Hepatoprotective Effect of Curcumin Nano-Lipid Carrier against Cypermethrin Toxicity by Countering the Oxidative, Inflammatory, and Apoptotic Changes in Wistar Rats
Abstract
1. Introduction
2. Results
2.1. Serum Biochemical Assay
2.2. Effect on Albumin and Total Protein
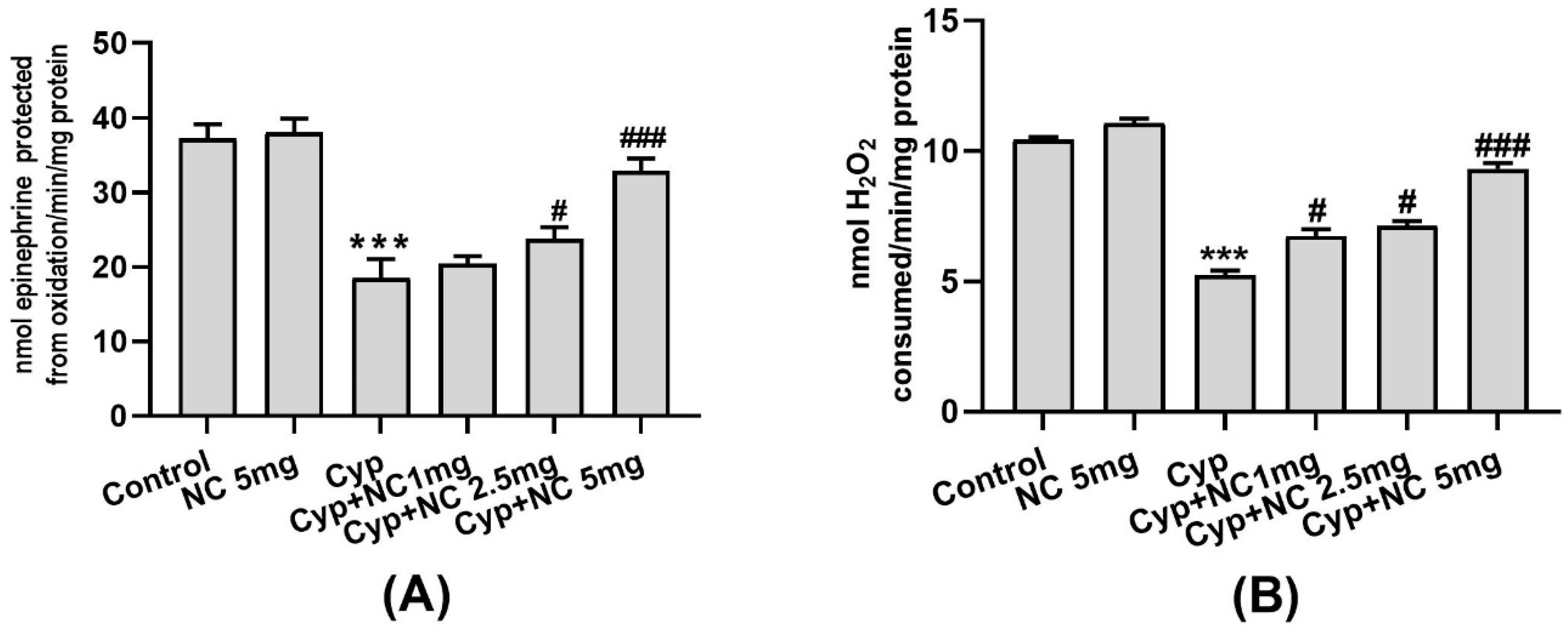
2.3. Effects on LPO and GSH
2.4. Effects on Antioxidative Enzymes (SOD and Catalase)
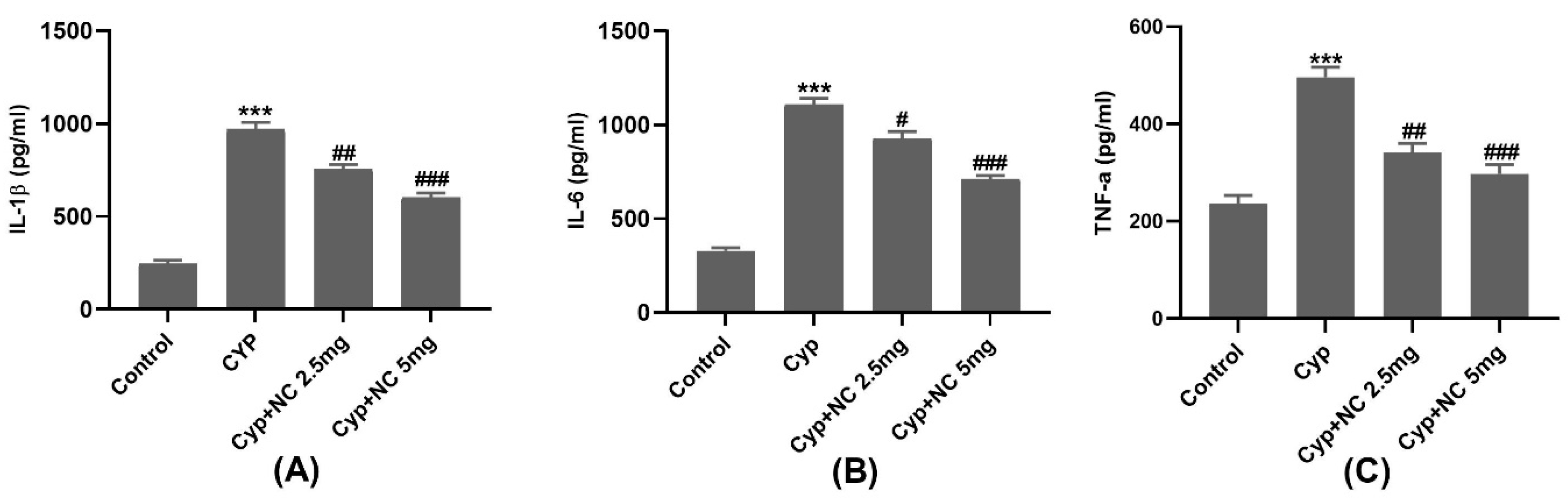
2.5. Inflammatory Markers
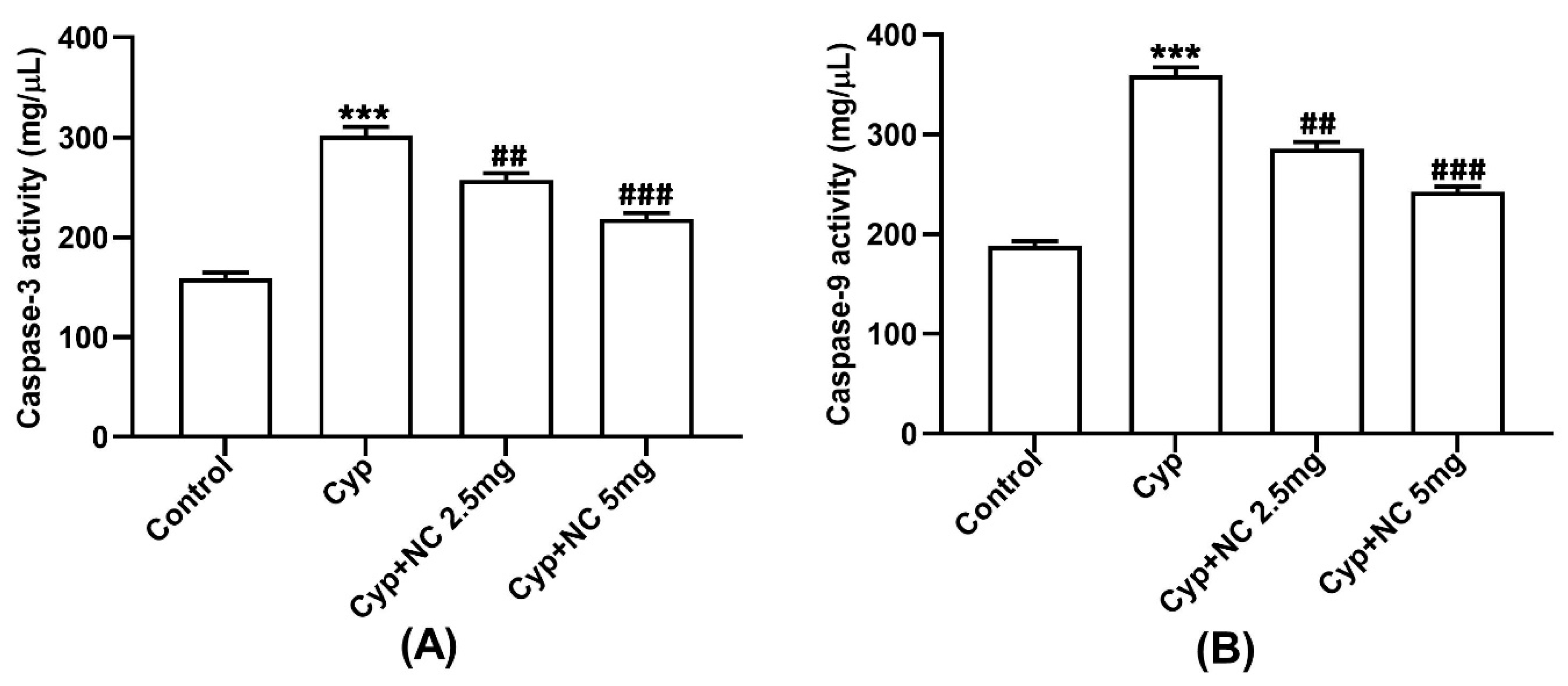
2.6. Assay on Caspases 3 and 9
2.7. Immunohistochemical Studies
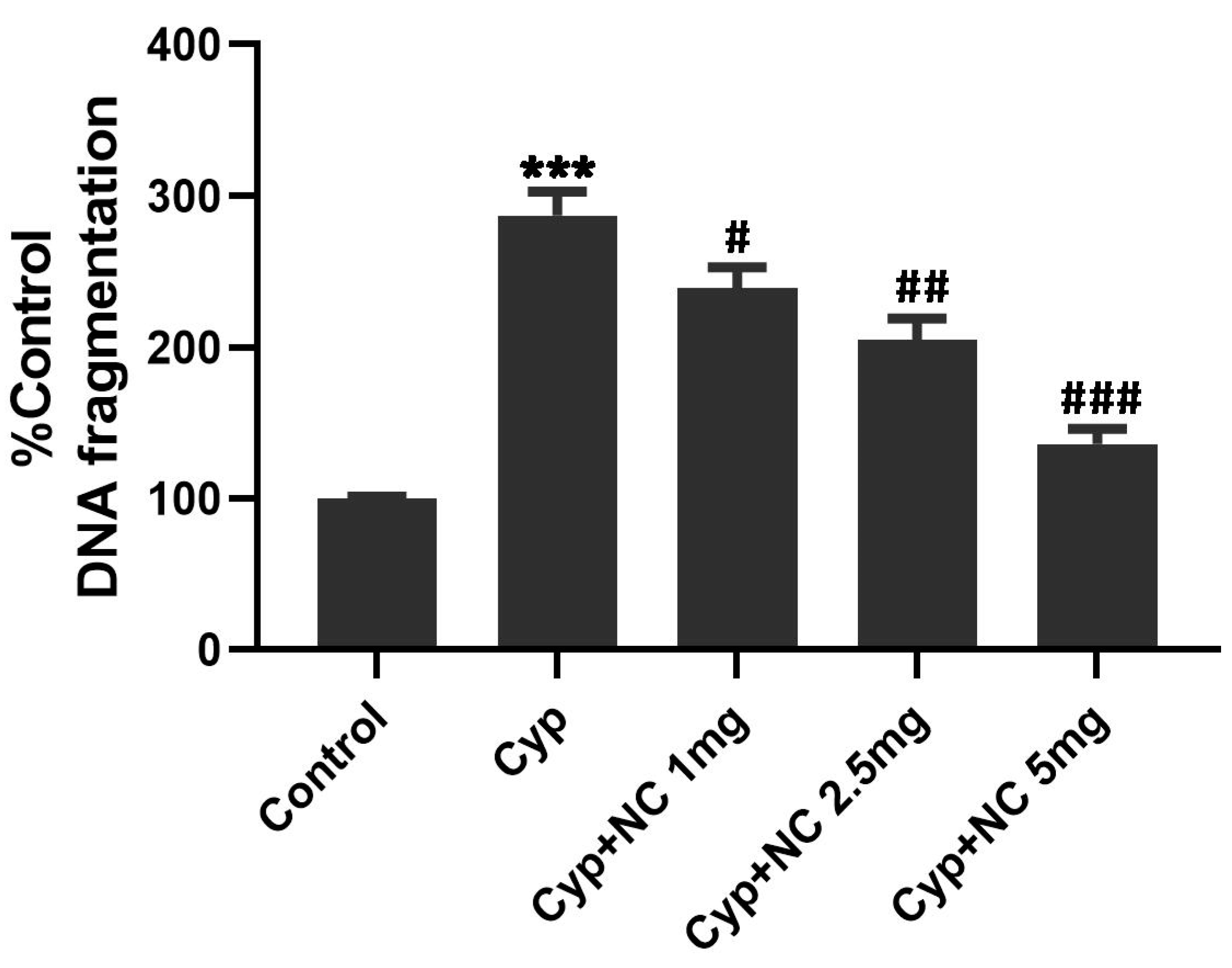
2.8. Quantitative DNA Estimation
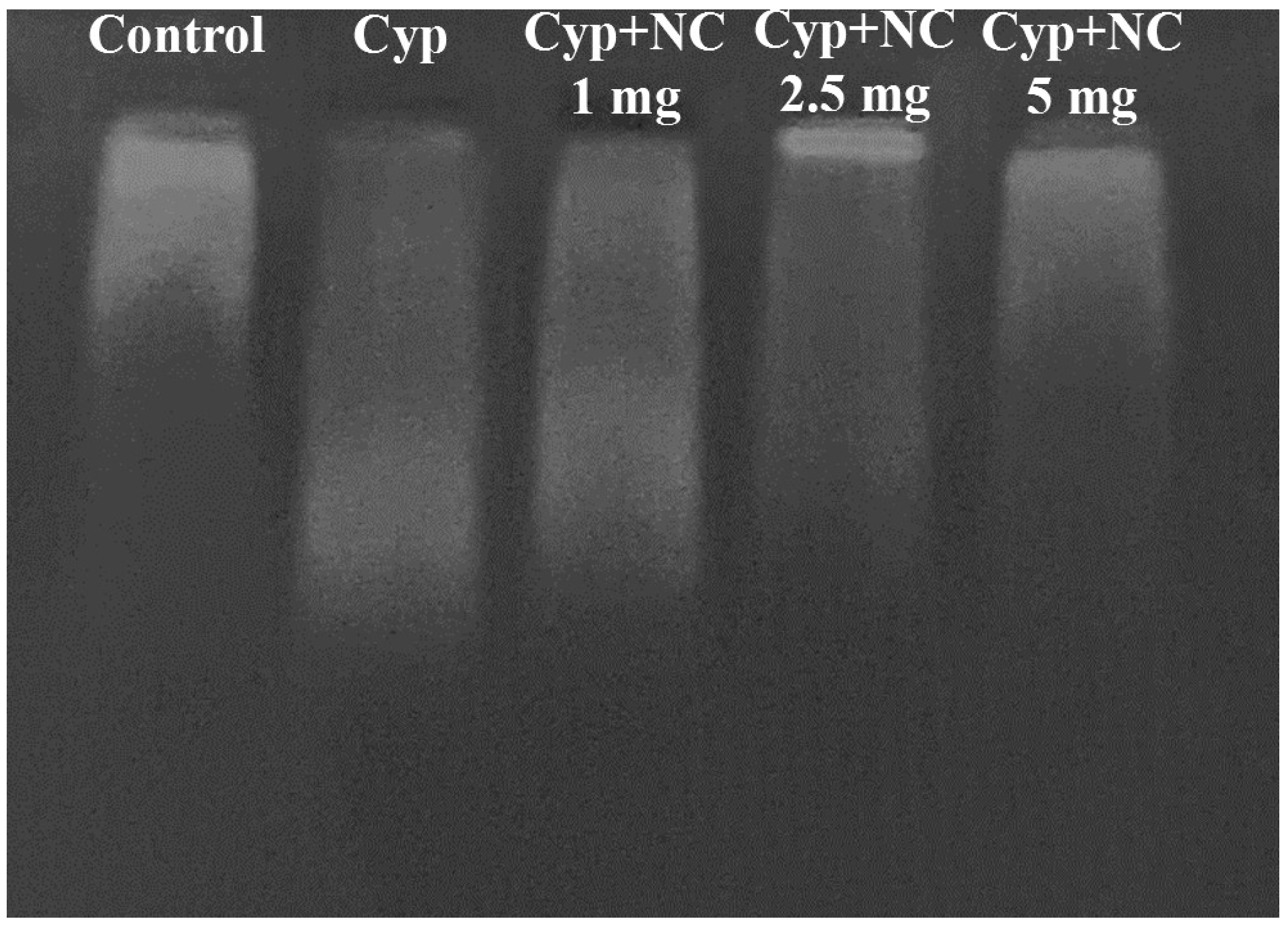
2.9. DNA Fragmentation by Agarose Gel Electrophoresis
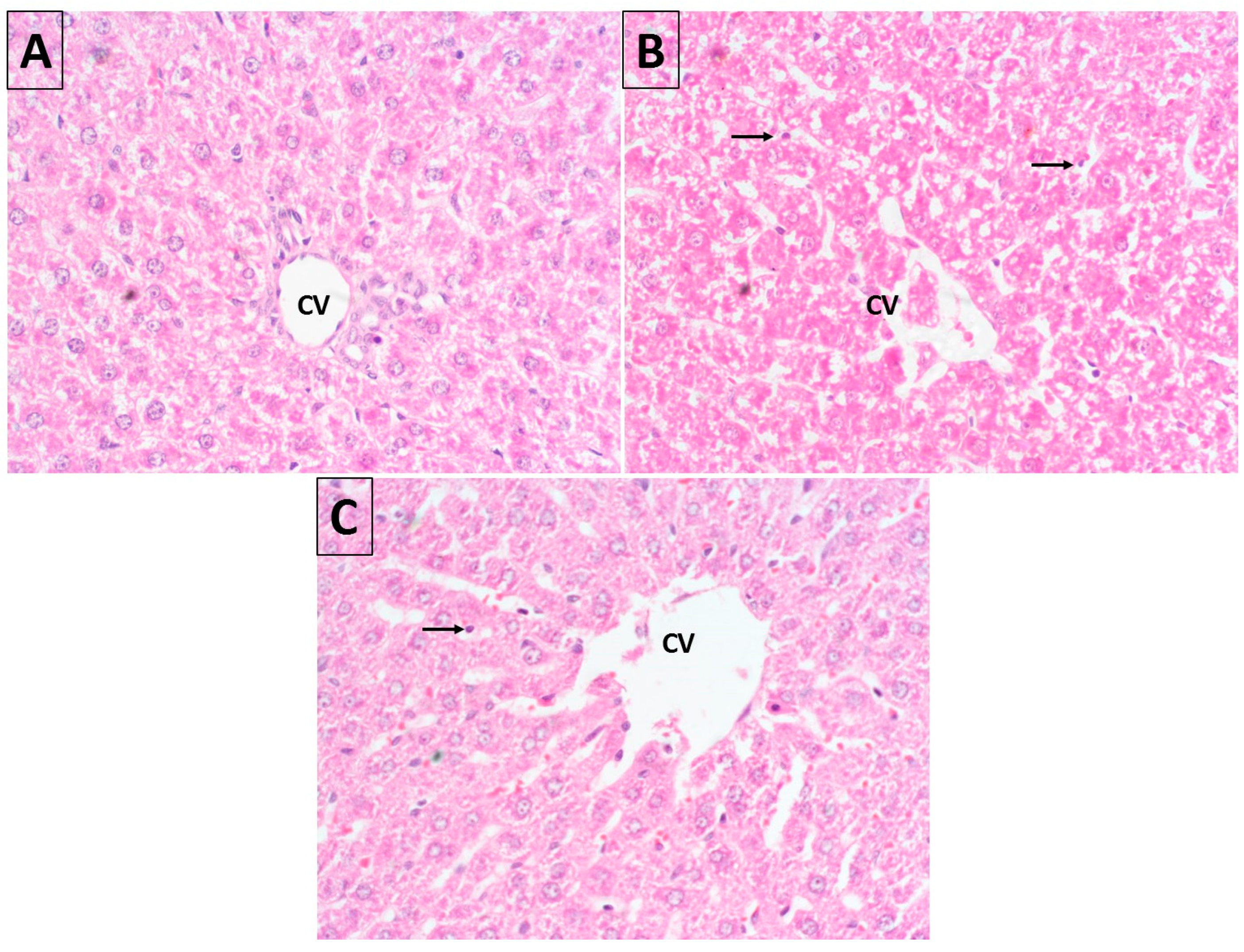
2.10. Histopathology
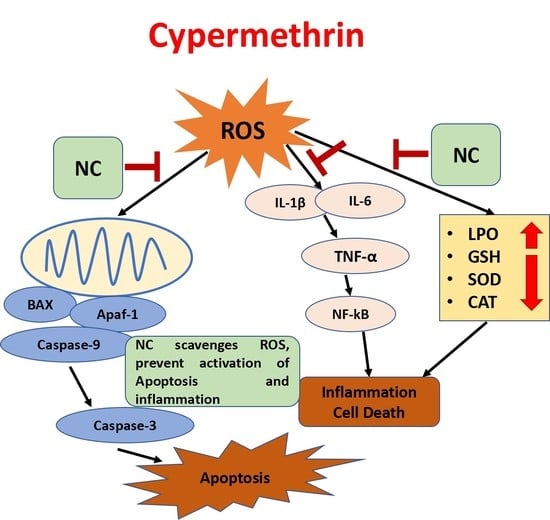
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Dose Selection
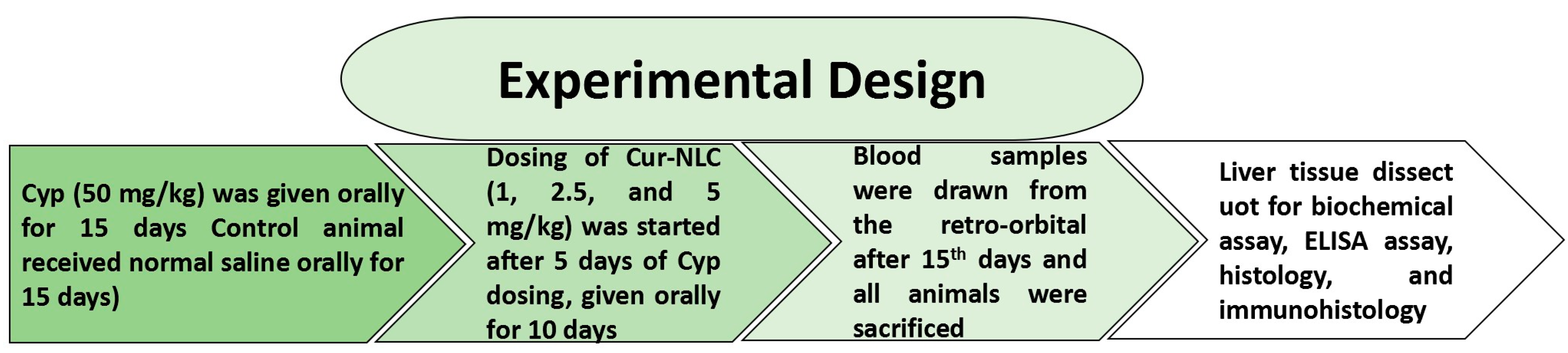
4.4. Experimental Design and Sample Preparation
4.5. Serum Biomarkers Assay
4.6. Estimation of LPO and GSH and Antioxidant Enzyme Activity
4.7. Inflammatory Markers
4.8. Apoptotic Markers Assay Caspases 3,9
4.9. Immunohistochemistry of 4-HNE, NF-κB, Bax, and Apaf-1
4.10. Quantitative DNA Assay and DNA Fragmentation by Agarose Gel Electrophoresis
4.11. Histopathological Study
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantalamessa, F. Acute toxicity of two pyrethroids, permethrin and cypermethrin in neonatal and adult rats. Arch. Toxicol. 1993, 67, 510–513. [Google Scholar] [CrossRef]
- Luty, S.; Latuszynska, J.; Obuchowska-Przebirowska, D.; Tokarska, M.; Haratym-Maj, A. Subacute toxicity of orally applied alpha-cypermethrin in Swiss mice. Ann. Agric. Environ. Med. 2000, 7, 33–41. [Google Scholar]
- Mcdaniel, K.L.; Moser, V.C. Utility of a neurobehavioral screening battery for differentiating the effects of two pyrethroids, permethrin and cypermethrin. Neurotoxicol. Teratol. 1993, 15, 71–83. [Google Scholar] [CrossRef]
- Cox, C. Insecticide factsheet, Cypermethrin. J. Pestic. Reform 1996, 16, 15–20. [Google Scholar]
- Michelangeli, F.; Robson, M.J.; East, J.M.; Lee, A.G. The conformation of pyrethroids bound to lipid bilayers. Biochim. Biophys. Acta 1990, 1028, 49–57. [Google Scholar] [CrossRef]
- Manna, S.; Bhattacharyya, D.; Mandal, T.K.; Das, S. Repeated dose toxicity of alfa-cypermethrin in rats. J. Vet. Sci. 2004, 5, 241–245. [Google Scholar] [CrossRef]
- Abdou, R.H.; Sayed, N. Antioxidant and Anti-Inflammatory Effects of Nano-Selenium against Cypermethrin-Induced Liver Toxicity. CellBio 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Kasuba, V.; Tariba Lovakovic, B.; Lucic Vrdoljak, A.; Katic, A.; Kopjar, N.; Micek, V.; Milic, M.; Pizent, A.; Zeljezic, D.; Zunec, S. Evaluation of Toxic Effects Induced by Sub-Acute Exposure to Low Doses of α-Cypermethrin in Adult Male Rats. Toxics 2022, 10, 717. [Google Scholar] [CrossRef]
- Mahna, D.; Puri, S.; Sharma, S. Cypermethrin Induced Liver Toxicity: Altered Gene Expression and DNA Methylation. Biochem. Mol. Biol. 2019, 33, 621–629. [Google Scholar] [CrossRef]
- Giray, B.; Gürbay, A.; Hincal, F. Cypermethrin-Induced Oxidative Stress in Rat Brain and Liver Is Prevented by Vitamin E or Allopurinol. Toxicol. Lett. 2001, 118, 139–146. [Google Scholar] [CrossRef]
- Abdou, H.M.; Hussien, H.M.; Yousef, M.I. Deleterious effects of cypermethrin on rat liver and kidney: Protective role of sesame oil. J. Environ. Sci. Health B 2012, 47, 306–314. [Google Scholar] [CrossRef]
- Ileriturk, M.; Kandemir, O.; Kandemir, F.M. Evaluation of protective effects of quercetin against cypermethrin-induced lung toxicity in rats via oxidative stress, inflammation, apoptosis, autophagy, and endoplasmic reticulum stress pathway. Environ. Toxicol. 2022, 37, 2639–2650. [Google Scholar] [CrossRef]
- Sankar, D.; Sambandam, G.; Ramakrishna Rao, M.; Pugalendi, K.V. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin. Chim. Acta 2005, 355, 97–104. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Afolabi, O.K.; Aderibigbe, F.A.; Folarin, D.T.; Arinola, A.; Wusu, A.D. Oxidative stress and inflammation following sub-lethal oral exposure of cypermethrin in rats: Mitigating potential of epicatechin. Heliyon 2019, 5, e02274. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Zafeer, M.F.; Javed, M.; Ahmad, M. Pendimethalin-induced oxidative stress, DNA damage and activation of anti-infammatory and apoptotic markers in male rats. Sci. Rep. 2018, 8, 817139. [Google Scholar] [CrossRef]
- Chan, W.; Stub, D.; Clark, D.J. Usefulness of transient and persistent no reflow to predict adverse clinical outcomes following percutaneous coronary intervention. Am. J. Cardiol. 2012, 109, 478–485. [Google Scholar] [CrossRef]
- Pashkow, F.J. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int. J. Inflam. 2011, 1–9, 514623. [Google Scholar] [CrossRef]
- Blum, A. Heart failure—New insights. Isr. Med. Assoc. J. 2009, 11, 105–111. [Google Scholar]
- Bulku, E.; Stohs, S.J.; Cicero, L.; Brooks, T.; Halley, H.; Ray, S.D. Curcumin exposure modulates multiple pro-apoptotic and Anti- apoptotic signaling pathways to antagonize acetaminophen-induced toxicity. Curr. Neurovasc. Res. 2012, 9, 58–71. [Google Scholar] [CrossRef]
- Hussien, H.M.; Abdou, H.M.; Yousef, M.I. Cypermethrin induced damage in genomic DNA and histopathological changes in brain and haematotoxicity in rats: The protective effect of sesame oil. Brain Res. Bull. 2013, 92, 76–83. [Google Scholar] [CrossRef]
- Aziz, F.M.; Maulood, I.M.; Chawsheen, M.A. Effects of melatonin, vitamin C and E alone or in combination on lead induced injury in liver and kidney organs of rats. Pak. J. Zool. 2014, 46, 1425–1431. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Choudhury, A.K. Antioxidants: Balancing the Good, the Bad and the Ugly. Nutr. Food Technol. 2016, 2, 1–4. [Google Scholar]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; Laino, L.; Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef]
- Pattanayak, R.; Basak, P.; Sen, S.; Bhattacharyya, M. Interaction of kras gquadruplex with natural polyphenols: A spectroscopic analysis with molecular modeling. Int. J. Biol. Macromol. 2016, 89, 228–237. [Google Scholar] [CrossRef]
- Elsayed, A.S.I. The curcumin as an antioxidant natural herb, with emphasize on its effects against some diseases. Int. J. App. Biol. Pharmacol. Technol. 2016, 7, 26–40. [Google Scholar]
- Sankar, P.; Telang, A.G.; Manimaran, A. Protective effect of curcumin on cypermethrin-induced oxidative stress in Wistar rats. Exp. Toxicol. Pathol. 2012, 64, 487–493. [Google Scholar] [CrossRef]
- Otuechere, C.A.; Abarikwu, S.O.; Olateju, V.I.; Animashaun, A.L.; Kale, O.E. Protective Effect of Curcumin against the Liver Toxicity Caused by Propanil in Rats. Int. Sch. Res. Not. 2014, 29, 853697. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Yousef, M.I.; Radwan, F.M.E. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem. Toxicol. 2009, 47, 249–254. [Google Scholar] [CrossRef]
- Cekmen, M.; Ilbey, Y.O.; Ozbek, E.; Simsek, A.; Somay, A.; Ersoz, C. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem. Toxicol. 2009, 47, 1480–1484. [Google Scholar] [CrossRef]
- Sankar, P.; Gopal Telang, A.; Kalaivanan, R.; Karunakaran, V.; Manikam, K.; Sarkar, S.N. Effects of nanoparticle-encapsulated curcumin on arsenic-induced liver toxicity in rats. Environ. Toxicol. 2015, 30, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Muhammad, F.; Akhtar, B.; Ur Rehman, S.; Saleemi, M.K. Nephroprotective effects of curcumin loaded chitosan nanoparticles in cypermethrin induced renal toxicity in rabbits. Environ. Sci. Pollut. Res. Int. 2020, 27, 14771–14779. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Bhattacharyya, D.; Basak, D.K.; Mandal, T.K. Single oral dose toxicity study of a-cypermethrin in rats. Indian J. Pharmacol. 2004, 36, 25–28. [Google Scholar]
- Hassanin, K.M.; Abd El-Kawi, S.H.; Hashem, K.S. The Prospective Protective Effect of Selenium Nanoparticles against Chromium-Induced Oxidative and Cellular Damage in Rat Thyroid. Int. J. Nanomed. 2013, 8, 1713–1720. [Google Scholar]
- Ali, S.I.; Gaafar, A.A.; Abdallah, A.A.; El-Daly, S.M.; ElBana, M.; Hussein, J. Mitigation of Alpha-Cypermethrin-Induced Hepatotoxicity in Rats by Tribulus terrestris Rich in Antioxidant Compounds. Jordan J. Biol. Sci. 2018, 11, 517–525. [Google Scholar]
- Yousef, M.I.; Omar, S.M.A.M.; El-Guendi, M.I.; Abdelmegid, L.A. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem. Toxicol. 2010, 48, 3246–3261. [Google Scholar] [CrossRef]
- Yousef, M.I.; El-Demerdash, F.M.; Kamel, K.I.; Al-Salhen, K.S. Changes in some hematological and biochemical indices of rabbits induced by isoflavones and cypermethrin. Toxicology 2003, 189, 223–234. [Google Scholar] [CrossRef]
- Rivarola, V.A.; Balegno, H.F. Effect of 2,4-dichlorophenxyacetic acid on polyamine synthesis in Chinese hamster ovary cells. Toxicol. Lett. 1991, 56, 151–157. [Google Scholar] [CrossRef]
- Sood, R. Medical Laboratory Technology Method and Interpretations, 5th ed.; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2006; p. 723. [Google Scholar]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.A. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Prüss-üstün, A.; Corvalan, C. Preventing Disease through Healthy Environments: Towards an Estimate of the Environmental Burden of Disease; World Health Organization: Geneva, Switzerland, 2006.
- Wei, J.; Liu, J.; Zhang, L.; Zhu, Y.; Li, X.; Zhou, G.; Zhao, Y.; Sun, Z.; Zhou, X. Endosulfan induces cardiotoxicity through apoptosis via unbalance of pro-survival and mitochondrial-mediated apoptotic pathways. Sci. Total Environ. 2020, 727, 138790. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A.; Rodrigo, R.; Araya, J.; Poniachik, J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liverdisease. Free Radic. Biol. Med. 2004, 37, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Nachiappan, V. Cassia auriculata flower extract attenuates hyperlipidemia in male Wistar rats by regulating the hepatic cholesterol metabolism. Biomed Pharm. 2017, 95, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functionalfoods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Pachauri, V. Arsenic, Free Radical and Oxidative Stress in Encyclopedia of Metalloproteins; Springer: New York, NY, USA, 2013; pp. 149–159. [Google Scholar]
- Mittal, R.; Gupta, R.L. In vitro antioxidant activity of piperine. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 271–274. [Google Scholar] [CrossRef]
- De Leo, V.; Di Gioia, S.; Milano, F.; Fini, P.; Comparelli, R.; Mancini, E.; Agostiano, A.; Conese, M.; Catucci, L. Eudragit s100 entrapped liposome for curcumin delivery: Anti-oxidative effect in Caco-2 cells. Coatings 2020, 10, 114. [Google Scholar] [CrossRef]
- Khan, S.M.; Sobti, R.C.; Kataria, L. Pesticide-induced alteration in mice hepatooxidative status and protective effects of black tea extract. Clin. Chim. Acta 2005, 358, 131–138. [Google Scholar] [CrossRef]
- Khan, A.; Faridi, H.A.M.; Ali, M.; Khan, M.Z.; Siddique, M.; Hussain, I. Effects of cypermethrin on some clinicohemato-biochemical and pathological parameters in male dwarf goats (Capra hircus). Exp. Toxicol. Pathol. 2009, 61, 151–160. [Google Scholar] [CrossRef]
- Khillare, Y.K.; Wagh, S.B. Acute toxicity of pesticides in the freshwater fish Barbus stigma: Histopathology of the stomach. Uttar Pradesh J. Zool. 1988, 8, 176–179. [Google Scholar]
- El-Hossary, G.G.; Mansour, S.M.; Mohamed, A.S. Neurotoxic effects of chlorpyrifos and the possible protective role of antioxidant supplements: An experimental study. J. Appl. Sci. Res. 2009, 5, 1218–1222. [Google Scholar]
- Corda, S.; Laplace, C.; Vicaut, E.; Duranteau, J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am. J. Respir. Cell Mol. Biol. 2001, 24, 762–768. [Google Scholar] [CrossRef]
- Mendes, A.F.; Caramona, M.M.; Carvalho, A.P.; Lopes, M.C. Hydrogen peroxide mediates interleukin-1betainduced AP-1 activation in articular chondrocytes: Implications for the regulation of iNOS expression. Cell Biol. Toxicol. 2003, 19, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hamida, M.; Moustafaa, N.; Abd Alla Asranb, A.M.A.; Mowafyb, L. Cypermethrin-induced histopathological, ultrastructural and biochemical changes in liver of albino rats: The protective role of propolis and curcumin. Beni-Suef. Univ. J. Basic Appl. Sci. 2017, 6, 160–173. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Liu, J.; Geletka, L.; Delaney, C.; Delproposto, J.; Desai, A.; Oatmen, K.; Santibanez, G.M.; Julius, A.; Garg, S.; et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J. Immunol. 2011, 187, 6208–6216. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Misra, H.P. Reactive oxygen species in in vitro pesticide-induced neuronal cell (SH-SY5Y) cytotoxicity: Role of NFkappaB and caspase-3. Free Radic. Biol. Med. 2007, 42, 288–298. [Google Scholar] [CrossRef]
- Li, G.; Chen, J.B.; Wang, C.; Xu, Z.; Nie, H.; Qin, X.Y.; Chen, X.M.; Gong, Q. Curcumin protects against acetaminophen-induced apoptosis in hepatic injury. World J. Gastroenterol. 2013, 19, 7440–7446. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, L.; Huang, J.; Lv, H.X.; Deng, X.; Ci, X. Pterostilbene reduces acetaminophen-induced liver injury by activating the Nrf2 antioxidative defense system via the AMPK/Akt/GSK3β pathway. Cell. Physiol. Biochem. 2018, 49, 1943–1958. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, N.; Lin, L. Curcumin Suppresses Apoptosis and Inflammation in Hypoxia/Reperfusion-Exposed Neurons via Wnt Signaling Pathway. Med. Sci. Monit. 2020, 26, e920445-1–e920445-8. [Google Scholar] [CrossRef]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Katragadda, V.; Adem, M.; Mohammad, R.A.; Bhasyam, S.S.; Battini, K. Testosterone recuperates deteriorated male fertility in cypermethrin intoxicated rats. Toxicol. Res. 2021, 37, 125–134. [Google Scholar] [CrossRef]
- Sangha, G.K.; Kaur, K.P.; Khera, K.S.; Singh, B. Toxicological Effects of Cypermethrin on Female Albino Rats. Toxicol. Int. 2011, 18, 5–8. [Google Scholar] [CrossRef]
- Hussain, S. Comparative efficacy of the curcumin against H2O2 induced ROS in cervical cancer biopsies and Hela cell line. Int. J. Adv. Pharm. Med. Bioallied Sci. 2017, 123, 123. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ashafaq, M.; Raza, S.S.; Khan, M.M.; Ahmad, A.; Javed, H.; Ahmad, M.E.; Tabassum, R.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Catechin hydrate ameliorates redox imbalance and limits inflammatory response in focal cerebral ischemia. Neurochem. Res. 2012, 37, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S.; Ashafaq, M.; Hussain, S.; Mohammed, M.; Sultan, M.; Jali, A.M.; Siddiqui, R.; Islam, F. Renoprotective effects of cinnamon oil against APAP-Induced nephrotoxicity by ameliorating oxidative stress, apoptosis and inflammation in rats. Saudi Pharm. J. 2021, 29, 194–200. [Google Scholar] [CrossRef]
- Makeen, H.A.; Mohan, S.; Al-Kasim, M.A.; Sultan, M.H.; Albarraq, A.A.; Ahmed, R.A.; Alhazmi, H.A.; Alam, M.I. Preparation, Characterization, and Anti-Cancer Activity of Nanostructured Lipid Carriers Containing Imatinib. Pharmaceutics 2021, 13, 1086. [Google Scholar] [CrossRef]
- Makeen, H.A.; Mohan, S.; Al-Kasim, M.A.; Ibraheem, M.A.; Ahmed, R.A.; Syed, N.K.; Sultan, M.H.; Al-Bratty, M.; Alhazmi, H.A.; Safhi, M.M.; et al. Gefitinib loaded nanostructured lipid carriers: Characterization, evaluation and anti-human colon cancer activity in vitro. Drug Delivery 2020, 271, 622–631. [Google Scholar] [CrossRef]


| Liver Markers | Control | CUR-NLC 5 mg | Cyp 50 mg | Cyp + CUR-NLC | ||
|---|---|---|---|---|---|---|
| 1 mg | 2.5 mg | 5 mg | ||||
| ALT (IU/L) | 45 ± 6 | 43 ± 7 | 189 ± 12 *** | 176 ± 10 # | 159 ± 10 ## | 106 ± 8 ### |
| AST (IU/L) | 36 ± 3 | 34 ± 4 | 89 ± 7 *** | 69 ± 6 ## | 56 ± 4 ## | 50 ± 5 ### |
| ALP (IU/L) | 89 ± 10 | 91 ± 11 | 172 ± 15 *** | 153 ± 12 ## | 147 ± 11 ### | 123 ± 12 ### |
| Total protein (g/dL) | 8 ± 1 | 8 ± 1 | 4 ± 1 *** | 5 ± 1 | 7 ± 1 # | 7 ± 1 ## |
| Albumin | 4 ± 1 | 4 ± 1 | 2 ± 2 *** | 3 ± 1 | 3 ± 1 ## | 4 ± 1 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Ashafaq, M.; Alshahrani, S.; Bokar, I.A.M.; Siddiqui, R.; Alam, M.I.; Taha, M.M.E.; Almoshari, Y.; Alqahtani, S.S.; Ahmed, R.A.; et al. Hepatoprotective Effect of Curcumin Nano-Lipid Carrier against Cypermethrin Toxicity by Countering the Oxidative, Inflammatory, and Apoptotic Changes in Wistar Rats. Molecules 2023, 28, 881. https://doi.org/10.3390/molecules28020881
Hussain S, Ashafaq M, Alshahrani S, Bokar IAM, Siddiqui R, Alam MI, Taha MME, Almoshari Y, Alqahtani SS, Ahmed RA, et al. Hepatoprotective Effect of Curcumin Nano-Lipid Carrier against Cypermethrin Toxicity by Countering the Oxidative, Inflammatory, and Apoptotic Changes in Wistar Rats. Molecules. 2023; 28(2):881. https://doi.org/10.3390/molecules28020881
Chicago/Turabian StyleHussain, Sohail, Mohammad Ashafaq, Saeed Alshahrani, Ibrahim A. M. Bokar, Rahimullah Siddiqui, Mohammad Intakhab Alam, Manal Mohamed Elhassan Taha, Yosif Almoshari, Saad S. Alqahtani, Rayan A. Ahmed, and et al. 2023. "Hepatoprotective Effect of Curcumin Nano-Lipid Carrier against Cypermethrin Toxicity by Countering the Oxidative, Inflammatory, and Apoptotic Changes in Wistar Rats" Molecules 28, no. 2: 881. https://doi.org/10.3390/molecules28020881
APA StyleHussain, S., Ashafaq, M., Alshahrani, S., Bokar, I. A. M., Siddiqui, R., Alam, M. I., Taha, M. M. E., Almoshari, Y., Alqahtani, S. S., Ahmed, R. A., Jali, A. M., & Qadri, M. (2023). Hepatoprotective Effect of Curcumin Nano-Lipid Carrier against Cypermethrin Toxicity by Countering the Oxidative, Inflammatory, and Apoptotic Changes in Wistar Rats. Molecules, 28(2), 881. https://doi.org/10.3390/molecules28020881