The Influence of Hard Coal Combustion in Individual Household Furnaces on the Atmosphere Quality in Pszczyna (Poland)
Abstract
:1. Introduction
2. Samples and Methods
2.1. Samples Collection
2.2. Mineral Composition
2.3. Chemical Composition
3. Results and Discussion
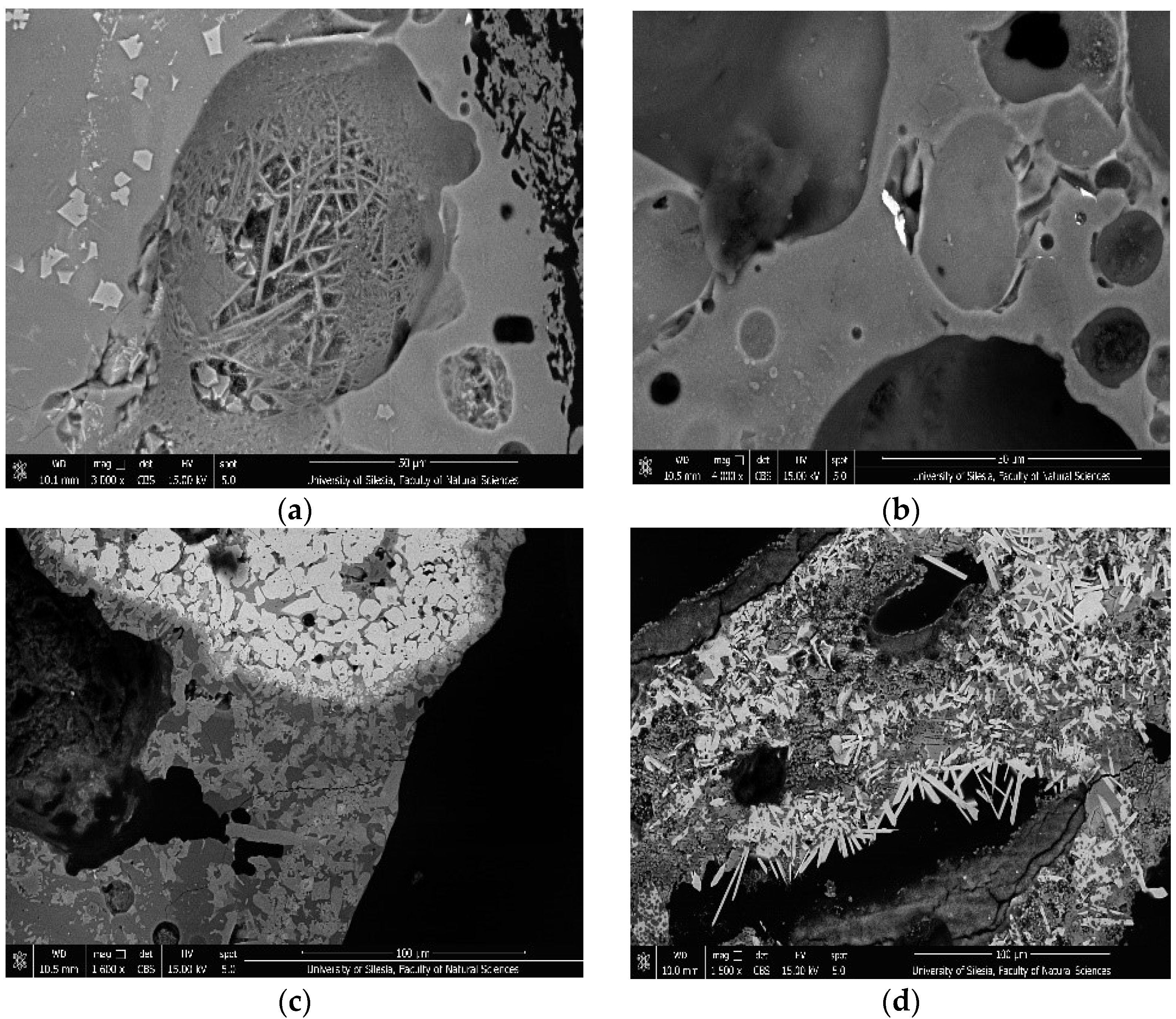
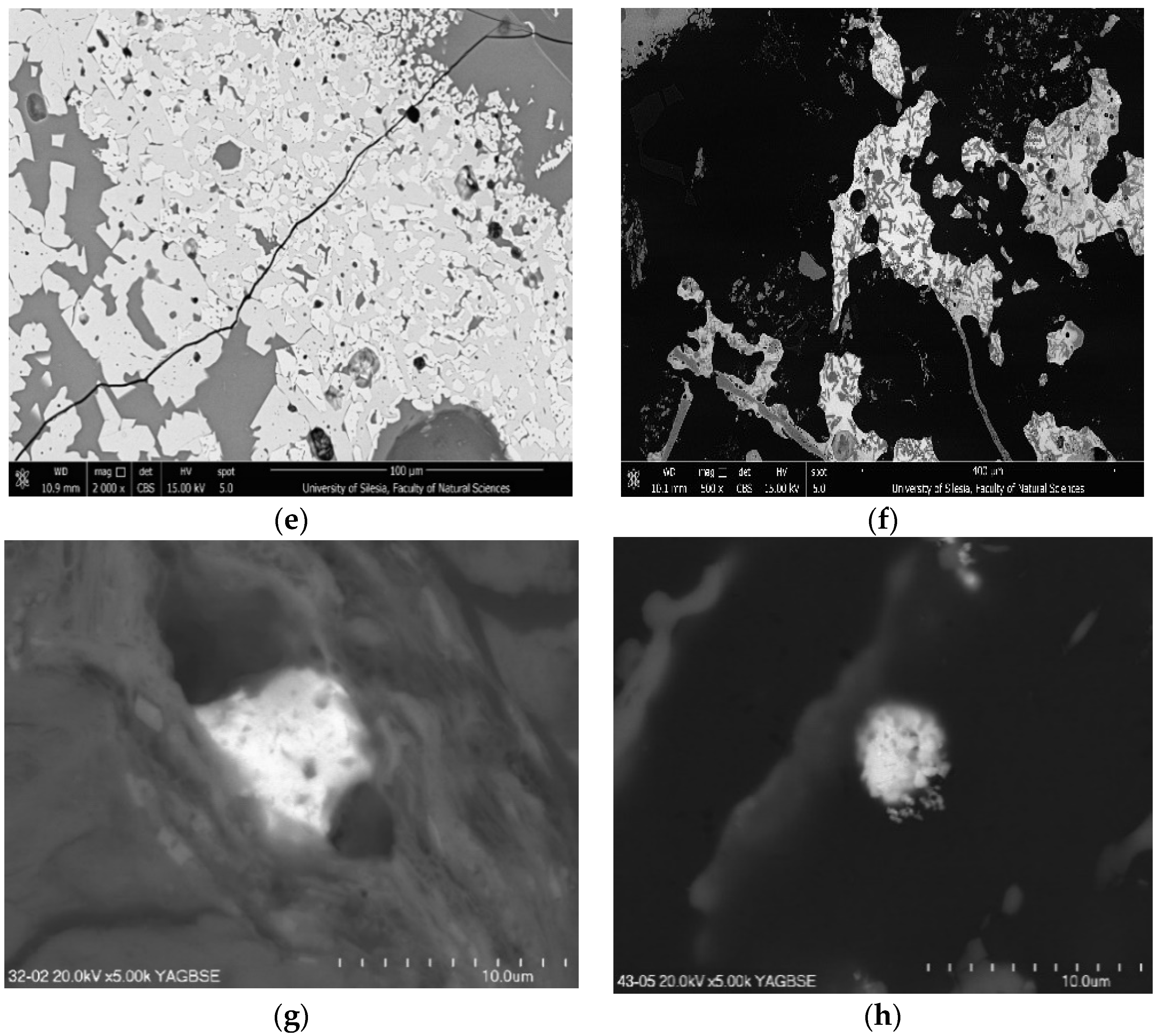
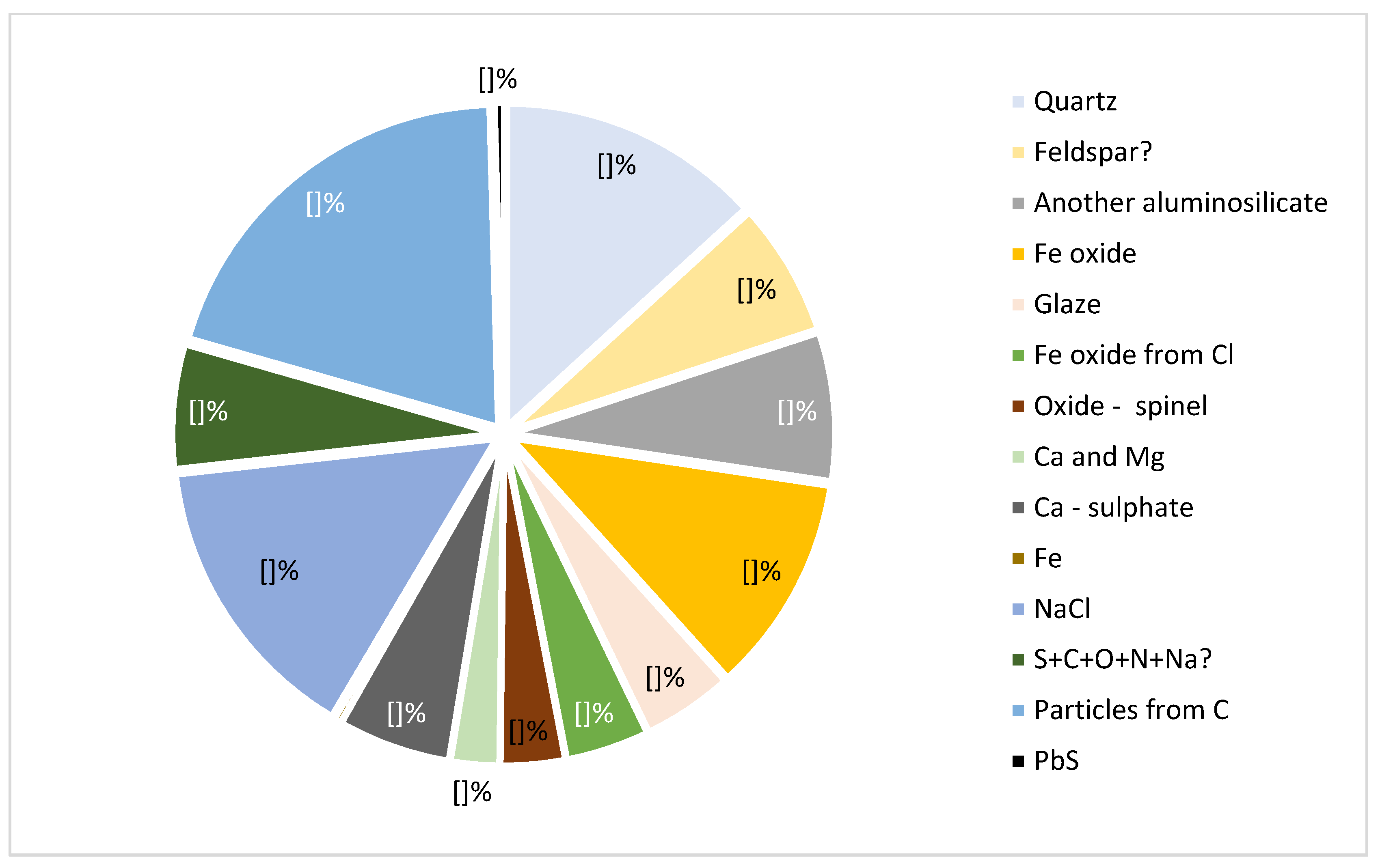
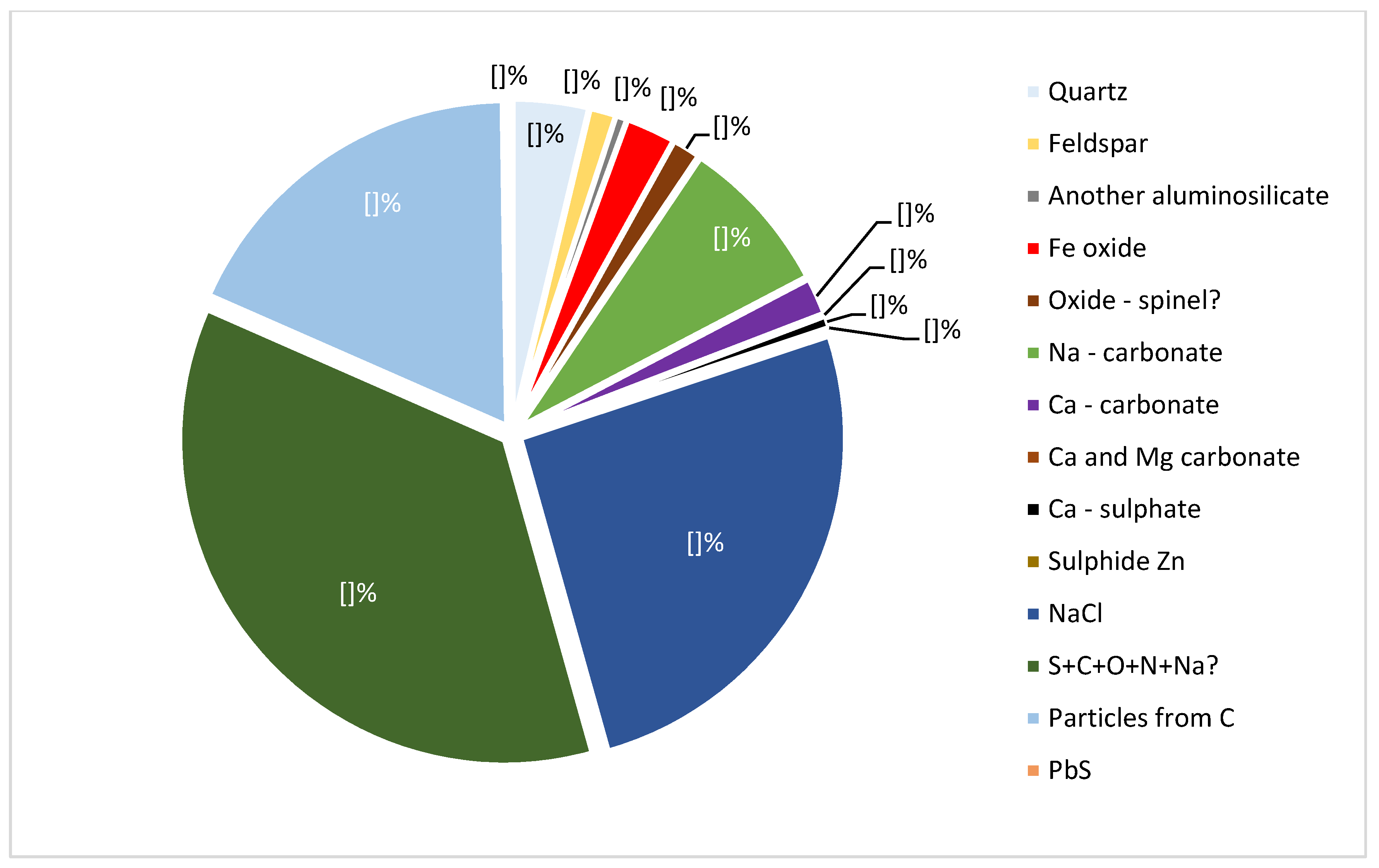
3.1. Mineral Composition of Ashes
3.2. Composition of Dust Mineral
3.3. Chemical Composition of Ashes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smil, V. Energy Transitions: Global and National Perspectives, 2nd ed.; ABC-CLIO, LLC: Santa Barbara, CA, USA, 2016. [Google Scholar]
- Munawer, M. Human health and environmental impacts of coal combustion and post-combustion wastes. J. Sustain. Min. 2018, 17, 87e96. [Google Scholar] [CrossRef]
- Pavlović, B.; Ivezić, D.; Živković, M. State and perspective of individual household heating in Serbia: A survey-based study. Energy Build. 2021, 247, 111128. [Google Scholar] [CrossRef]
- Olufemi, A.C.; Bello, P.O.; Mja, A. Conflict implications of coal mining and environmental pollution in South Africa: Lessons from Niger delta, Nigeria. Afr. J. Confl. Resolut. 2018, 18, 7–35. [Google Scholar]
- Hendryx, M.; Islam, M.S.; Dong, G.H.; Paul, G. Air pollution emissions 2008–2018 from Australian coal mining: Implications for public and occupational health. Int. J. Environ. Res. Public Health 2020, 17, 1570. [Google Scholar] [CrossRef] [Green Version]
- Kubica, K.; Paradiz, B.; Dilara, P. Small Combustion Installations: Techniques, Emissions and Measures for Emission Reduction; JRC Scientific and Technical Report; EUR 23214 EN—Joint Research Centre—Institute for Environment and Sustainability, Office for Official Publications of the European Communities: Luxembourg, 2007. [Google Scholar] [CrossRef]
- Zierold, K.M.; Odoh, C. A review on fly ash from coal-fired power plants: Chemical composition, regulations, and health evidence. Rev. Environ. Health 2020, 35, 401–418. [Google Scholar] [CrossRef]
- Ye, J.; Zubair, M.; Wang, S.; Cai, Y.; Zhang, P. Power production waste. Water Environ. Res. 2020, 91, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Flues, M.; Sato, I.M.; Scapin, M.A.; Cotrim, M.E.B.; Camargo, I.M.C. Toxic elements mobility in coal and ashes of Figueira cola power plant, Brazil. Fuel 2013, 103, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Perera, F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: Solutions exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, M.; Li, P.; Ma, R.; Shan, M.; Yang, X. Air pollutant emission factors of solid fuel stoves and estimated emission amounts in rural Beijing. Environ. Int. 2020, 138, 106608. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qi, J.; Jiang, J.; Wu, J.; Duan, L.; Wang, S.; Hao, J. Significant reduction in air pollutant emissions from household cooking stoves by replacing raw solid fuels with their carbonized products. Sci. Total Environ. 2019, 650, 653–660. [Google Scholar] [CrossRef]
- Nádudvari, Á.; Fabiańska, M.J.; Marynowski, L.; Kozielska, B.; Konieczyński, J.; Smołka-Danielowska, D.; Ćmiel, S. Distribution of coal and coal combustion related organic pollutants in the environment of the Upper Silesian Industrial Region. Sci. Total Environ. 2018, 628–629, 1462–1488. [Google Scholar] [CrossRef]
- Fabiańska, M.J.; Smołka-Danielowska, D. Biomarker compounds in ash from coal combustion in domestic furnaces (Upper Silesia Coal Basin, Poland). Fuel 2012, 102, 333–344. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Wang, S.; Rumchev, K.; Mead-Hunter, R.; Morawska, L.; Hao, J. Impacts of household coal and biomass combustion on indoor and ambient air quality in China: Current status and implication. Sci. Total Environ. 2017, 576, 347–361. [Google Scholar] [CrossRef] [Green Version]
- GUS—Główny Urząd Stystyczny. Statistical Yearbook of the Republic of Poland; Energy Consumption in Households in 2018; Statistics Poland: Warsaw, Poland, 2019. [Google Scholar]
- Report IQ Air. World Air Quality Report 2020; Region and City PM2.5 Ranking; IQAir: Goldach, Switzerland, 2021. [Google Scholar]
- Zhao, N.; Li, B.; Li, H.; Li, G.; Wu, R.; Hong, Q.; Mperejekumana, P.; Li, S.; Zhou, Y.; Ahmad, R.; et al. The potential co-benefits for health, economy and climate by substituting raw coal with waste cooking oil as a winter heating fuel in rural households of northern China. Environ. Res. 2021, 194, 110683. [Google Scholar] [CrossRef] [PubMed]
- Tsiridis, V.; Petala, M.; Samaras, P.; Kungolos, A.; Sakellaropoulos, G.P. Environmental hazard assessment of coal fly ashes using leaching and ecotoxicity test. Ecotoxicol. Environ. Saf. 2012, 84, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kicińska, A. Chemical and mineral composition of fly ashes from home furnaces, and health and environmental risk related to their presence in the environment. Chemosphere 2019, 215, 574–585. [Google Scholar] [CrossRef]
- Smołka-Danielowska, D. Trace elements and mineral composition of waste produced in the process of combustion of solid fuels in individual household furnaces in the Upper Silesian Industrial Region (Poland). Environ. Socio-Econ. Stud. 2015, 3, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Smolka-Danielowska, D.; Fiedor, D. Potentially toxic elements in fly ash dependently of applied technology of hard coal combustion. Environ. Sci. Pollut. Res. 2018, 25, 25091–25097. [Google Scholar] [CrossRef] [PubMed]
- GIOŚ—Główny Inspektorat Ochrony Środowiska w Polsce. Stan Środowiska 404 W Województwie Śląskim; Raport; Chief Inspectorate of Environmental Protection: Katowice, Poland, 2020. [Google Scholar]
- PN-EN 303-5:2012. Kotły Grzewcze—Część 5: Kotły Grzewcze Na Paliwa Stałe Z Ręcznym I Automatycznym Zasypem Paliwa O Mocy Nominalnej Do 500 kW—Terminologia, Wymagania, Badania I Oznakowanie; Polish Committee for Standardization: Warszawa, Poland, 2012; ICS 01.040.91.140.10. [Google Scholar]
- Commission Regulation (EU) 2015/1185 of 24 April 2015 Implementing Directive 2009/125/EC of the European Parliament and of the Council with Regard to Ecodesign Requirements for Solid Fuel Local Space Heaters (Official Journal of the European Union); Publications Office of the European Union: Luxembourg, 2015.
- Jończy, I.; Wieczorek, A.; Podwórny, J.; Gerle, A.; Staszuk, M.; Szweblik, J. Characteristic of hard coal and its mixtures with water subjected to friction. Gospod. Surowcami Miner.—Miner. Resour. Manag. 2020, 36, 185–202. [Google Scholar] [CrossRef]
- Parzentny, H.R.; Róg, L. The role of mineral matter in concentrating uranium and thorium in coal and combustion residues from power plants in Poland. Minerals 2019, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.L.S.; Ward, C.R.; Sampaio, C.H.; Querol, X.; Cutruneo, C.M.N.L.; Taffarel, S.R.; Silva, L.F.O. Partitioning of mineralogical and inorganic geochemical components of coals from Santa Catarina, Brazil, by industrial beneficiation processes. Int. J. Geol. 2013, 116–117, 75–92. [Google Scholar] [CrossRef]
- Juda-Rezler, K.; Toczko, B. Pyły Drobne W Atmosferze. Kompendium Wiedzy O Zanieczyszczeniu Powietrza Pyłem Zawieszonym W Polsce; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2016. [Google Scholar]
- Gieré, R.; Querol, X. Solid particulate matter in the atmosphere. Elements 2010, 6, 215–222. [Google Scholar] [CrossRef]
- Jablonska, M.; Rietmeijer, F.J.M.; Janeczek, J. Fine-grained barite in coal fly ash from the Upper Silesian industrial region. Environ. Geol. 2001, 40, 941–948. [Google Scholar] [CrossRef]
- Rietmeijer, F.J.M.; Janeczek, J. An analytical electron microscope study of airborne industrial particle in Sosnowiec, Poland. Atmos. Environ. 1997, 31, 1941–1951. [Google Scholar] [CrossRef]
- Gieré, R.; Blackford, M.G.; Smith, K.L. TEM study of PM2.5 emitted from coal and tire combustion in a thermal power station. Environ. Sci. Technol. 2006, 40, 6235–6240. [Google Scholar] [CrossRef]
- Report. Officially Reported Emission Data. 2021. Available online: https://www.ceip.at/webdab-emission-database/reportedemissiondata; https://www.ceip.at/status-of-reporting-and-review-results/2021-submissions (accessed on 12 September 2021).
- Xing, Y.; Xu, Y.; Shi, M.; Lian, Y. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef]
- Zahran, A.; Ibrahim, M.I.; Ramadan, L.; Ibrahim, M.M. Air quality indices, sources and impact on human health of PM10 and PM2.5 in Alexandria Governorate, Egypt. J. Environ. Prot. 2018, 9, 1237–1261. [Google Scholar] [CrossRef] [Green Version]
- Jabłońska, M.; Janeczek, J.; Smieja-Król, B. The impact of ambient atmospheric mineral-dust particles on the calcification of lungs. Minerals 2020, 11, 125. [Google Scholar] [CrossRef]
- Kostrz, M.; Satora, P. Związki odpowiedzialne za zanieczyszczenie powietrza. Inżynieria Ekol. (Ecol. Eng.) 2017, 18, 89–95. [Google Scholar] [CrossRef]
- Shi, Y.; Ji, Y.; Sun, H.; Hui, F.; Hu, J.; Wu, Y.; Fang, J.; Lin, H.; Wang, J.; Duan, H.; et al. Nanocsale characterization of PM2.5 airborne pollutants reveals high adhesiveness and aggregation capability of soot particles. Sci. Rep. 2015, 5, 11232. [Google Scholar] [CrossRef]
- Colbeck, I.; Lazaridis, M. Aerosols and environmental pollution. Naturwissenschaften 2010, 97, 117–131. [Google Scholar] [CrossRef]
- Reizer, M. Co to Jest pył zawieszony? W: Pyły Drobne W Atmosferze. Kompendium Wiedzy O Zanieczyszczeniu Powietrza Pyłem Zawieszonym W Polsce, Juda-Rezler, K. and Toczko B; Biblioteka Monitoringu Środowiska, Główny Inspektorat Ochrony Środowiska: Chief Inspectorate of Environmental Protection: Warszawa, Poland, 2016; pp. 11–21. [Google Scholar]
- Lewińska-Preis, L.; Fabiańska, M.J.; Ćmiel, S.; Kita, A. Geochemical distribution of trace elements in Kaffioyra and Longyearbyen coals, Spitsbergen, Norway. Int. J. Coal Geol. 2009, 80, 211. [Google Scholar] [CrossRef]
- Swaine, D.J. Why trace elements are important. Fuel Process. Technol. 2000, 65–66, 21–33. [Google Scholar] [CrossRef]
- Srinivasa, R.M.; Basha, S.; Joshi, H.V.; Jha, B. Evaluation of the emission characteristics of trace metals from coal and oil fired power plants and their fate during combustion. J. Hazard. Mater. 2005, 123, 242–249. [Google Scholar] [CrossRef]
- Singh, R.N.; Porter, J. Incidence of barium pollution in mine water discharge in an underground coal mine. In Proceedings of the Johannesburg, International Mine Water Association Symposium; 1998; pp. 433–447. [Google Scholar]
- Jabłońska, M.; Kramarczyk, M.; Smieja-Król, B. Barium concentration in cost roe deer antlers related to air pollution caused by burning of barium—enriched coal in southern Poland. Environ. Sci. Pollut. Res. 2016, 23, 5978–5982. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhou, X.; Wang, Q.; Li, X.; Zhang, L.; Wang, D.; He, T.; Cao, Y.; Feng, X. Behavior of tallium in pulverized coal utility boiler installations in Southwest China. J. Air Waste Manag. Assoc. 2021, 71, 488–500. [Google Scholar] [CrossRef]
- Yudovich, Y.E.; Ketris, M.P. Selenium in coal: A review. Int. J. Coal Geol. 2006, 1–2, 112–126. [Google Scholar] [CrossRef]
- Coleman, L.; Bragg, L.J.; Finkelman, R.B. Distribution and mode of occurrence of selenium in US coals. Environ. Geochem. Health 1993, 15, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Arbuzov, S.L.; Maslar, S.G.; Volostnov, A.V.; II’enok, S.S.; Arkhipov, V.S. Modes of occurrence of uranium and thorium in coals and peats of Northern Asia. Solid Fuel Chem. 2012, 46, 52–66. [Google Scholar] [CrossRef]
- Chen, J.; Chen, P.; Yao, D.; Huang, W.; Tong, S.; Wang, K.; Liu, W.; Hu, J.; Li, Q.; Wang, R. Geochemistry of uranium in Chinese coals and the emission invertory of coal-fired power plants in China. Int. Geol. Rev. 2018, 60, 621–637. [Google Scholar] [CrossRef]
- Meij, R. Trace Element Behavior in Coal-Fired Power Plants. Fuel Process. Technol. 1994, 39. [Google Scholar] [CrossRef]
- Meij, R.; Winkel, H. The emissions of heavy metals and persistent organic pollutants from modern coal-fired power stations. Atmos. Environ. 2007, 41, 9262–9272. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Klejnowski, K.; Rogula-Kopiec, P.; Mathews, B.; Szopa, S. A study on the seasonal mass closure of ambient fine and coarse dusts in Zabrze, Poland. Bull. Environ. Contam. Toxicol. 2012, 88, 722–729. [Google Scholar] [CrossRef]
- Tao, Z.; Guo, Q.; Wei, R.; Dong, X.; Han, X.; Guo, Z. Atmospheric lead pollution in a typical megacity: Evidence from lead isotopes. Sci. Total Environ. 2016, 778, 145810. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, S.; Jensen, K.A.; Keller, G.J.; Ewing, J.C. Direct identification of trace metals in fine and ultrafine particles in the Detroit urban atmosphere. Environ. Sci. Technol. 2004, 38, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 Relating to Arsenic, Cadmium, Mercury, Nickel and Polycyclic Aromatic Hydrocarbons in Ambient Air; Publications Office of the European Union: Luxembourg, 2005; p. 3.
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe; Publications Office of the European Union: Luxembourg, 2008; p. 1.
- Meng, C.C.; Wang, L.T.; Zhang, F.F.; Wei, Z.; Ma, S.M.; Ma, X.; Yang, J. Characteristics of concentrations and water-soluble inorganic ions in PM2.5 in Handan City, Hebei province, China. Atmos. Res. 2016, 171, 133–146. [Google Scholar] [CrossRef]






| Component | Formula | Hard Coal | Ash | Eco-Pea Coal | Ash |
|---|---|---|---|---|---|
| Quartz | SiO2 | x | x | x | x |
| Cristobalite | SiO2 | x | x | - | - |
| Corundum | Al2O3 | - | x | - | - |
| Sillimanite | Al2SiO5 | - | x | - | - |
| Mullite | Al6Si2O13 | x | x | x | x |
| Calcite | CaCO3 | - | x | - | - |
| Magnetite | Fe3O4 | x | x | - | - |
| Hematite | Fe2O3 | x | x | x | x |
| Spinel | MgAl2O4 | - | x | - | x |
| Goethite | FeO(OH) | x | - | - | - |
| Gypsum | CaSO4·2H2O | - | x | - | x |
| Bassanite | CaSO4·½ H2O | - | x | - | - |
| Diopside | CaMg[Si2O6] | - | - | x | x |
| Cordierite | Mg2Al3[AlSi5O18] | - | - | - | x |
| Pyrite | FeS2 | x | - | - | - |
| Kaolinite | Al4[Si4O10](OH)8 | x | - | - | - |
| Sphalerite | ZnS | x | - | x | - |
| Galena | PbS | x | - | - | - |
| Feldspars (albite-anorthite) | KAlSi3O8 (NaAlSi3O8–CaAl2Si2O8) | - | x | - | x |
| Measurement | PM1.0 (μg/m3) | PM2.5 (μg/m3) | PM10 (μg/m3) | TVOC (ppb) | TSP (μg/m3) | Soot (ng/m3) |
| Meteorological conditions: temperature min. 2.5 °C; temperature max. 3.0 °C; humidity 54–61%; atmospheric pressure 987 hPa (15 January 2021) | ||||||
| Min. | 71.63 | 81.24 | 98.02 | 40 | 185 | 3217 |
| Max. | 76.32 | 88.49 | 172.59 | 70 | 222 | 17,078 |
| Mean | 73.9 | 84.2 | 123.5 | 53 | 204 | 10,162 |
| Meteorological conditions: temperature min. 0 °C; temperature max. 2.0 °C; humidity 66–72%; atmospheric pressure 987 hPa (10 February 2021) | ||||||
| Measurement | PM1.0 (μg/m3) | PM2.5 (μg/m3) | PM10 (μg/m3) | TVOC (ppb) | TSP (μg/m3) | Soot (ng/m3) |
| Min. | 114.57 | 151.59 | 173.70 | 439 | 98 | 1247 |
| Max. | 118.85 | 154.46 | 187.83 | 536 | 320 | 22,858 |
| Mean | 116.5 | 152.6 | 179.6 | 498 | 243 | 11,779 |
| Sample | Element (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Pb | Ba | Se | Ni | Tl | Zn | Th | U | |
| Hard coal Mean | 4.9 (0.45) | 0.4 (0.083) | 77.4 (2.83) | 103 (15.9) | 0.7 (0.095) | 18.2 (1.99) | 1.41 (0.13) | 87.6 (2.20) | 10.2 (0.83) | 5.2 (0.27) |
| Min. | 4.4 | 0.2 | 69.4 | 87 | 0.4 | 17.3 | 1.12 | 79.3 | 9.8 | 4.9 |
| Max. | 5.3 | 0.4 | 86.2 | 128 | 0.8 | 19.7 | 1.51 | 96.2 | 10.9 | 5.4 |
| Ash Mean | 20.2 (0.87) | 7.1 (6.83) | 36.1 (13.31) | 101.4 (121.5) | 6.4 (0.69) | 122.5 (14.58) | 6.6 (0.42) | 2016.8 (31.39) | 12.4 (1.17) | 6.1 (0.33) |
| Min. | 19.6 | 3.4 | 29.4 | 76.3 | 5.7 | 109.4 | 5.5 | 1983.3 | 11.8 | 5.9 |
| Max. | 21.3 | 8.6 | 43.8 | 128.4 | 6.9 | 131.6 | 7.1 | 2173.2 | 12.9 | 6.3 |
| RF | 1.0 | 4.5 | 0.1 | 0.2 | 2.3 | 1.7 | 1.2 | 5.8 | 0.3 | 0.3 |
| Eco-pea coal Mean | 1.1 (0.54) | 0.6 (0.35) | 60.2 (2.38) | 940 (20.3) | 0.2 (0.058) | 43.1 (0.98) | 0.13 (0.04) | 933.6 (17.67) | 0.6 (0.18) | 0.2 (0.016) |
| Min. | 0.9 | 0.5 | 56.4 | 845 | 0.2 | 39.6 | 0.11 | 879.2 | 0.4 | 0.2 |
| Max. | 1.3 | 0.7 | 63.8 | 969 | 0.3 | 48.1 | 0.14 | 951.6 | 0.7 | 0.3 |
| Ash Mean | 5.5 (0.23) | 3.5 (0.30) | 8.4 (0.43) | 213 (313.3) | 0.6 (0.20) | 11.5 (2.36) | 0.22 (0.06) | 1386.3 (32.22) | 2.5 (0.36) | 1.3 (0.62) |
| Min. | 4.9 | 3.3 | 7.8 | 167 | 0.4 | 10.8 | 0.20 | 1312.7 | 2.3 | 0.9 |
| Max. | 5.7 | 3.7 | 8.6 | 242 | 0.8 | 11.9 | 0.26 | 1434.9 | 2.7 | 1.4 |
| RF | 0.6 | 0.6 | 0.02 | 0.02 | 0.3 | 0.03 | 0.2 | 0.2 | 0.5 | 0.7 |
| Literature data 1 | Comparison of trace elements contents in coal from different countries | |||||||||
| 0.5–80 | 0.1–4.31 | 2.69–80 | 0.2–4360 | 0.2–4.7 | 0.5–50 | 0.1–0.34 | 5–300 | 2.4–3.5 | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smołka-Danielowska, D.; Jabłońska, M.; Godziek, S. The Influence of Hard Coal Combustion in Individual Household Furnaces on the Atmosphere Quality in Pszczyna (Poland). Minerals 2021, 11, 1155. https://doi.org/10.3390/min11111155
Smołka-Danielowska D, Jabłońska M, Godziek S. The Influence of Hard Coal Combustion in Individual Household Furnaces on the Atmosphere Quality in Pszczyna (Poland). Minerals. 2021; 11(11):1155. https://doi.org/10.3390/min11111155
Chicago/Turabian StyleSmołka-Danielowska, Danuta, Mariola Jabłońska, and Sandra Godziek. 2021. "The Influence of Hard Coal Combustion in Individual Household Furnaces on the Atmosphere Quality in Pszczyna (Poland)" Minerals 11, no. 11: 1155. https://doi.org/10.3390/min11111155
APA StyleSmołka-Danielowska, D., Jabłońska, M., & Godziek, S. (2021). The Influence of Hard Coal Combustion in Individual Household Furnaces on the Atmosphere Quality in Pszczyna (Poland). Minerals, 11(11), 1155. https://doi.org/10.3390/min11111155






