Fermented Deer Blood Ameliorates Intense Exercise-Induced Fatigue via Modulating Small Intestine Microbiota and Metabolites in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Medium
2.2. Preparation of Fermented Deer Blood
2.3. Peptide and Amino Acid Content of Fermented Deer Blood
2.4. Antioxidant Activity Assay of Fermented Deer Blood
2.5. Animal Experimental Design
2.6. Serum and Liver Biochemical Analysis
2.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.8. Gut Microbiota Analysis of Small Intestine and Cecal Contents
2.9. Metabolomic Analysis of Urine and Intestinal Content
2.10. Statistical Analysis
3. Results
3.1. Compositions and Antioxidant Activity of Fermented Deer Blood
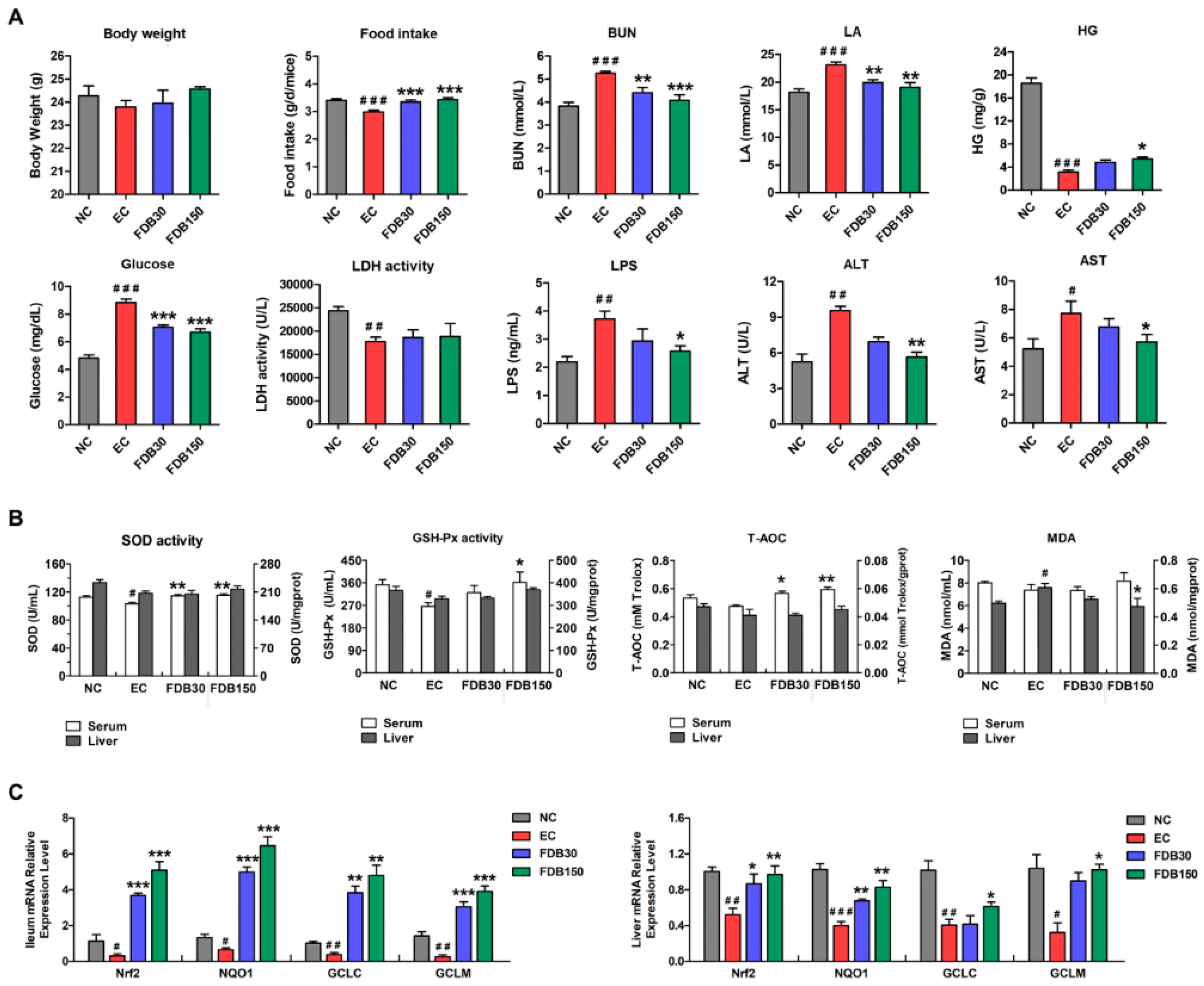
3.2. FDB Alleviated Intense Exercise-Induced Fatigue and Oxidative Stress Response
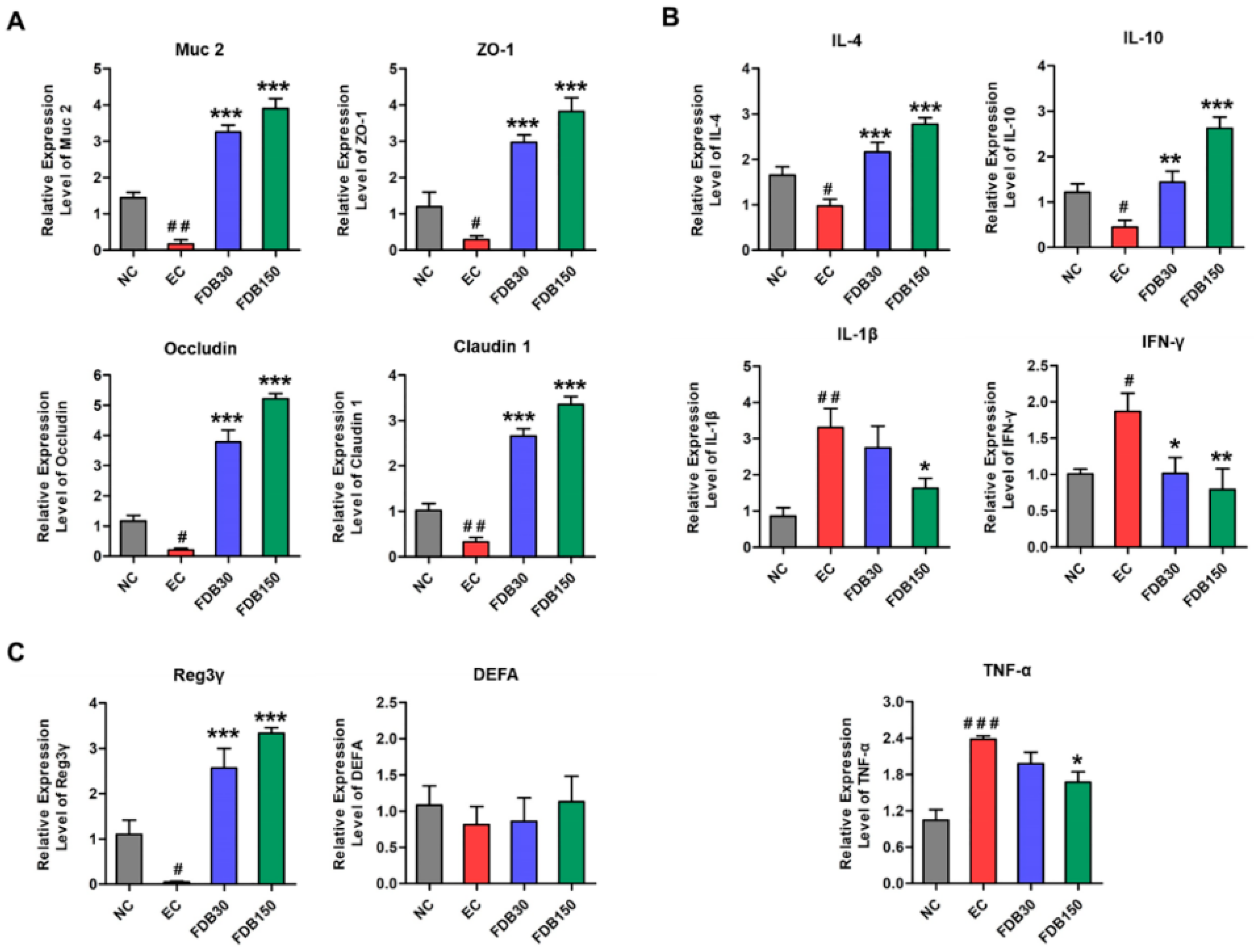
3.3. FDB Improved Intestinal Integrity and Inflammatory Response
3.4. FDB Modulated the Gut Microbiota of Fatigued Mice under Intense Exercise
3.5. Effects of FDB on the Metabolome of Urine and Intestine
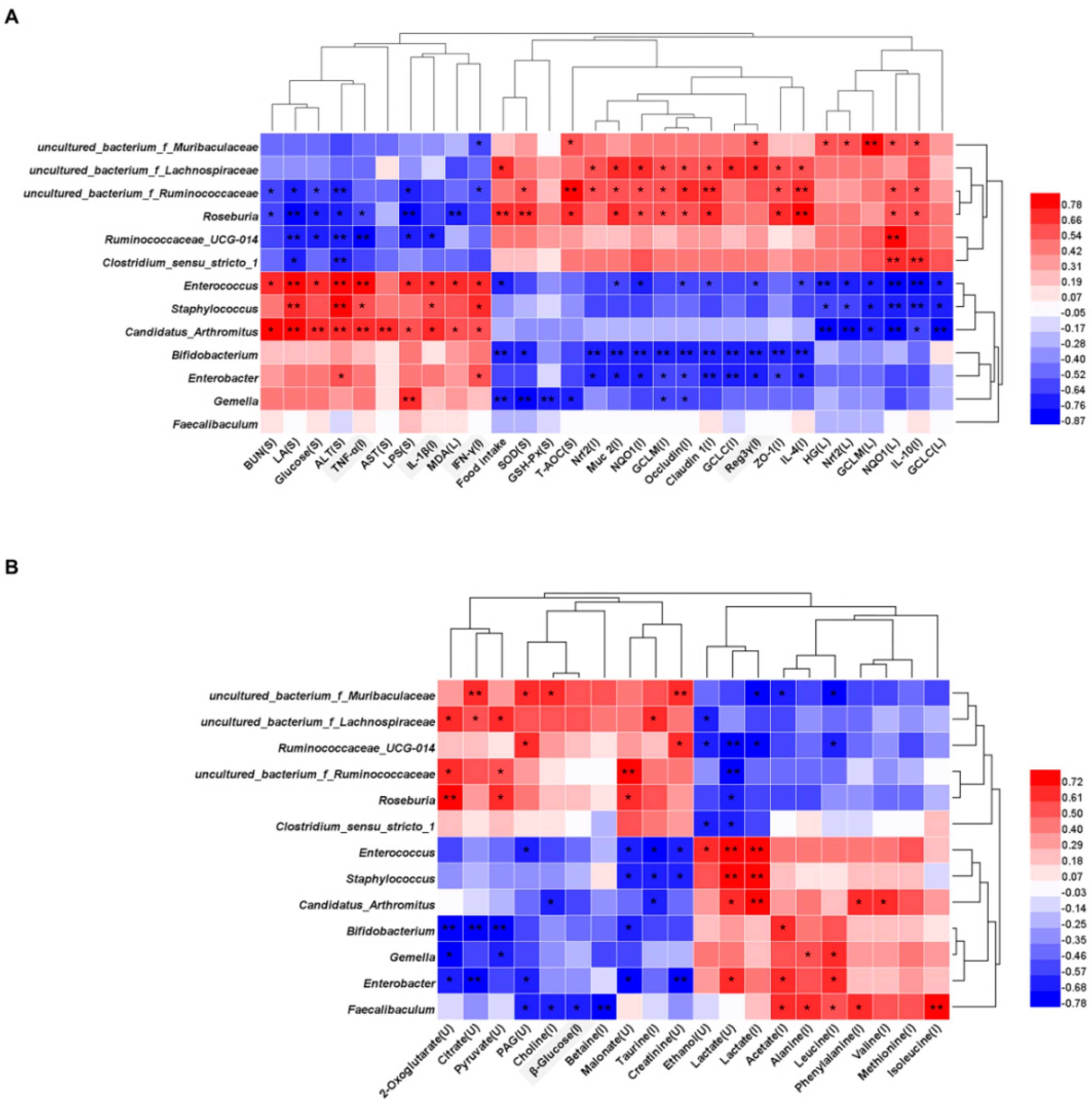
3.6. Association of Gut Microbial Dysbiosis with Fatigue Related Parameters and Dysregulation Metabolites
4. Discussion
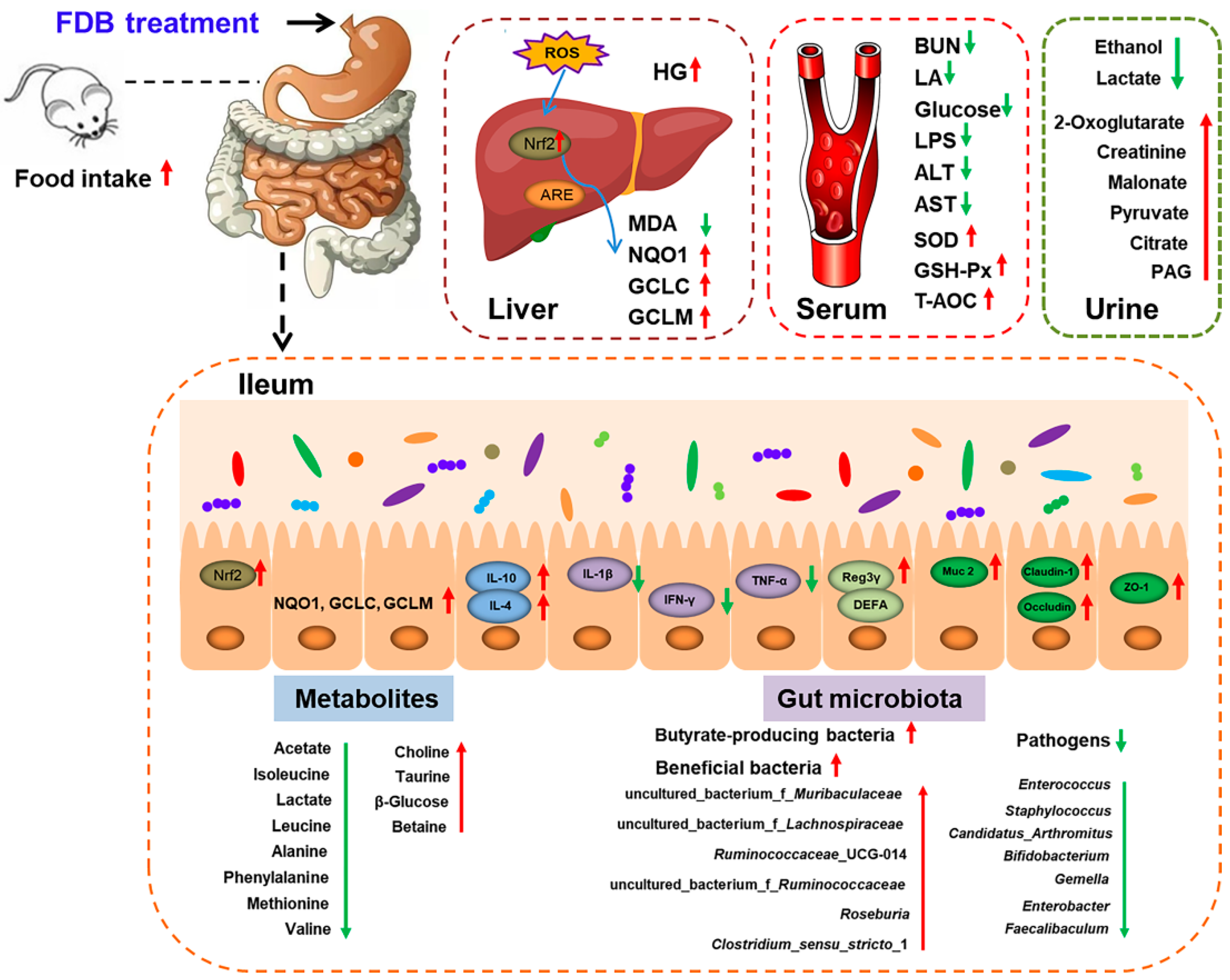
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petriz, B.A.; Castro, A.P.; Almeida, J.A.; Gomes, C.P.; Fernandes, G.R.; Kruger, R.H.; Pereira, R.W.; Franco, O.L. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genom. 2014, 15, 511. [Google Scholar] [CrossRef] [Green Version]
- Roberts, C.K.; Barnard, R.J. Effects of exercise and diet on chronic disease. J. Appl. Physiol. 2005, 98, 3–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Mao, X.; Li, R.W.; Hou, E.; Wang, Y.; Xue, C.; Tang, Q. Neoagarotetraose protects mice against intense exercise-induced fatigue damage by modulating gut microbial composition and function. Mol. Nutr. Food Res. 2017, 61, 1600585. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Ma, N.; Zheng, H.; Ma, G.; Zhao, L.; Hu, Q. Tuber indicum polysaccharide relieves fatigue by regulating gut microbiota in mice. J. Funct. Foods 2019, 63, 103580. [Google Scholar] [CrossRef]
- Frémont, M.; Coomans, D.; Massart, S.; De Meirleir, K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 2013, 22, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Berk, M.; Galecki, P.; Walder, K.; Maes, M. The neuro-immune pathophysiology of central and peripheral fatigue in systemic immune-inflammatory and neuro-immune diseases. Mol. Neurobiol. 2016, 53, 1195–1219. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr. Neuropharmacol. 2014, 12, 168–185. [Google Scholar] [CrossRef] [Green Version]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise modifies the gut microbiota with positive health effects. Oxid. Med. Cell. Longev. 2017, 2017, 383197. [Google Scholar] [CrossRef]
- Chakaroun, R.M.; Massier, L.; Kovacs, P. Gut microbiome, intestinal permeability, and tissue bacteria in metabolic disease: Perpetrators or bystanders? Nutrients 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, E.B.; Martinez-Guryn, K. Small intestinal microbiota: The neglected stepchild needed for fat digestion and absorption. Gut Microbes 2019, 10, 235–240. [Google Scholar] [CrossRef] [Green Version]
- El Aidy, S.; Van Den Bogert, B.; Kleerebezem, M. The small intestine microbiota, nutritional modulation and relevance for health. Curr. Opin. Biotechnol. 2015, 32, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Leser, T.D.; Mølbak, L. Better living through microbial action: The benefits of the mammalian gastrointestinal microbiota on the host. Environ. Microbiol. 2009, 11, 2194–2206. [Google Scholar] [CrossRef]
- Cui, J.; Xia, P.; Zhang, L.; Hu, Y.; Xie, Q.; Xiang, H. A novel fermented soybean, inoculated with selected Bacillus, Lactobacillus and Hansenula strains, showed strong antioxidant and anti-fatigue potential activity. Food Chem. 2020, 333, 127527. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lin, D.; Guo, J.; Zhang, Y.; Zheng, B. In vitro antioxidant activity and in vivo anti-fatigue effect of sea horse (hippocampus) peptides. Molecules 2017, 22, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Y Falavigna, G.; de Araújo Junior, J.A.; Rogero, M.M.; de Oliveira Pires, I.S.; Pedrosa, R.G.; Junior, E.M.; de Castro, I.A.; Tirapegui, J. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nutrients 2012, 4, 1767–1780. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Lin, H.; Deng, S.G. Study of anti-fatigue effect in rats of ferrous chelates including hairtail protein hydrolysates. Nutrients 2015, 7, 9860–9871. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.L.; van der Linden, D.S.; Sugiarto, H.; Anderson, R.C. Antimicrobial peptides isolated from the blood of farm animals. Anim. Prod. Sci. 2010, 50, 660–669. [Google Scholar] [CrossRef]
- Bah, C.S.; Carne, A.; McConnell, M.A.; Mros, S.; Bekhit, A.E.D.A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016, 202, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Mora, L.; Reig, M. New insights into meat by-product utilization. Meat Sci. 2016, 120, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Liu, Q.; Zhao, L.; Yu, J.; Wang, S.; Cao, T.; Gao, X.; Wei, Y. Identification and antihypertension study of novel angiotensin I-converting enzyme inhibitory peptides from the skirt of Chlamys farreri fermented with Bacillus natto. J. Agric. Food Chem. 2021, 69, 146–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, P.; Xie, Y.; Yang, P.; Zheng, S.; Tian, Y.; Li, J.; Feng, D. DNA damage protection and antioxidant activities of peptides isolated from sour meat co-fermented by P. pentosaceus SWU73571 and L. curvatus LAB26. Cyta J. Food 2020, 18, 375–382. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Rufián-Henares, J.Á.; Martínez, R.G.; Miralles, B.; Bergillos, T.; Navarro-Alarcón, M.; Jauregi, P. Antioxidant, ACE-inhibitory and antimicrobial activity of fermented goat milk: Activity and physicochemical property relationship of the peptide components. Food Funct. 2017, 8, 2783–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, K.N.; Barry, D.T.; Rees, R.C.; Dodi, I.A.; McArdle, S.E.; Creaser, C.S.; Bonner, P.L. Estimation of peptide concentration by a modified bicinchoninic acid assay. Anal. Biochem. 2009, 393, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1-6. J. Funct. Foods 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Martins, N.O.; de Brito, I.M.; Araújo, S.S.O.; Negri, G.; de Araújo Carlini, E.; Mendes, F.R. Antioxidant, anticholinesterase and antifatigue effects of Trichilia catigua (catuaba). BMC Complementary Altern. Med. 2018, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Q.; Ma, W.; Ning, K.; Xiang, J.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020, 104, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiang, J.Y.; Xiang, H.; Xie, Q. Cecal butyrate (not propionate) was connected with metabolism-related chemicals of mice, based on the different effects of the two inonotus obliquus extracts on obesity and their mechanisms. ACS Omega 2020, 5, 16690–16700. [Google Scholar] [CrossRef]
- Jia, J.M.; Wu, C.F. Antifatigue Activity of Tissue Culture Extracts of Saussurea involucrata. Pharm. Biol. 2008, 46, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Praphatsorn, P.; Thong-Ngam, D.; Kulaputana, O.; Klaikeaw, N. Effects of intense exercise on biochemical and histological changes in rat liver and pancreas. Asian Biomed. 2010, 4, 619–625. [Google Scholar] [CrossRef] [Green Version]
- Duan, F.; Guo, Y.; Li, J.; Yuan, K. Antifatigue effect of luteolin-6-C-neohesperidoside on oxidative stress injury induced by forced swimming of rats through modulation of Nrf2/ARE signaling pathways. Oxid. Med. Cell. Longev. 2017, 2017, 3159358. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cao, W.; Xiang, D.; Hu, Y.P.; Luo, B.; Chen, P. Exercise induces tissue hypoxia and HIF-1α redistribution in the small intestine. J. Sport Health 2020, 9, 82–89. [Google Scholar] [CrossRef]
- Xie, S.Z.; Liu, B.; Ye, H.Y.; Li, Q.M.; Pan, L.H.; Zha, X.Q.; Liu, J.; Duan, J.; Luo, J.P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Yuan, Z.-W.; Qu, C.; Yu, X.-T.; Huang, T.; Chen, P.-V.; Su, Z.-R.; Dou, Y.-X.; Wu, J.-Z.; Zeng, H.-F.; et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol. Res. 2018, 137, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Watanabe, K.; Kimura, I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Sun, R.; Qiao, X.J.; Xu, C.C.; Shang, X.Y.; Niu, W.N. Protective effect of glutamine on intestinal injury and bacterial community in rats exposed to hypobaric hypoxia environment. World J. Gastroenterol. 2014, 20, 4662–4674. [Google Scholar] [CrossRef]
- Chaves, F.M.; Baptista, I.L.; Simabuco, F.M.; Quaresma, P.G.F.; Pena, F.L.; Bezerra, R.M.N.; Pauli, J.R.; da Cunha, D.T.; Campos-Ferraz, P.L.; Antunes, A.E.C. High-intensity- exercise-induced intestinal damage is protected by fermented milk supplemented with whey protein, probiotic and pomegranate (Punica granatum L.). Br. J. Nutr. 2018, 119, 896–909. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Macfarlane, G.T.; Cummings, J.H. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut 1993, 34, 437–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sartor, R.B. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology 2010, 139, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, J.R.; Wettenhall, R.E.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; McGregor, N.; Tapleton, D.I.; Butt, H.; De Meirleir, K. Increased d-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 2009, 23, 621–628. [Google Scholar] [PubMed]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sports Med. 2014, 44, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Zeng, S.L.; Li, S.Z.; Xiao, P.T.; Cai, Y.Y.; Chu, C.; Chen, B.Z.; Li, P.; Li, J.; Liu, E.H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef] [Green Version]
- Pundir, C.S.; Kumar, P.; Jaiwal, R. Biosensing methods for determination of creatinine: A review. Biosens. Bioelectron. 2019, 126, 707–724. [Google Scholar] [CrossRef]
- Heng, X.; Liu, W.; Chu, W. Identification of choline-degrading bacteria from healthy human feces and used for screening of trimethylamine (TMA)-lyase inhibitors. Microb. Pathog. 2020, 152, 104658. [Google Scholar] [CrossRef]
- Maulidiani, M.; Abas, F.; Rudiyanto, R.; Abd Kadir, N.H.; Zolkeflee, N.K.Z.; Lajis, N.H. Analysis of urinary metabolic alteration in type 2 diabetic rats treated with metformin using the metabolomics of quantitative spectral deconvolution 1H NMR spectroscopy. Microchem. J. 2020, 153, 104513. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zheng, H.; Lu, R.; Huang, H.; Zhu, H.; Yin, C.; Mo, Y.; Wu, J.; Liu, X.; Deng, M.; et al. Intervening effects of total alkaloids of Corydalis saxicola Bunting on rats with antibiotic-induced gut microbiota dysbiosis based on 16S rRNA gene sequencing and untargeted metabolomics analyses. Front. Microb. Immunol. 2019, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Shi, C.; Xia, P.; Ning, K.; Xiang, H.; Xie, Q. Fermented Deer Blood Ameliorates Intense Exercise-Induced Fatigue via Modulating Small Intestine Microbiota and Metabolites in Mice. Nutrients 2021, 13, 1543. https://doi.org/10.3390/nu13051543
Cui J, Shi C, Xia P, Ning K, Xiang H, Xie Q. Fermented Deer Blood Ameliorates Intense Exercise-Induced Fatigue via Modulating Small Intestine Microbiota and Metabolites in Mice. Nutrients. 2021; 13(5):1543. https://doi.org/10.3390/nu13051543
Chicago/Turabian StyleCui, Jingwen, Chao Shi, Peibin Xia, Ke Ning, Hongyu Xiang, and Qiuhong Xie. 2021. "Fermented Deer Blood Ameliorates Intense Exercise-Induced Fatigue via Modulating Small Intestine Microbiota and Metabolites in Mice" Nutrients 13, no. 5: 1543. https://doi.org/10.3390/nu13051543





