Research and Application of Kupffer Cell Thresholds for BSA Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of BSA Nanoparticles
2.2. Characterization of Threshold In Vitro
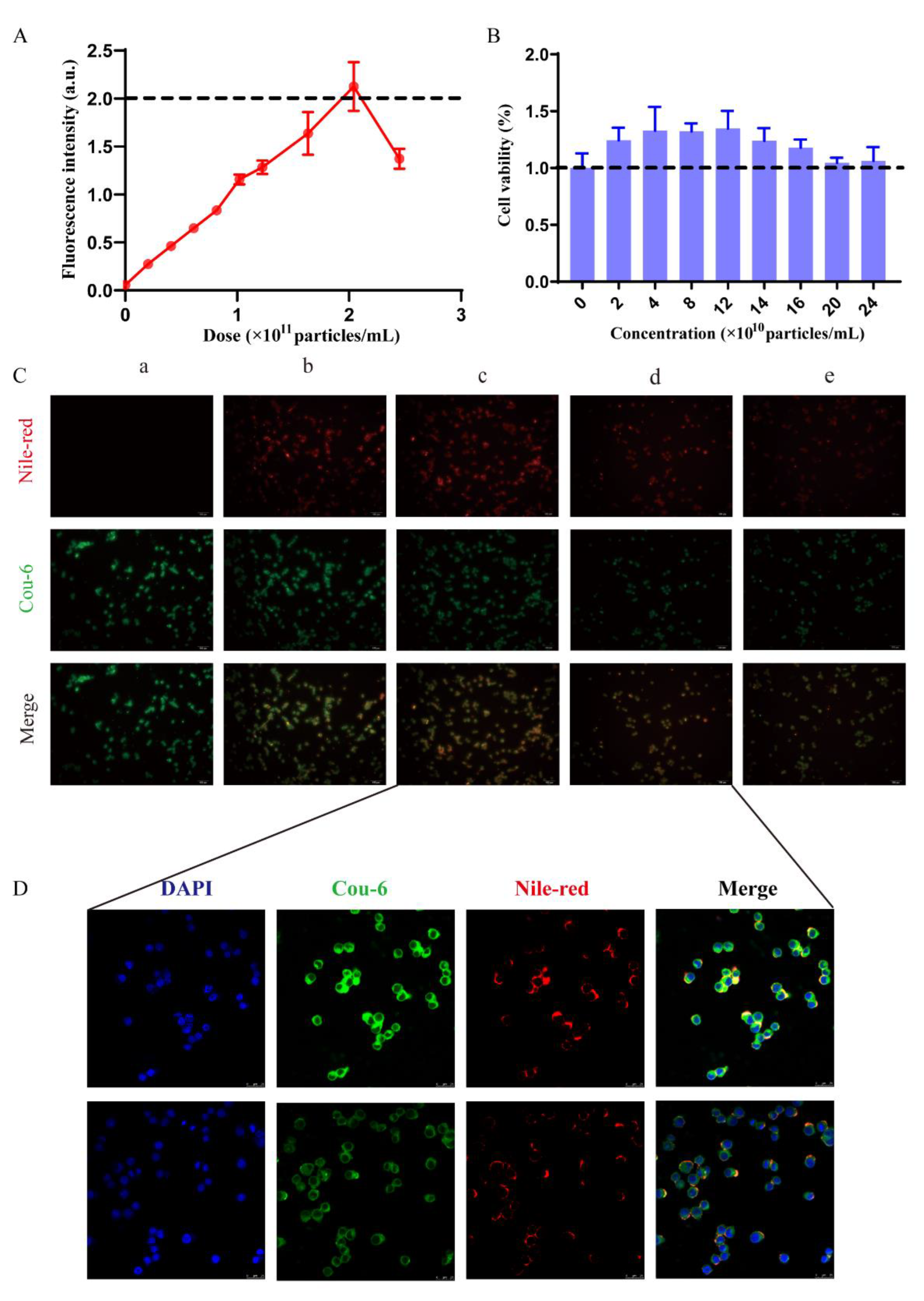
2.2.1. Macrophage Uptake Threshold and Safety Assessment In Vitro
2.2.2. Characterization of Saturation Phenomena In Vitro
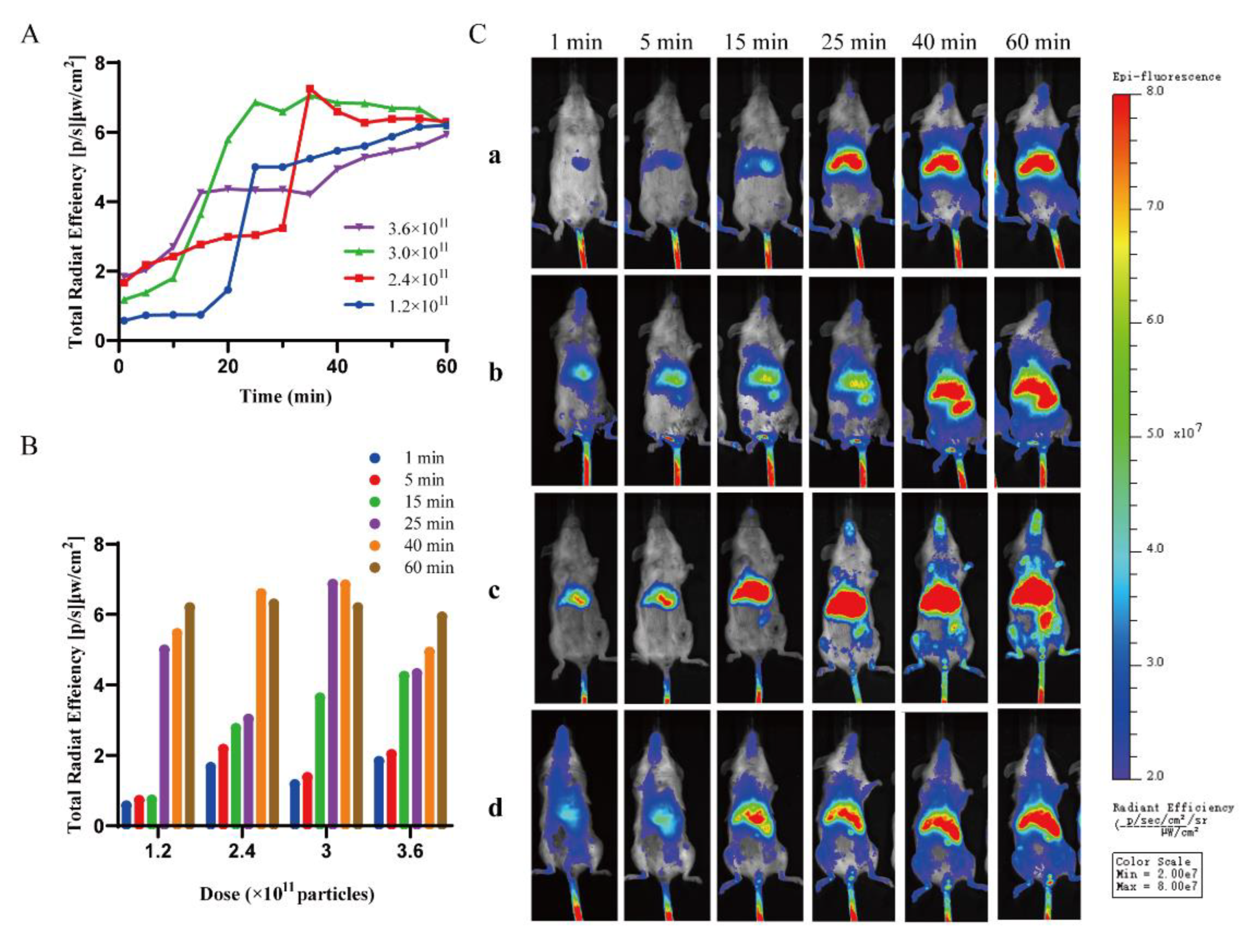
2.3. Characterization of Saturation Phenomena In Vivo
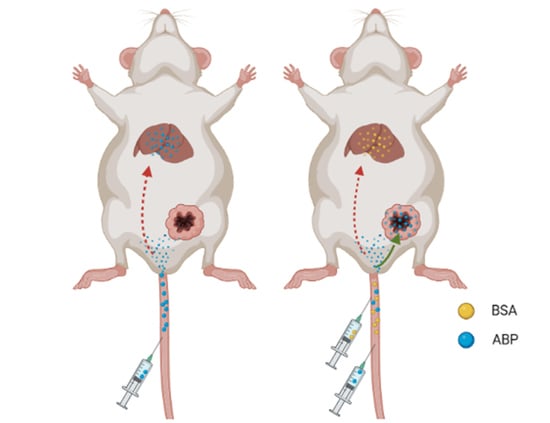
2.4. Application of Saturated Doses of BSA Nanoparticles in Antitumor Efficacy
2.5. Evaluation of the Safety of BSA Saturation Doses in Antitumor Efficacy
3. Materials and Methods
3.1. Materials and Reagents
3.2. Cell Lines and Animals
3.3. Preparation of the BSA Nanoparticles
3.4. Characterization of the BSA Nanoparticles
3.5. Macrophage Uptake Threshold and Safety Assessment In Vitro
3.6. Characterization of Saturation Phenomena In Vitro
3.7. Tumor Inoculation
3.8. Characterization of Saturation Phenomena In Vivo
3.9. Application of Saturated Dose of BSA Nanoparticles in Antitumor Efficacy
3.10. Evaluation of the Safety of BSA Saturation Dose in Antitumor Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Klochkov, S.; Neganova, M.; Nikolenko, V.; Chen, K.; Somasundaram, S.; Kirkland, C.; Aliev, G. Implications of nanotechnology for the treatment of cancer: Recent advances. Semin. Cancer Biol. 2021, 69, 190–199. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Chan, W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Sindhwani, S.; Chan, W.C.W. Nanotechnology for modern medicine: Next step towards clinical translation. J. Intern. Med. 2021, 290, 486–498. [Google Scholar] [CrossRef]
- Poon, W.; Kingston, B.R.; Ouyang, B.; Ngo, W.; Chan, W.C. A framework for designing delivery systems. Nat. Nanotechnol. 2020, 15, 819–829. [Google Scholar] [CrossRef]
- Ngo, W.; Ahmed, S.; Blackadar, C.; Bussin, B.; Ji, Q.; Mladjenovic, S.M.; Sepahi, Z.; Chan, W.C.W. Why nanoparticles prefer liver macrophage cell uptake in vivo. Adv. Drug Deliv. Rev. 2022, 185, 114238. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-Liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Zhou, H.; Tang, X.; Wu, Y.; Jiang, W.; Tian, Z.; Zhou, X.; Yang, X.; Wang, Y. Chemotaxis-Driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy. Nat. Commun. 2020, 11, 1126. [Google Scholar] [CrossRef]
- Tsoi, K.; MacParland, S.; Ma, X.; Spetzler, V.; Echeverri, J.; Ouyang, B.; Fadel, S.; Sykes, E.; Goldaracena, N.; Kaths, J.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Scott, C.L. Liver macrophages in health and disease. Immunity 2022, 55, 1515–1529. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G.J.N. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Han, Z.; Gong, C.; Li, J.; Guo, H.; Chen, X.; Jin, Y.; Gao, S.; Tai, Z. Immunologically modified enzyme-responsive micelles regulate the tumor microenvironment for cancer immunotherapy. Mater. Today Bio 2022, 13, 100170. [Google Scholar] [CrossRef]
- Chen, C.; Song, M.; Du, Y.; Yu, Y.; Li, C.; Han, Y.; Yan, F.; Shi, Z.; Feng, S. Tumor-Associated-Macrophage-Membrane-Coated Nanoparticles for Improved Photodynamic Immunotherapy. Nano Lett. 2021, 21, 5522–5531. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Huang, H.; Chen, L.; Sun, W.; Du, H.; Dong, S.; Ahmed, A.; Cao, D.; Cui, J.; Zhang, Y.; Cao, Q.J.T. Collagenase IV and clusterin-modified polycaprolactone-polyethylene glycol nanoparticles for penetrating dense tumor tissues. Theranostics 2021, 11, 906–924. [Google Scholar] [CrossRef]
- Ramesh, A.; Kumar, S.; Nandi, D.; Kulkarni, A. CSF1R- and SHP2-Inhibitor-Loaded Nanoparticles Enhance Cytotoxic Activity and Phagocytosis in Tumor-Associated Macrophages. Adv. Mater. 2019, 31, e1904364. [Google Scholar] [CrossRef]
- Jang, D.; Moon, C.; Oh, E.J.B.; Biomedecine, P. Improved tumor targeting and antitumor activity of camptothecin loaded solid lipid nanoparticles by preinjection of blank solid lipid nanoparticles. Biomed. Pharmacother. 2016, 80, 162–172. [Google Scholar] [CrossRef]
- Yin, J.; Wang, P.; Yin, Y.; Hou, Y.; Song, X. Optimization on biodistribution and antitumor activity of tripterine using polymeric nanoparticles through RES saturation. Drug Deliv. 2017, 24, 1891–1897. [Google Scholar] [CrossRef]
- Proffitt, R.; Williams, L.; Presant, C.; Tin, G.; Uliana, J.; Gamble, R.; Baldeschwieler, J.J.S. Liposomal blockade of the reticuloendothelial system: Improved tumor imaging with small unilamellar vesicles. Science 1983, 220, 502–505. [Google Scholar] [CrossRef]
- Ouyang, B.; Poon, W.; Zhang, Y.N.; Lin, Z.P.; Kingston, B.R.; Tavares, A.J.; Zhang, Y.; Chen, J.; Valic, M.S.; Syed, A.M.; et al. The dose threshold for nanoparticle tumour delivery. Nat. Mater. 2020, 19, 1362–1371. [Google Scholar] [CrossRef]
- Sofias, A.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef]
- Shourni, S.; Javadi, A.; Hosseinpour, N.; Bahramian, A.; Raoufi, M. Characterization of protein corona formation on nanoparticles via the analysis of dynamic interfacial properties: Bovine serum albumin—Silica particle interaction. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128273. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Lazarovits, J.; Chan, W. An Analysis of the Binding Function and Structural Organization of the Protein Corona. J. Am. Chem. Soc. 2020, 142, 8827–8836. [Google Scholar] [CrossRef]
- Desai, N.; Trieu, V.; Yao, Z.; Louie, L.; Ci, S.; Yang, A.; Tao, C.; De, T.; Beals, B.; Dykes, D.; et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 1317–1324. [Google Scholar] [CrossRef]
- Bouwens, L.; Baekeland, M.; De Zanger, R.; Wisse, E.J.H. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology 1986, 6, 718–722. [Google Scholar] [CrossRef]


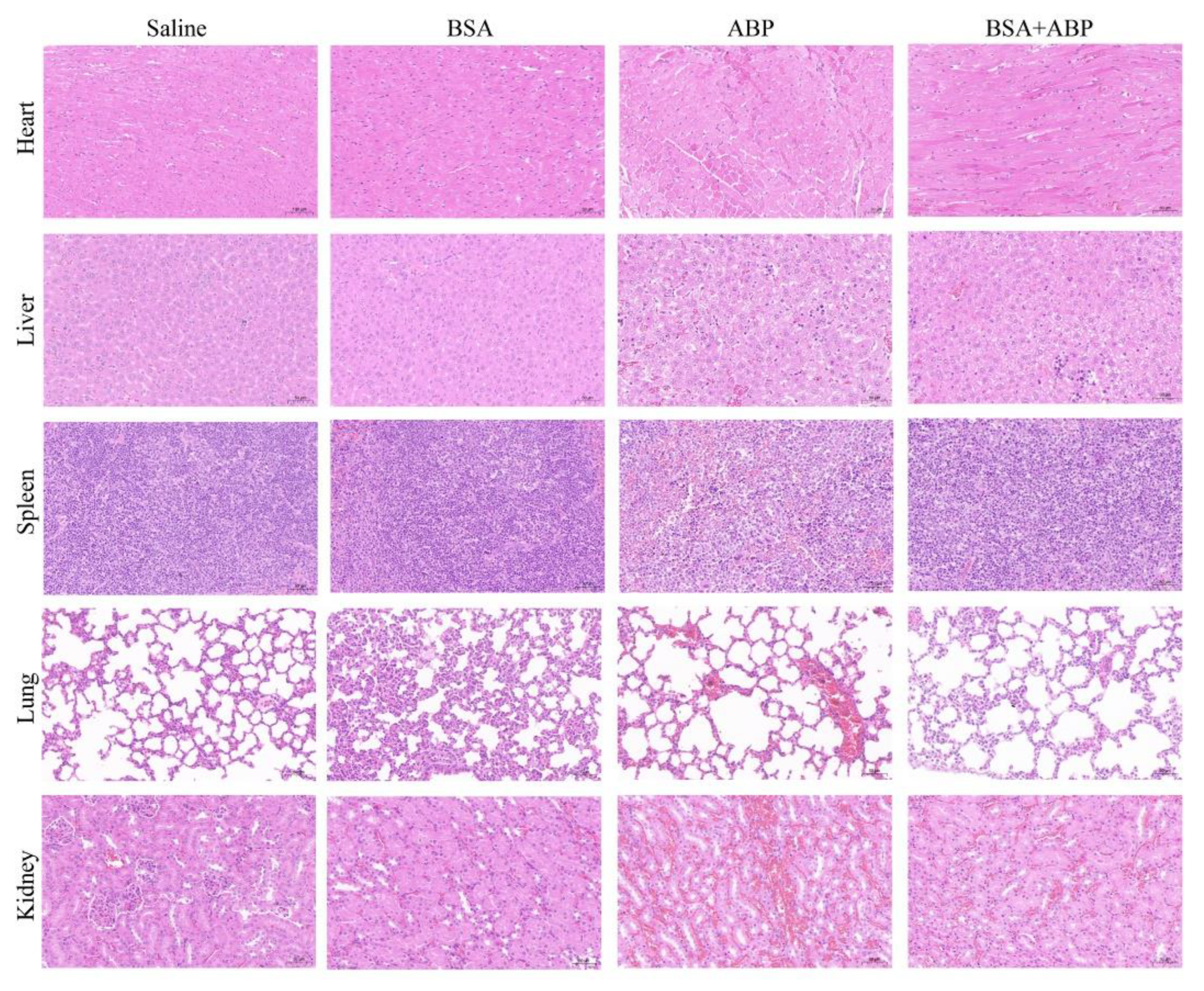
| Group | ALT (U/L) | AST (U/L) | ALB (g/L) | BUN (mg/dL) | CREA (umol/L) |
|---|---|---|---|---|---|
| Saline | 45.32 ± 3.07 | 175.01 ± 27.1 | 32.72 ± 1.44 | 27.31 ± 1.53 | 48.71 ± 8.35 |
| BSA | 41.87 ± 8.53 | 178.24 ± 31.50 | 31.03 ± 2.05 | 18.24 ± 1.72 | 49.52 ± 5.58 |
| ABP | 36.64 ± 9.72 | 283.11 ± 122.60 | 30.48 ± 2.24 | 40.24 ± 11.89 | 34.56 ± 9.40 |
| BSA + ABP | 35.72 ± 7.00 | 186.91 ± 21.50 | 34.13 ± 1.64 | 23.71 ± 1.24 | 29.20 ± 5.94 |
| Normal | 10.06–96.47 | 36.31–235.48 | 21.22–39.15 | 10.81–34.74 | 10.91–85.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Tai, Z.; Liu, F.; Tian, J.; Ding, N.; Chen, Z.; Gao, S. Research and Application of Kupffer Cell Thresholds for BSA Nanoparticles. Molecules 2023, 28, 880. https://doi.org/10.3390/molecules28020880
Guo H, Tai Z, Liu F, Tian J, Ding N, Chen Z, Gao S. Research and Application of Kupffer Cell Thresholds for BSA Nanoparticles. Molecules. 2023; 28(2):880. https://doi.org/10.3390/molecules28020880
Chicago/Turabian StyleGuo, Huanhuan, Zongguang Tai, Fang Liu, Jing Tian, Nan Ding, Zhongjian Chen, and Shen Gao. 2023. "Research and Application of Kupffer Cell Thresholds for BSA Nanoparticles" Molecules 28, no. 2: 880. https://doi.org/10.3390/molecules28020880
APA StyleGuo, H., Tai, Z., Liu, F., Tian, J., Ding, N., Chen, Z., & Gao, S. (2023). Research and Application of Kupffer Cell Thresholds for BSA Nanoparticles. Molecules, 28(2), 880. https://doi.org/10.3390/molecules28020880