Elucidation of Natural Components of Gardenia thunbergia Thunb. Leaves: Effect of Methanol Extract and Rutin on Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Results
2.1. LC–ESI–MS/MS Analysis of G. thunbergia Methanol Extract of Leaves (GME)
2.2. Identification of Compounds’ Structures Isolated from G. thunbergia Methanol Extract of Leaves
2.2.1. Spectroscopic Analysis
Compound (1):
Compound (2):
Compound (3):
Compound (4):
2.3. Effect of Rutin and Leaves Methanol Extract on Liver Index and Liver Function Tests of Mice
2.3.1. Effect of Rutin and Leaves Methanol Extract on the Lipid Profile of Mice
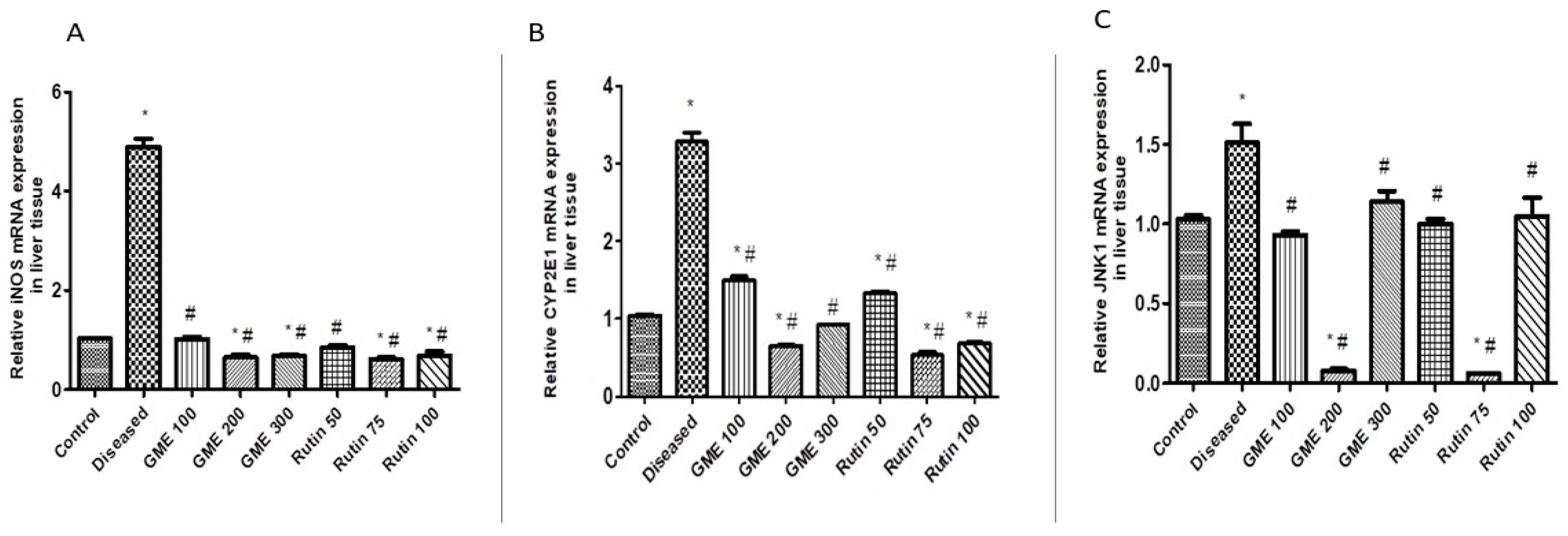
2.3.2. Effect of Rutin and Methanolic Extract on Oxidative Stress-Related Gene Expression
2.3.3. Effect of Rutin and Total Leaves Extract on Apoptotic Kinase (c-JNK1) Gene Expression
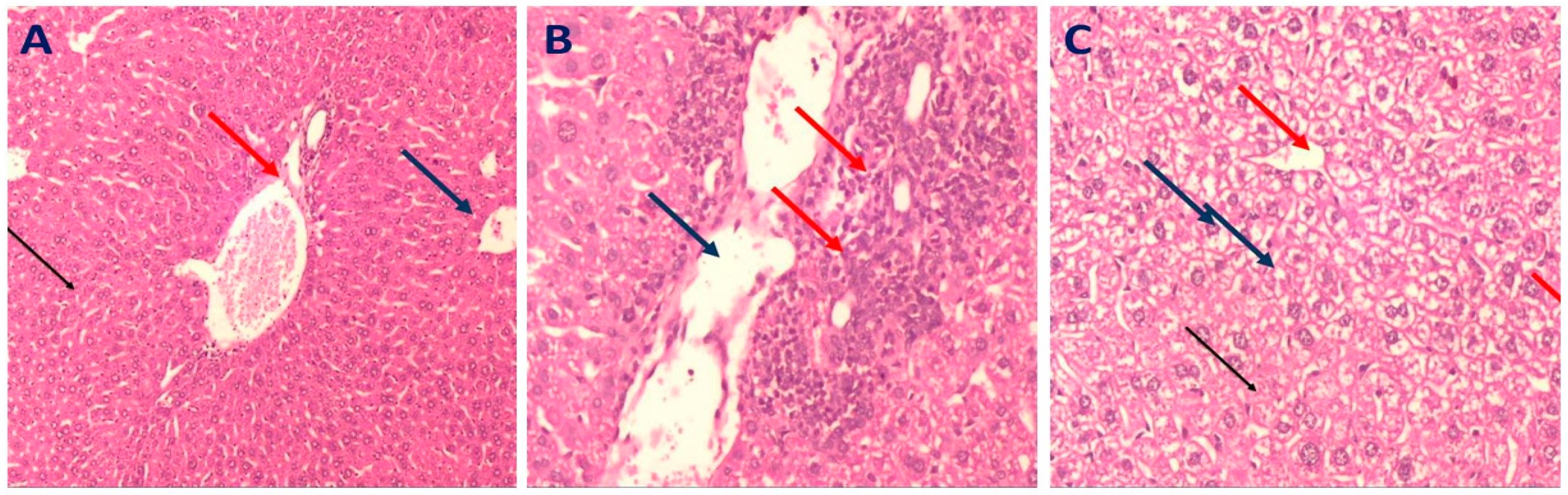
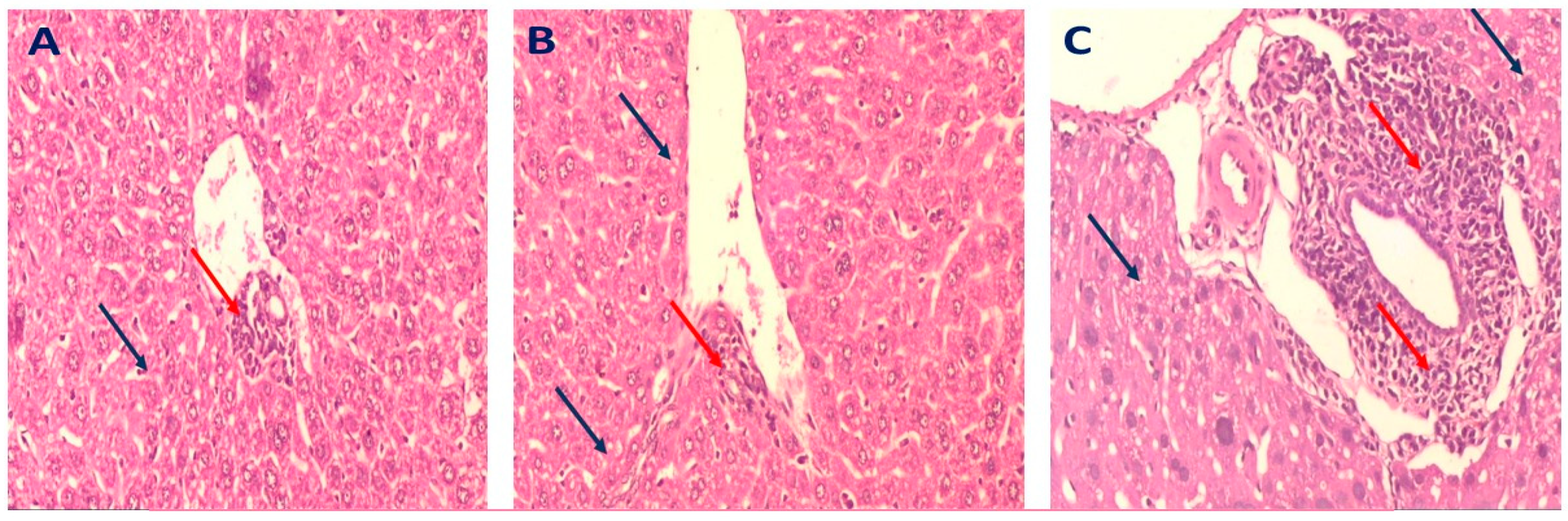
2.3.4. Histopathology of Liver in Different Mice Groups
3. Discussion
4. Materials and Methods
4.1. Plant Material
Extraction and Isolation Procedure
4.2. LC–ESI–MS/MS for Metabolite Analysis
4.2.1. Sample Preparation
4.2.2. Acquisition Method and Analytical Parameters
4.3. Experimental Animals
4.3.1. Method of Assessment of the Effect of Methanol Extract and Rutin on Non-Alcoholic Fatty Liver Disease
4.3.2. Determination of Liver Index and Liver Function Tests
4.3.3. Determination of Lipid Profile in the Serum
4.3.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) for Liver Tissue
4.3.5. Histopathological Examination of Liver Tissue
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yao, H.; Qiao, Y.-J.; Zhao, Y.-L.; Tao, X.-F.; Xu, L.-N.; Yin, L.-H.; Qi, Y.; Peng, J.-Y. Herbal medicines and nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 6890. [Google Scholar] [CrossRef]
- Nower, A.A.; Hamza, E.M. Production of Gardenia jasminoides scions via tissue culture for grafting on Gardenia thunbergia under greenhouse conditions. J. Appl. Sci. Res. 2013, 9, 3118–3128. [Google Scholar]
- Debnath, T.; Park, P.-J.; Nath, N.C.D.; Samad, N.B.; Park, H.W.; Lim, B.O. Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chem. 2011, 128, 697–703. [Google Scholar] [CrossRef]
- Yin, F.; Liu, J.-h. Research and application progress of Gardenia jasminoides. Chin. Herb. Med. 2018, 10, 362–370. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Zeng, J.-L.; Ho, S.-T.; Xu, J.-W.; Li, S.; Wu, J.-H. Anti-NAFLD effect of Djulis hull and its major compound, rutin, in mice with high-fat diet (HFD)-induced obesity. Antioxidants 2021, 10, 1694. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.G.; El-Sherbeni, S.A.; El-Kadem, A.H.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Negm, W.A. Elucidation of the Metabolite Profile of Yucca gigantea and Assessment of its Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities. Molecules 2022, 27, 1329. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Shastri, Y.; Al Otaibi, M.; Buchel, E.; Saleh, H.; Ahmad, R.; Ahmed, H.; Al Idris, F.; Ahmed, S.; Guda, M. Expert opinion on the management of Non-alcoholic fatty liver disease (NAFLD) in the Middle east with a focus on the use of silymarin. Gastroenterol. Insights 2021, 12, 155–165. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, D.; Zhao, A.; Huang, F.; Kuang, J.; Ren, Z.; Chen, T.; Lei, J.; Lin, J.; Wang, X. Theabrownin and Poria cocos polysaccharide improve lipid metabolism via modulation of bile acid and fatty acid metabolism. Front. Pharmacol. 2022, 2439. [Google Scholar] [CrossRef]
- Ratziu, V. Non-pharmacological interventions in non-alcoholic fatty liver disease patients. Liver Int. 2017, 37, 90–96. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Pasarín, M.; Abraldes, J.G.; Liguori, E.; Kok, B.; La Mura, V. Intrahepatic vascular changes in non-alcoholic fatty liver disease: Potential role of insulin-resistance and endothelial dysfunction. World J. Gastroenterol. 2017, 23, 6777. [Google Scholar] [CrossRef]
- Harjumäki, R.; Pridgeon, C.S.; Ingelman-Sundberg, M. CYP2E1 in alcoholic and non-alcoholic liver injury. Roles of ROS, reactive intermediates and lipid overload. Int. J. Mol. Sci. 2021, 22, 8221. [Google Scholar] [CrossRef]
- de Carvalho Tavares, I.M.; Lago-Vanzela, E.S.; Rebello, L.P.G.; Ramos, A.M.; Gomez-Alonso, S.; Garcia-Romero, E.; Da-Silva, R.; Hermosin-Gutierrez, I. Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini (L.) Skeels). Food Res. Int. 2016, 82, 1–13. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, B.; Li, Z.; Hong, T.; Chen, M.; Tan, Y.; Jiang, J.; Huang, C. Metabolite identification of myricetin in rats using HPLC coupled with ESI-MS. Chromatographia 2012, 75, 655–660. [Google Scholar] [CrossRef]
- Slimestad, R.; Hostettmann, K. Characterisation of phenolic constituents from juvenile and mature needles of Norway spruce by means of high performance liquid chromatography–mass spectrometry. Phytochem. Anal. 1996, 7, 42–48. [Google Scholar] [CrossRef]
- Fang, N.; Yu, S.; Prior, R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002, 50, 3579–3585. [Google Scholar] [CrossRef] [PubMed]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio-Protocol 2019, 9, e3230. [Google Scholar] [CrossRef]
- Shen, J.; Jia, Q.; Huang, X.; Yao, G.; Ma, W.; Zhang, H.; Ouyang, H.; He, J. Development of a HPLC-MS/MS method to determine the 13 elements of semen cuscutae and application to a pharmacokinetic study in rats. Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Nuzul, M.I.; Jong, V.Y.M.; Koo, L.F.; Chan, T.H.; Ang, C.H.; Idris, J.; Husen, R.; Wong, S.W. Effects of Extraction Methods on Phenolic Content in the Young Bamboo Culm Extracts of Bambusa beecheyana Munro. Molecules 2022, 27, 2359. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, M.; Ksouda, K.; Dhouibi, R.; Charfi, S.; Turki, M.; Hammami, S.; Ayedi, F.; Sahnoun, Z.; Zeghal, K.M.; Affes, H. LC-MS/MS analysis and hepatoprotective activity of artichoke (Cynara Scolymus L.) leaves extract against high fat diet-induced obesity in rats. BioMed Res. Int. 2019, 2019. [Google Scholar]
- El Sayed, A.M.; Basam, S.M.; Marzouk, H.S.; El-Hawary, S. LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in pollen typhae for transformation rule exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef]
- Robotham, S.A.; Brodbelt, J.S. Identification of Flavone Glucuronide Isomers by Metal Complexation and Tandem Mass Spectrometry: Regioselectivity of Uridine 5′-Diphosphate–Glucuronosyltransferase Isozymes in the Biotransformation of Flavones. J. Agric. Food Chem. 2013, 61, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Torskangerpoll, K.; Nørbæk, R.; Nodland, E.; Øvstedal, D.O.; Andersen, Ø.M. Anthocyanin content of Tulipa species and cultivars and its impact on tepal colours. Biochem. Syst. Ecol. 2005, 33, 499–510. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Wu, Z.; Pei, J.; Chen, Y.; Huang, X.; Gao, S.; Kan, R.; Zhang, W.; Xie, S. Gardenia jasminoides Ellis Fruit Extracts Attenuated Colitis in 2, 4, 6-Trinitrobenzenesulfonic Acid-Induced Rats. Evid. Based Complement. Altern. Med. 2021, 2021. [Google Scholar] [CrossRef]
- Alotaibi, B.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elharty, M.E.; Saleh, A.; Abdelkader, D.H.; Mokhtar, F.A. Antibacterial activity of nano zinc oxide green-synthesised from Gardenia thailandica triveng. Leaves against Pseudomonas aeruginosa clinical isolates: In vitro and in vivo study. Artif. Cells Nanomed. Biotechnol. 2022, 50, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, E.I.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Attallah, N.G.; Altwaijry, N.; Batiha, G.E.-S.; El-Sherbeni, S.A. Antidiarrheal and antibacterial activities of Monterey cypress phytochemicals: In vivo and in vitro approach. Molecules 2022, 27, 346. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography− negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef]
- Hanganu, D.; Vlase, L.; Olah, N. LC/MS analysis of isoflavones from Fabaceae species extracts. Farmacia 2010, 58, 177–183. [Google Scholar]
- Mahrous, F.S.M.; Mohammed, H.; Sabour, R. LC-ESI-QTOF-MS/MS of Holoptelea integrifolia (Roxb.) Planch. leaves and In silico study of phenolic compounds’ antiviral activity against the HSV1 virus. Azhar Int. J. Pharm. Med Sci. 2021, 1, 91–101. [Google Scholar] [CrossRef]
- Beesk, N.; Perner, H.; Schwarz, D.; George, E.; Kroh, L.W.; Rohn, S. Distribution of quercetin-3, 4′-O-diglucoside, quercetin-4′-O-monoglucoside, and quercetin in different parts of the onion bulb (Allium cepa L.) influenced by genotype. Food Chem. 2010, 122, 566–571. [Google Scholar] [CrossRef]
- Cappiello, A.; Famiglini, G.; Mangani, F.; Tirillini, B. Analysis of coumarins by micro high-performance liquid chromatography—mass spectrometry with a particle beam interface. J. Am. Soc. Mass Spectrom. 1995, 6, 132–139. [Google Scholar] [CrossRef]
- Osakabe, K.; Tsao, C.C.; Li, L.; Popko, J.L.; Umezawa, T.; Carraway, D.T.; Smeltzer, R.H.; Joshi, C.P.; Chiang, V.L. Coniferyl aldehyde 5-hydroxylation and methylation direct syringyl lignin biosynthesis in angiosperms. Proc. Natl. Acad. Sci. 1999, 96, 8955–8960. [Google Scholar] [CrossRef]
- Carlsen, S.C.; Mortensen, A.G.; Oleszek, W.; Piacente, S.; Stochmal, A.; Fomsgaard, I.S. Variation in flavonoids in leaves, stems and flowers of white clover cultivars. Nat. Prod. Commun. 2008, 3. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, M.; Dhanalakshmi, R.; Nandhini, N. GC-MS analysis of phytochemicals, fatty acid profile, antimicrobial activity of Gossypium seeds. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 273–276. [Google Scholar]
- Saravanakumar, K.; Park, S.; Sathiyaseelan, A.; Kim, K.-N.; Cho, S.-H.; Mariadoss, A.V.A.; Wang, M.-H. Metabolite profiling of methanolic extract of Gardenia jaminoides by LC-MS/MS and GC-MS and its anti-diabetic, and anti-oxidant activities. Pharmaceuticals 2021, 14, 102. [Google Scholar] [CrossRef]
- Chang, C.-S.; Yeh, T.S. Detection of 10 sweeteners in various foods by liquid chromatography/tandem mass spectrometry. J. Food Drug Anal. 2014, 22, 318–328. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Kim, M.-S.; Choi, J.S. Anti-adipogenic activity of the edible brown alga Ecklonia stolonifera and its constituent fucosterol in 3T3-L1 adipocytes. Arch. Pharmacal Res. 2014, 37, 713–720. [Google Scholar] [CrossRef]
- Arora, M.; Kalia, A. Isolation and characterization of stigmasterol and β-sitosterol-D-glycoside from ethanolic extract of the stems of Salvadora persica linn. Int. J. Pharm. Pharm. Sci. 2013, 5, 245–249. [Google Scholar]
- El-shiekh, R.A.; Al-Mahdy, D.A.; Hifnawy, M.S.; Tzanova, T.; Evain-Bana, E.; Philippot, S.; Bagrel, D.; Abdelsattar, E.A. Chemical and biological investigation of Ochrosia elliptica Labill. cultivated in Egypt. Rec. Nat. Prod 2017, 11, 552–557. [Google Scholar] [CrossRef]
- Tshilanda, D.D.; Onyamboko, D.N.; Babady-Bila, P.; Ngbolua, K.-t.-N.; Tshibangu, D.S.; Mpiana, P.T. Anti-sickling activity of ursolic acid isolated from the leaves of Ocimum gratissimum L.(Lamiaceae). Nat. Prod. Bioprospecting 2015, 5, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.H.; Lee, H.J.; Kim, S.B.; Jung, J.S.; Lim, S.S.; Suh, H.W. Antinociceptive effect of single components isolated from Agrimonia pilosa Ledeb. Extract. Sci. Pharm. 2019, 87, 18. [Google Scholar] [CrossRef]
- Srinivasan, R.; Natarajan, D.; Shivakumar, M.S. Antioxidant Compound Quercetin-3-O-α-L-rhamnoside (1→ 6)-β-D-glucose (Rutin) isolated from ethyl acetate leaf extracts of Memecylon edule Roxb (Melastamataceae). Free. Radic. Antioxid. 2015, 5, 35–42. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Siqueira, E.P.; Nunes, Y.R.; Cota, B.B. Flavonoids from leaves of Mauritia flexuosa. Rev. Bras. De Farmacogn. 2013, 23, 614–620. [Google Scholar] [CrossRef]
- Nwanodi, O. Nutraceuticals: Curative integrative cancer treatment. Altern. Integr. Med. 2017, 6, 1000240. [Google Scholar] [CrossRef]
- Fabbrini, M.; D’Amico, F.; Barone, M.; Conti, G.; Mengoli, M.; Brigidi, P.; Turroni, S. Polyphenol and tannin nutraceuticals and their metabolites: How the human gut microbiota influences their properties. Biomolecules 2022, 12, 875. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Sang, S.; Lapsley, K.; Rosen, R.T.; Ho, C.-T. New prenylated benzoic acid and other constituents from almond hulls (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 607–609. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, C.; Liu, X.; Li, M.; Gao, M.; Liu, X.; Fang, F.; Chang, Y. Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Mol. Metab. 2017, 6, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.-A.; Fromenty, B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: Mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.A.; Cederbaum, A.I. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 27. [Google Scholar] [CrossRef]
- Song, B.; Cederbaum, A.I. Ethanol-inducible cytochrome P450 (CYP2E1): Biochemistry, molecular biology and clinical relevance: 1996 update. Alcoholism: Clin. Exp. Res. 1996, 20, 138a–146a. [Google Scholar] [CrossRef] [PubMed]
- Aljomah, G.; Baker, S.S.; Liu, W.; Kozielski, R.; Oluwole, J.; Lupu, B.; Baker, R.D.; Zhu, L. Induction of CYP2E1 in non-alcoholic fatty liver diseases. Exp. Mol. Pathol. 2015, 99, 677–681. [Google Scholar] [CrossRef]
- Leung, T.-M.; Nieto, N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 395–398. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free. Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Banerjee, A.; Yoo, S.-H.; Jang, S.; Gonzalez, F.J.; Song, B.-J. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J. Hepatol. 2012, 57, 860–866. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Singh, R.; Wang, Y.; Lefkowitch, J.H.; Rigoli, R.M.; Scherer, P.E.; Czaja, M.J. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology 2006, 43, 163–172. [Google Scholar] [CrossRef]
- Singh, R.; Wang, Y.; Schattenberg, J.M.; Xiang, Y.; Czaja, M.J. Chronic oxidative stress sensitizes hepatocytes to death from 4-hydroxynonenal by JNK/c-Jun overactivation. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G907–G917. [Google Scholar] [CrossRef]
- Iyer, S.; Upadhyay, P.K.; Majumdar, S.S.; Nagarajan, P. Animal models correlating immune cells for the development of NAFLD/NASH. J. Clin. Exp. Hepatol. 2015, 5, 239–245. [Google Scholar] [CrossRef]
- Cicuéndez, B.; Ruiz-Garrido, I.; Mora, A.; Sabio, G. Stress kinases in the development of liver steatosis and hepatocellular carcinoma. Mol. Metab. 2021, 50, 101190. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kubota, N.; Yamauchi, T.; Kadowaki, T. Role of insulin resistance in MAFLD. Int. J. Mol. Sci. 2021, 22, 4156. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.G.; Negm, W.A.; Elekhnawy, E.; Elmongy, E.I.; Altwaijry, N.; El-Haroun, H.; El-Masry, T.A.; El-Sherbeni, S.A. Elucidation of phytochemical content of Cupressus macrocarpa leaves: In vitro and in vivo antibacterial effect against methicillin-resistant Staphylococcus aureus clinical isolates. Antibiotics 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.M.; Metwaly, A.M.; Mostafa, A.E.; Radwan, M.M.; Mehany, A.B.M.; Ahmed, E.; Enany, S.; Magdeldin, S.; Afifi, W.M.; ElSohly, M.A. Biological and chemical evaluation of some African plants belonging to Kalanchoe species: Antitrypanosomal, cytotoxic, antitopoisomerase I activities and chemical profiling using ultra-performance liquid chromatography/quadrupole-time-of-flight mass spectrometer. Pharmacogn. Mag. 2021, 17, 6. [Google Scholar]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S. Phytochemical profiling, in vitro and in silico anti-microbial and anti-cancer activity evaluations and Staph GyraseB and h-TOP-IIβ receptor-docking studies of major constituents of Zygophyllum coccineum L. Aqueous-ethanolic extract and its subsequent fractions: An approach to validate traditional phytomedicinal knowledge. Molecules 2021, 26, 577. [Google Scholar]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Fathy, M.; Khalifa, E.M.; Fawzy, M.A. Modulation of inducible nitric oxide synthase pathway by eugenol and telmisartan in carbon tetrachloride-induced liver injury in rats. Life Sci. 2019, 216, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Mardaneh, J.; Ahouran, M.; Mohammadzadeh, A.; Ardebili, A.; Yousefi, M. Diagnosis of cattle brucellosis by PCR and serological methods: Comparison of diagnostic tests. Biomed. Pharmacol. J. 2017, 14, 881–888. [Google Scholar] [CrossRef]
- Ni, X.; Wang, H. Silymarin attenuated hepatic steatosis through regulation of lipid metabolism and oxidative stress in a mouse model of nonalcoholic fatty liver disease (NAFLD). Am. J. Transl. Res. 2016, 8, 1073. [Google Scholar] [PubMed]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.-S.; Mestry, S.N.; Gursahani, M.S.; Juvekar, A.R. Plumbagin reduces obesity and nonalcoholic fatty liver disease induced by fructose in rats through regulation of lipid metabolism, inflammation and oxidative stress. Biomed. Pharmacother. 2019, 111, 686–694. [Google Scholar] [CrossRef] [PubMed]
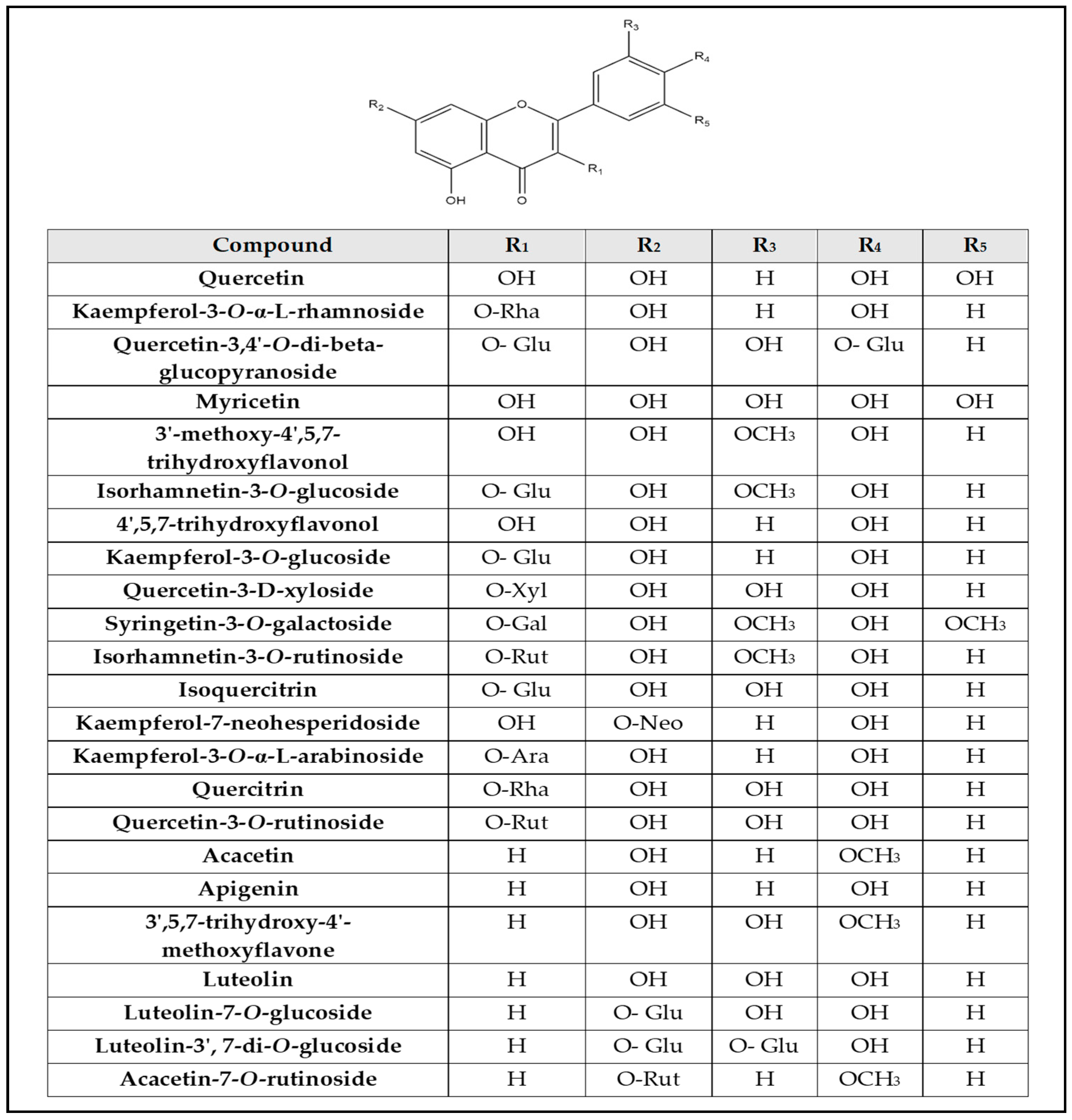
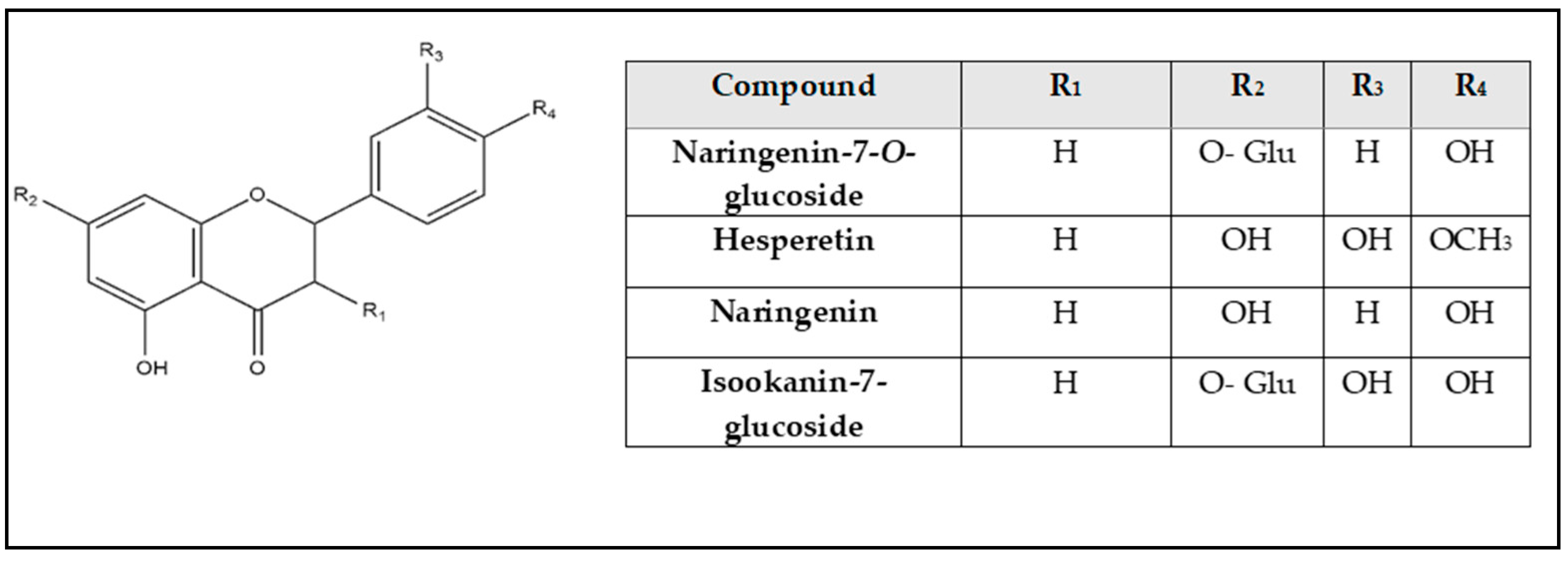
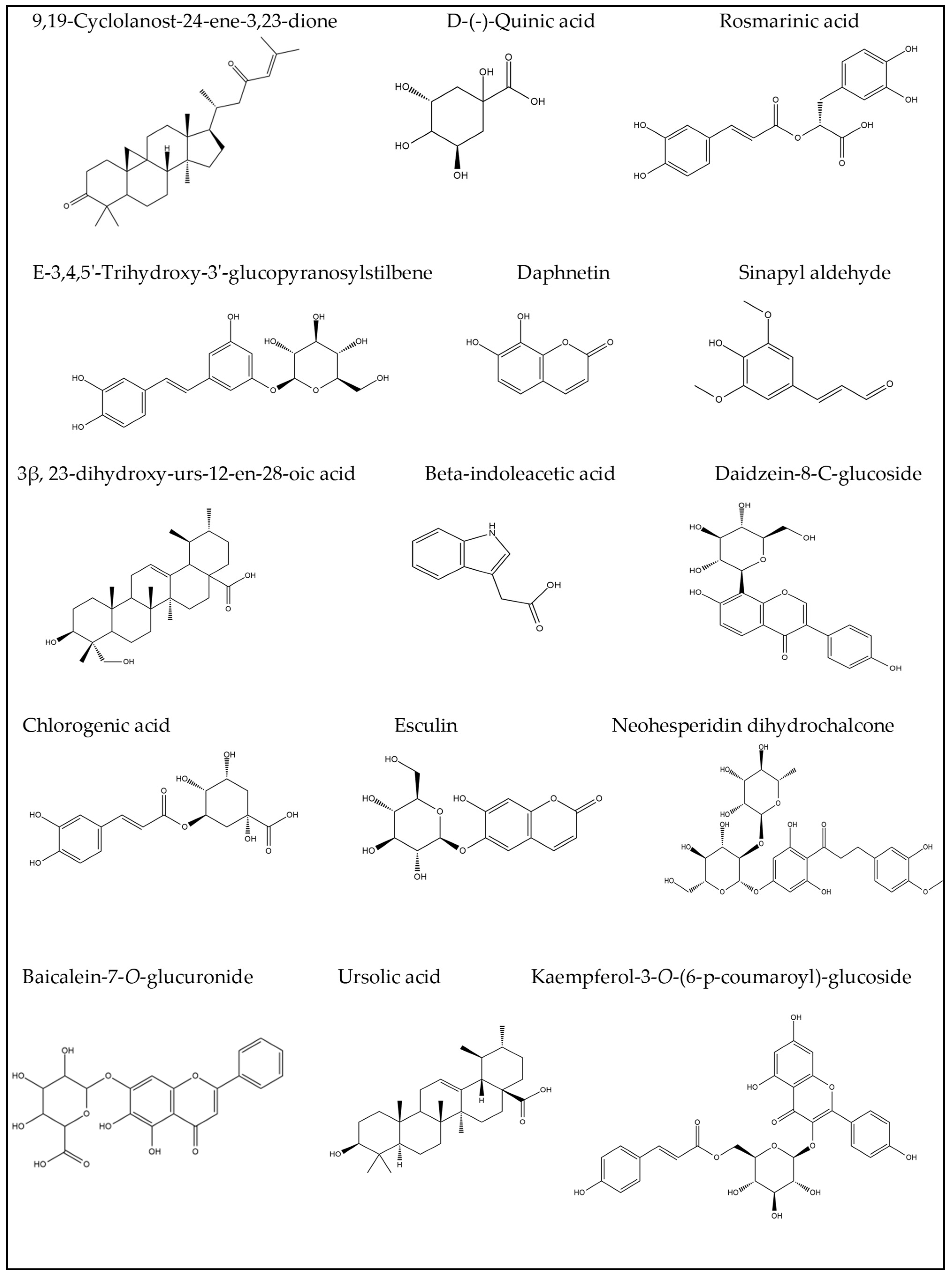
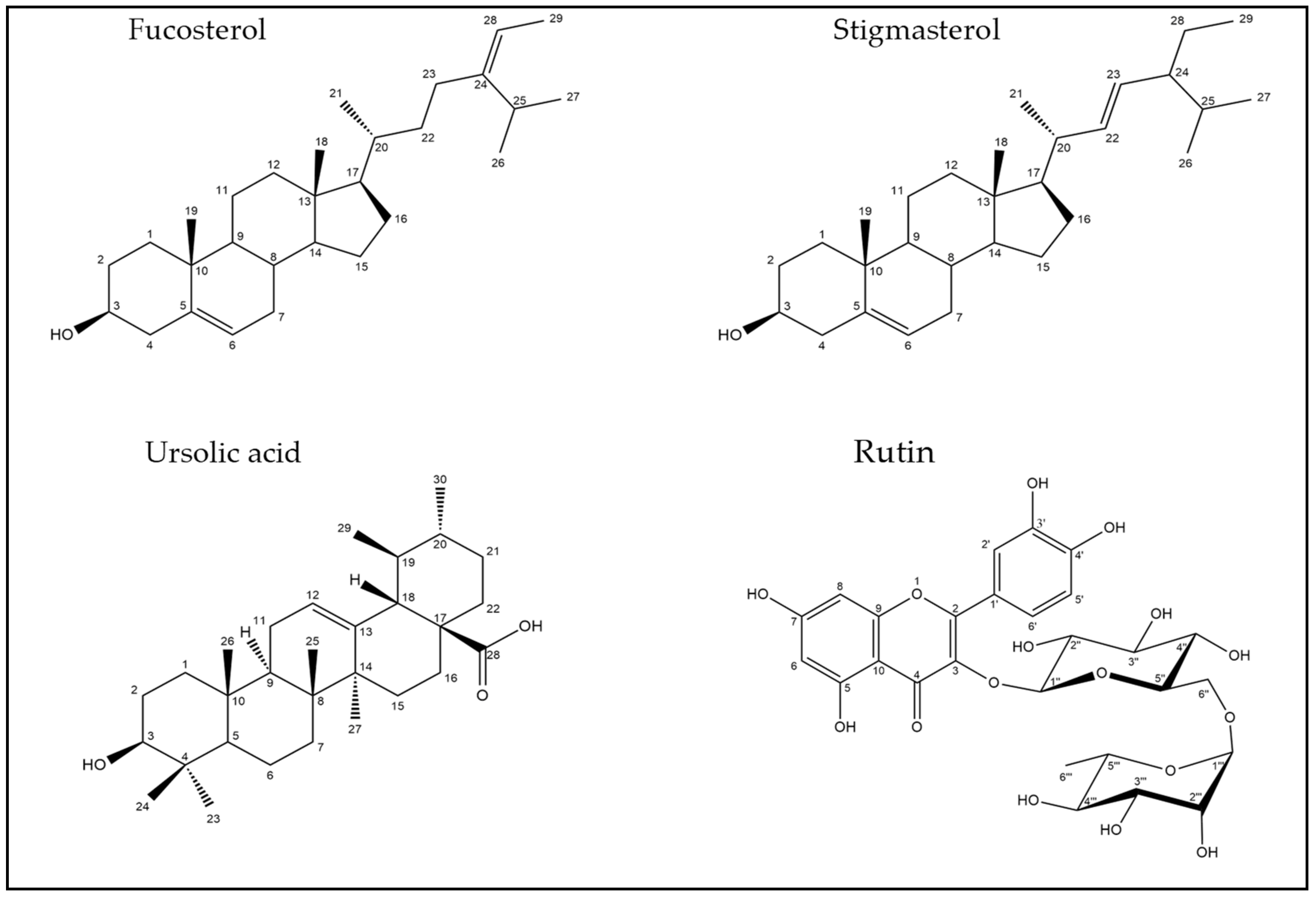

| No. | Rt (min.) | [M − H]¯ m/z | Error PPM | Matching Score | MS2 m/z | Formula | Identification | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.05 | 178.969 | 9.1 | 76.1 | 179.054, 161.043 | C9H8O4 | Caffeic acid | [6] |
| 2 | 1.07 | 507.049 | −0.2 | 72.7 | 507.050, 444.951 | C23H24O13 | Syringetin-3-O-galactoside | [13] |
| 3 | 1.08 | 317.050 | 1.1 | 77 | 317.056, 273.071, 249.004, 213.035, 191.055, 80.963 | C15H10O8 | Myricetin | [14] |
| 4 | 1.08 | 405.098 | 2.5 | 71.9 | 405.099, 404.874, 299.083, 191.055 | C20H22O9 | E-3,4,5′-Trihydroxy-3′-glucopyranosylstilbene (Astringin) | [15] |
| 5 | 1.10 | 191.055 | 2.6 | 85.2 | 191.055, 190.445, 173.040, 171.028, 153.020, 137.023 | C7H12O6 | D-(-)-Quinic acid | [16] |
| 6 | 1.16 | 174.077 | −0.3 | 81.9 | 174.074, 144.068, 128.066 | C10H9NO2 | β-indoleacetic acid | [17] |
| 7 | 1.24 | 447.112 | −0.9 | 86.3 | 447.077, 446.996, 379.080, 191.053, 102.954 | C21H20O11 | Quercitrin | [18] |
| 8 | 1.29 | 163.06 | −3.3 | 86.2 | 163.060, 119.041 | C9H8O3 | 3-(4-hydroxyphenyl)prop-2-enoic acid | [19] |
| 9 | 1.45 | 593.153 | −2.4 | 82.5 | 593.112, 285.301, 248.955 | C27H30O15 | Kaempferol-7-neohesperidoside | [6] |
| 10 | 1.81 | 353.088 | −0.7 | 86.1 | 353.065, 191.055, 179.037, 161.023 | C16H18O9 | Chlorogenic acid | [20] |
| 11 | 3.70 | 339.104 | −21.9 | 88.1 | 339.112, 177.014 | C15H16O9 | Esculin | [21] |
| 12 | 4.14 | 445.135 | −0.4 | 81.1 | 445.135, 269.425, 161.032 | C21H18O11 | Baicalein-7-O-glucuronide | [22,23] |
| 13 | 5.09 | 315.108 | 1 | 77.1 | 315.108, 269.098 | C16H12O7 | 3′-methoxy-4′,5,7- trihydroxyflavonol (Isorhamnetin) | [6] |
| 14 | 5.44 | 431.189 | 4.1 | 84.2 | 431.189, 385.187, 335.081, 285.524, 223.133, 205.128 | C21H20O10 | Kaempferol-3-O-α-L-rhamnoside (Afzelin) | [6] |
| 15 | 6.24 | 609.174 | −46 | 91.9 | 609.143, 607.169, 343.025, 301.033, 300.027, 299.059, 272.036 | C27H31O16 | Quercetin-3-O-rutinoside (Rutin) | [22] |
| 16 | 6.25 | 609.143 | 3.1 | 92.1 | 609.143, 607.169, 343.025, 302.415, 301.033, 300.027, 299.059 | C27H31O16 | Delphinidin-3-O-(6″-O-α-rhamnopyranosyl-β-glucopyranoside) | [24] |
| 17 | 6.58 | 463.088 | −0.9 | 92.7 | 463.085, 461.594, 445.094, 343.051, 301.038, 300.026 | C21H20O12 | Isoquercitrin | [25] |
| 18 | 6.78 | 449.104 | −6.2 | 86.4 | 449.111, 287.040, 151.006 | C21H22O11 | Isookanin-7-glucoside | [26] |
| 19 | 6.86 | 593.152 | −1 | 91.3 | 593.150, 591.226, 285.039, 284.005, 255.031 | C30H26O13 | Kaempferol-3-O-(6-p-coumaroyl)-glucoside | [6] |
| 20 | 6.88 | 433.076 | −0.2 | 84.9 | 433.056, 301.078 | C20H18O11 | Quercetin-3-D-xyloside | [27] |
| 21 | 6.99 | 623.162 | −0.6 | 85.5 | 623.155, 315.036 | C28H32O16 | Isorhamnetin-3-O-rutinoside | [28] |
| 22 | 7.00 | 447.095 | −3.9 | 85.1 | 447.093, 285.043, 284.031, 255.028 | C21H20O11 | Luteolin-7-O-glucoside | [20] |
| 23 | 7.2 | 447.092 | 0.2 | 87 | 447.096, 445.646, 285.038, 284.033, 256.033, 255.033 | C21H20O11 | Kaempferol-3-O-glucoside (astragalin) | [29] |
| 24 | 7.30 | 415.197 | −0.6 | 77.6 | 415.189, 392.128, 253.524, 179.055, 170.951 | C21H20O9 | Daidzein-8-C-glucoside (Puerarin) | [30] |
| 25 | 7.46 | 477.106 | −2.5 | 80.8 | 477.097, 409.160, 315.530 | C22H22O12 | Isorhamnetin-3-O-glucoside | [28] |
| 26 | 7.68 | 433.111 | 3.4 | 81.4 | 433.118, 387.207, 271.056 | C21H22O10 | Naringenin-7-O-glucoside | [31] |
| 27 | 8.70 | 193.049 | −3.3 | 86.2 | 193.050, 178.030, 162.036, 161.022 | C10H10O4 | 3-(4-hydroxy-3-methoxyphenyl) prop-2-enoic acid | [32] |
| 28 | 9.51 | 301.032 | 9.1 | 79.4 | 301.035, 178.998, 150.994 | C15H10O7 | Quercetin | [28] |
| 29 | 9.55 | 359.170 | 1.6 | 71.2 | 359.132, 331.070 | C18H16O8 | Rosmarinic acid | [21] |
| 30 | 9.79 | 417.156 | −38.6 | 83.5 | 417.160, 285.412 | C20H18O10 | Kaempferol-3-O-α-L-arabinoside | [31] |
| 31 | 9.89 | 625.153 | 1.4 | 80.5 | 625.148, 579.297, 301.634 | C27H30O17 | Quercetin-3,4′-O-di-β-glucopyranoside (QDG) | [32] |
| 32 | 10.25 | 271.061 | 0.3 | 80.7 | 271.063, 252.033 | C15H12O5 | Naringenin | [24] |
| 33 | 10.38 | 177.054 | 2.6 | 73.3 | 177.055, 162.021, 145.030, 134.038, 121.022, 118.041 | C9H6O4 | Daphnetin | [33] |
| 34 | 10.60 | 609.160 | −23.6 | 85.4 | 609.149, 285.152 | C27H30O16 | Luteolin-3′,7-di-O-glucoside | [34,35] |
| 35 | 10.76 | 207.065 | 0.7 | 89 | 207.066, 192.041, 177.025, 133.028 | C11H12O4 | Sinapyl aldehyde | [36] |
| 36 | 10.81 | 285.040 | 0.8 | 83 | 285.036 | C15H10O6 | Luteolin | [28] |
| 37 | 12.31 | 301.072 | 0.8 | 71.8 | 301.072, 165.008, 135.044, 134.036 | C16H14O6 | Hesperetin | [6] |
| 38 | 13.57 | 269.080 | 2.6 | 85.4 | 269.079 | C15H10O5 | Apigenin | [28] |
| 39 | 13.80 | 285.076 | 1 | 77.1 | 285.076, 191.036, 165.014, 119.041 | C15H10O6 | 4′,5,7-trihydroxyflavonol | [6] |
| 40 | 14.00 | 283.062 | −2.4 | 84.9 | 283.060, 282.158, 268.037, 240.039, 149.996 | C16H12O5 | Acacetin | [6] |
| 41 | 14.20 | 299.055 | 0.9 | 74.2 | 299.056, 284.032, 271.044, 256.030, 243.068, 240.032 | C16H12O6 | 3′,5,7-trihydroxy-4’-methoxyflavone | [6] |
| 42 | 14.40 | 591.197 | −35.1 | 86.6 | 591.174, 283.065 | C28H32O14 | Acacetin-7-O-rutinoside (Linarin) | [31] |
| 43 | 16.10 | 471.347 | 0 | 77.9 | 471.345 | C30H48O4 | 3β,23-dihydroxy-urs-12-en-28-oic acid | [37] |
| 44 | 16.87 | 469.328 | 6 | 79.3 | 469.329 | C30H46O4 | 9,19-Cyclolanost-24-ene-3,23-dione | [25] |
| 45 | 21.33 | 279.233 | −0.9 | 81.8 | 279.234 | C18H32O2 | 9–12-Octadecadienoic acid | [38] |
| 46 | 22.63 | 455.353 | -0.9 | 79.3 | 455.320 | C30H48O3 | Ursolic acid | [25] |
| 47 | 23.12 | 255.233 | −0.4 | 83 | 255.231 | C16H32O2 | Palmitic acid | [38,39] |
| 48 | 24.00 | 611.154 | −3.4 | 84.3 | 611.147 | C28H36O15 | Neohesperidin dihydrochalcone | [39] |
| Groups | Liver Index (%) | ALT (U/L) | AST (U/L) |
|---|---|---|---|
| Control (NC) | 3.50 ± 0.76 | 21.59 ± 0.49 | 25.96 ± 0.51 |
| Diseased group (DG) | 4.84 ± 0.86 a | 110.25 ± 0.86 a | 86.91 ± 0.73 a |
| Simvastatin | 3.66 ± 0.64 b | 76.98 ± 0.66 b | 76.89 ± 0.66 b |
| Rutin 50 mg/kg | 4.76 ± 0.79 | 89.63 ± 0.74 b | 79.34 ± 0.67 b |
| Rutin 75 mg/kg | 2.71 ± 0.63 b,c | 60.71 ± 0.51 b,c | 71.29 ± 0.66 b,c |
| Rutin 100 mg/kg | 4.21 ± 0.69 | 102.30 ± 0.79 b | 84.36 ± 0.71 b |
| G. thunbergia methanol extract of leaves 100 mg/kg | 3.92 ± 0.51 b | 95.84 ± 0.69 b | 80.99 ± 0.61 b |
| G. thunbergia methanol extract of leaves 200 mg/kg | 2.85 ± 0.53 b,c | 80.36 ± 0.69 b | 78.63 ± 0.65 b |
| G. thunbergia methanol extract of leaves 300 mg/kg | 4.76 ± 0.73 | 110.60 ± 0.88 | 86.01 ± 0.63 |
| Groups | TC (mmole/L) | TG (mmole/L) | HDL-C (mmole/L) | LDL-C (mmole/L) |
|---|---|---|---|---|
| NC | 2.55 ± 0.56 | 1.56 ± 0.44 | 2.11 ± 0.66 | 2.09 ± 0.76 |
| DG | 10.40 ± 0.74 a | 2.65 ± 0.71 a | 1.36 ± 0.33 a | 4.96 ± 0.88 a |
| Simvastatin | 3.97 ± 0.41 b | 1.58 ± 0.66 b | 2.25 ± 0.52 b | 2.11 ± 0.51 b |
| Rutin 50 mg/kg | 6.84 ± 0.64 b | 2.01 ± 0.37 | 1.50 ± 0.43 | 4.80 ± 0.86 |
| Rutin 75 mg/kg | 4.36 ± 0.48 b | 1.66 ± 0.58 b | 2.34 ± 0.48 b | 2.99 ± 0.66 b |
| Rutin 100 mg/kg | 7.90 ± 0.76 b | 2.33 ± 0.76 | 1.53 ± 0.36 | 3.76 ± 0.41 b |
| G. thunbergia methanol extract of leaves 100/kg | 5.33 ± 0.61 b | 2.05 ± 0.86 | 1.62 ± 0.35 | 3.99 ± 0.43 b |
| G. thunbergia methanol extract of leaves 200/kg | 4.97 ± 0.53 b | 1.69 ± 0.74 b | 2.30 ± 0.66 b | 3.01 ± 0.56 b |
| G. thunbergia methanol extract of leaves 300/kg | 6.01 ± 0.71 b | 2.31 ± 0.85 | 2.40 ± 0.57 b | 4.76 ± 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
EL-Shial, E.M.; Kabbash, A.; El-Aasr, M.; El-Feky, O.A.; El-Sherbeni, S.A. Elucidation of Natural Components of Gardenia thunbergia Thunb. Leaves: Effect of Methanol Extract and Rutin on Non-Alcoholic Fatty Liver Disease. Molecules 2023, 28, 879. https://doi.org/10.3390/molecules28020879
EL-Shial EM, Kabbash A, El-Aasr M, El-Feky OA, El-Sherbeni SA. Elucidation of Natural Components of Gardenia thunbergia Thunb. Leaves: Effect of Methanol Extract and Rutin on Non-Alcoholic Fatty Liver Disease. Molecules. 2023; 28(2):879. https://doi.org/10.3390/molecules28020879
Chicago/Turabian StyleEL-Shial, Eman M., Amal Kabbash, Mona El-Aasr, Ola A. El-Feky, and Suzy A. El-Sherbeni. 2023. "Elucidation of Natural Components of Gardenia thunbergia Thunb. Leaves: Effect of Methanol Extract and Rutin on Non-Alcoholic Fatty Liver Disease" Molecules 28, no. 2: 879. https://doi.org/10.3390/molecules28020879
APA StyleEL-Shial, E. M., Kabbash, A., El-Aasr, M., El-Feky, O. A., & El-Sherbeni, S. A. (2023). Elucidation of Natural Components of Gardenia thunbergia Thunb. Leaves: Effect of Methanol Extract and Rutin on Non-Alcoholic Fatty Liver Disease. Molecules, 28(2), 879. https://doi.org/10.3390/molecules28020879







