Estrogenic Activity of Mycoestrogen (3β,5α,22E)-Ergost-22-en-3-ol via Estrogen Receptor α-Dependent Signaling Pathways in MCF-7 Cells
Abstract
:1. Introduction
2. Results
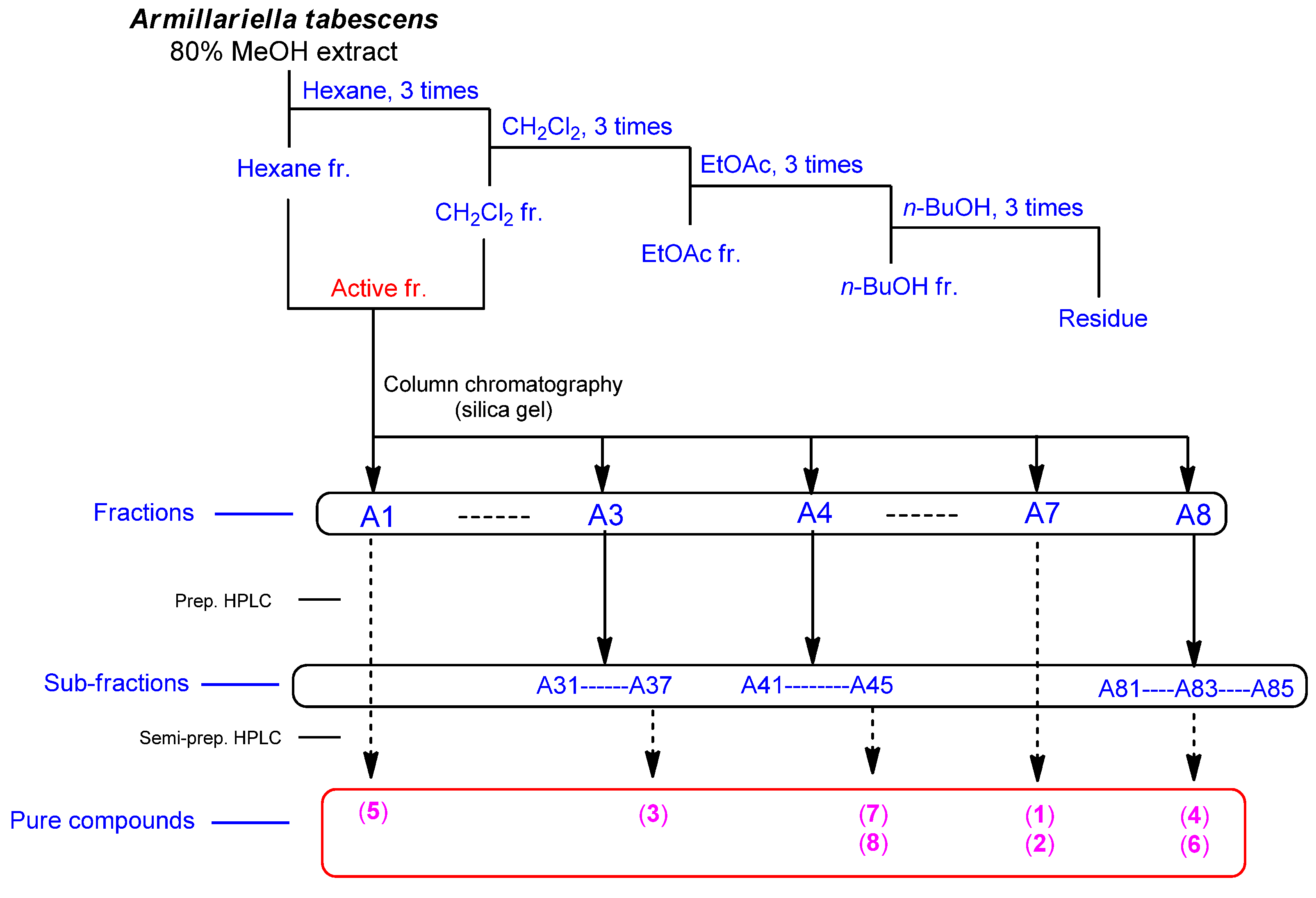
2.1. Bioactivity-Guided Fractionation of the MeOH Extract of A. tabescens
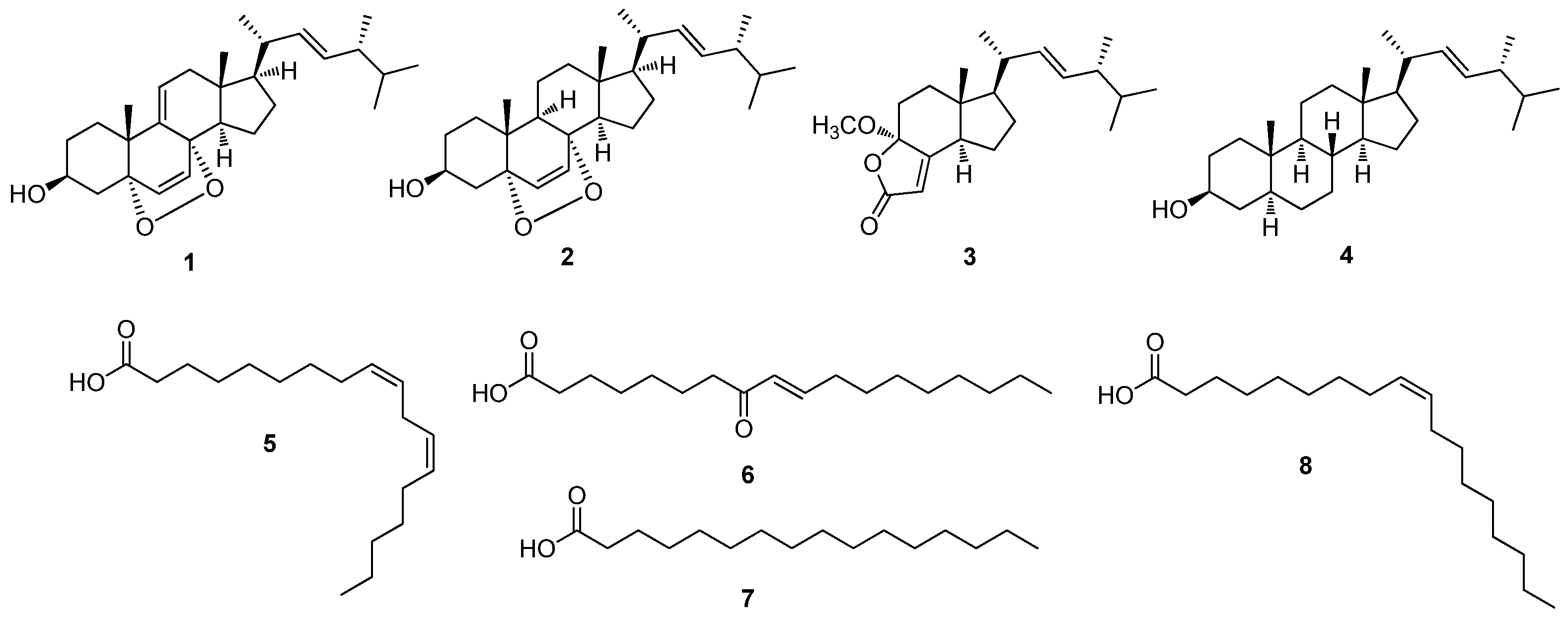
2.2. Isolation and Identification of Compounds from the Active Fraction
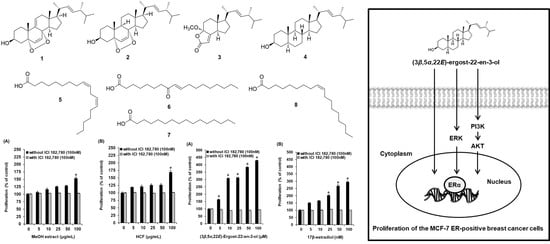
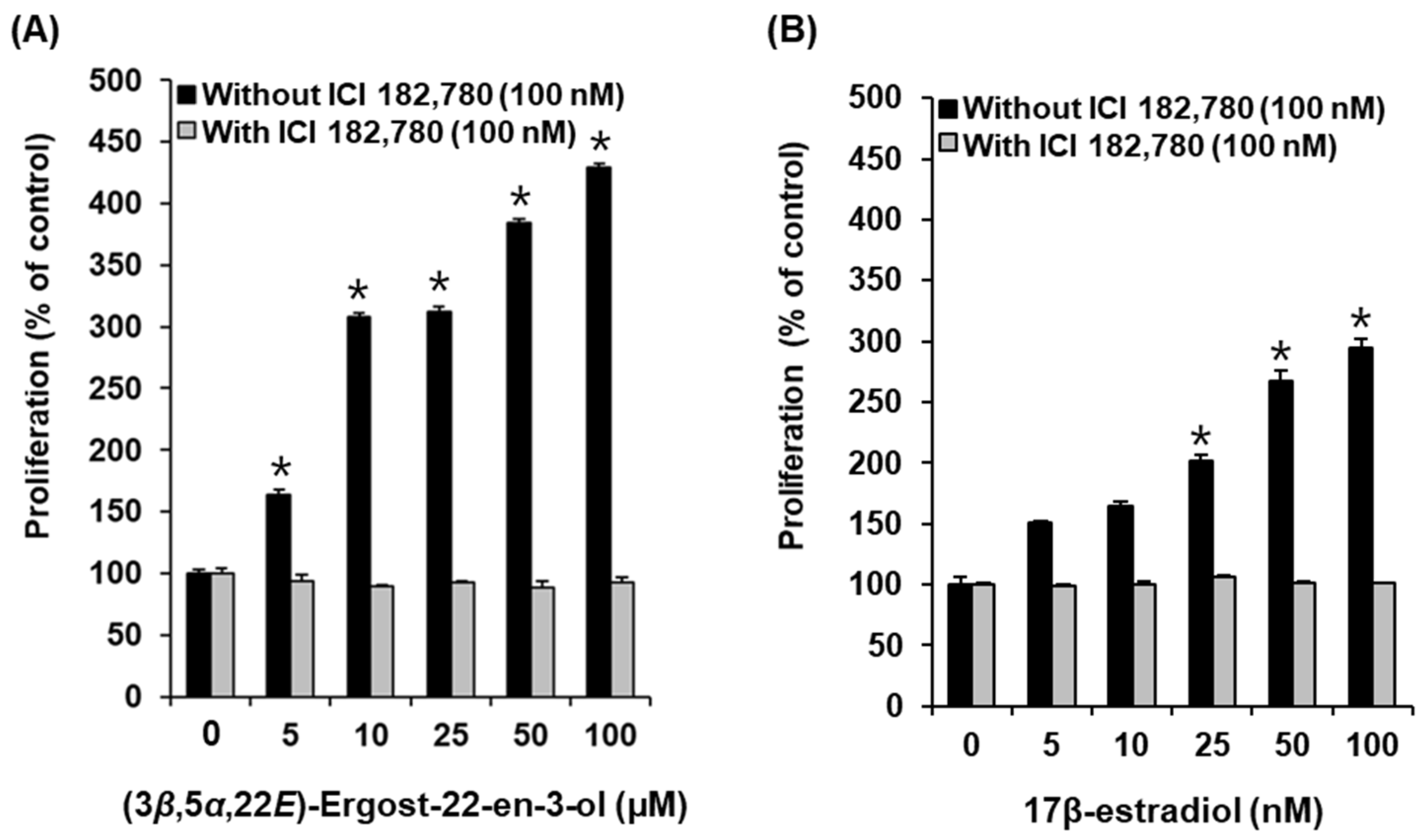
2.3. Effects of Compounds on the Proliferation of MCF-7 Cells
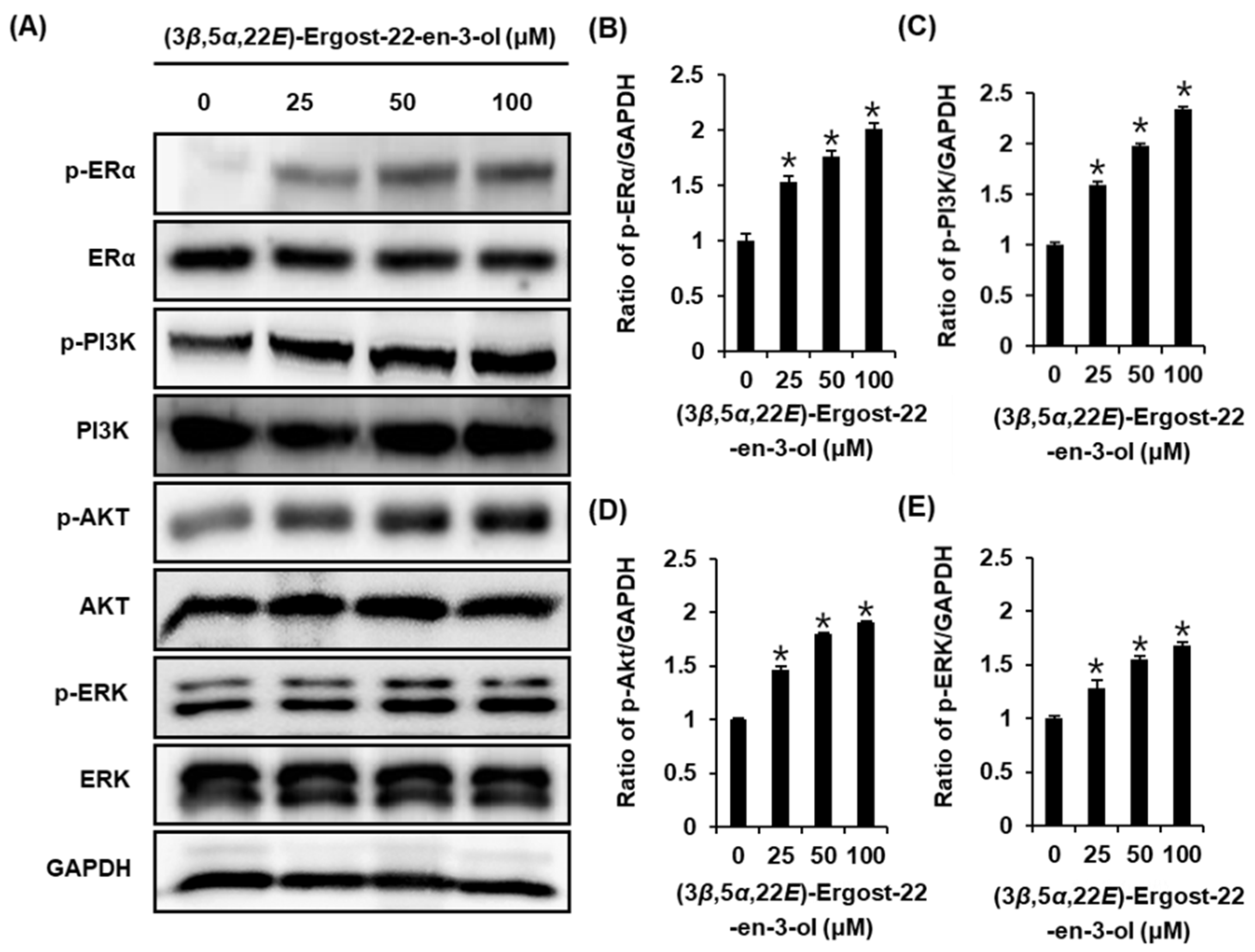
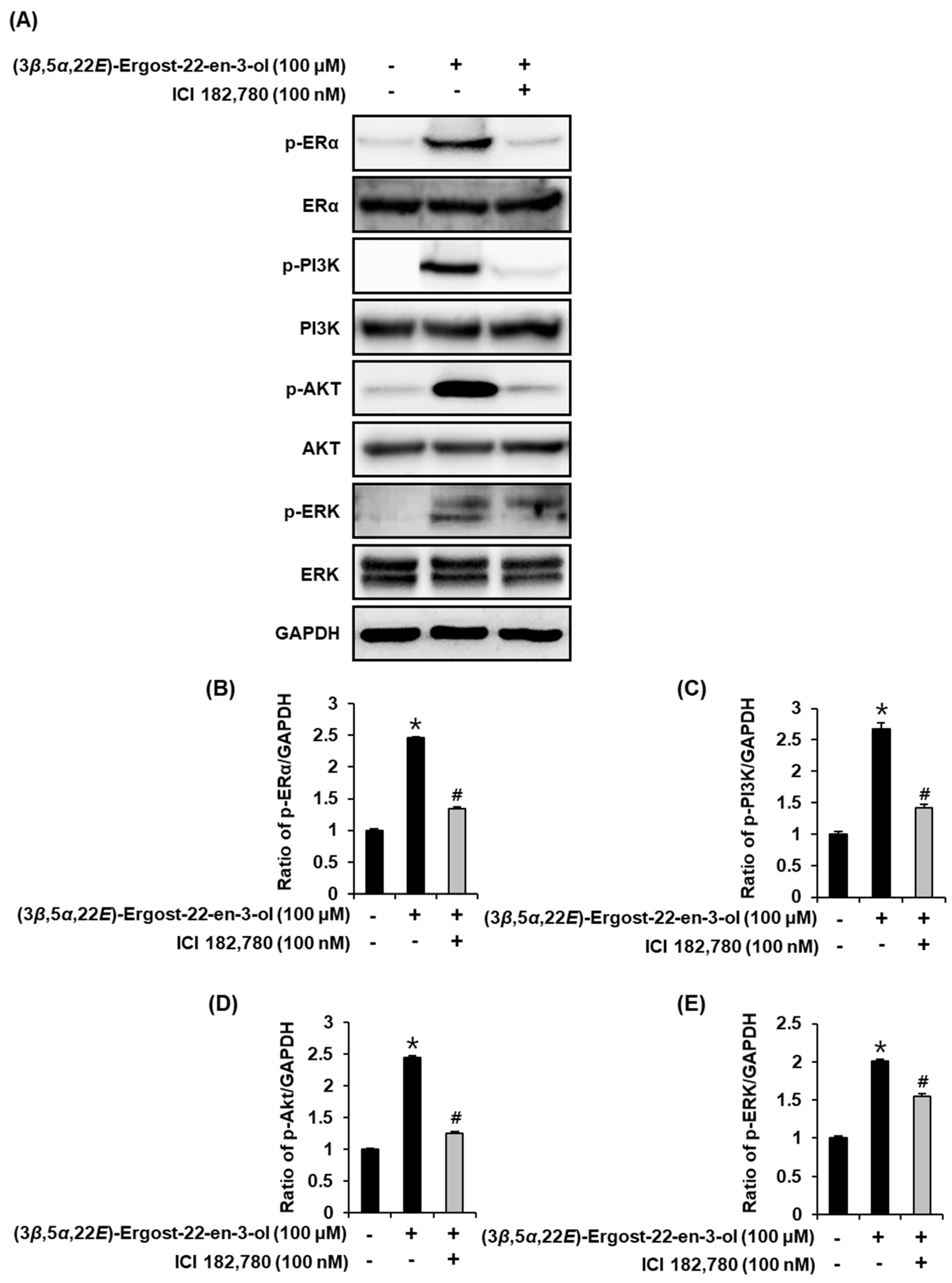
2.4. Effect of (3β,5α,22E)-Ergost-22-en-3-ol on the Protein Expression of Phospho-PI3K, PI3K, Phospho-Akt, Akt, Phospho-ERα, and ERα
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungus Material
4.3. Extraction and Isolation
4.4. Cell Culture
4.5. E-Screen Assay
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- World Health Organization. Research on the Menopause in the 1990s: Report of a WHO Scientific Group; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Hill, K. The demography of menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef]
- Kim, M.Y.; Im, S.-W.; Park, H.M. The demographic changes of menopausal and geripausal women in Korea. J. Bone Metab. 2015, 22, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurzer, M. Soy consumption for reduction of menopausal symptoms. Inflammopharmacology 2008, 16, 227–229. [Google Scholar] [CrossRef]
- Whiteley, J.; DiBonaventura, M.D.; Wagner, J.-S.; Alvir, J.; Shah, S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J. Women’s Health 2013, 22, 983–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton, J.L. Menopausal hormone therapy, age, and chronic diseases: Perspectives on statistical trends. Chem. Res. Toxicol. 2016, 29, 1583–1590. [Google Scholar] [CrossRef]
- Glazier, M.G.; Bowman, M.A. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch. Intern. Med. 2001, 161, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Fait, T. Menopause hormone therapy: Latest developments and clinical practice. Drugs Context. 2019, 8, 212551. [Google Scholar] [CrossRef]
- Barrett-Connor, E.L. The risks and benefits of long-term estrogen replacement therapy. Public Health Rep. 1989, 104, 62. [Google Scholar]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [Green Version]
- Cos, P.; De Bruyne, T.; Apers, S.; Berghe, D.V.; Pieters, L.; Vlietinck, A.J. Phytoestrogens: Recent developments. Planta Med. 2003, 69, 589–599. [Google Scholar]
- Carusi, D. Phytoestrogens as hormone replacement therapy: An evidence-based approach. Prim. Care Update OB/GYNS 2000, 7, 253–259. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef]
- Richardson, K.E.; Hagler, W.M., Jr.; Mirocha, C.J. Production of zearalenone,. alpha.-and. beta.-zearalenol, and. alpha.-and. beta.-zearalanol by Fusarium spp. in rice culture. J. Agric. Food Chem. 1985, 33, 862–866. [Google Scholar] [CrossRef]
- Ding, X.; Lichti, K.; Staudinger, J.L. The mycoestrogen zearalenone induces CYP3A through activation of the pregnane X receptor. Toxicol. Sci. 2006, 91, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Rathore, H.; Sharma, S.; Yadav, A. Medicinal mushrooms as a source of novel functional food. Int. J. Food Sci. Nutr. Diet. 2015, 4, 221–225. [Google Scholar]
- Shimizu, K.; Yamanaka, M.; Gyokusen, M.; Kaneko, S.; Tsutsui, M.; Sato, J.; Sato, I.; Sato, M.; Kondo, R. Estrogen-like activity and prevention effect of bone loss in calcium deficient ovariectomized rats by the extract of Pleurotus eryngii. Phytother. Res. 2006, 20, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yu, J.S.; Ryoo, R.; Kim, J.-C.; Jang, T.S.; Kang, K.S.; Kim, K.H. Pulveraven A from the fruiting bodies of Pulveroboletus ravenelii induces apoptosis in breast cancer cell via extrinsic apoptotic signaling pathway. J. Antibiot. 2021, 74, 752–757. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Ryoo, R.; Kim, J.-C.; Park, H.B.; Kang, K.S.; Kim, K.H. Calvatianone, a sterol possessing a 6/5/6/5-fused ring system with a contracted tetrahydrofuran B-ring, from the fruiting bodies of Calvatia nipponica. J. Nat. Prod. 2020, 83, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Lee, D.; Lee, B.S.; Ryoo, R.; Pang, C.; Kang, K.S.; Kim, K.H. Phallac acids A and B, new sesquiterpenes from the fruiting bodies of Phallus luteus. J. Antibiot. 2020, 73, 729–732. [Google Scholar] [CrossRef]
- Lee, D.; Lee, W.-Y.; Jung, K.; Kwon, Y.S.; Kim, D.; Hwang, G.S.; Kim, C.-E.; Lee, S.; Kang, K.S. The inhibitory effect of cordycepin on the proliferation of MCF-7 breast cancer cells, and its mechanism: An investigation using network pharmacology-based analysis. Biomolecules 2019, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, D.; Lee, J.C.; Kang, K.S.; Ryoo, R.; Park, H.J.; Kim, K.H. Bioactivity-guided isolation of anti-inflammatory constituents of the rare mushroom Calvatia nipponica in LPS-stimulated RAW 264.7 macrophages. Chem. Biodivers. 2018, 15, e1800203. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.; Park, J.Y.; Seok, S.; Jang, T.S.; Park, H.B.; Shim, S.H.; Kang, K.S.; Kim, K.H. Antigastritis effects of Armillariella tabescens (Scop.) Sing. and the identification of its anti-inflammatory metabolites. J. Pharm. Pharmacol. 2018, 70, 404–412. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.Y.; Lee, D.; Seok, S.; Kwon, Y.J.; Jang, T.S.; Kang, K.S.; Kim, K.H. Chemical constituents from the rare mushroom Calvatia nipponica inhibit the promotion of angiogenesis in HUVECs. Bioorg. Med. Chem. Lett. 2017, 27, 4122–4127. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, D.; Lee, H.-J.; Noh, H.J.; Jung, K.; Kang, K.S.; Kim, K.H. Renoprotective chemical constituents from an edible mushroom, Pleurotus cornucopiae in cisplatin-induced nephrotoxicity. Bioorg Chem. 2017, 71, 67–73. [Google Scholar] [CrossRef]
- Shen, J.-W.; Ma, B.-J.; Li, W.; Yu, H.-Y.; Wu, T.-T.; Ruan, Y. Activity of armillarisin B in vitro against plant pathogenic fungi. Z. Nat. C J. Biosci. 2009, 64, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Li, P.; Tang, Y.; Fawcett, J.P.; Gu, J. Quantitation of Armillarisin A in human plasma by liquid chromatography–electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Cai, F. Study on the isolation, purification and antitumor activity in vivo of polysaccharide extracted from Armillariella tabescens. Jiyinzuxue Yu Yingyong Shengwuxue 2013, 32, 767–770. [Google Scholar]
- Kiho, T.; Shiose, Y.; Nagai, K.; Ukai, S. Polysaccharides in fungi. XXX. Antitumor and immunomodulating activities of two polysaccharides from the fruiting bodies of Armillariella tabescens. Chem. Pharm. Bull. 1992, 40, 2110–2114. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-K.; Kuo, Y.-H.; Chiang, B.-H.; Lo, J.-M.; Sheen, L.-Y. Cytotoxic activities of 9,11-dehydroergosterol peroxide and ergosterol peroxide from the fermentation mycelia of Ganoderma lucidum cultivated in the medium containing leguminous plants on Hep 3B cells. J. Agric. Food Chem. 2009, 57, 5713–5719. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Mangoni, A.; Pansini, M. Incisterols, a new class of highly degraded sterols from the marine sponge Dictyonella incisa. J. Am. Chem. Soc. 1990, 112, 3505–3509. [Google Scholar] [CrossRef]
- Shirane, N.; Takenaka, H.; Ueda, K.; Hashimoto, Y.; Katoh, K.; Ishii, H. Sterol analysis of DMI-resistant and-sensitive strains of Venturia inaequalis. Phytochemistry 1996, 41, 1301–1308. [Google Scholar] [CrossRef]
- Marwah, R.G.; Fatope, M.O.; Deadman, M.L.; Al-Maqbali, Y.M.; Husband, J. Musanahol: A new aureonitol-related metabolite from a Chaetomium sp. Tetrahedron 2007, 63, 8174–8180. [Google Scholar] [CrossRef]
- Kawagishi, H.; Miyazawa, T.; Kume, H.; Arimoto, Y.; Inakuma, T. Aldehyde Dehydrogenase Inhibitors from the Mushroom Clitocybe clavipes. J. Nat. Prod. 2002, 65, 1712–1714. [Google Scholar] [CrossRef]
- Sujun, Y.; Jingyu, S.; Longmei, Z.; Lijuan, L.; Yanhong, W. Chemical constituents of alga Halimeda incrassata (II). Trop. Oceanogr. 2002, 21, 92–95. [Google Scholar]
- Akita, C.; Kawaguchi, T.; Kaneko, F.; Yamamoto, H.; Suzuki, M. Solid-state13C NMR study on order→disorder phase transition in oleic acid. J. Phys. Chem. B 2004, 108, 4862–4868. [Google Scholar] [CrossRef]
- Macêdo, D.S.; Sanders, L.L.O.; das Candeias, R.; Montenegro, C.D.F.; de Lucena, D.F.; Chaves Filho, A.J.M.; Seeman, M.V.; Monte, A.S.G. Protein-Coupled Estrogen Receptor 1 (GPER) as a Novel Target for Schizophrenia Drug Treatment. Schizophr. Bull. Open 2020, 1, sgaa062. [Google Scholar] [CrossRef]
- Pepermans, R.A.; Sharma, G.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor in Cancer and Stromal Cells: Functions and Novel Therapeutic Perspectives. Cells 2021, 10, 672. [Google Scholar] [CrossRef]
- Bratton, M.R.; Antoon, J.W.; Duong, B.N.; Frigo, D.E.; Tilghman, S.; Collins-Burow, B.M.; Elliott, S.; Tang, Y.; Melnik, L.I.; Lai, L. Gαo potentiates estrogen receptor α activity via the ERK signaling pathway. J. Endocrinol. 2012, 214, 45. [Google Scholar] [CrossRef] [Green Version]
- Kazi, A.A.; Molitoris, K.H.; Koos, R.D. Estrogen rapidly activates the PI3K/AKT pathway and hypoxia-inducible factor 1 and induces vascular endothelial growth factor A expression in luminal epithelial cells of the rat uterus. Biol. Reprod. 2009, 81, 378–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivastav, A.; Murphy, L. Interactions of PI3K/Akt/mTOR and estrogen receptor signaling in breast cancer. Breast Cancer Manag. 2012, 1, 235–249. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, D.-D.; Fang, L.; Wu, F.; Kwong, W.H.; Xu, J. Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim. Biophys. Acta 2012, 1820, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Ma, J.; Si, L.; Ren, B.; Chen, X.; Wang, D.; Hao, W.; Tang, X.; Li, D.; Zheng, Q. Low dose of acacetin promotes breast cancer MCF-7 cells proliferation through the activation of ERK/PI3K/AKT and cyclin signaling pathway. Recent Pat. Anticancer Drug Discov. 2018, 13, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Barlas, N.; Özer, S.; Karabulut, G. The estrogenic effects of apigenin, phloretin and myricetin based on uterotrophic assay in immature Wistar albino rats. Toxicol. Lett. 2014, 226, 35–42. [Google Scholar] [CrossRef]
- Wei, Y.; Yuan, P.; Zhang, Q.; Fu, Y.; Hou, Y.; Gao, L.; Zheng, X.; Feng, W. Acacetin improves endothelial dysfunction and aortic fibrosis in insulin-resistant SHR rats by estrogen receptors. Mol. Biol. Rep. 2020, 47, 6899–6918. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Ko, Y.; Pang, C.; Ko, Y.-J.; Choi, Y.-K.; Kim, K.H.; Kang, K.S. Estrogenic Activity of Mycoestrogen (3β,5α,22E)-Ergost-22-en-3-ol via Estrogen Receptor α-Dependent Signaling Pathways in MCF-7 Cells. Molecules 2022, 27, 36. https://doi.org/10.3390/molecules27010036
Lee D, Ko Y, Pang C, Ko Y-J, Choi Y-K, Kim KH, Kang KS. Estrogenic Activity of Mycoestrogen (3β,5α,22E)-Ergost-22-en-3-ol via Estrogen Receptor α-Dependent Signaling Pathways in MCF-7 Cells. Molecules. 2022; 27(1):36. https://doi.org/10.3390/molecules27010036
Chicago/Turabian StyleLee, Dahae, Yuri Ko, Changhyun Pang, Yoon-Joo Ko, You-Kyoung Choi, Ki Hyun Kim, and Ki Sung Kang. 2022. "Estrogenic Activity of Mycoestrogen (3β,5α,22E)-Ergost-22-en-3-ol via Estrogen Receptor α-Dependent Signaling Pathways in MCF-7 Cells" Molecules 27, no. 1: 36. https://doi.org/10.3390/molecules27010036
APA StyleLee, D., Ko, Y., Pang, C., Ko, Y.-J., Choi, Y.-K., Kim, K. H., & Kang, K. S. (2022). Estrogenic Activity of Mycoestrogen (3β,5α,22E)-Ergost-22-en-3-ol via Estrogen Receptor α-Dependent Signaling Pathways in MCF-7 Cells. Molecules, 27(1), 36. https://doi.org/10.3390/molecules27010036