Vapor Phase Alkylation of Isomeric Cresols with Tert-Butyl Alcohol over Perlite Supported Sulfated Zirconia Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SZP Catalysts
2.3. Characterization Techniques
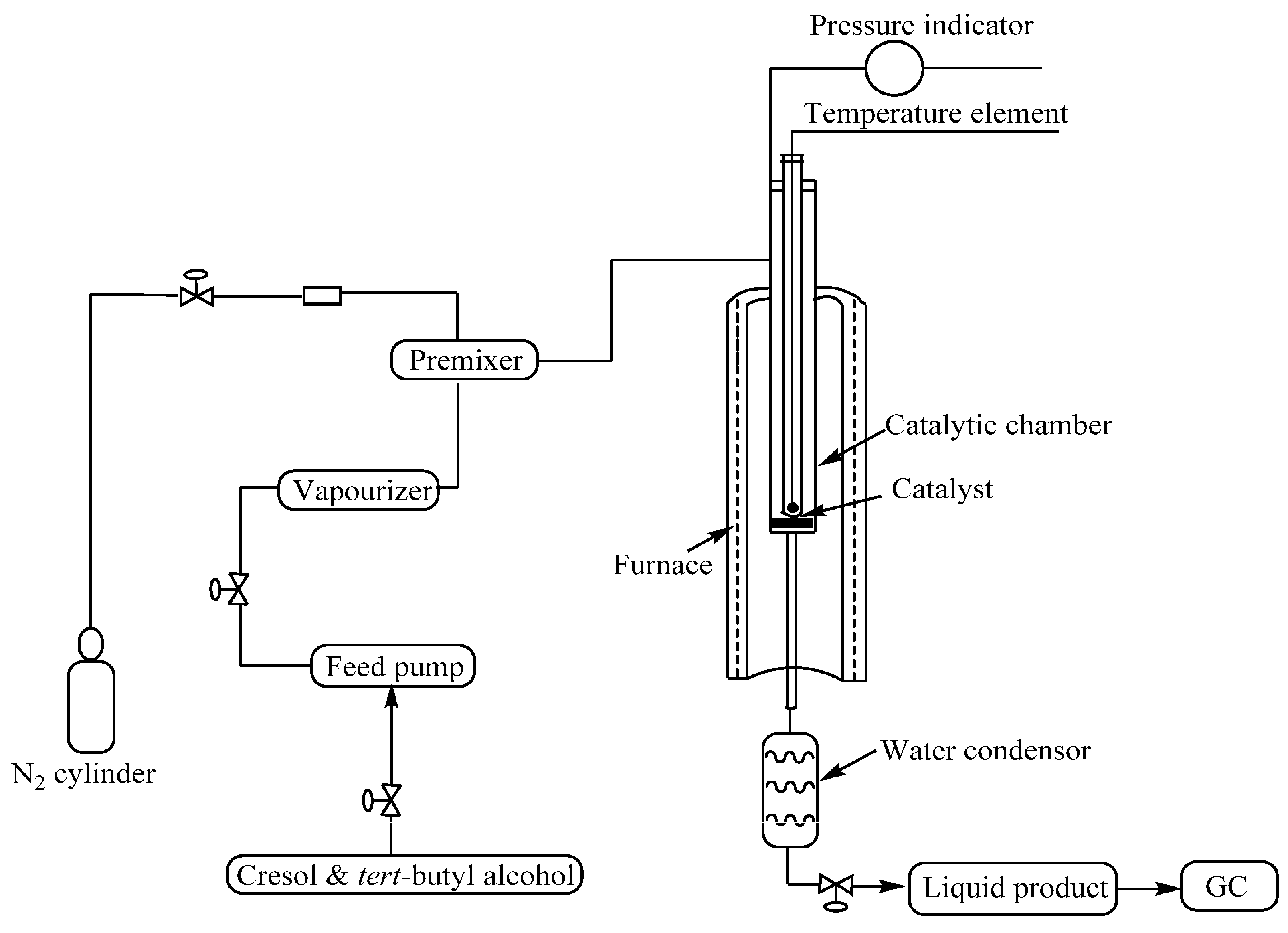
2.4. Catalytic Reactions
3. Results
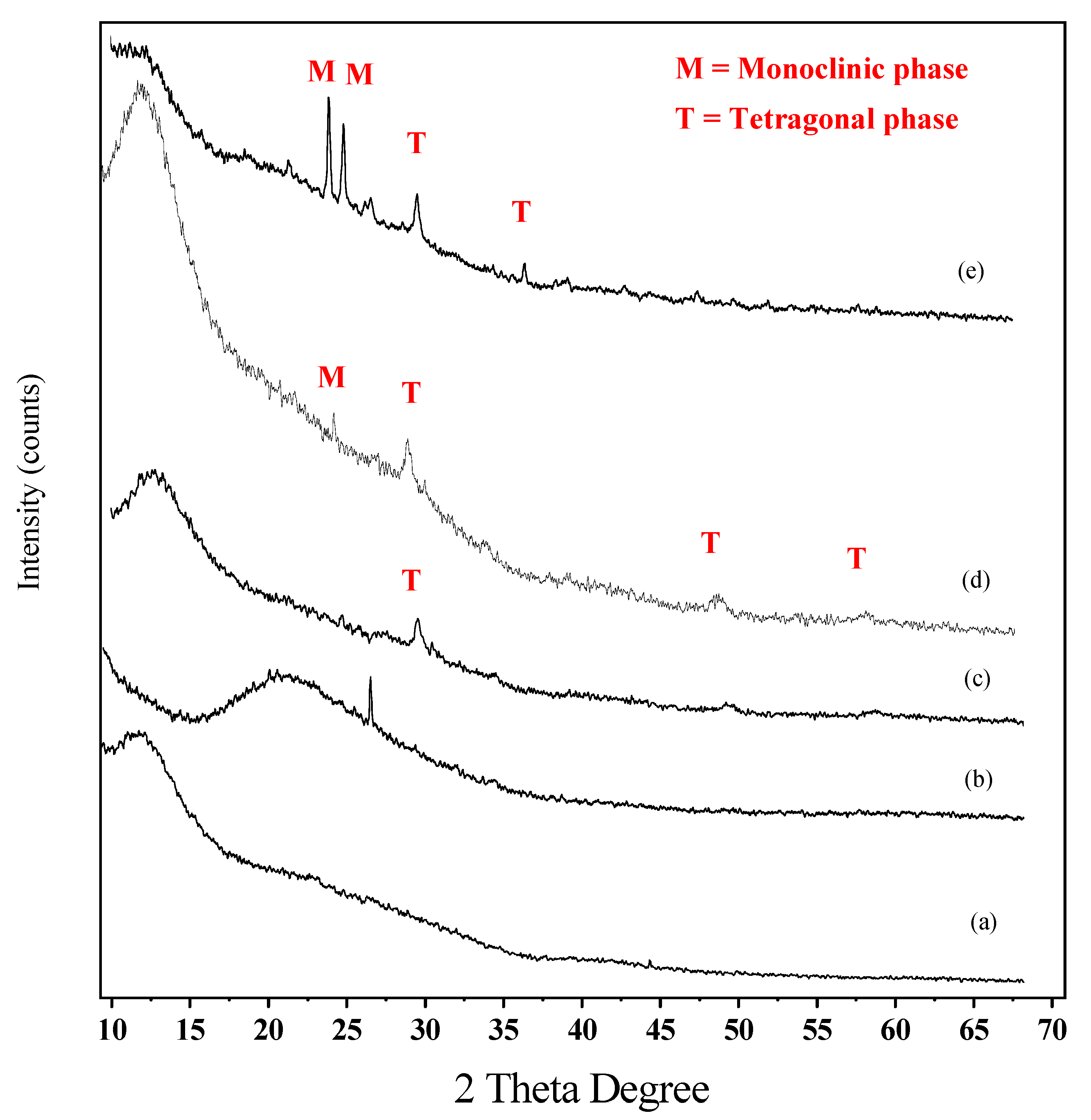
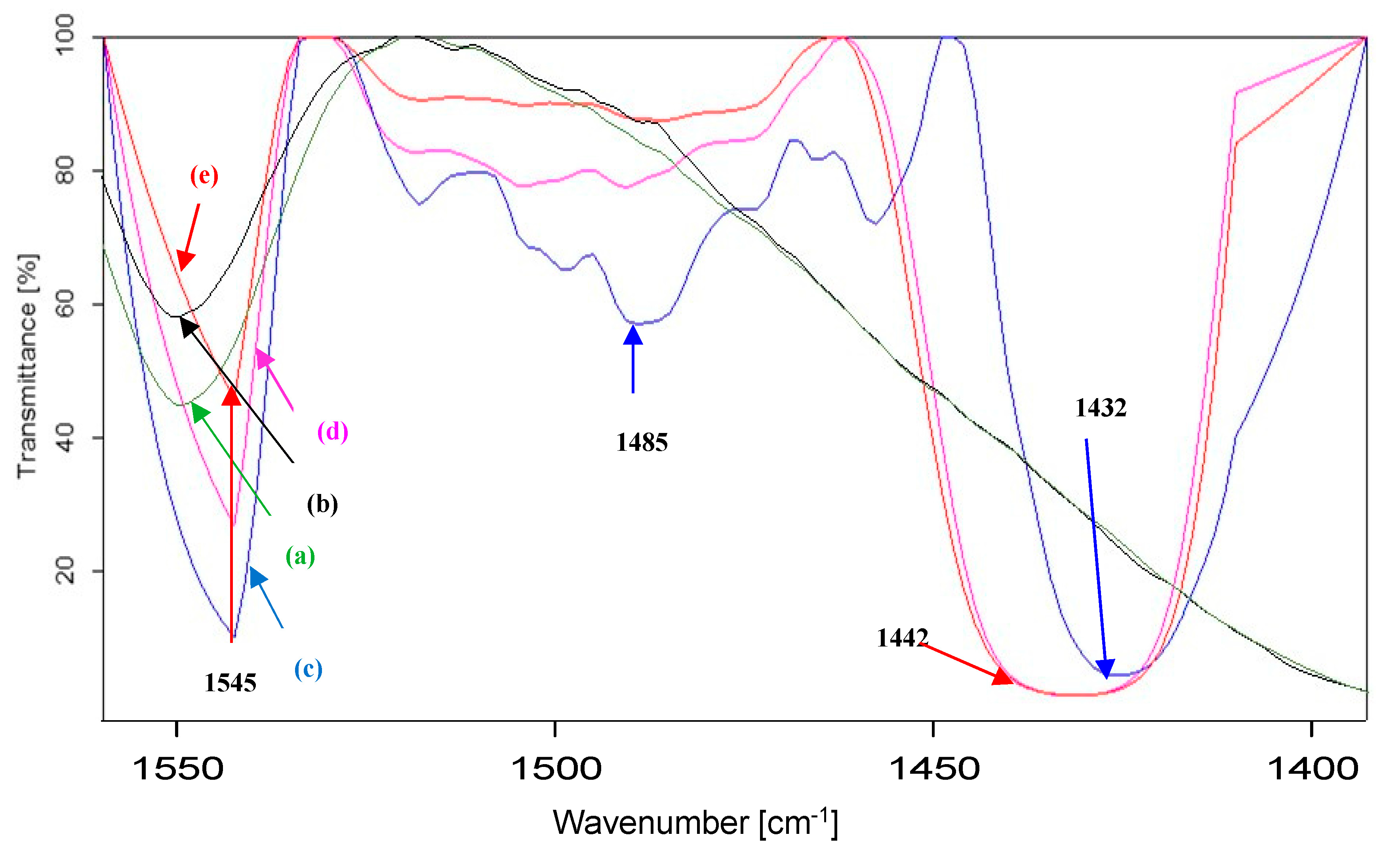
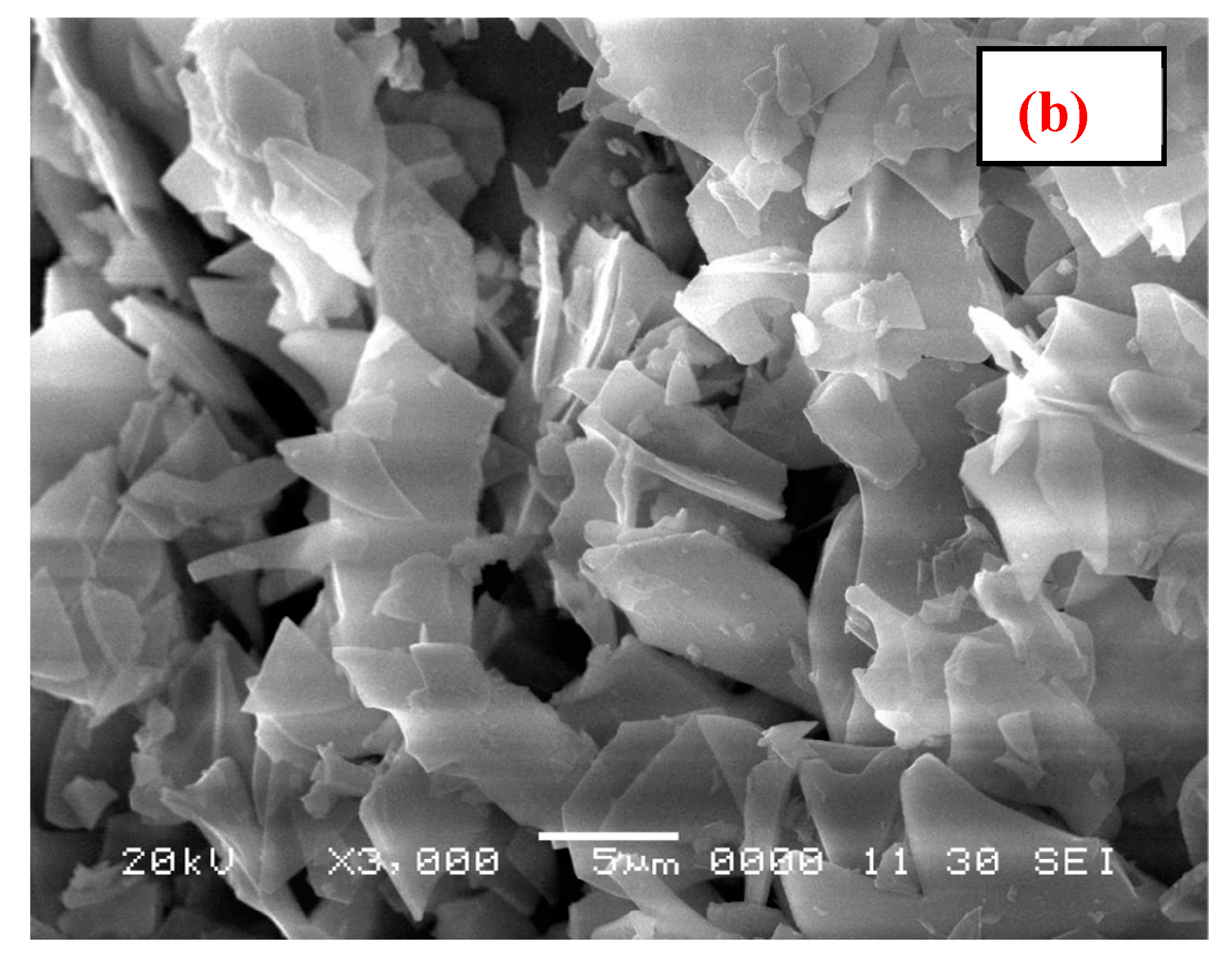
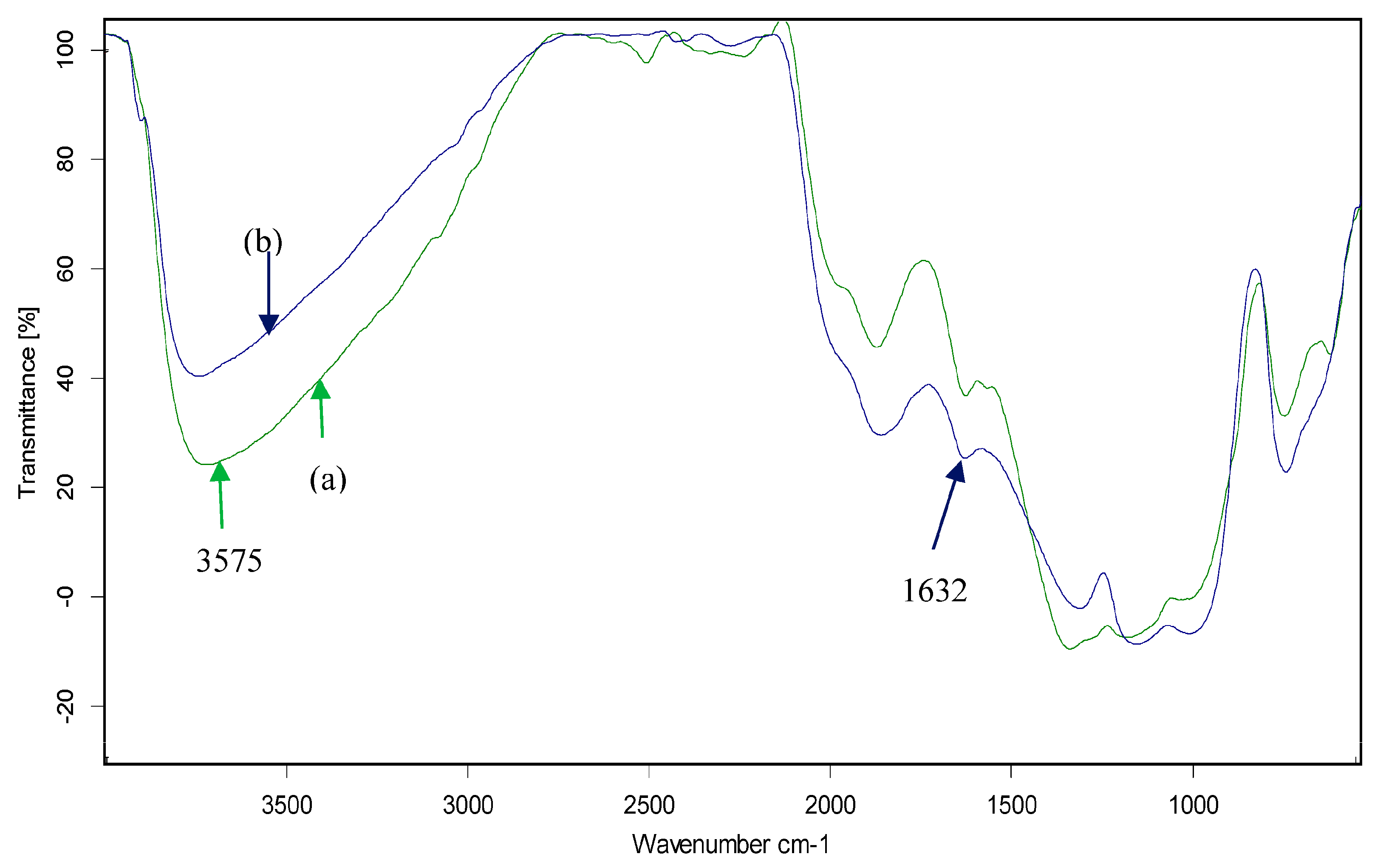
3.1. Characterization
3.2. Catalytic Reaction
3.2.1. Effect of Reaction Temperature
3.2.2. Effect of Molar Ratio
3.2.3. Comparison with Other Reported Catalysts
3.3. Catalyst Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakir, A.; Bessiere, J.; Kacemi, K.E.L.; Marouf, B. A comparative study of the removal of trivalent chromium from aqueous solutions by bentonite and expanded perlite. J. Hazard. Mater. 2002, 95, 29–46. [Google Scholar] [CrossRef]
- Koumanova, B.; Peeva-Antova, P. Adsorption of p-chlorophenol from aqueous solutions on bentonite and perlite. J. Hazard. Mater. 2002, 90, 229–234. [Google Scholar] [CrossRef]
- Alihosseini, A.; Taghikhani, V.; Safekordi, A.A.; Bastani, D. Equilibrium sorption of crude oil by expanded perlite using different adsorption isotherms at 298.15 k. Int. J. Environ. Sci. Technol. 2010, 7, 591–598. [Google Scholar] [CrossRef]
- Celik, A.G.; Kilic, A.M.; Cakal, G.O. Expanded perlite aggregate characterization for use as a lightweight construction raw material. Physicochem. Probl. Miner. Process. 2013, 49, 689–700. [Google Scholar]
- Saufi, H.; El Alouani, M.; Alehyen, S.; El Achouri, M.; Aride, J.; Taibi, M. Photocatalytic Degradation of Methylene Blue from Aqueous Medium onto Perlite-Based Geopolymer. Int. J. Chem. Eng. 2020, 2020, 9498349. [Google Scholar] [CrossRef]
- Silber, A.; Bar-Yosef, B.; Levkovitch, I.; Kautzky, L.; Minz, D. Kinetics and mechanisms of pH-dependent Mn(II) reactions in plant-growth medium. Soil Biol. Biochem. 2008, 40, 2787–2795. [Google Scholar] [CrossRef]
- Kalus, K.; Opaliński, S.; Maurer, D.; Rice, S.; Koziel, J.A.; Korczyński, M.; Dobrzański, Z.; Kołacz, R.; Gutarowska, B. Odour reducing microbial-mineral additive for poultry manure treatment. Front. Environ. Sci. Eng. 2017, 11, 1–9. [Google Scholar] [CrossRef]
- Joiner, A. Review of the extrinsic stain removal and enamel/dentine abrasion by a calcium carbonate and perlite containing whitening toothpaste. Int. Dent. J. 2006, 56, 175–180. [Google Scholar] [CrossRef]
- Puga, A.; Rosales, E.; Pazos, M.; Sanromán, M.A. Prompt removal of antibiotic by adsorption/electro-Fenton degradation using an iron-doped perlite as heterogeneous catalyst. Process Saf. Environ. Prot. 2020, 144, 100–110. [Google Scholar] [CrossRef]
- Esmaielpour, M.; Akhlaghinia, B.; Jahanshahi, R. Green and efficient synthesis of aryl/alkylbis(indolyl)methanes using Expanded Perlite-PPA as a heterogeneous solid acid catalyst in aqueous media. J. Chem. Sci. 2017, 129, 313–328. [Google Scholar] [CrossRef]
- Xue, H.; Jiang, Y.; Yuan, K.; Yang, T.; Hou, J.; Cao, C.; Feng, K.; Wang, X. Floating photocatalyst of B-N-TiO2/expanded perlite: A sol-gel synthesis with optimized mesoporous and high photocatalytic activity. Sci. Rep. 2016, 6, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.M.; Kolvari, E.; Vahidian, M.; Bagheri, R. Nano perlite sulfuric acid: An inexpensive heterogeneous acid catalyst for the synthesis of 1, 8-dioxo-octahydroxanthenes and tetrahydrobenzoxanthenes under solvent-free conditions. J. Appl. Chem. 2017, 11, 109–118. [Google Scholar]
- Modiba, E.; Osifo, P.; Rutto, H. The use of impregnated perlite as a heterogeneous catalyst for biodiesel production from marula oil. Chem. Pap. 2014, 68, 1341–1349. [Google Scholar] [CrossRef]
- Abrouki, Y.; Anouzla, A.; Loukili, H.; Chakir, A.; Abrouki, K.; Loukili, M.; Rayadh, A.; Bahlaoui, M.A.; Zahouily, M.; Kacemi, K.E.; et al. Investigation of the basis for catalytic activity of expanded Perlite in Knoevnagel condensation. Adv. Res. J. Chem. Mater. Sci. 2013, 1, 1–7. [Google Scholar]
- Shokrollahzadeh, S.; Abassi, M.; Ranjbar, M. A new nano-ZnO/perlite as an efficient catalyst for catalytic ozonation of azo dye. Environ. Eng. Res. 2019, 24, 513–520. [Google Scholar] [CrossRef]
- Radonjić, V.D.; Krstić, J.B.; Lončarević, D.; Vukelić, N.; Jovanović, D.M. Mg-ni supported on perlite as hydrogenation catalyst: Influence of mg and ni content. Chem. Ind. Chem. Eng. Q. 2019, 25, 193–206. [Google Scholar] [CrossRef]
- Malpani, S.K.; Goyal, D.; Katara, S.; Rani, A. Turkish perlite supported nickel oxide as the heterogeneous acid catalyst for a series of Claisen–Schmidt condensation reactions. Turk. J. Chem. 2021, 45, 1097–1114. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Priyanka; Hada, R.; Katara, S.; Bhatia, A.; Kabra Malpani, S. Development of green, effective, and cost-efficient perlite supported solid base catalyst and application in condensation reactions. Mater. Today Proc. 2021, 49, 3717–3725. [Google Scholar] [CrossRef]
- Goyal, D.; Saikia, H.; Hada, R.; Katara, S.; Bhatia, A.; Malpani, S.K. Perlite supported cobalt oxide catalyst for a series of liquidphase esterification reactions. Macromol. Symp. 2022, 402, 2100369. [Google Scholar] [CrossRef]
- Umamaheswari, V.; Palanichamy, M.; Arabindoo, B.; Murugesan, V. Regioselective t-butylation of m-cresol over mesoporous Al-MCM-41 molecular sieves. Indian J. Chem.—Sect. A Inorg. Phys. Theor. Anal. Chem. 2000, 39A, 1241–1247. [Google Scholar]
- Yadav, G.D.; Pathre, G.S. Novel mesoporous solid superacids for selective C-alkylation of m-cresol with tert-butanol. Microporous Mesoporous Mater. 2006, 89, 16–24. [Google Scholar] [CrossRef]
- Muraleedharan, L.; Chandrashekara, M.; Prakash, S.J.; Bhat, S. Clay-Based Solid Acid Catalyst for the Alkylation of p-Cresol with tert-Butyl Alcohol. ChemistrySelect 2018, 3, 801–808. [Google Scholar] [CrossRef]
- Bhatt, N.; Patel, A. Liquid phase tert-butylation of cresols catalysed by 12-tungstophosphoricacid and 12-tungstosilicicacid supported onto neutral alumina. Catal. Lett. 2007, 113, 99–103. [Google Scholar] [CrossRef]
- Chandra, K.G.; Sharma, M.M. Alkylation of phenol with MTBE and other tert-butyl ethers: Cation exchange resins as catalysts. Catal. Lett. 1993, 19, 309–317. [Google Scholar] [CrossRef]
- Su, Z.R.; Wang, T.J. 12-Tungstophosphoric acid immobilized on modified macroporous phenol-furfural sulfonic acid resin for tert-butylation of p-cresol. React. Funct. Polym. 1995, 28, 97–102. [Google Scholar] [CrossRef]
- Devassy, B.M.; Shanbhag, G.V.; Lefebvre, F.; Halligudi, S.B. Alkylation of p-cresol with tert-butanol catalyzed by heteropoly acid supported on zirconia catalyst. J. Mol. Catal. A Chem. 2004, 210, 125–130. [Google Scholar] [CrossRef]
- Campbell, C.B.; Onopchenko, A.; Young, D.C. Amberlyst- 15t-Catalyzed Alkylation of Phenolics with Branched Alkenes. Rearrangement of tert -Alkylphenols and Catechols to sec -Alkyl Isomers. Ind. Eng. Chem. Res. 1990, 29, 642–647. [Google Scholar] [CrossRef]
- Yadav, G.D.; Thorat, T.S. Kinetics of alkylation of p-cresol with isobutylene catalyzed by sulfated zirconia. Ind. Eng. Chem. Res. 1996, 35, 721–731. [Google Scholar] [CrossRef]
- Rahman, N.J.A.; Ramli, A.; Jumbri, K.; Uemura, Y. Tailoring the surface area and the acid–base properties of ZrO2 for biodiesel production from Nannochloropsis sp. Sci. Rep. 2019, 9, 16223. [Google Scholar] [CrossRef] [PubMed]
- Berrones, R.; Camas, K.; Pérez, Y.; Ramírez, E.; Pérez, A.; Eapen, D.; Sebastian, P.J. Synthesis and performance of sulfated zirconia catalyst in esterification of oleic acid. J. New Mater. Electrochem. Syst. 2014, 17, 99–104. [Google Scholar] [CrossRef]
- Kauppi, E.I.; Honkala, K.; Krause, A.O.I.; Kanervo, J.M.; Lefferts, L. ZrO2 Acting as a Redox Catalyst. Top. Catal. 2016, 59, 823–832. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Fujitani, T. Catalytic conversion of levulinic acid and its esters to γ-valerolactone over silica-supported zirconia catalysts. Bull. Chem. Soc. Jpn. 2014, 87, 1252–1254. [Google Scholar] [CrossRef]
- Ward, A.J.; Pujari, A.A.; Costanzo, L.; Masters, A.F.; Maschmeyer, T. Ionic liquid-templated preparation of mesoporous silica embedded with nanocrystalline sulfated zirconia. Nanoscale Res. Lett. 2011, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Abdel Salam, M.S.; Betiha, M.A.; Shaban, S.A.; Elsabagh, A.M.; Abd El-Aal, R.M.; El kady, F.Y. Synthesis and characterization of MCM-41-supported nano zirconia catalysts. Egypt. J. Pet. 2015, 24, 49–57. [Google Scholar] [CrossRef]
- Tamizhdurai, P.; Sakthinathan, S.; Santhana Krishnan, P.; Ramesh, A.; Mangesh, V.L.; Abilarasu, A.; Narayanan, S.; Shanthi, K.; Chiu, T.W. Catalytic activity of ratio-dependent SBA-15 supported zirconia catalysts for highly selective oxidation of benzyl alcohol to benzaldehyde and environmental pollutant heavy metal ions detection. J. Mol. Struct. 2019, 1176, 650–661. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, M.; Dai, B. Synthesis, Characterization and Application of ZS/HMS Catalyst in the Esterification of Gossypol. Green Sustain. Chem. 2012, 2, 8–13. [Google Scholar] [CrossRef][Green Version]
- Kim, K.D.; Kim, J.; Teoh, W.Y.; Kim, J.C.; Huang, J.; Ryoo, R. Cascade reaction engineering on zirconia-supported mesoporous MFI zeolites with tunable Lewis-Brønsted acid sites: A case of the one-pot conversion of furfural to γ-valerolactone. RSC Adv. 2020, 10, 35318–35328. [Google Scholar] [CrossRef]
- Bikmetova, L.I.; Kazantsev, K.V.; Zatolokina, E.V.; Dzhikiya, O.V.; Smolikov, M.D.; Belyi, A.S. Sulfated zirconia catalysts supported on alumina for hexane isomerization. AIP Conf. Proc. 2020, 2301, 030002. [Google Scholar]
- Khatri, C.; Mishra, M.K.; Rani, A. Synthesis and characterization of fly ash supported sulfated zirconia catalyst for benzylation reactions. Fuel Process. Technol. 2010, 91, 1288–1295. [Google Scholar] [CrossRef]
- Mishra, M.; Devi, K.R.S.; Pinheiro, D.; Nizam, A. Zirconia Supported on Rice Husk Silica from Biowaste: A Novel, Efficient, and Recoverable Nanocatalyst for the Green Synthesis of Tetrahydro-1-benzopyrans. Russ. J. Org. Chem. 2020, 56, 1784–1789. [Google Scholar] [CrossRef]
- Mishra, M.K.; Tyagi, B.; Jasra, R.V. Synthesis and characterization of nano-crystalline sulfated zirconia by sol-gel method. J. Mol. Catal. A Chem. 2004, 223, 61–65. [Google Scholar] [CrossRef]
- Yurdakoç, M.; Akçay, M.; Tonbul, Y.; Yurdakoç, K. Acidity of silica-alumina catalysts by amine titration using Hammett indicators and FT-IR study of pyridine adsorption. Turk. J. Chem. 1999, 23, 319–327. [Google Scholar]
- Akkari, R.; Ghorbel, A.; Essayem, N.; Figueras, F. Synthesis and characterization of mesoporous silica-supported nano-crystalline sulfated zirconia catalysts prepared by a sol-gel process: Effect of the S/Zr molar ratio. Appl. Catal. A Gen. 2007, 328, 43–51. [Google Scholar] [CrossRef]
- Devi, K.R.S.; Jayashree, S. Eco friendly nitration of toluene using modified zirconia. Bull. Chem. React. Eng. Catal. 2013, 7, 205–214. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A.; Shultz, J. A simple method for production of pure silica from rice hull ash. Fuel Energy Abstr. 2001, 42, 45. [Google Scholar] [CrossRef]
- Jain, D.; Khatri, C.; Rani, A. Synthesis and characterization of novel solid base catalyst from fly ash. Fuel 2011, 90, 2083–2088. [Google Scholar] [CrossRef]
- Liu, E.; Locke, A.J.; Frost, R.L.; Martens, W.N. Sulfated fibrous ZrO2/Al2O3 core and shell nanocomposites: A novel strong acid catalyst with hierarchically macro-mesoporous nanostructure. J. Mol. Catal. A Chem. 2012, 353–354, 95–105. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Shanbhag, G.V.; Halgeri, A.B. Sulfated zirconia; An efficient and reusable acid catalyst for the selective synthesis of 4-phenyl-1,3-dioxane by Prins cyclization of styrene. Appl. Catal. A Gen. 2013, 451, 71–78. [Google Scholar] [CrossRef]
- Delarmelina, M.; Deshmukh, G.; Goguet, A.; Catlow, C.R.A.; Manyar, H. Role of Sulfation of Zirconia Catalysts in Vapor Phase Ketonization of Acetic Acid. J. Phys. Chem. C 2021, 125, 27578–27595. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.J.; Parthasarathy, G. Fourier Transform Infrared Spectroscopic Characterization of Kaolinite from Assam and Meghalaya, Northeastern India. J. Mod. Phys. 2010, 1, 206–210. [Google Scholar] [CrossRef]
- Sharma, S.D.; Singh, S. Synthesis and Characterization of Highly Effective Nano Sulfated Zirconia over Silica: Core-Shell Catalyst by Ultrasonic Irradiation. Am. J. Chem. 2013, 2013, 96–104. [Google Scholar]
- Yadav, G.D.; Ajgaonkar, N.P.; Varma, A. Preparation of highly superacidic sulfated zirconia via combustion synthesis and its application in Pechmann condensation of resorcinol with ethyl acetoacetate. J. Catal. 2012, 292, 99–110. [Google Scholar] [CrossRef]
- Rodríguez-Castellón, E.; Jiménez-López, A.; Maireles-Torres, P.; Jones, D.J.; Rozière, J.; Trombetta, M.; Busca, G.; Lenarda, M.; Storaro, L. Textural and structural properties and surface acidity characterization of mesoporous silica-zirconia molecular sieves. J. Solid State Chem. 2003, 175, 159–169. [Google Scholar] [CrossRef]
- Sepehrian, H.; Khanchi, A.R.; Rofouei, M.K.; Husain, S.W. Non-thermal synthesis of mesoporous zirconium silicate and its characterization. J. Iran. Chem. Soc. 2006, 3, 253–257. [Google Scholar] [CrossRef]
- Parry, E.P. An Infrared Study of Pyridine Adsorbed on Acidic Solids. Characterization of Surface Acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Reddy, C.R.; Bhat, Y.S.; Nagendrappa, G.; Prakash, B.S.J. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160. [Google Scholar] [CrossRef]
- Musthofa, M.; Karim, A.H.; Ahmad Fadzlillaah, N.; Rozali Annuar, N.H.; Abdul Jalil, A.; Triwahyono, S. Determination of Lewis and Brönsted acid sites by gas flow-injection technique. Malays. J. Fundam. Appl. Sci. 2014, 6, 127–131. [Google Scholar] [CrossRef]
- Wang, Z.; Navarrete, J. Keggin Structure and Surface Acidity of 12-Phosphotungstic Acid Grafted Zr-MCM-48 Mesoporous Molecular Sieves. World J. Nano Sci. Eng. 2012, 2, 134–141. [Google Scholar] [CrossRef]
- Malpani, S.K.; Goyal, D.; Katara, S.; Rani, A. Green, efficient and economical coal fly ash based phosphomolybdic acid catalysts: Preparation, characterization and application. Chem. Pap. 2021, 75, 3017–3034. [Google Scholar] [CrossRef]
- Hussain, T.S.; Mazhar, M.; Gul, S. Increase in concentration and strength of acidic catalytic sites on sulfated zirconia by doping with silica. Cuihua Xuebao/Chin. J. Catal. 2007, 28, 622–626. [Google Scholar] [CrossRef]
- Ahmed, M.A. Surface characterization and catalytic activity of sulfated-hafnia promoted zirconia catalysts for n-butane isomerization. Fuel Process. Technol. 2011, 92, 1121–1128. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, L.; Ma, S.; Han, Y.; Zhao, L.; Wang, W.; Chen, C.L.; Xiao, F.S. Improved catalytic activity and stability of mesostructured sulfated zirconia by Al promoter. Appl. Catal. A Gen. 2004, 268, 17–24. [Google Scholar] [CrossRef]
- Rachmat, A.; Trisunaryanti, W.; Wijaya, K. Synthesis and characterization of sulfated zirconia mesopore and its application on lauric acid esterification. Mater. Renew. Sustain. Energy 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Bautista, P.; Faraldos, M.; Yates, M.; Bahamonde, A. Influence of sulphate doping on Pd/zirconia based catalysts for the selective catalytic reduction of nitrogen oxides with methane. Appl. Catal. B Environ. 2007, 71, 254–261. [Google Scholar] [CrossRef]
- Genov, K.; Georgiev, V.; Batakliev, T.; Sarker, D.K. Ozone decomposition over silver-loaded perlite. World Acad. Sci. Eng. Technol. 2011, 80, 1015–1018. [Google Scholar]
- Negrón-Silva, G.; Hernández-Reyes, C.X.; Angeles-Beltrán, D.; Lomas-Romero, L.; González-Zamora, E.; Méndez-Vivar, J. Comparative study of the regioselective synthesis of β-aminoalcohols under solventless conditions catalyzed by sulfated zirconia and SZ/MCM-41. Molecules 2007, 12, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.A.; Chen, L.F.; Noreña, L.E.; Navarrete, J.; Llanos, M.E.; Contreras, J.L.; Novaro, O. Mesoporous structure, surface acidity and catalytic properties of Pt/Zr-MCM-41 catalysts promoted with 12-tungstophosphoric acid. Microporous Mesoporous Mater. 2008, 112, 61–76. [Google Scholar] [CrossRef]
- Kondamudi, K.; Elavarasan, P.; Dyson, P.J.; Upadhyayula, S. Alkylation of p-cresol with tert-butyl alcohol using benign Bronsted acidic ionic liquid catalyst. J. Mol. Catal. A Chem. 2010, 321, 34–41. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Molnár, Á.; Papp, A. Catalyst recycling—A survey of recent progress and current status. Coord. Chem. Rev. 2017, 349, 1–65. [Google Scholar] [CrossRef]


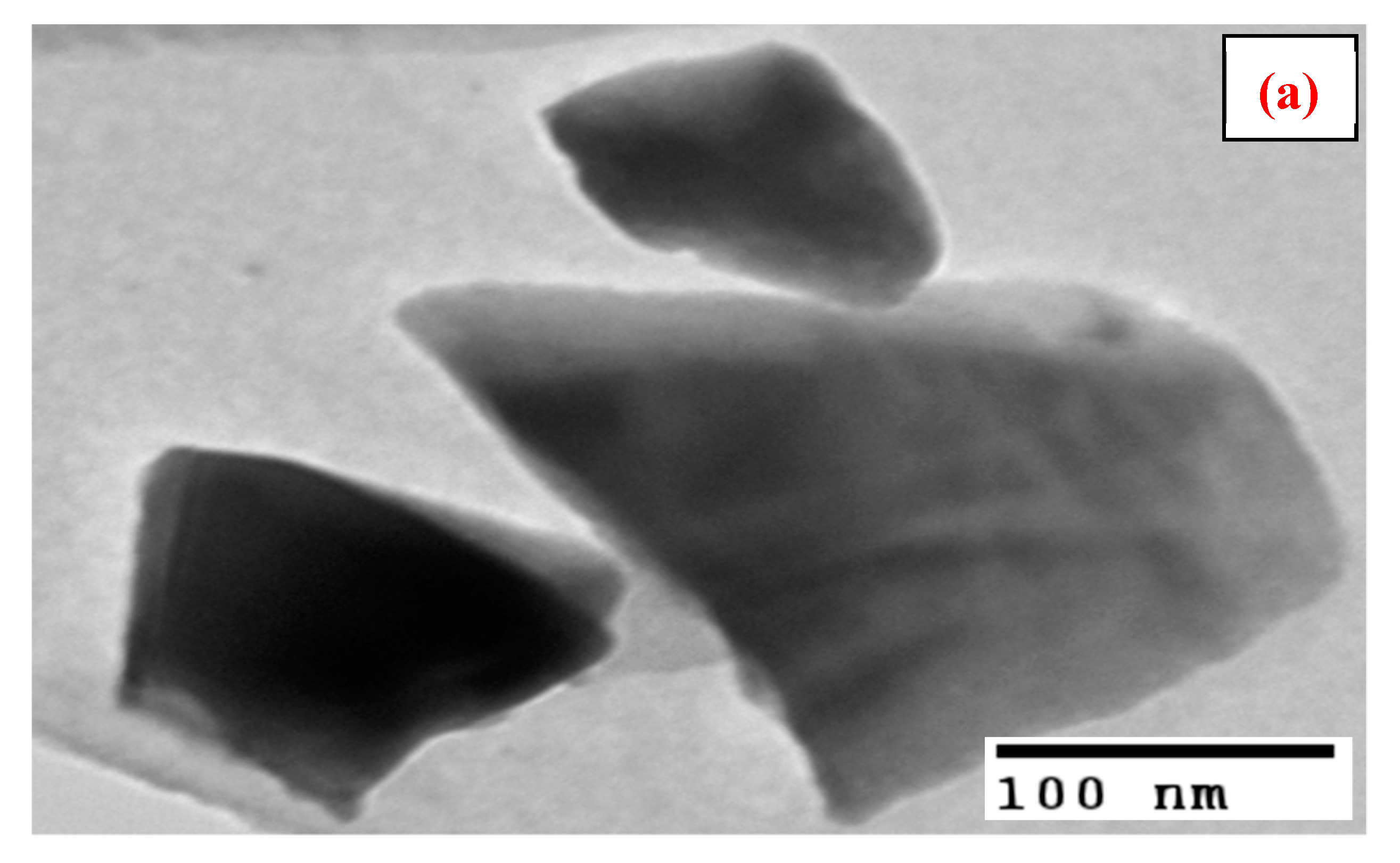
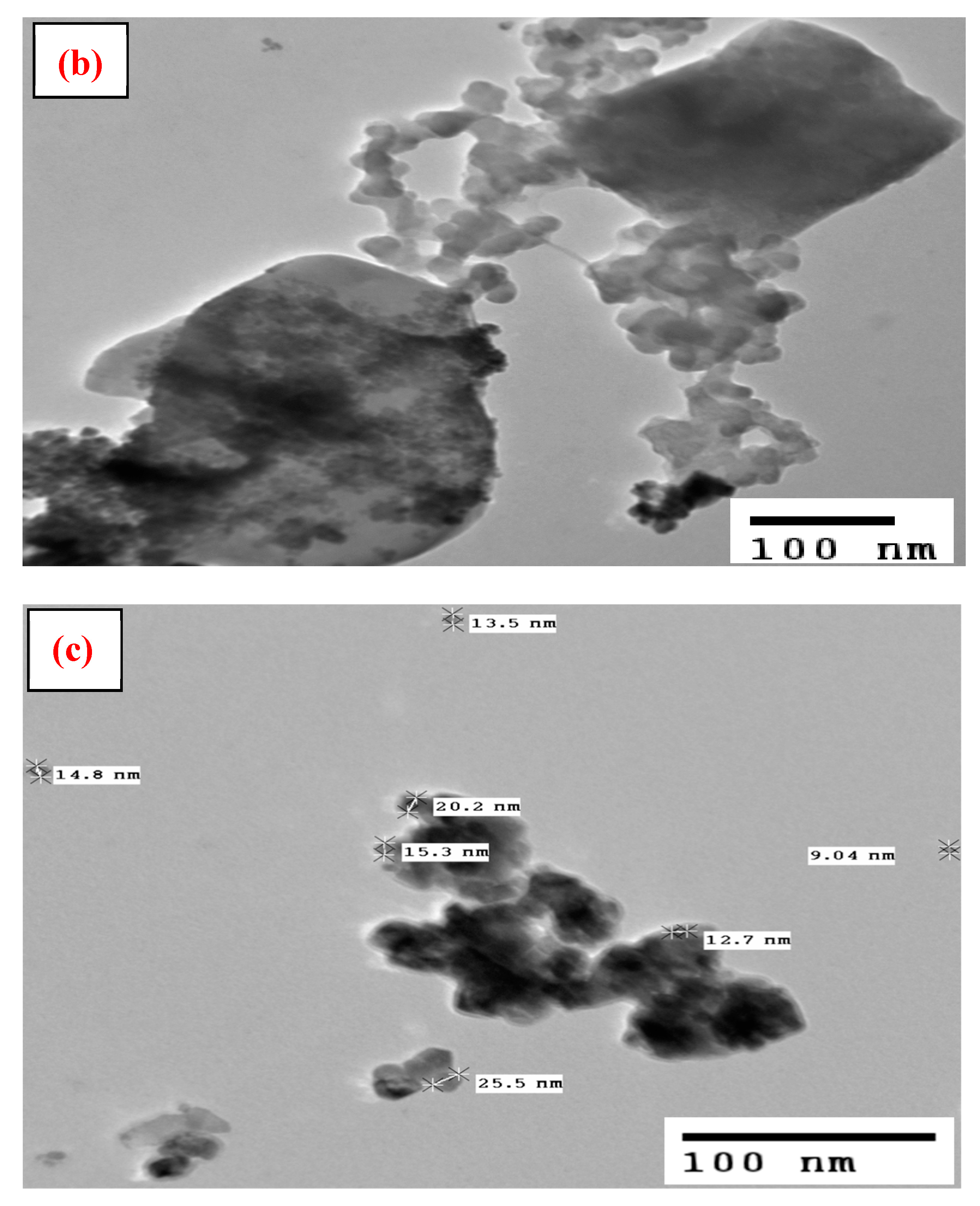
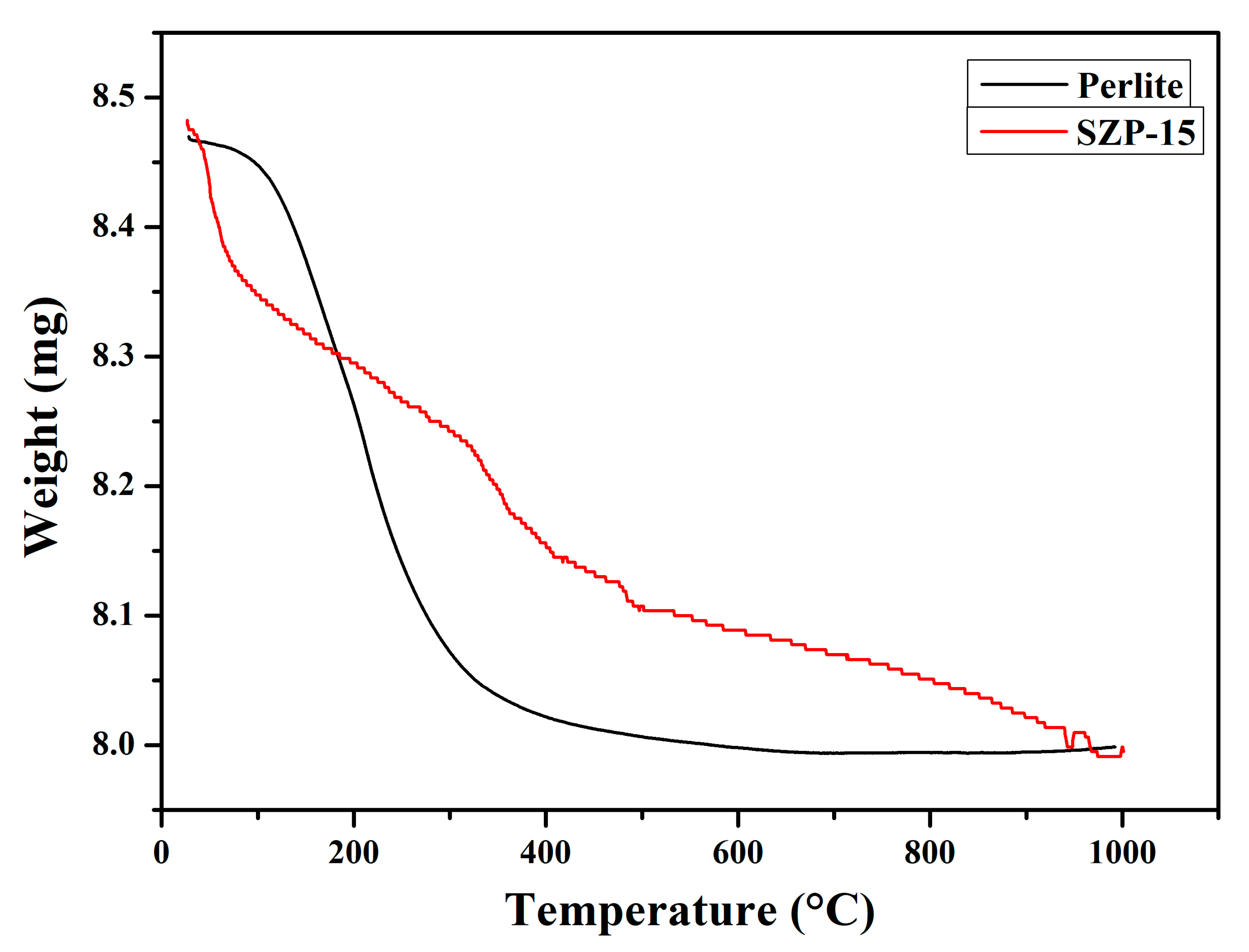

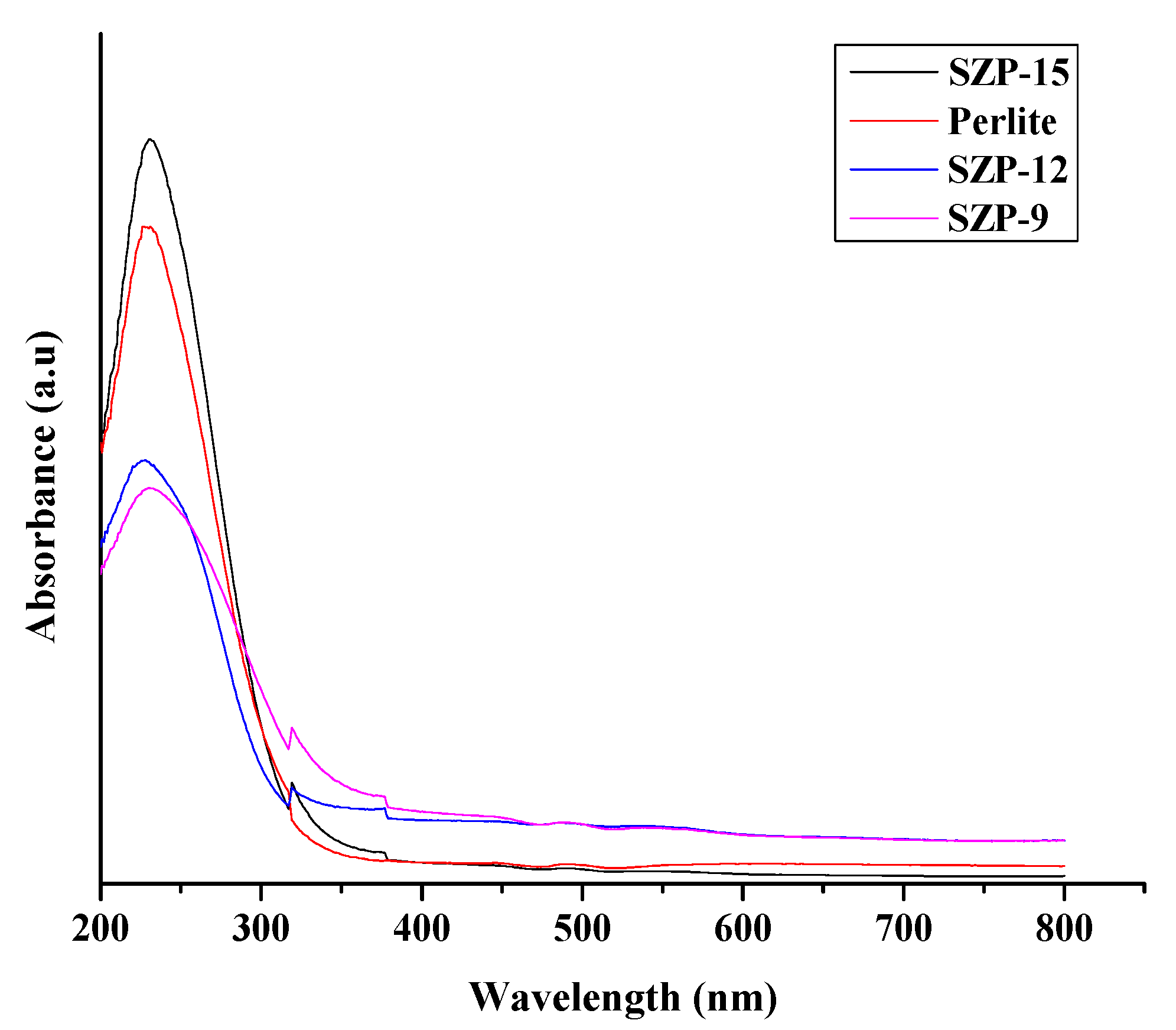


| Samples | BET Surface Area (m2/g) |
|---|---|
| Perlite | 3 |
| TAP | 2 |
| SZP-9 | 49 |
| SZP-12 | 66 |
| SZP-15 | 80 |
| Surface area, SBET (m2/g) | 80 |
| Sulfur content (wt.%) | 5.46 |
| Total pore volume, Vp at (p/po = 0.95) (cm3/g) | 0.03 |
| Average pore diameter, Dp (nm) | 4.8 |
| Crystallite size (nm) | Cannot be measured |
| Particle size (nm) | 9–25 |
| Samples | SiO2 (wt%) | Al2O3 (wt%) | K2O (wt%) | Na2O (wt%) | ZnO (wt%) | FeO (wt%) | TiO2 (wt%) | SO3 (wt%) | ZrO2 (wt%) |
|---|---|---|---|---|---|---|---|---|---|
| Perlite | 72.74 | 14.79 | 7.02 | 2.10 | 2.04 | 0.91 | 0.40 | - | - |
| SZP-9 | 77.27 | 16.64 | - | 1.40 | - | - | 0.40 | 1.31 | 2.98 |
| SZP-12 | 74.51 | 16.63 | - | 1.67 | - | - | 0.32 | 1.24 | 5.63 |
| SZP-15 | 69.71 | 14.45 | - | 1.83 | - | - | - | 2.03 | 11.98 |
| Catalysts | Conversion (%) |
|---|---|
| Without catalyst | Negligible |
| Perlite | Negligible |
| TAP | Negligible |
| SZP-9 | 78 ± 1.8 |
| SZP-12 | 86 ± 0.7 |
| SZP-15 | 92 ± 0.7 |
| Reaction Temperature (°C) | Conversion (%) of o-Cresol | mb (%) | Product Selectivity (%) | ||
|---|---|---|---|---|---|
| 6-TBOC | 4-TBOC | 4,6-DTBOC | |||
| 195 | 59 ± 0.7 | 97 | 83 ± 0.3 | 13 ± 0.15 | 04 |
| 215 | 75 ± 0.7 | 96 | 90 ± 0.3 | 09 ± 0.3 | 01 |
| 235 | 94 ± 0.7 | 95.2 | 95 ± 0.06 | 05 | - |
| 255 | 88 ± 0.8 | 95.5 | 95 ± 0.3 | 05 | - |
| 275 | 83 ± 0.3 | 95.7 | 92 ± 0.3 | 08 ± 0.4 | - |
| Reaction Temperature (°C) | Conversion (%) of m-Cresol | mb (%) | Product Selectivity (%) | ||
|---|---|---|---|---|---|
| 6-TBMC | 4,6-DTBMC | 4-TBMC | |||
| 205 | 70 ± 0.3 | 98.9 | 88 ± 0.3 | 10 ± 0.15 | 02 |
| 225 | 85 ± 0.3 | 98.8 | 92 ± 0.1 | 06 ± 0.06 | 02 |
| 245 | 98 ± 0.7 | 98.3 | 100 ± 0.05 | - | - |
| 265 | 90 ± 0.7 | 98.6 | 100 ± 0.06 | - | - |
| 285 | 88 ± 0.7 | 98.6 | 95 ± 0.3 | 05 | - |
| Reaction Temperature (°C) | Conversion (%) of p-Cresol | mb (%) | Product Selectivity (%) | ||
|---|---|---|---|---|---|
| 2-TBPC | 2,6-DTBPC | TBPCE | |||
| 205 | 71 ± 0.8 | 98.3 | 90 ± 0.1 | 07 ± 0.4 | 03 |
| 225 | 83 ± 0.7 | 97.8 | 89 ± 0.1 | 08 ± 0.15 | 03 |
| 245 | 92 ± 0.6 | 97.9 | 98 ± 0.05 | 02 | - |
| 265 | 85 ± 0.7 | 97.4 | 90 ± 0.1 | 10 ± 0.3 | - |
| 285 | 79 ± 0.3 | 97.6 | 90 ± 0.1 | 10 ± 0.3 | - |
| Molar Ratio | Conversion (%) of o-Cresol | mb (%) | Product Selectivity (%) | ||
|---|---|---|---|---|---|
| 6-TBOC | 4,6-DTBOC | 4-TBOC | |||
| 1:1 | 80 ± 0.7 | 95.9 | 85 ± 0.1 | 13 ± 0.5 | 02 |
| 1:2 | 95 ± 0.6 | 96.5 | 95 ± 0.05 | 05 | - |
| 1:3 | 78 ± 0.8 | 97.7 | 88 ± 0.1 | 10 ± 0.1 | 02 |
| 1:4 | 68 ± 0.9 | 98 | 72 ± 0.15 | 20 ± 0.3 | 08 ± 0.1 |
| 2:1 | 86 ± 0.7 | 97.2 | 90 ± 0.06 | 07 ± 0.1 | 03 |
| Molar Ratio | Conversion (%) of m-Cresol | mb (%) | Product Selectivity (%) | ||
|---|---|---|---|---|---|
| 6-TBMC | 4,6-DTBMC | 4-TBMC | |||
| 1:1 | 90 ± 0.6 | 98.8 | 90 ± 0.1 | 04 | 06 ± 0.03 |
| 1:2 | 96 ± 0.6 | 98.5 | 100 ± 0.05 | - | - |
| 1:3 | 88 ± 0.6 | 98.3 | 90 ± 0.1 | 08 ± 0.06 | 02 |
| 1:4 | 54 ± 0.7 | 98.7 | 78 ± 0.1 | 18 ± 0.1 | 04 |
| 2:1 | 72 ± 0.8 | 98.5 | 84 ± 0.1 | 09 ± 0.2 | 07 ±0.3 |
| Molar Ratio | Conversion (%) of p-Cresol | mb (%) | Product Selectivity (%) | ||
|---|---|---|---|---|---|
| 2-TBPC | 2,6-DTBPC | TBPCE | |||
| 1:1 | 92 ± 0.7 | 98.2 | 98 ± 0.02 | 02 | - |
| 1:2 | 83 ± 0.7 | 98.4 | 89 ± 0.05 | 08 ± 0.12 | 03 |
| 1:3 | 78 ± 0.7 | 98.8 | 82 ± 0.06 | 12 ± 0.08 | 06 ± 0.1 |
| 1:4 | 52 ± 0.3 | 98.1 | 70 ± 0.08 | 20 ± 0.05 | 10 ± 0.1 |
| 2:1 | 68 ± 0.8 | 98.5 | 78 ± 0.07 | 14 ± 0.07 | 08 ± 0.1 |
| Catalysts | Conversion (%) of p-Cresol in First Reaction Cycle | Conversion (%) of p-Cresol in Last Reaction Cycle | Reusability of Catalyst (No. of Runs) | Reaction Medium | References |
|---|---|---|---|---|---|
| 15% TPA/ZrO2 a | ~61 | - | - | Vapor phase | [26] |
| D3-MMT b | 72.84 | 49.40 | 03 | Microwave irradiations | [22] |
| Bronsted acidic ionic liquid (IL-1) c | 80.7 | 77.9 | 05 | Conventional (autoclave) | [68] |
| SZP-15 d | 92 | 85 | 05 | Vapor phase | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malpani, S.K.; Goyal, D.; Chinnam, S.; Sharma, S.K.; Katara, S.; Rani, A. Vapor Phase Alkylation of Isomeric Cresols with Tert-Butyl Alcohol over Perlite Supported Sulfated Zirconia Catalyst. Sustainability 2022, 14, 5149. https://doi.org/10.3390/su14095149
Malpani SK, Goyal D, Chinnam S, Sharma SK, Katara S, Rani A. Vapor Phase Alkylation of Isomeric Cresols with Tert-Butyl Alcohol over Perlite Supported Sulfated Zirconia Catalyst. Sustainability. 2022; 14(9):5149. https://doi.org/10.3390/su14095149
Chicago/Turabian StyleMalpani, Sakshi Kabra, Deepti Goyal, Sampath Chinnam, Sunil K. Sharma, Stuti Katara, and Ashu Rani. 2022. "Vapor Phase Alkylation of Isomeric Cresols with Tert-Butyl Alcohol over Perlite Supported Sulfated Zirconia Catalyst" Sustainability 14, no. 9: 5149. https://doi.org/10.3390/su14095149
APA StyleMalpani, S. K., Goyal, D., Chinnam, S., Sharma, S. K., Katara, S., & Rani, A. (2022). Vapor Phase Alkylation of Isomeric Cresols with Tert-Butyl Alcohol over Perlite Supported Sulfated Zirconia Catalyst. Sustainability, 14(9), 5149. https://doi.org/10.3390/su14095149








