Colored Surfaces Made of Synthetic Eumelanin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silicon Wafer Characteristics
2.2. Poly(L-DOPA) Coating Process
3. Results and Discussion
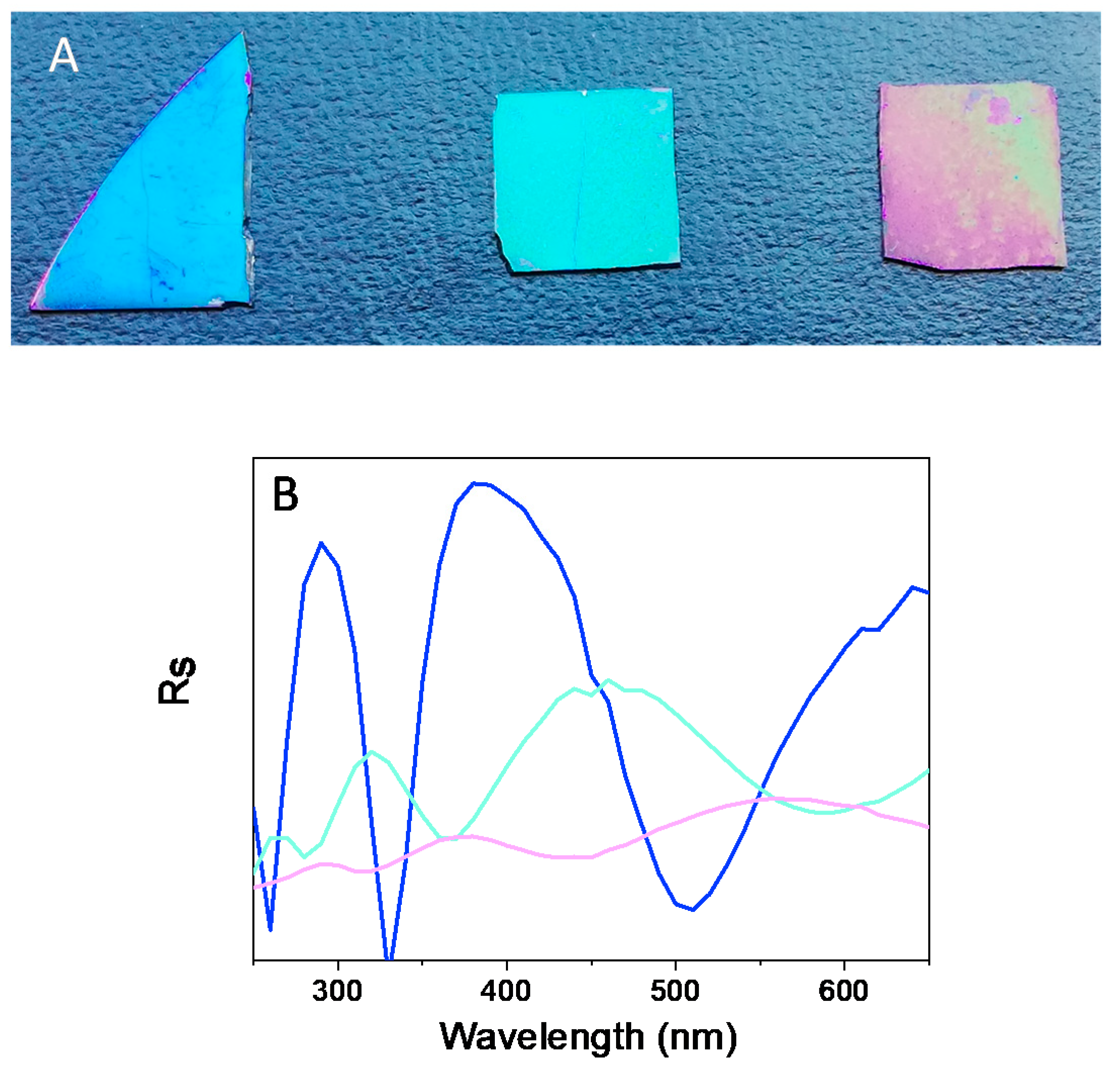
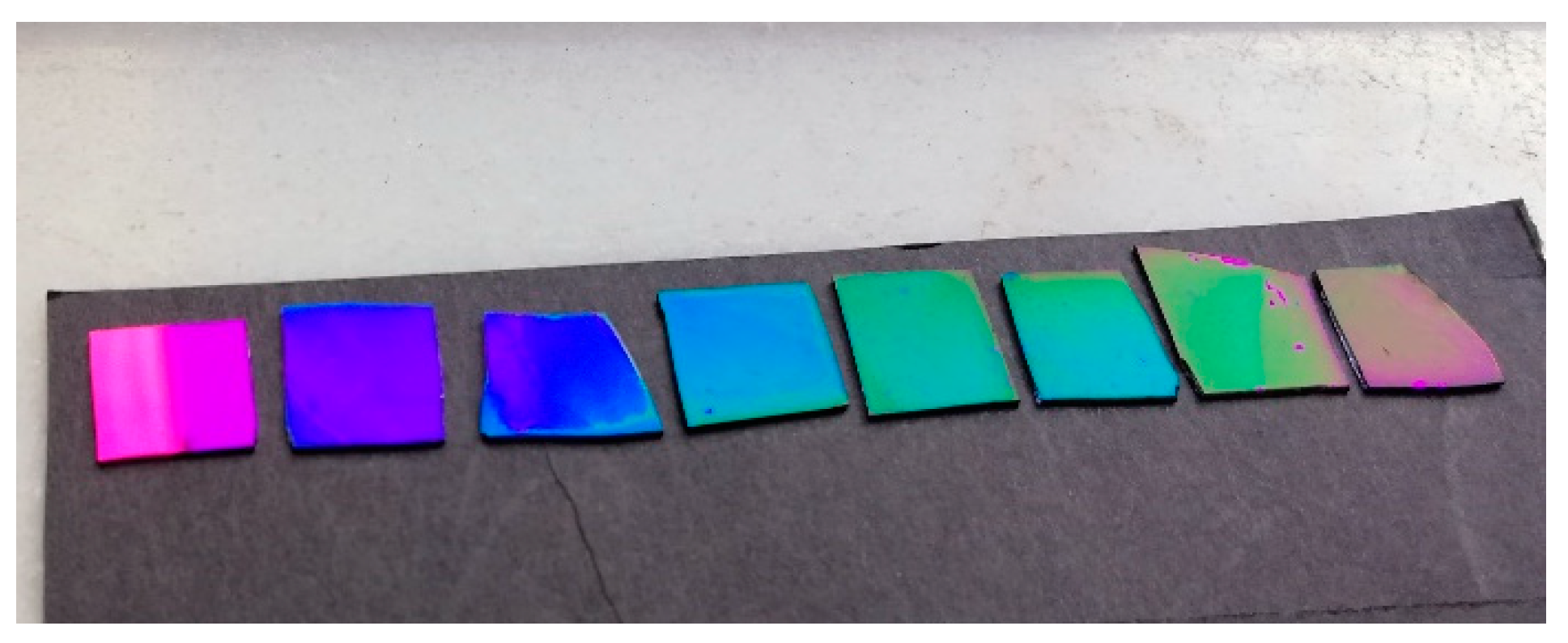
3.1. Effect of NaCl Concentration on Film Growth and Color
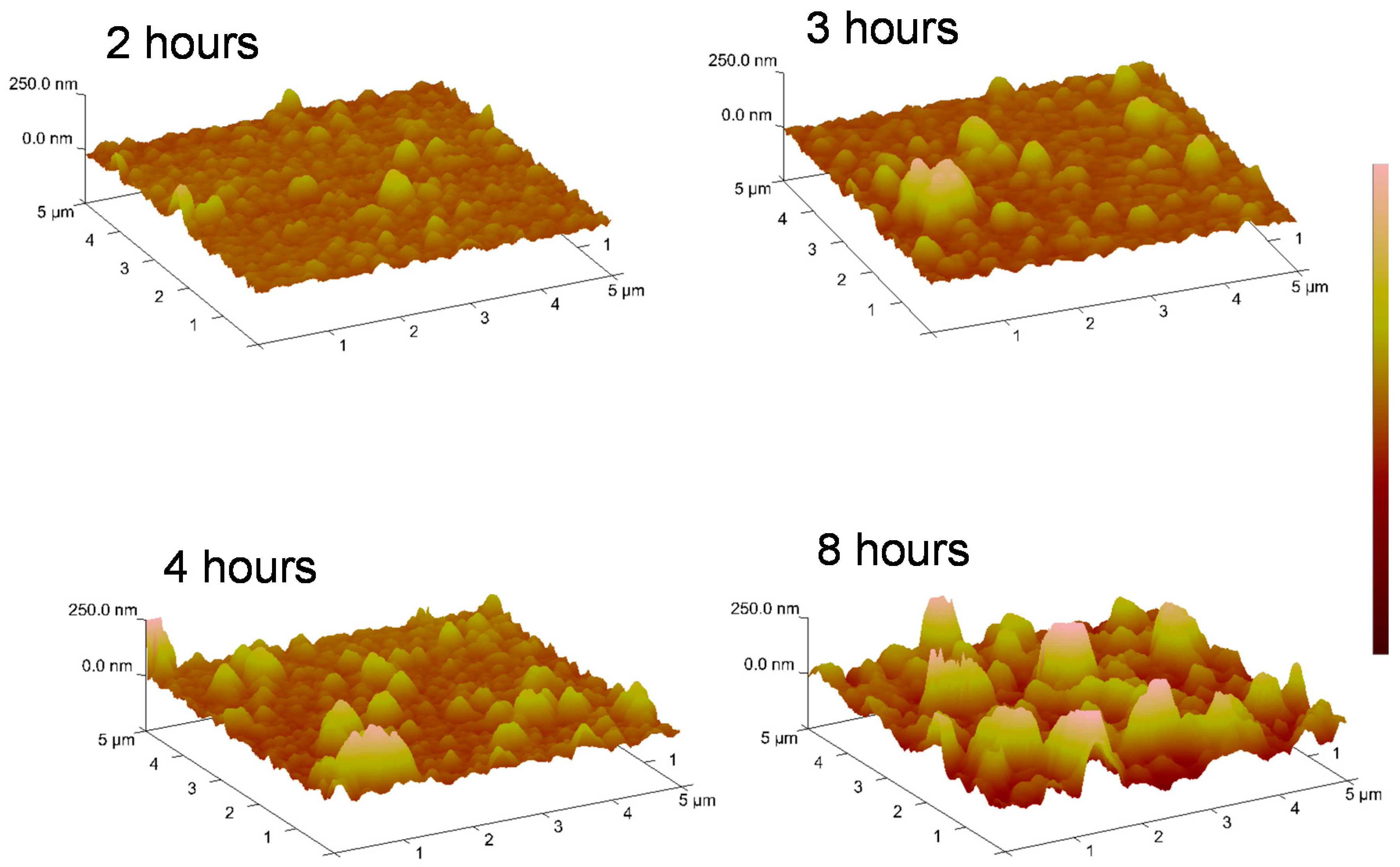
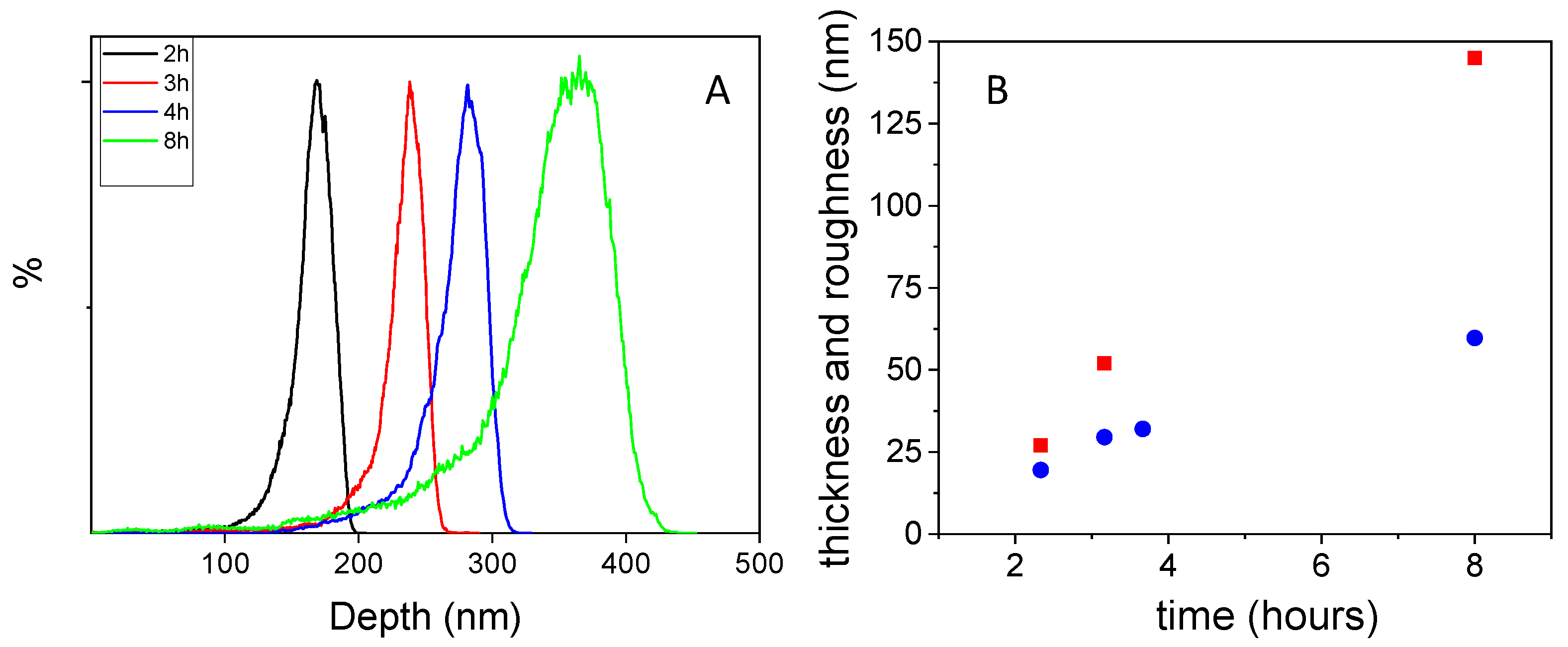
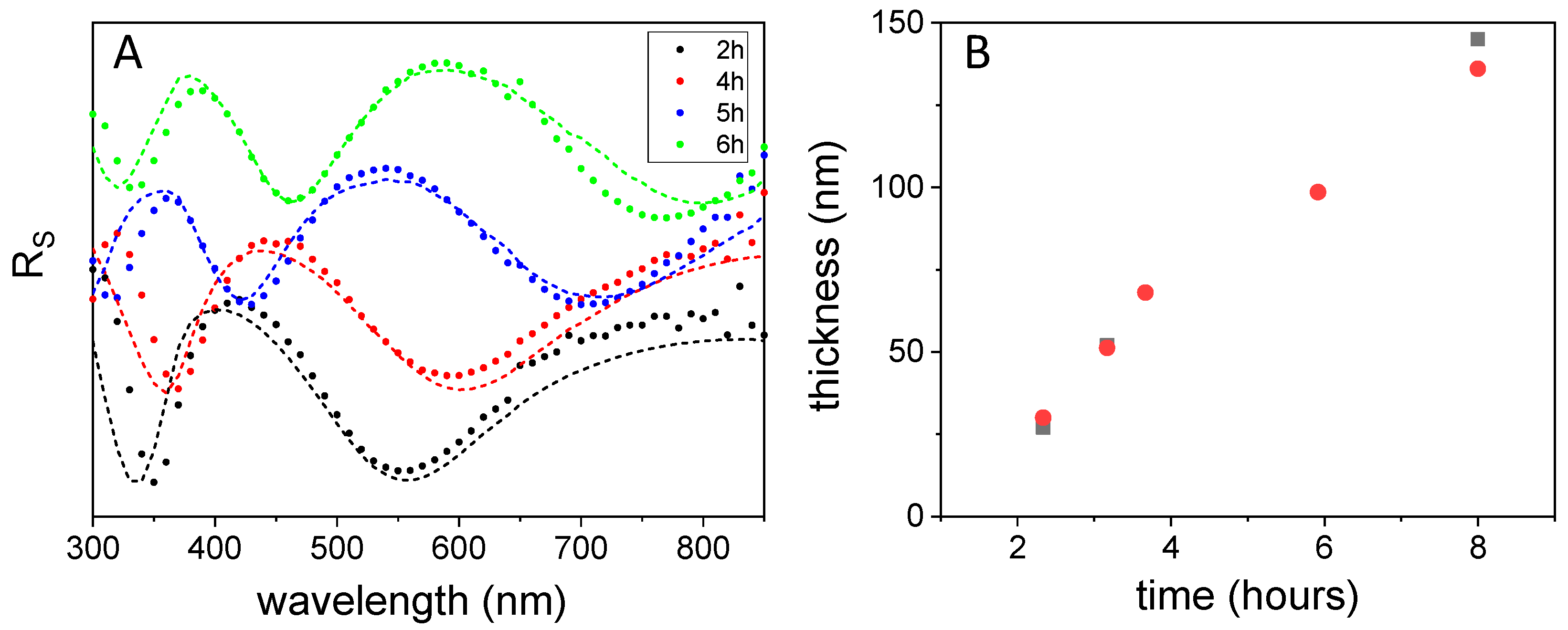
3.2. Effect of Polymerization Time on Film Growth and Color
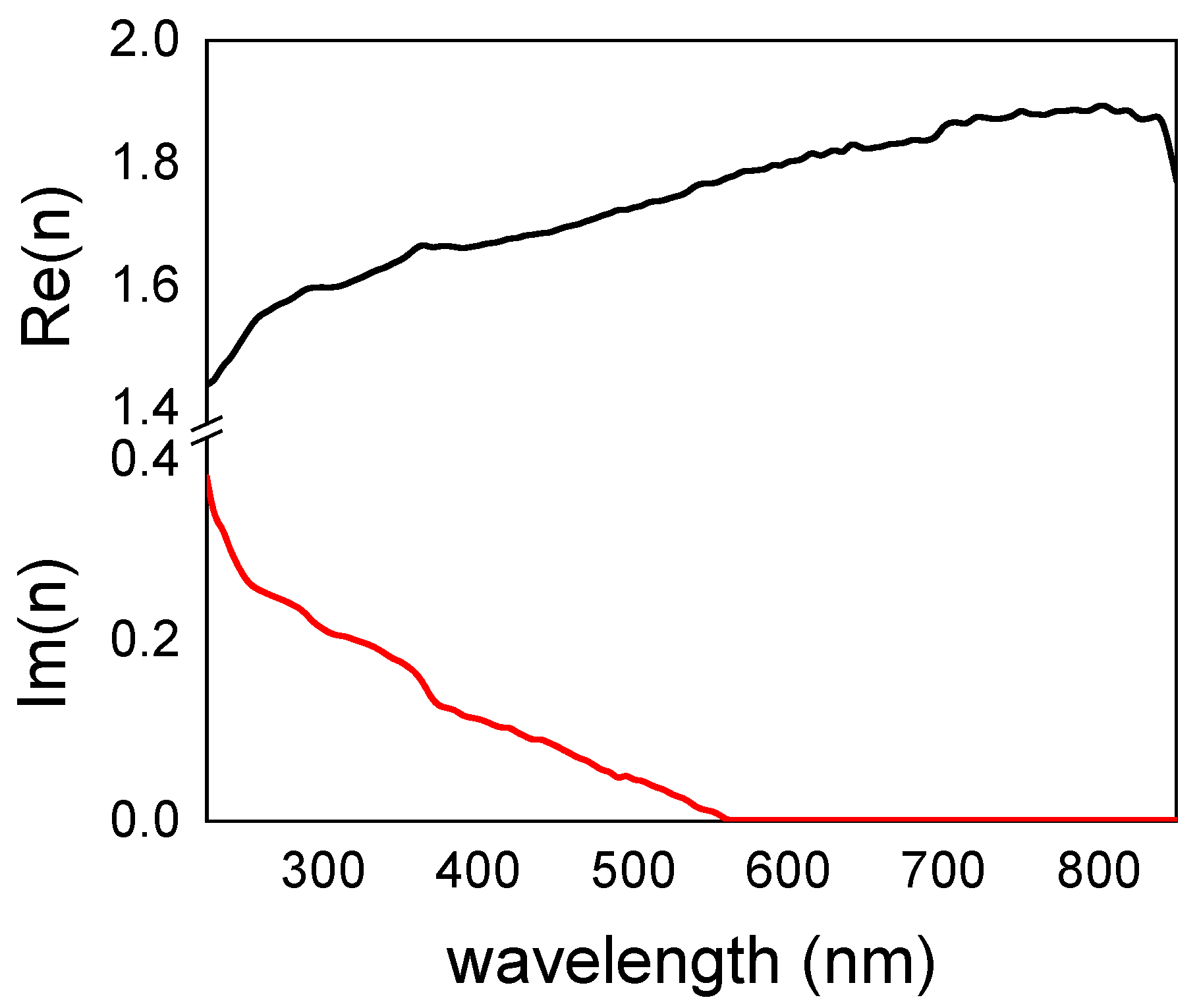
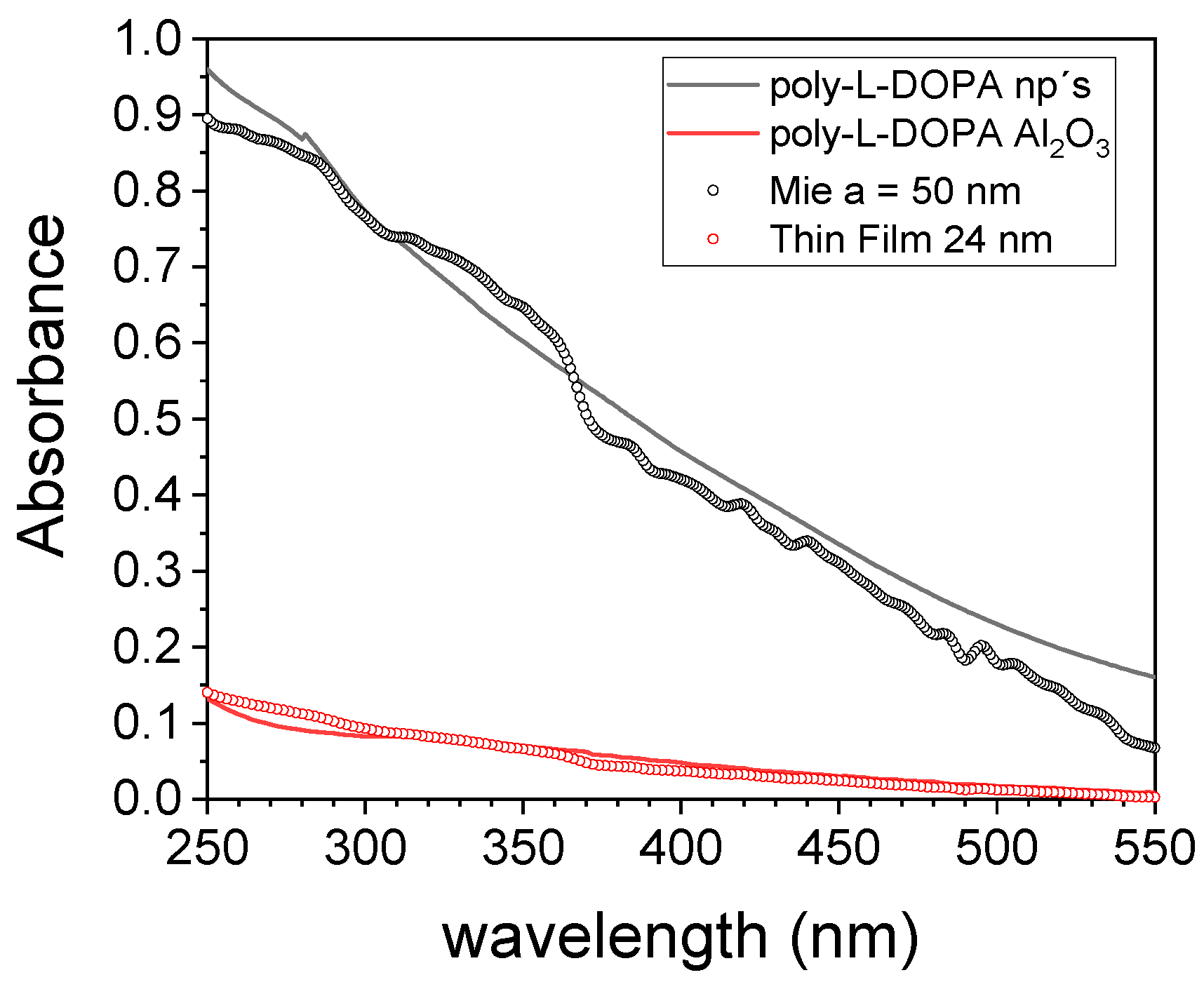
3.3. Optical Characterization: Refractive Index Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tadepalli, S.; Slocik, J.M.; Gupta, M.K.; Naik, R.R.; Singamaneni, S. Bio-optics and bio-inspired optical materials. Chem. Rev. 2017, 117, 12705–12763. [Google Scholar] [CrossRef]
- Richard, J.D.T. The production of colour by reflection. In Colour and the Optical Properties of Materials; Wiley: Hoboken, NJ, USA, 2010; pp. 91–128. [Google Scholar]
- Kats, M.; Blanchard, R.; Genevet, P.; Capasso, F. Nanometre optical coatings based on strong interference effects in highly absorbing media. Nat. Mater. 2012, 12, 20–24. [Google Scholar] [CrossRef]
- Fu, Y.; Tippets, C.A.; Donev, E.U.; Lopez, R. Structural colors: From natural to artificial systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2016, 8, 758–775. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Leertouwer, H.; Osorio, D.; Wilts, B. High refractive index of melanin in shiny occipital feathers of a bird of paradise. Light. Sci. Appl. 2015, 4, e243. [Google Scholar] [CrossRef]
- Xiao, M.; Shawkey, M.D.; Dhinojwala, A. Bioinspired melanin-based optically active materials. Adv. Opt. Mater. 2020, 8. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.; Messersmith, P. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Ju, K.-Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.-K. Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.-H.; Du, Y.; Ma, M.-Q.; Xu, Z.-K. Mussel-inspired polydopamine coatings for large-scale and angle-independent structural colors. J. Mater. Chem. C 2017, 5, 3898–3902. [Google Scholar] [CrossRef]
- Wu, T.-F.; Hong, J.-D. Dopamine-melanin nanofilms for biomimetic structural coloration. Biomacromolecules 2015, 16, 660–666. [Google Scholar] [CrossRef]
- Pi, J.-K.; Yang, J.; Zhong, Q.; Wu, M.; Yang, H.-C.; Schwartzkopf, M.; Roth, S.V.; Müller-Buschbaum, P.; Xu, Z.-K. Dual-layer nanofilms via mussel-inspiration and silication for non-iridescent structural color spectrum in flexible displays. ACS Appl. Nano Mater. 2019, 2, 4556–4566. [Google Scholar] [CrossRef]
- Vega, M.; Del Valle, E.M.M.; Pérez, M.; Pecharromán, C.; Marcelo, G. Color engineering of silicon nitride surfaces to characterize the polydopamine refractive index. Chem. Phys. Chem. 2018, 19, 3418–3424. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.D.; Peles, D.N. The red and the black. Acc. Chem. Res. 2010, 43, 1452–1460. [Google Scholar] [CrossRef] [Green Version]
- Watt, A.; Bothma, J.P.; Meredith, P. The supramolecular structure of melanin. Soft Matter 2009, 5, 3754–3760. [Google Scholar] [CrossRef]
- Felix, C.C.; Hyde, J.S.; Sarna, T.; Sealy, R.C. Interactions of melanin with metal ions. Electron spin resonance evidence for chelate complexes of metal ions with free radicals. J. Am. Chem. Soc. 1978, 100, 3922–3926. [Google Scholar] [CrossRef]
- Kuang, J.; Guo, J.L.; Messersmith, P.B. High ionic strength formation of DOPA-melanin coating for loading and release of cationic antimicrobial compounds. Adv. Mater. Interfaces 2014, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, A.; Hamre, B.; Frette, O.; Stamnes, K.; Stamnes, J.J. Modeling optical properties of human skin using Mie theory for particles with different size distributions and refractive indices. Opt. Express 2011, 19, 14549–14567. [Google Scholar] [CrossRef]
- Xi, Z.-Y.; Xu, Y.-Y.; Zhu, L.-P.; Wang, Y.; Zhu, B.-K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Huang, Z.; Lui, H.; Chen, X.K.; Alajlan, A.; McLean, D.I.; Zeng, H. Raman spectroscopy of in vivo cutaneous melanin. J. Biomed. Opt. 2004, 9, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G.; Leertouwer, H.L.; Hariyama, T.; De Raedt, H.A.; Wilts, B. Sexual dichromatism of the damselfly calopteryx japonica caused by a melanin-chitin multilayer in the male wing veins. PLoS ONE 2012, 7, e49743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Patil, A.; Zhou, X.; Wang, Z.; Xiao, M.; Shawkey, M.D.; Gianneschi, N.C.; Dhinojwala, A. Characterization of broadband complex refractive index of synthetic melanin coatings and their changes after ultraviolet irradiation. Appl. Phys. Lett. 2020, 117, 203701. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. Principles of Optics, Electromagnetic Theory of Propagation, Interference, and Diffraction of Light; Pergamon Press: Oxford, UK, 1964. [Google Scholar]
- Wakamatsu, K.; Ito, S. Advanced chemical methods in melanin determination. Pigment. Cell Res. 2002, 15, 174–183. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment. Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and scattering by a sphere. In Absorption and Scattering of Light by Small Particles; Wiley: Hoboken, NJ, USA, 1998; pp. 82–129. [Google Scholar]
- Matzler, C. Matlab Codes Foe Mie Scattering and Absorption; Institut für Angewandte Physik: Bern, Switzerland, 2002; pp. 125–128. [Google Scholar]
- Ito, S.; Kikuta, M.; Koike, S.; Szewczyk, G.; Sarna, M.; Zadlo, A.; Sarna, T.; Wakamatsu, K. Roles of reactive oxygen species in UVA-induced oxidation of 5,6-dihydroxyindole-2-carboxylic acid-melanin as studied by differential spectrophotometric method. Pigment. Cell Melanoma Res. 2016, 29, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Molteni, E.; Cappellini, G.; Onida, G.; Mula, G. Extensive stacking of DHI-like monomers as a model of out-of-plane complexity in eumelanin protomolecules: Chemical and structural sensitivity of optical absorption spectra. Chem. Phys. 2019, 524, 92–100. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and eumelanin: From structure–property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcelo, G.; López-González, M.d.M.; Vega, M.; Pecharromán, C. Colored Surfaces Made of Synthetic Eumelanin. Nanomaterials 2021, 11, 2320. https://doi.org/10.3390/nano11092320
Marcelo G, López-González MdM, Vega M, Pecharromán C. Colored Surfaces Made of Synthetic Eumelanin. Nanomaterials. 2021; 11(9):2320. https://doi.org/10.3390/nano11092320
Chicago/Turabian StyleMarcelo, Gema, María del Mar López-González, Milena Vega, and Carlos Pecharromán. 2021. "Colored Surfaces Made of Synthetic Eumelanin" Nanomaterials 11, no. 9: 2320. https://doi.org/10.3390/nano11092320







