Imipenem Resistance Mediated by blaOXA-913 Gene in Pseudomonas aeruginosa
Abstract
:1. Introduction
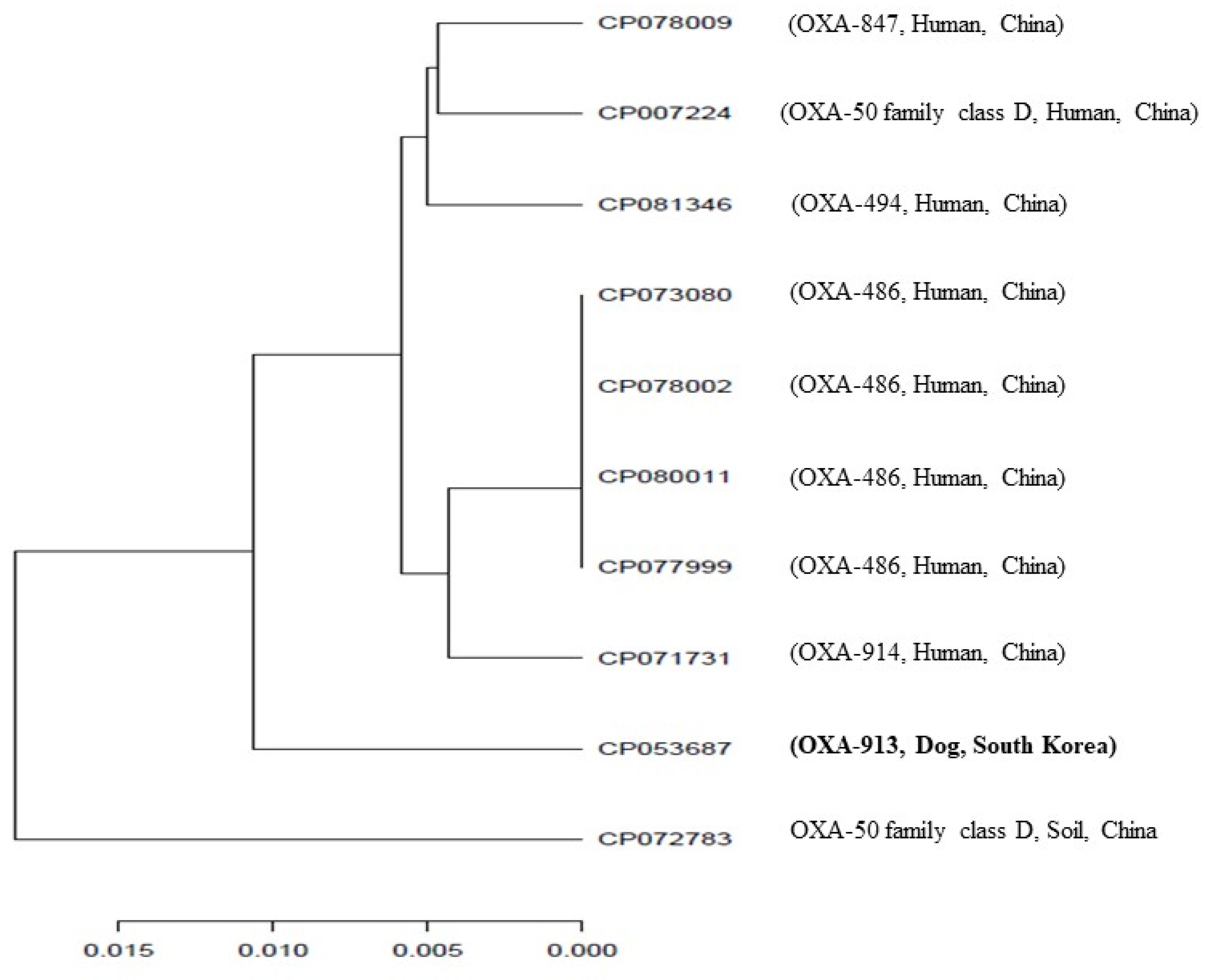
2. Results and Discussion
3. Materials and Methods
3.1. Isolation and Identification of P. aeruginosa
3.2. Antimicrobial Susceptibility Testing
3.3. Polymerase Chain Reaction (PCR) and Whole-Genome Sequencing
3.4. Peptide-Peptide Nucleic Acid Conjugation
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, M.; Sellera, F.; Moura, Q.; Carvalho, M.P.; Rosato, P.N.; Cerdeira, L.; Lincopan, N. Zooanthroponotic Transmission of Drug-Resistant Pseudomonas aeruginosa, Brazil. Emerg. Infect. Dis. 2018, 24, 1160–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramíez-Estrada, S.; Borgatta, B.; Rello, J. Pseudomonas aeruginosa Ventilator-associated Pneumonia Management. Infect. Drug Resist. 2016, 9, 7–18. [Google Scholar]
- Nicoletti, G.; Russo, G.; Bonfiglio, G. Recent developments in carbapenems. Expert Opin. Investig. Drugs 2002, 11, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Bonomo, R.A. Increasing prevalence of carbapenem-resistant Enterobacteriaceae and strategies to avert a looming crisis. Expert Rev. Anti-Infect. Ther. 2013, 11, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.Y.; Jeong, J.W.; Choi, J.H.; Lee, K.P.; Youn, H.Y.; Maeng, H.J.; Song, K.H.; Koo, T.S.; Seo, K.W. Pharmacokinetic study of meropenem in healthy beagle dogs receiving intermittent hemodialysis. J. Vet. Pharmacol. Ther. 2016, 39, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G. Antibiotic Treatment of Resistant Infections in Small Animals. Vet. Clin. North Am. Small Anim. Pr. 2013, 43, 1091–1107. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Wiebe, R.; Dilay, L.; Thomson, K.; Rubinstein, E.; Hoban, D.J.; Noreddin, A.M.; Karlowsky, J.A. Comparative Review of the Carbapenems. Drugs 2007, 67, 1027–1052. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Szabo, D. Mechanisms of Multidrug Resistance in Acinetobacter Species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006, 43, S49–S56. [Google Scholar] [CrossRef] [Green Version]
- Meunier, D.; Doumith, M.; Findlay, J.; Mustafa, N.; Mallard, K.; Anson, J.; Panagea, S.; Pike, R.; Wright, L.; Woodford, N.; et al. Carbapenem resistance mediated by blaOXA-181 in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2016, 71, 2056–2057. [Google Scholar] [CrossRef] [Green Version]
- Mathers, A.J.; Hazen, K.C.; Carroll, J.; Yeh, A.J.; Cox, H.L.; Bonomo, R.A.; Sifri, C.D. First Clinical Cases of OXA-48-Producing Carbapenem-Resistant Klebsiella pneumoniae in the United States: The “Menace” Arrives in the New World. J. Clin. Microbiol. 2012, 51, 680–683. [Google Scholar] [CrossRef] [Green Version]
- Borah, V.; Saikia, K.K.; Hazarika, N.K. First report on the detection of OXA-48 β-lactamase gene in Escherichia coli and Pseudomonas aeruginosa co-infection isolated from a patient in a Tertiary Care Hospital in Assam. Indian J. Med. Microbiol. 2016, 34, 252–253. [Google Scholar] [CrossRef]
- El Garch, F.; Bogaerts, P.; Bebrone, C.; Galleni, M.; Glupczynski, Y. OXA-198, an Acquired Carbapenem-Hydrolyzing Class D β-Lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 4828–4833. [Google Scholar] [CrossRef] [Green Version]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevillano, E.; Gallego, L.; García-Lobo, J. First detection of the OXA-40 carbapenemase in P. aeruginosa isolates, located on a plasmid also found in A. baumannii. Pathol. Biol. 2009, 57, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.N.A.; Desa, M.N.M.; Aziz, M.N.; Taib, N.M. Antimicrobial susceptibility pattern and distribution of exoU and exoS in clinical isolates of Pseudomonas aeruginosa at a Malaysian hospital. Southeast Asian J. Trop. Med. Public Health 2012, 43, 116–123. [Google Scholar] [PubMed]
- Bradbury, R.; Roddam, L.; Merritt, A.; Reid, D.; Champion, A.C. Virulence gene distribution in clinical, nosocomial and environmental isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 2010, 59, 881–890. [Google Scholar] [CrossRef]
- Bocharova, Y.; Savinova, T.; Shagin, D.; Shelenkov, A.A.; Mayanskiy, N.A.; Chebotar, I.V. Inactivation of the oprD porin gene by a novel insertion sequence ISPa195 associated with large deletion in a carbapenem-resistant Pseudomonas aeruginosa clinical isolate. J. Glob. Antimicrob. Resist. 2019, 17, 309–311. [Google Scholar] [CrossRef]
- Cho, H.H.; Kwon, K.C.; Kim, S.; Koo, S.H. Correlation Between Virulence Genotype and Fluoroquinolone Resistance in Carbapenem-Resistant Pseudomonas aeruginosa. Ann. Lab. Med. 2014, 34, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Libisch, B.; Watine, J.; Balogh, B.; Gacs, M.; Muzslay, M.; Szabó, G.; Füzi, M. Molecular typing indicates an important role for two international clonal complexes in dissemination of VIM-producing Pseudomonas aeruginosa clinical isolates in Hungary. Res. Microbiol. 2008, 159, 162–168. [Google Scholar] [CrossRef]
- De Groote, V.N.; Fauvarrt, M.; Kint, C.I.; Verstraeten, N.; Jans, A.; Cornelis, P.; Michiles, J. Pseudomonas aureginosa fosfomycin resistance mechanisms affect non-inherited fluoroquinolone tolerance. J. Med. Microbiol. 2011, 60, 329–336. [Google Scholar] [CrossRef]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [Green Version]
- Bonnin, R.A.; Bogaerts, P.; Girlich, D.; Huang, T.-D.; Dortet, L.; Glupczynski, Y.; Naas, T. Molecular Characterization of OXA-198 Carbapenemase-Producing Pseudomonas aeruginosa Clinical Isolates. Antimicrob. Agents Chemother. 2018, 62, e02496-17. [Google Scholar] [CrossRef] [Green Version]
- Grandjean, T.; Le Guern, R.; Duployez, C.; Faure, K.; Kipnis, E.; Dessein, R. Draft Genome Sequences of Two Pseudomonas aeruginosa Multidrug-Resistant Clinical Isolates, PAL0.1 and PAL1.1. Microbiol. Resour. Announc. 2018, 7, e00940-18. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Suliman, M.; Ahmed, A.; Altayb, H.; Elneima, E. Draft Genome Sequence of a Multidrug-Resistant Pseudomonas aeruginosa Strain Isolated from a Patient with a Urinary Tract Infection in Khartoum, Sudan. Genome Announc. 2017, 5, e00203-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madaha, E.L.; Mienie, C.; Gonsu, H.K.; Bughe, R.N.; Fonkoua, M.C.; Mbacham, W.F.; Alayande, K.A.; Bezuidenhout, C.C.; Ateba, C.N. Whole-genome sequence of multi-drug resistant Pseudomonas aeruginosa strains UY1PSABAL and UY1PSABAL2 isolated from human broncho-alveolar lavage, Yaoundé, Cameroon. PLoS ONE 2020, 15, e0238390. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, A.; Nielsen, P.E. Potent Antibacterial Antisense Peptide–Peptide Nucleic Acid Conjugates Against Pseudomonas aeruginosa. Nucleic Acid Ther. 2012, 22, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, K.; Azuma, M.; Okuno, Y.; Tsukamoto, T.; Nishiguchi, K.; Setsukinai, K.-I.; Maki, H.; Numata, Y.; Takemoto, H.; Rokushima, M. Antisense peptide nucleic acid–peptide conjugates for functional analyses of genes in Pseudomonas aeruginosa. Bioorg. Med. Chem. 2015, 23, 7234–7239. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Nordén, B. Peptide nucleic acid (PNA): Its medical and biotechnical applications and promise for the future. FASEB J. 2000, 14, 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, A.K.; Sahni, R.D.; Anandan, S.; Sharma, A.; Gopi, R.; Hadibasha, N.; Gunasekaran, P.; Veeraraghavan, B. A Pilot Study on Carbapenemase Detection: Do We See the Same Level of Agreement as with the CLSI Observations. J. Clin. Diagn. Res. 2016, 10, DC09–DC13. [Google Scholar] [CrossRef] [PubMed]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Moon, D.; Kim, S.-J.; Mechesso, A.; Kang, H.; Song, H.-J.; Choi, J.-H.; Yoon, S.-S.; Lim, S.-K. Mobile Colistin Resistance Gene mcr-1 Detected on an IncI2 Plasmid in Salmonella Typhimurium Sequence Type 19 from a Healthy Pig in South Korea. Microorganisms 2021, 9, 398. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus Sequence Typing of Total-Genome-Sequenced Bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, L.; Glover, R.H.; Humphris, S.; Elphinstone, J.G.; Toth, I.K. Genomics and Taxonomy in Diagnostics for Food Security: Soft-rotting Enterobacterial Plant Pathogens. Anal. Methods 2016, 8, 12–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.-C.; Mechesso, A.F.; Kang, H.-Y.; Kim, S.-J.; Choi, J.-H.; Song, H.-J.; Yoon, S.-S.; Lim, S.-K. Imipenem Resistance Mediated by blaOXA-913 Gene in Pseudomonas aeruginosa. Antibiotics 2021, 10, 1188. https://doi.org/10.3390/antibiotics10101188
Moon D-C, Mechesso AF, Kang H-Y, Kim S-J, Choi J-H, Song H-J, Yoon S-S, Lim S-K. Imipenem Resistance Mediated by blaOXA-913 Gene in Pseudomonas aeruginosa. Antibiotics. 2021; 10(10):1188. https://doi.org/10.3390/antibiotics10101188
Chicago/Turabian StyleMoon, Dong-Chan, Abraham Fikru Mechesso, Hee-Young Kang, Su-Jeong Kim, Ji-Hyun Choi, Hyun-Ju Song, Soon-Seek Yoon, and Suk-Kyung Lim. 2021. "Imipenem Resistance Mediated by blaOXA-913 Gene in Pseudomonas aeruginosa" Antibiotics 10, no. 10: 1188. https://doi.org/10.3390/antibiotics10101188
APA StyleMoon, D.-C., Mechesso, A. F., Kang, H.-Y., Kim, S.-J., Choi, J.-H., Song, H.-J., Yoon, S.-S., & Lim, S.-K. (2021). Imipenem Resistance Mediated by blaOXA-913 Gene in Pseudomonas aeruginosa. Antibiotics, 10(10), 1188. https://doi.org/10.3390/antibiotics10101188






