Impact of Sprouting under Potassium Nitrate Priming on Nitrogen Assimilation and Bioactivity of Three Medicago Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Analysis of Mineral Contents
2.3. Determination of Leaf Pigments
2.4. Determination of Amino Acids
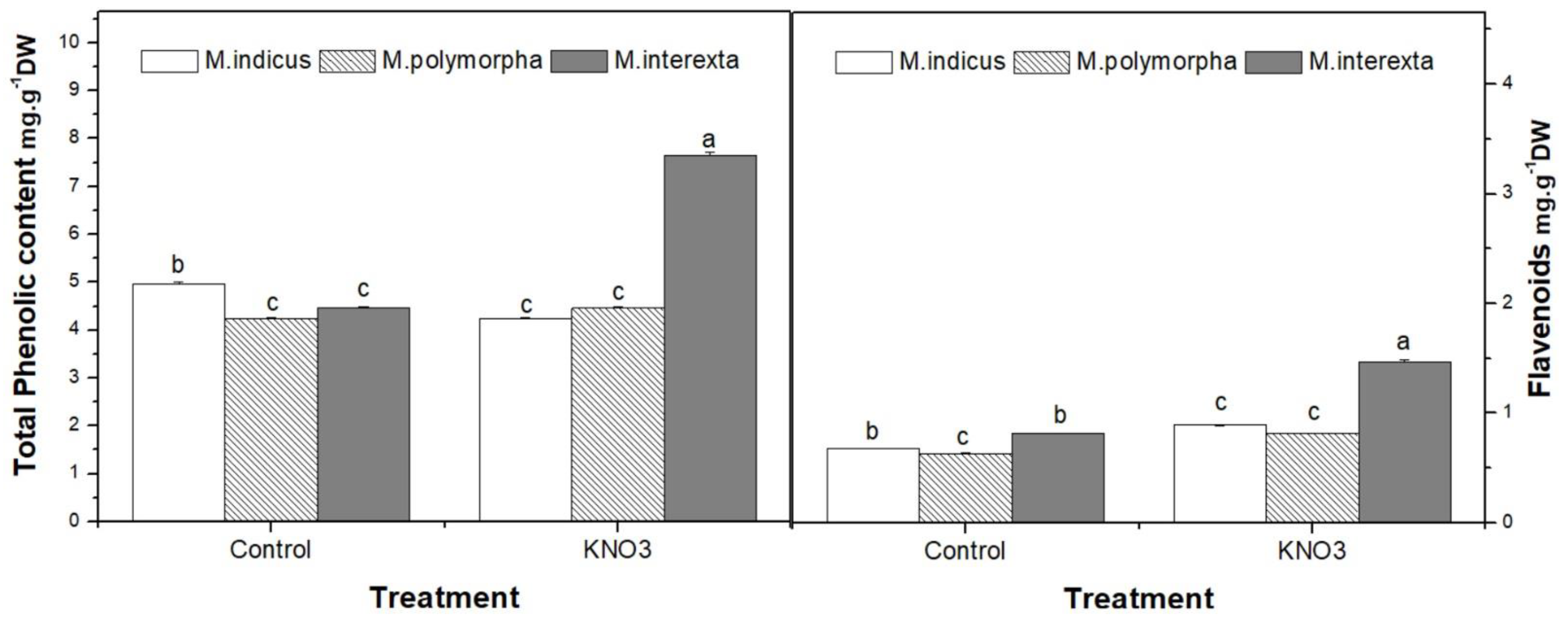
2.5. Determination of Polyphenols and Flavonoid Contents
2.6. Determination of Vitamin Contents
2.7. Determination of Total Proteins
2.8. Determination of N, Ammonium, and Nitrate Contents
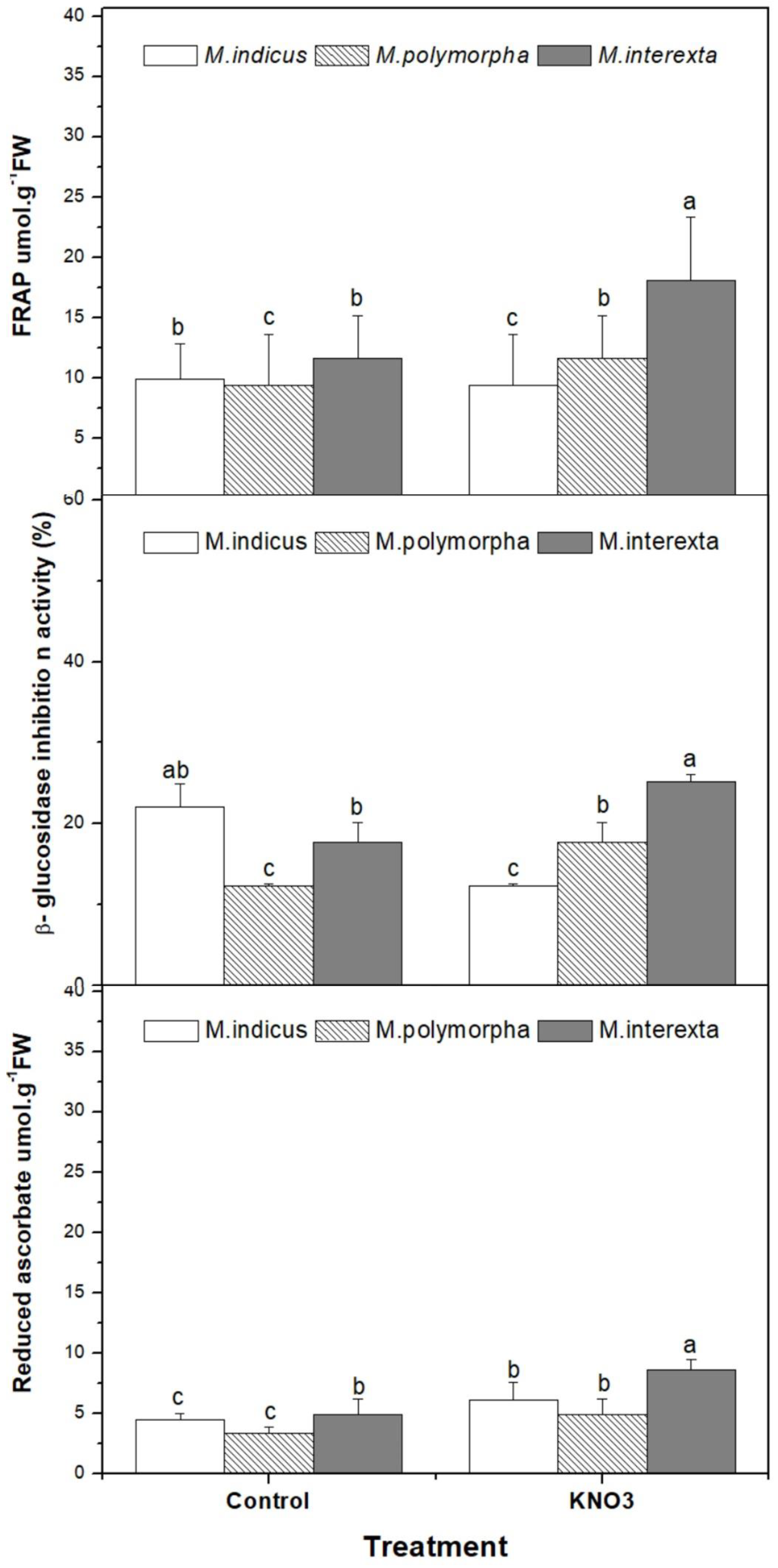
2.9. Determination of Antioxidant and Antidiabetic Activities
2.9.1. Antioxidant Activity
2.9.2. Antidiabetic Activity
α-Amylase Inhibition Assay
α-Glucosidase Inhibition Assay
2.9.3. Glycemic Index GI
2.10. Statistical Analyses
3. Results
3.1. Growth and Photosynthetic Pigments of Medicago Sprouts
3.2. Improvement of Nutritive Values: Mineral Content, Vitamins, and Antioxidant Activities
3.3. Amino Acid Metabolism
3.4. Antioxidant and Antidiabetic Avtivities
3.4.1. Antioxidant Metabolites and Free Radical Scavenging Activity of Medicago Sprouts
3.4.2. Antidiabetic Activity
3.5. Principal Component Analysis (PCA)
4. Discussion
4.1. KNO3 Priming Increased Biomass Accumulation in Medicago Sprouts
4.2. KNO3 Priming Increases Nutritive Values of Medicago Sprouts
4.3. KNO3 Priming Promotes N assimilation in Medicago Sprouts
4.4. Antioxidant Metabolites Accumulation Increased Antioxidant Biological Activity of Medicago Sprouts Extracts
4.5. Species-Specific Responses to KNO3 Priming
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Plaza, L.; De Ancos, B.; Cano, M.P. Nutritional and health-related compounds in sprouts and seeds of soybean (Glycine max), wheat (Triticum aestivum L.) and alfalfa (Medicago sativa) treated by a new drying method. Eur. Food Res. Technol. 2003, 216, 138–144. [Google Scholar] [CrossRef]
- Marton, M.; Mandoki, Z.; Caspo, J.; Caspo-Kiss, Z.; Marton, M.; Mándoki, Z.; Marton, M.; Mandoki, Z.; Caspo, J.; Caspo-Kiss, Z. The role of sprouts in human nutrition. A review. Aliment. Hungarian Univ. Transylvania 2010, 3, 81–117. [Google Scholar]
- Gabrovská, D.; Paulíčková, I.; Mašková, E.; Fiedlerová, V.; Kocurová, K.; Průchová, J.; Strohalm, J.; Houška, M. Changes in selected vitamins, microorganism counts, and sensory quality during storage of pressurised sprouted seed of alfalfa (Medicago sativa L.). Czech. J. Food Sci. 2005, 23, 246–250. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Martino, M.; Ruggeri, S.; Marconi, O.; Sileoni, V.; Falcinelli, B.; Castellini, C.; Benincasa, P. Alfalfa and flax sprouts supplementation enriches the content of bioactive compounds and lowers the cholesterol in hen egg. J. Funct. Foods 2016, 22, 454–462. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Walczak-Skierska, J.; Mozeński, C.; Buszewski, B. Extraction and Determination of Polar Bioactive Compounds from Alfalfa (Medicago sativa L.) Using Supercritical Techniques. Molecules 2019, 24, 4608. [Google Scholar] [CrossRef] [PubMed]
- Apostol, L.; Iorga, S.; Mosoiu, C.; Racovita, R.C.; Niculae, O.M.; Vlasceanu, G. Alfalfa Concentrate—A Rich Source of Nutrients for Use in Food Products. Int. Sci. Publ. 2017, 5, 66–73. [Google Scholar]
- Pandey, S.; Shenmare, K. Review on nutritional profile of Medicago sativa seeds. Asian J. Adv. Res. 2021, 7, 28–31. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Zrig, A.; Tounekti, T.; BenMohamed, H.; Abdelgawad, H.; Vadel, A.M.; Valero, D.; Khemira, H. Differential response of two almond rootstocks to chloride salt mixtures in the growing medium. Russ. J. Plant Physiol. 2016, 63, 143–151. [Google Scholar] [CrossRef]
- Nacry, P.; Bouguyon, E.; Gojon, A. Nitrogen acquisition by roots: Physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 2013, 370, 1–29. [Google Scholar] [CrossRef]
- Reddy, M.M.; Ulaganathan, K. Nitrogen Nutrition, Its Regulation and Biotechnological Approaches to Improve Crop Productivity. Am. J. Plant Sci. 2015, 6, 2745–2798. [Google Scholar] [CrossRef][Green Version]
- Balotf, S.; Kavoosi, G. Differential nitrate accumulation, nitrate reduction, nitrate reductase activity, protein production and carbohydrate biosynthesis in response to potassium and sodium nitrate. Afr. J. Biotechnol. 2011, 10, 17973–17980. [Google Scholar] [CrossRef]
- Yang, S.; Zu, Y.; Li, B.; Bi, Y.; Jia, L.; He, Y.; Li, Y. Response and intraspecific differences in nitrogen metabolism of alfalfa (Medicago sativa L.) under cadmium stress. Chemosphere 2019, 220, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Zhou, H.X. Protein folding and binding in confined spaces and in crowded solutions. J. Mol. Recognit. 2004, 17, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Tanha, A.; Golzardi, F.; Mostafavi, K. Seed Priming to Overcome Autotoxicity of Alfalfa (Medicago sativa). World J. Environ. Biosci. 2017, 6, 1–5. [Google Scholar]
- Lara, T.S.; Lira, J.M.S.; Rodrigues, A.C.; Rakocevic, M.; Alvarenga, A.A. Potassium Nitrate Priming Affects the Activity of Nitrate Reductase and Antioxidant Enzymes in Tomato Germination. J. Agric. Sci. 2014, 6, 72. [Google Scholar] [CrossRef]
- Ali, M.M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of seed priming with potassium nitrate on the performance of tomato. Agriculture 2020, 10, 498. [Google Scholar] [CrossRef]
- Abdelgawad, H.; De Vos, D.; Zinta, G.; Domagalska, M.A.; Beemster, G.T.S.; Asard, H. Grassland species differentially regulate proline concentrations under future climate conditions: An integrated biochemical and modelling approach. New Phytol. 2015, 208, 354–369. [Google Scholar] [CrossRef]
- Hu, X.; Tanaka, A.; Tanaka, R. Simple extraction methods that prevent the artifactual conversion of chlorophyll to chlorophyllide during pigment isolation from leaf samples. Plant Methods 2013, 1, 9–19. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Kamal, J. Quantification of alkaloids, phenols and flavonoids in sunflower (Helianthus annuus L.). Afr. J. Biotechnol. 2011, 10, 3149–3151. [Google Scholar] [CrossRef]
- Sykes, M.; Croucher, J.; Smith, R.A. Proficiency testing has improved the quality of data of total vitamin B2 analysis in liquid dietary supplement. Anal. Bioanal. Chem. 2011, 400, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. Canadian Journal of Biochemistry and Physiology. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Haroon, M.H.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Hamad, I.; Abdelgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic analysis of various date palm fruit (Phoenix dactylifera L.) cultivars from Saudi Arabia to assess their nutritional quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef]
- Zinta, G.; Abdelgawad, H.; Peshev, D.; Weedon, J.T.; Van Den Ende, W.; Nijs, I.; Janssens, I.A.; Beemster, G.T.S.; Asard, H. Dynamics of metabolic responses to periods of combined heat and drought in Arabidopsis thaliana under ambient and elevated atmospheric CO2. J. Exp. Bot. 2018, 69, 2159–2170. [Google Scholar] [CrossRef]
- Farooq, M.; Irfan, M.; Aziz, T.; Ahmad, I.; Cheema, S.A. Seed Priming with Ascorbic Acid Improves Drought Resistance of Wheat. J. Agron. Crop Sci. 2013, 199, 12–22. [Google Scholar] [CrossRef]
- Shah, S.A.; Zeb, A.; Masood, T.; Noreen, N.; Abbas, S.J.; Samiullah, M.; Alim, M.A.; Muhammad, A. Effects of sprouting time on biochemical and nutritional qualities of Mungbean varieties. Afr. J. Agric. Res. 2011, 6, 5091–5098. [Google Scholar] [CrossRef]
- Anwar, A.; Yu, X.; Li, Y. Seed priming as a promising technique to improve growth, chlorophyll, photosynthesis and nutrient contents in cucumber seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 116–127. [Google Scholar] [CrossRef]
- Ahmadvand, G.; Soleimani, F.; Saadatian, B.; Pouya, M. Effect of Seed Priming with Potassium Nitrate on Germination and Emergence Traits of Two Soybean Cultivars under Salinity Stress Conditions. Am. J. Agric. Environ. Sci. 2012, 12, 769–774. [Google Scholar] [CrossRef]
- Iskender, T.; Mustafa, K.A.; Mahmut, K. Rapid and enhanced germination at low temperature of alfalfa and white clover seeds following osmotic priming. Trop. Grasslands 2009, 43, 171–177. [Google Scholar]
- Brooks, S.; Athinuwat, D.; Chiangmai, P.N. Enhancing germination and seedling vigor of upland rice seed under salinity and water stresses by osmopriming. Sci. Technol. Asia 2020, 25, 63–74. [Google Scholar] [CrossRef]
- Mapongmetsem, P.M.; Duguma, B.; Nkongmeneck, B.A.; Selegny, E. The effect of various seed pretreatments to improve germination in eight indigenous tree species in the forests of Cameroon. Ann. For. Sci. 1999, 56, 679–684. [Google Scholar] [CrossRef]
- Gaweł, E. Chemical composition of lucerne leaf extract (EFL) and its applications as a phytobiotic in human nutrition. Acta Sci. Pol. Technol. Aliment. 2012, 11, 303–309. [Google Scholar]
- Yanar, M.; Erçen, Z.; Özlüer Hunt, A.; Büyükçapar, H.M. The use of alfalfa, Medicago sativa as a natural carotenoid source in diets of goldfish, Carassius auratus. Aquaculture 2008, 284, 196–200. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Kim, H.Y.; Shin, H.S.; Park, H.; Kim, Y.C.; Yun, Y.G.; Park, S.; Shin, H.J.; Kim, K. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J. Clin. Virol. 2008, 41, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Soto-Zarazúa, M.G.; Bah, M.; Costa, A.S.G.; Rodrigues, F.; Pimentel, F.B.; Rojas-Molina, I.; Rojas, A.; Oliveira, M.B.P.P. Nutraceutical Potential of New Alfalfa (Medicago sativa) Ingredients for Beverage Preparations. J. Med. Food 2017, 20, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Brennan, L.; Bender, K. Amino acid metabolism, β-cell function, and diabetes. Diabetes 2006, 55, 39–47. [Google Scholar] [CrossRef]
- Gaweł, E.; Grzelak, M.; Janyszek, M. Lucerne (Medicago sativa L.) in the human diet—Case reports and short reports. J. Herb. Med. 2017, 10, 8–16. [Google Scholar] [CrossRef]
- Jabeen, N.; Ahmad, R. Foliar application of potassium nitrate affects the growth and nitrate reductase activity in sunflower and safflower leaves under salinity. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 172–178. [Google Scholar] [CrossRef][Green Version]
- León-López, L.; Escobar-Zúñiga, Y.; Salazar-Salas, N.Y.; Mora Rochín, S.; Cuevas-Rodríguez, E.O.; Reyes-Moreno, C.; Milán-Carrillo, J. Improving Polyphenolic Compounds: Antioxidant Activity in Chickpea Sprouts through Elicitation with Hydrogen Peroxide. Foods 2020, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Caunii, A.; Pribac, G.; Grozea, I.; Gaitin, D.; Samfira, I. Design of optimal solvent for extraction of bio-active ingredients from six varieties of Medicago sativa. Chem. Cent. J. 2012, 6, 1–8. [Google Scholar] [CrossRef]
- Salih, B.A.; Azeez, K.O. Antidiabetic Action of Alfalfa (Medicago sativa) Leaves Powder on Type II Diabetic Patients. Polytech. J. 2019, 9, 23–25. [Google Scholar] [CrossRef]
- Helal, E.G.E.; Abd El-Wahab, S.M.; Atia, T.A. Hypoglycemic Effect of the Aqueous Extracts of Lupinus Albus, Medicago sativa (Seeds) and their Mixture on Diabetic Rats. Egypt. J. Hosp. Med. 2013, 685–698. [Google Scholar] [CrossRef]
- Julier, B.; Huyghe, C.; Ecalle, C. Within- and Among-Cultivar Genetic Variation in Alfalfa: Forage Quality, Morphology, and Yield. Crop Sci. 2000, 369, 365–369. [Google Scholar] [CrossRef]
- Basbag, M.; Demirel, R.; Avci, M. Determination of some agronomical and quality properties of wild alfalfa (Medicago sativa L.) clones in Turkey Determination of some agronomical and quality properties of wild alfalfa (Medicago sativa L.) clones in Turkey. J. Food Agric. Environ. 2009, 7, 357–359. [Google Scholar]




| Parameters | Control | KNO3 Priming | ||||
|---|---|---|---|---|---|---|
| M. indicus | M. polymorpha | M. interexta | M. indicus | M. polymorpha | M. interexta | |
| Ca mg·g−1 dw | 17.57 ± 2.35 b | 12.68 ± 1.87 b | 15.79 ± 3.5 b | 27.79 ± 6.69 a | 19.63 ± 3.48 a | 25.17 ± 0.46 a |
| Cu μg·g−1 dw | 2.26 ± 0.71 a | 2.42 ± 0.79 a | 2.57 ± 1.06 b | 2.87 ± 0.28 a | 3.963 ± 0.86 a | 4.38 ± 1.13 a |
| Fe μg·g−1 dw | 3.99 ± 0.238 b | 4.80 ± 1.06 b | 3.15 ± 0.78 b | 5.48 ± 1.02 a | 6.798 ± 1.69 a | 5.76 ± 0.44 a |
| Zn μg·g−1 dw | 22.62 ± 2.08 b | 13.49 ± 1.10 b | 36.61 ± 3.25 a | 35.8 ± 3.21 a | 17.24 ± 1.50 a | 35.71 ± 3.16 a |
| Mn μg·g−1 dw | 0.25 ± 0.12 a | 0.19 ± 0.13 b | 0.13 ± 0.13 b | 0.27 ± 0.12 a | 0.30 ± 0.12 a | 0.27 ± 0.12 a |
| Mg mg·g−1 dw | 2.90 ± 0.160 a | 2.38 ± 0.10 b | 2.69 ± 0.14 b | 2.67 ± 0.12 a | 3.81 ± 0.22 a | 3.65 ± 0.22 a |
| K mg·g−1 dw | 15.7 ± 1.311 b | 20.8 ± 1.76 b | 12.0 ± 0.98 b | 40.6 ± 3.57 a | 60.5 ± 5.50 a | 67.3 ± 6.02 a |
| P mg·g−1 dw | 5.8 ± 0.57 b | 7.6 ± 0.595 b | 6.5 ± 0.46 b | 10.4 ± 0.84 a | 11.2 ± 0.93 a | 13.6 ± 1.13 a |
| Parameters | Control | KNO3 Priming | ||||
|---|---|---|---|---|---|---|
| M. indicus | M. polymorpha | M. interexta | M. indicus | M. polymorpha | M. interexta | |
| Vit C (mg·g−1 FW) | 7.81 ± 1.35 b | 7.67 ± 1.33 b | 7.31 ± 1.26 b | 8.15 ± 2.39 a | 9.48 ± 1.37 a | 13.92 ± 0.76 a |
| Vit E (mg·g−1 FW) | 47.46 ± 1.17 a | 38.05 ± 1.09 b | 44.57 ± 1.58 b | 48.47 ± 4.40 a | 59.72 ± 2.43 a | 61.92 ± 3.86 a |
| Thiamin (mg·g−1 FW) | 0.10 ± 0.00 b | 0.13 ± 0.02 a | 0.06 ± 0.02 b | 0.13 ± 0.02 a | 0.13 ± 0.07 a | 0.14 ± 0.06 a |
| Riboflavin (mg·g−1 FW) | 0.30 ± 0.05 b | 0.52 ± 0.09 b | 0.75 ± 0.22 b | 0.46 ± 0.22 a | 0.87 ± 0.07 a | 0.96 ± 0.46 a |
| Parameters | Control | KNO3 Priming | ||||
|---|---|---|---|---|---|---|
| M. indicus | M. polymorpha | M. interexta | M. indicus | M. polymorpha | M. interexta | |
| Amino acids (mg·g−1 fw) | ||||||
| Asparagine | 1.64 ± 0.29 a | 1.71 ± 0.38 b | 1.9 ± 0.35 b | 1.79 ± 1.52 a | 1.99 ± 1.73 a | 2.2 ± 1.93 a |
| Glutamine | 1.86 ± 0.41 b | 1.96 ± 0.45 b | 2.17 ± 0.53 b | 3.56 ± 3.32 a | 4.07 ± 3.95 a | 4.27 ± 4.23 a |
| Serine | 1.37 ± 0.04 b | 1.43 ± 0.15 a | 1.59 ± 0.08 b | 2.03 ± 2.16 a | 1.25 ± 1.35 b | 2.02 ± 2.48 a |
| Glycine | 1.56 ± 0.28 a | 1.68 ± 0.41 a | 1.83 ± 0.28 b | 1.18 ± 1.16 b | 1.88 ± 1.62 a | 2.02 ± 1.86 a |
| Arginine | 0.31 ± 0.1 b | 0.35 ± 0.15 b | 0.37 ± 0.17 b | 0.88 ± 0.78 a | 0.84 ± 0.81 a | 0.41 ± 0.61 a |
| Alanine | 0.58 ± 0.1 a | 0.71 ± 0.03 a | 0.68 ± 0.12 a | 0.51 ± 0.5 a | 0.57 ± 0.5 a | 0.65 ± 0.61 a |
| Proline | 1.19 ± 0.44 b | 1.34 ± 0.46 b | 1.59 ± 0.59 b | 2.67 ± 3.11 a | 2.37 ± 3.26 a | 3.09 ± 3.42 a |
| Histidine | 0.74 ± 0.11 a | 0.81 ± 0.11 a | 0.89 ± 0.11 a | 0.58 ± 0.58 b | 0.41 ± 0.59 b | 0.52 ± 0.74 b |
| Valine | 0.89 ± 0.38 a | 0.97 ± 0.43 a | 1.05 ± 0.48 a | 0.79 ± 0.54 a | 0.65 ± 0.55 b | 0.96 ± 0.71 b |
| Methionine | 0.47 ± 0 b | 0.55 ± 0 b | 0.63 ± 0 b | 0.85 ± 0.89 a | 0.86 ± 0.89 a | 0.86 ± 0.89 a |
| Cystine | 0.87 ± 0.06 b | 0.89 ± 0.03 b | 0.92 ± 0.06 b | 1.44 ± 1.17 a | 1.45 ± 1.26 a | 1.41 ± 1.37 a |
| Isoleucine | 1.11 ± 0.72 b | 1.12 ± 0.76 b | 1.14 ± 0.8 b | 1.39 ± 1.13 a | 1.46 ± 1.17 a | 1.28 ± 0.95 a |
| Leucine | 1.04 ± 0.15 a | 1.01 ± 0.29 a | 0.98 ± 0.43 b | 1.02 ± 0.86 a | 1.15 ± 0.87 a | 1.34 ± 1 a |
| Phenylalanine | 1.98 ± 1.08 a | 1.82 ± 1.17 a | 1.65 ± 1.45 b | 1.88 ± 2.14 a | 1.13 ± 2.11 b | 2.27 ± 2.44 a |
| Tyrosine | 0.31 ± 0.08 b | 0.32 ± 0.05 b | 0.33 ± 0.05 b | 0.4 ± 0.35 a | 0.44 ± 0.34 a | 0.47 ± 0.41 a |
| Lysine | 0.77 ± 0.14 b | 0.84 ± 0.1 b | 0.92 ± 0.07 a | 1.07 ± 0.92 a | 1 ± 0.9 a | 0.85 ± 0.82 b |
| Threonine | 1.33 ± 0.28 a | 1.39 ± 0.26 a | 1.45 ± 0.24 a | 1.47 ± 1.25 a | 1.23 ± 1.26 a | 1.59 ± 1.46 a |
| Tryptophan | 0.78 ± 0.21 b | 0.84 ± 0.23 b | 0.9 ± 0.24 b | 1.11 ± 0.92 a | 1.32 ± 1.04 a | 1.36 ± 1.1 a |
| Nitrogen content and metabolism | ||||||
| Nitrogen (g/100 g) | 23.39 ± 0.89 b | 19.69 ± 0.82 b | 15.72 ± 0.53 b | 28.11 ± 1.24 a | 24.95 ± 0.72 a | 19.20 ± 0.8 a |
| Total protein (mg/g FW) | 169.5 ± 1.9 a | 153.61 ± 8.86 a | 118.0 ± 3.16 b | 99.6 ± 2.27 b | 129.0 ± 8.47 b | 136.8 ± 2.81 a |
| Nitrate reductase μmol nitrite/mg protein.min | 45.24 ± 0.03 a | 49.53 ± 2.47 b | 86.19 ± 5.45 b | 33.11 ± 2.23 b | 56.36 ± 0.91 a | 118 ± 11.27 a |
| GDH μmol NADH/mg protein.min | 4.14 ± 0.21 a | 4.10 ± 0.09 a | 6.99 ± 0.48 b | 4.14 ± 0.21 a | 4.10 ± 0.09 a | 10.33 ± 0.48 a |
| GOGAT μmol NADH/mg protein.min | 7.83 ± 0.28 a | 11.16 ± 0.45 b | 14.35 ± 0.45 b | 6.35 ± 0.29 b | 14.70 ± 0.52 a | 21.38 ± 1.83 a |
| GS μmol γ-glutamyl hydroxamate/mg protein.min | 16.12 ± 0.91 b | 23.09 ± 1.24 b | 26.16 ± 0.45 b | 23.00 ± 1.24 a | 29.2 ± 0.77 a | 32.55 ± 0.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zrig, A.; Saleh, A.; Hamouda, F.; Okla, M.K.; Al-Qahtani, W.H.; Alwasel, Y.A.; Al-Hashimi, A.; Hegab, M.Y.; Hassan, A.H.A.; AbdElgawad, H. Impact of Sprouting under Potassium Nitrate Priming on Nitrogen Assimilation and Bioactivity of Three Medicago Species. Plants 2022, 11, 71. https://doi.org/10.3390/plants11010071
Zrig A, Saleh A, Hamouda F, Okla MK, Al-Qahtani WH, Alwasel YA, Al-Hashimi A, Hegab MY, Hassan AHA, AbdElgawad H. Impact of Sprouting under Potassium Nitrate Priming on Nitrogen Assimilation and Bioactivity of Three Medicago Species. Plants. 2022; 11(1):71. https://doi.org/10.3390/plants11010071
Chicago/Turabian StyleZrig, Ahlem, Ahmed Saleh, Foued Hamouda, Mohammad K. Okla, Wahidah H. Al-Qahtani, Yasmeen A. Alwasel, Abdulrahman Al-Hashimi, Momtaz Y. Hegab, Abdelrahim H. A. Hassan, and Hamada AbdElgawad. 2022. "Impact of Sprouting under Potassium Nitrate Priming on Nitrogen Assimilation and Bioactivity of Three Medicago Species" Plants 11, no. 1: 71. https://doi.org/10.3390/plants11010071
APA StyleZrig, A., Saleh, A., Hamouda, F., Okla, M. K., Al-Qahtani, W. H., Alwasel, Y. A., Al-Hashimi, A., Hegab, M. Y., Hassan, A. H. A., & AbdElgawad, H. (2022). Impact of Sprouting under Potassium Nitrate Priming on Nitrogen Assimilation and Bioactivity of Three Medicago Species. Plants, 11(1), 71. https://doi.org/10.3390/plants11010071