [Green Version]
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for Foodborne Disease Outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef]
- Butler, A.J.; Thomas, M.K.; Pintar, K.D.M. Expert Elicitation as a Means to Attribute 28 Enteric Pathogens to Foodborne, Waterborne, Animal Contact, and Person-to-Person Transmission Routes in Canada. Foodborne Pathog. Dis. 2015, 12, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Christidis, T.; Hurst, M.; Rudnick, W.; Pintar, K.D.M.; Pollari, F. A comparative exposure assessment of foodborne, animal contact and waterborne transmission routes of Salmonella in Canada. Food Control 2020, 109, 106899. [Google Scholar] [CrossRef]
- Feasey, N.A.; Archer, B.N.; Heyderman, R.S.; Sooka, A.; Dennis, B.; Gordon, M.A.; Keddy, K.H. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg. Infect. Dis. 2010, 16, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S.; Owusu-Dabo, E. Research on Invasive Nontyphoidal Salmonella Disease and Developments Towards Better Understanding of Epidemiology, Management, and Control Strategies. Clin. Infect. Dis. 2020, 71, S127–S129. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Sjolund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [Green Version]
- Takkinsatian, P.; Silpskulsuk, C.; Prommalikit, O. Clinical features and antibiotic susceptibility of Salmonella gastroenteritis in children: A ten-year review. Med. J. Malays. 2020, 75, 672–676. [Google Scholar]
- Lounis, Y.; Hugo, J.; Demarche, M.; Seghaye, M.C. Influence of age on clinical presentation, diagnosis delay and outcome in pre-school children with acute appendicitis. BMC Pediatr. 2020, 20, 151. [Google Scholar] [CrossRef] [Green Version]
- Park, S.E.; Pak, G.D.; Aaby, P.; Adu-Sarkodie, Y.; Ali, M.; Aseffa, A.; Biggs, H.M.; Bjerregaard-Andersen, M.; Breiman, R.F.; Crump, J.A.; et al. The Relationship Between Invasive Nontyphoidal Salmonella Disease, Other Bacterial Bloodstream Infections, and Malaria in Sub-Saharan Africa. Clin. Infect. Dis. 2016, 62 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.H.; Huang, Y.C.; Lin, T.Y.; Huang, Y.L.; Kuo, C.C.; Chiu, C.H. Reappraisal of parenteral antimicrobial therapy for nontyphoidal Salmonella enteric infection in children. Clin. Microbiol. Infect. 2011, 17, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.I.; Bartlett, J.A.; Corey, G.R. Extra-intestinal manifestations of salmonella infections. Medicine 1987, 66, 349–388. [Google Scholar] [CrossRef]
- Wang, J.H.; Liu, Y.C.; Yen, M.Y.; Wang, J.H.; Chen, Y.S.; Wann, S.R.; Cheng, D.L. Mycotic aneurysm due to non-typhi salmonella: Report of 16 cases. Clin. Infect. Dis. 1996, 23, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, E.; Bachur, R.; Harper, M. Non-typhi Salmonella bacteremia in children. Pediatr Infect. Dis. J. 1999, 18, 1073–1077. [Google Scholar] [CrossRef]
- Mohan, A.; Munusamy, C.; Tan, Y.C.; Muthuvelu, S.; Hashim, R.; Chien, S.L.; Wong, M.K.; Khairuddin, N.A.; Podin, Y.; Lau, P.S.; et al. Invasive Salmonella infections among children in Bintulu, Sarawak, Malaysian Borneo: A 6-year retrospective review. BMC Infect. Dis. 2019, 19, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uche, I.V.; MacLennan, C.A.; Saul, A. A Systematic Review of the Incidence, Risk Factors and Case Fatality Rates of Invasive Nontyphoidal Salmonella (iNTS) Disease in Africa (1966 to 2014). PLoS Negl. Trop. Dis. 2017, 11, e0005118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehrnbecher, T.; Laws, H.J. Infectious complications in pediatric cancer patients. Klin. Padiatr. 2005, 217 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, I.F.; Kao, C.H.; Lee, W.Y.; Chang, M.F.; Chen, Y.S.; Wu, K.S.; Hu, H.H.; Hsieh, K.S.; Chiou, C.C. Clinical manifestations of nontyphoid salmonellosis in children younger than 2 years old—Experiences of a tertiary hospital in southern Taiwan. Pediatr. Neonatol. 2012, 53, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.M.; Huang, W.Y.; Lee, M.L.; Yang, A.D.; Chaou, K.P.; Hsieh, L.Y. Clinical features, acute complications, and outcome of Salmonella meningitis in children under one year of age in Taiwan. BMC Infect. Dis. 2011, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Puthucheary, S.D.; Parasakthi, N. Extra-intestinal non-typhoidal Salmonella infections in children. Ann. Trop. Paediatr. 2000, 20, 125–129. [Google Scholar] [CrossRef]
- Barbara, G.; Stanghellini, V.; Berti-Ceroni, C.; De Giorgio, R.; Salvioli, B.; Corradi, F.; Cremon, C.; Corinaldesi, R. Role of antibiotic therapy on long-term germ excretion in faeces and digestive symptoms after Salmonella infection. Aliment. Pharmacol. Ther. 2000, 14, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Gal-Mor, O. Persistent Infection and Long-Term Carriage of Typhoidal and Nontyphoidal Salmonellae. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzel, A.; Desai, P.T.; Goren, A.; Schorr, Y.I.; Nissan, I.; Porwollik, S.; Valinsky, L.; McClelland, M.; Rahav, G.; Gal-Mor, O. Persistent Infections by Nontyphoidal Salmonella in Humans: Epidemiology and Genetics. Clin. Infect. Dis. 2016, 62, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Buchwald, D.S.; Blaser, M.J. A review of human salmonellosis: II. Duration of excretion following infection with nontyphi Salmonella. Rev. Infect. Dis. 1984, 6, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Murray, C.J. Salmonella carriage rate amongst school children—A three year study. Southeast Asian J. Trop. Med. Public Health 1991, 22, 357–361. [Google Scholar] [PubMed]
- Balasubramanian, R.; Im, J.; Lee, J.S.; Jeon, H.J.; Mogeni, O.D.; Kim, J.H.; Rakotozandrindrainy, R.; Baker, S.; Marks, F. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Hum. Vaccines Immunother. 2019, 15, 1421–1426. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Pijnacker, R.; Duijster, J.; Heck, M.; Wit, B.; Veldman, K.; Franz, E. Changing epidemiology of invasive non-typhoid Salmonella infection: A nationwide population-based registry study. Clin. Microbiol. Infect. 2020, 26, 941.e9–941.e14. [Google Scholar] [CrossRef]
- Marchello, C.S.; Fiorino, F.; Pettini, E.; Crump, J.A.; Vacc-i, N.T.S.C.C. Incidence of non-typhoidal Salmonella invasive disease: A systematic review and meta-analysis. J. Infect. 2021, in press. [Google Scholar] [CrossRef]
- Macdonald, W.B.; Friday, F.; Mc, E.M. The effect of chloramphenicol in Salmonella enteritis of infancy. Arch. Dis. Child. 1954, 29, 238–241. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J.M. Effect of antibiotic treatment on duration of excretion of Salmonella typhimurium by children. Br. Med. J. 1965, 2, 1343–1345. [Google Scholar] [CrossRef] [Green Version]
- Onwuezobe, I.A.; Oshun, P.O.; Odigwe, C.C. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst. Rev. 2012, 11, CD001167. [Google Scholar] [CrossRef]
- Huang, I.F.; Wagener, M.M.; Hsieh, K.S.; Liu, Y.C.; Wu, T.C.; Lee, W.Y.; Chiou, C.C. Nontyphoid salmonellosis in taiwan children: Clinical manifestations, outcome and antibiotic resistance. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 518–523. [Google Scholar] [CrossRef]
- Duff, N.; Steele, A.D.; Garrett, D. Global Action for Local Impact: The 11th International Conference on Typhoid and Other Invasive Salmonelloses. Clin. Infect. Dis. 2020, 71, S59–S63. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Yamada, M.; Muto, T.; Matsushima, A.; Yamai, S. Fecal excretion of Salmonella enterica serovar typhimurium following a food-borne outbreak. J. Clin. Microbiol. 2000, 38, 3495–3497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenstein, B.J. Salmonellosis in infants and children. J. Pediatr. 1967, 70, 1–7. [Google Scholar] [CrossRef]
- Wen, S.C.; Best, E.; Nourse, C. Non-typhoidal Salmonella infections in children: Review of literature and recommendations for management. J. Paediatr. Child. Health 2017, 53, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 31 August 2021).
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.H.; Laing Brown, G.; Latham Brown, D.; Emond, R.T.D.; Galpine, J.F.; Jamieson, S.R.; Lamb, S.G.; Lambert, H.P.; McKendrick, G.D.W.; Medlock, J.M.; et al. Effect of neomycin in non-invasive salmonella infections of the gastrointestinal tract. Joint Project by Members of the Association for the Study of Infectious Disease. Lancet 1970, 2, 1159–1161. [Google Scholar]
- Kazemi, M.; Gumpert, T.G.; Marks, M.I. A controlled trial comparing sulfametboxazole-trimethoprim, ampicillin, and no therapy in the treatment of salmonella gastroenteritis in children. J. Pediatr. 1973, 83, 646–650. [Google Scholar] [CrossRef]
- Nelson, J.D.; Kusmiesz, H.; Jackson, L.H.; Woodman, E. Treatment of Salmonella gastroenteritis with ampicillin, amoxicillin, or placebo. Pediatrics 1980, 65, 1125–1130. [Google Scholar]
- Stögmann, W.; Blümel, P. Salmonellosen im Kindersalter—Ein aktuelles Problem (Salmonella enteritis in childhood—A topical problem). Wien. Klin. Wochenschr. 1982, 94, 86–89. [Google Scholar] [PubMed]
- Chiu, C.H.; Lin, T.Y.; Ou, J.T. A clinical trial comparing oral azithromycin, cefixime and no antibiotics in the treatment of acute uncomplicated Salmonella enteritis in children. J. Paediatr. Child. Health 1999, 35, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chiu, C.H.; Lin, P.Y.; Wang, M.H.; Su, L.H.; Lin, T.Y. Short-term ceftriaxone therapy for treatment of severe non-typhoidal Salmonella enterocolitis. Acta Paediatr. 2003, 92, 537–540. [Google Scholar] [CrossRef]
- Ho, P.Y.; Chen, W.L.; Cheng, M.F.; Shen, Y.T.; Hu, H.H.; Sheu, S.K.; Huang, I.F. Factors affecting fecal excretion time in pediatric nontyphoid Salmonella infection. Pediatr. Neonatol. 2021, 62, 387–393. [Google Scholar] [CrossRef]
- Chiu, C.H.; Lin, T.Y.; Ou, J.T. A pilot study of seven days of ceftriaxone therapy for children with Salmonella enterocolitis. Changgeng Yi Xue Za Zhi 1997, 20, 115–121. [Google Scholar]
- Kazemi, M.; Gumpert, G.; Marks, M.I. Clinical spectrum and carrier state of nontyphoidal salmonella infections in infants and children. Can. Med. Assoc. J. 1974, 110, 1253–1257. [Google Scholar]
- Ruiz, M.; Rodriguez, J.C.; Escribano, I.; Royo, G. Available options in the management of non-typhi Salmonella. Expert Opin. Pharmacother. 2004, 5, 1737–1743. [Google Scholar] [CrossRef]
- Robinson, J.L. Salmonella infections in Canadian children. Paediatr. Child. Health 2019, 24, 50–51. [Google Scholar] [CrossRef] [Green Version]
- Cohen, R.; Raymond, J.; Gendrel, D. Antimicrobial treatment of diarrhea/acute gastroenteritis in children. Arch. Pediatr. 2017, 24, S26–S29. [Google Scholar] [CrossRef]
- Büttcher, M.; Flieger, A.; Fruth, A.; Simon, S.; Huppertz, H.-I. Salmonellose. In Handbook of the German Society for Pediatric Infectious Diseases—DGPI, 7th ed.; Berner, R., Bialek, R., Forster, J., Eds.; Thieme: Stuttgart, Germany, 2018; pp. 719–723. [Google Scholar]
- AAP. Salmonella infections. In Red Book—Report of the Committee on Infectious Diseases, 32th ed.; Kimberlin, D.W., Barnett, E.D., Lynfield, R., Sawyer, M.H., Eds.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2021; pp. 655–662. [Google Scholar]
- Tack, B.; Vanaenrode, J.; Verbakel, J.Y.; Toelen, J.; Jacobs, J. Invasive non-typhoidal Salmonella infections in sub-Saharan Africa: A systematic review on antimicrobial resistance and treatment. BMC Med. 2020, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Aserkoff, B.; Bennett, J.V. Effect of antibiotic therapy in acute salmonellosis on the fecal excretion of salmonellae. N. Engl. J. Med. 1969, 281, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Pitkäjärvi, T.; Kujanne, E.; Sillantaka, I.; Lumio, J. Norfloxacin and Salmonella excretion in acute gastroenteritis--a 6-month follow-up study. Scand. J. Infect. Dis. 1996, 28, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Garcia-Restoy, E.; Garau, J.; Bella, F.; Freixas, N.; Simo, M.; Lite, J.; Sanchez, P.; Espejo, E.; Cobo, E.; et al. Ciprofloxacin and trimethoprim-sulfamethoxazole versus placebo in acute uncomplicated Salmonella enteritis: A double-blind trial. J. Infect. Dis. 1993, 168, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Badley, B.W. Treatment of Salmonella enteritis and its effect on the carrier state. Can. Med. Assoc. J. 1971, 104, 1004–1006. [Google Scholar] [PubMed]
- Shen, Y.; Huang, I.; Hu, H.; Chang, M.; Sheu, S. Whether Antimicrobial Therapy Affect Fecal Excretion Time In Paediatric Patients Of Nontyphoid Salmonellosis With Different Severity. Arch. Dis. Child. 2014, 99, A293. [Google Scholar] [CrossRef] [Green Version]
- Yeung, C.Y.; Lee, H.C.; Chao, Y.N.; Chiu, N.C.; Huang, F.Y.; Hsieh, M.A. Effect of Antibiotic Therapy on Salmonella Fecal Excretion Time. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 249–250. [Google Scholar] [CrossRef]
- Bula-Rudas, F.J.; Rathore, M.H.; Maraqa, N.F. Salmonella Infections in Childhood. Adv. Pediatr. 2015, 62, 29–58. [Google Scholar] [CrossRef]
- Carlstedt, G.; Dahl, P.; Niklasson, P.M.; Gullberg, K.; Banck, G.; Kahlmeter, G. Norfloxacin treatment of salmonellosis does not shorten the carrier stage. Scand. J. Infect. Dis. 1990, 22, 553–556. [Google Scholar] [CrossRef]
- Neill, M.A.; Opal, S.M.; Heelan, J.; Giusti, R.; Cassidy, J.E.; White, R.; Mayer, K.H. Failure of ciprofloxacin to eradicate convalescent fecal excretion after acute salmonellosis: Experience during an outbreak in health care workers. Ann. Int. Med. 1991, 114, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumta, N.; Roberts, J.A.; Lipman, J.; Wong, W.T.; Joynt, G.M.; Cotta, M.O. A Systematic Review of Studies Reporting Antibiotic Pharmacokinetic Data in the Cerebrospinal Fluid of Critically Ill Patients with Uninflamed Meninges. Antimicrob. Agents Chemother. 2020, 65, e01998-20. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Reynolds, J.; Karp, B.E.; Tate, H.; Fedorka-Cray, P.J.; Plumblee, J.R.; Hoekstra, R.M.; Whichard, J.M.; Mahon, B.E. Ceftriaxone-Resistant Nontyphoidal Salmonella from Humans, Retail Meats, and Food Animals in the United States, 1996–2013. Foodborne Pathog. Dis. 2017, 14, 74–83. [Google Scholar] [CrossRef]
- Su, L.H.; Chiu, C.H.; Chu, C.; Ou, J.T. Antimicrobial resistance in nontyphoid Salmonella serotypes: A global challenge. Clin. Infect. Dis. 2004, 39, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Kariuki, S.; Gordon, M.A.; Feasey, N.; Parry, C.M. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 2015, 33 (Suppl. S3), C21–C29. [Google Scholar] [CrossRef] [Green Version]
- Sharland, M.; Pulcini, C.; Harbarth, S.; Zeng, M.; Gandra, S.; Mathur, S.; Magrini, N.; 21st WHO Expert Committee on Selection and Use of Essential Medicines. Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect. Dis. 2018, 18, 18–20. [Google Scholar] [CrossRef] [Green Version]
- Baliban, S.M.; Lu, Y.J.; Malley, R. Overview of the Nontyphoidal and Paratyphoidal Salmonella Vaccine Pipeline: Current Status and Future Prospects. Clin. Infect. Dis. 2020, 71, S151–S154. [Google Scholar] [CrossRef]
| First Author, Year of Publication | Country | Type of Study | Pathogen | Patients (Children) 1 | Antibiotics | Influence of Antibiotic Treatment on Excretion | Influence of Antibiotic Treatment on Clinical Course |
|---|---|---|---|---|---|---|---|
| Macdonald, 1954 [31] | Australia | RCS | S. typhimurium 2 | 51 (100%) | CHL | no influence | no influence |
| Dixon, 1965 [32] | England/Wales | RCS | S. typhimurium | 127 (100%) | NEO, STR, AMP, TET, CHL | prolongation | NA |
| Rosenstein, 1967 [37] | USA | RCS | NTS | 70 (100%) | AMP, NEO, CHL | prolongation | NA |
| Joint Group, 1970 [41] | England | RCT | NTS | 168 (64%) 4 | NEO | prolongation | no difference in duration of symptoms |
| Kazemi, 1973 [42] | Canada | RCT | NTS | 36 (100%) | SXT, AMP | no influence | no difference in clinical features or duration of symptoms |
| Kazemi, 1974 [49] | Canada | RCS | NTS | 117 (100%) | AMP, PEN, CHL, SXT | prolongation | slight increase in morbidity |
| Nelson, 1980 [43] | USA | RCT | NTS 3 | 44 (100%) | AMP, AMX | no influence, but more frequent bacteriologic relapse | no influence on symptoms |
| Stögmann, 1982 [44] | Austria | RCS | NTS | 148 (100%) | SXT and/or AMP | no influence | NA |
| Chiu, 1997 [48] | Taiwan | PCT | NTS | 30 (100%) | CRO | shortening | no difference in duration of symptoms |
| Chiu, 1999 [45] | Taiwan | RCT | NTS | 42 (100%) | AZM, CFM | no influence | no difference in duration of symptoms |
| Barbara, 2000 [23] | Italy | PCS | NTS | 1543 (95.1%) | PENs, SXT, CEFs | no influence | higher frequency of persistent symptoms after antibiotic treatment |
| Lin, 2003 [46] | Taiwan | PCT | NTS | 73 (100%) | CRO | no influence | longer duration of fever but rapid defervescence after antibiotic treatment |
| Tsai, 2011 [13] | Taiwan | RCS | NTS | 683 (100%) | AMP, CHL, SXT, CIP, CRO, FLO, CMF, IPM | NA | shorter hospitalization and duration of fever after treatment with CIP or CRO |
| Ho, 2021 [47] | Taiwan | PCS | NTS | 141 (100%) | CRO, SXT, AMP | no influence after appropriate treatment | worse clinical outcome in children with inappropriate antibiotics 5 |
| Findings | Conclusions | |
|---|---|---|
| Influence of antibiotic treatment on NTS excretion | Prolongation in four studies (all published before 1975) | Most recent studies did not observe a prolongation of NTS excretion. |
| Prolongation only after inappropriate antibiotic treatment in one study | ||
| Shortening in one study | ||
| No influence in six studies | ||
| Influence of antibiotic treatment on the clinical course of NTS infection | Higher frequency of persistent symptoms in one study | Variable results. Most recent studies did not observe a negative influence of antibiotic treatment on the clinical course. |
| Worse clinical outcome only after inappropriate antibiotic treatment in one study | ||
| Positive effects in two studies | ||
| No influence in six studies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leinert, J.L.; Weichert, S.; Jordan, A.J.; Adam, R. Non-Typhoidal Salmonella Infection in Children: Influence of Antibiotic Therapy on Postconvalescent Excretion and Clinical Course—A Systematic Review. Antibiotics 2021, 10, 1187. https://doi.org/10.3390/antibiotics10101187
Leinert JL, Weichert S, Jordan AJ, Adam R. Non-Typhoidal Salmonella Infection in Children: Influence of Antibiotic Therapy on Postconvalescent Excretion and Clinical Course—A Systematic Review. Antibiotics. 2021; 10(10):1187. https://doi.org/10.3390/antibiotics10101187
Chicago/Turabian StyleLeinert, Johanna L., Stefan Weichert, Alexander J. Jordan, and Rüdiger Adam. 2021. "Non-Typhoidal Salmonella Infection in Children: Influence of Antibiotic Therapy on Postconvalescent Excretion and Clinical Course—A Systematic Review" Antibiotics 10, no. 10: 1187. https://doi.org/10.3390/antibiotics10101187





