Effects of Environmental Factors on the Leaching and Immobilization Behavior of Arsenic from Mudstone by Laboratory and In Situ Column Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rock Sample Collection and Characterization
2.2. Natural Adsorbent and Immobilizer Collection and Characterization
2.3. Batch Adsorption Experiments
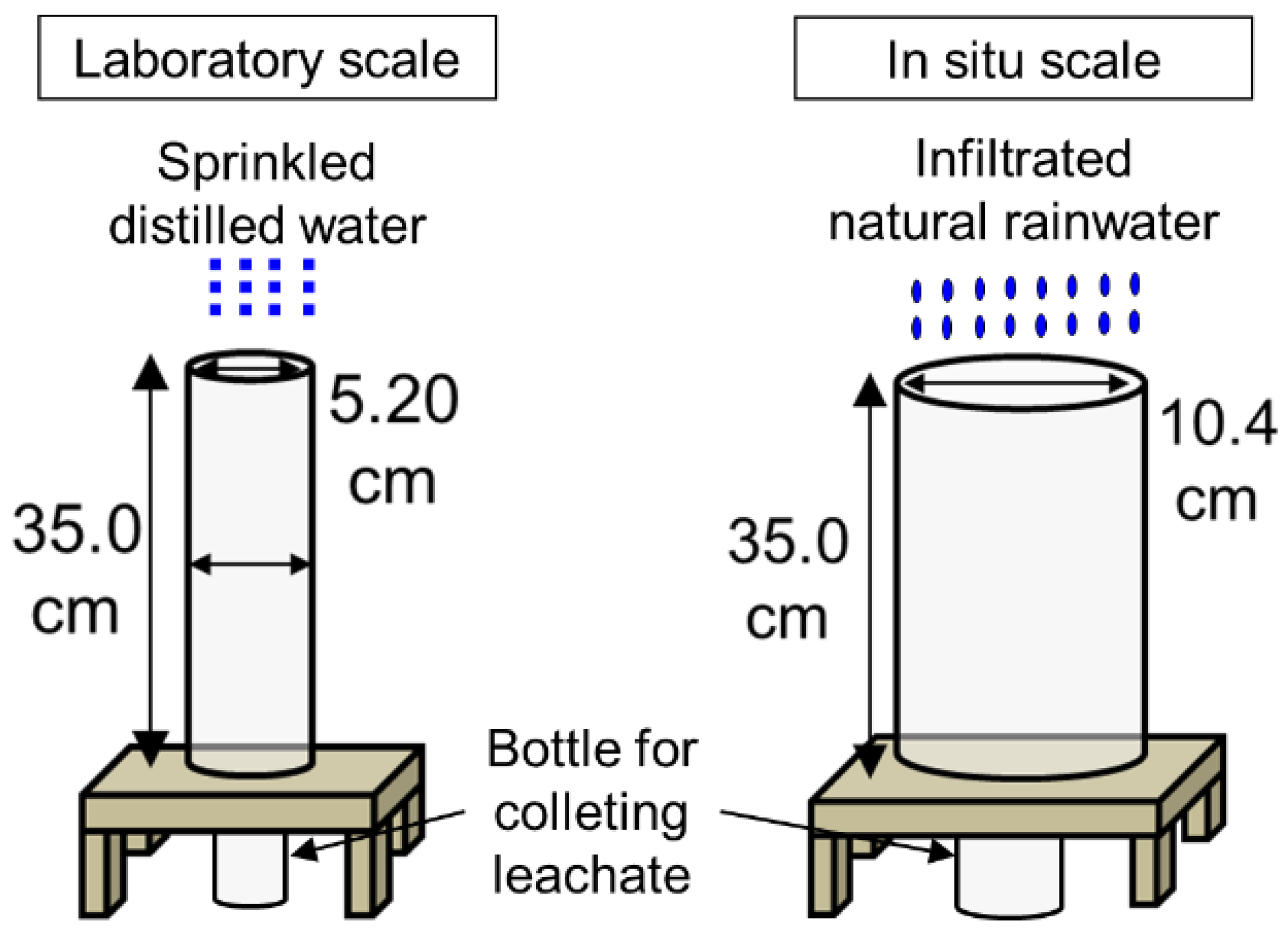
2.4. Laboratory and In Situ Column Experiments
- L-T1: crushed T1 sample only in laboratory column,
- L-T1-AL: crushed T1 sample with bottom adsorption layer in laboratory column,
- I-T1: crushed T1 sample only in situ column,
- I-T1-AL: crushed T1 sample with bottom adsorption layer in situ column,
- L-T2: crushed T2 sample only in laboratory column,
- L-T2-Im: crushed T2 sample mixed with RS as immobilizer in laboratory column,
- I-T2: crushed T2 sample only in situ column,
- I-T2-Im: crushed T2 sample mixed with RS as immobilizer in situ column.
2.5. Chemical Analysis
3. Results and Discussion
3.1. Chemical and Mineralogical Properties of Rock Samples and River Sediment
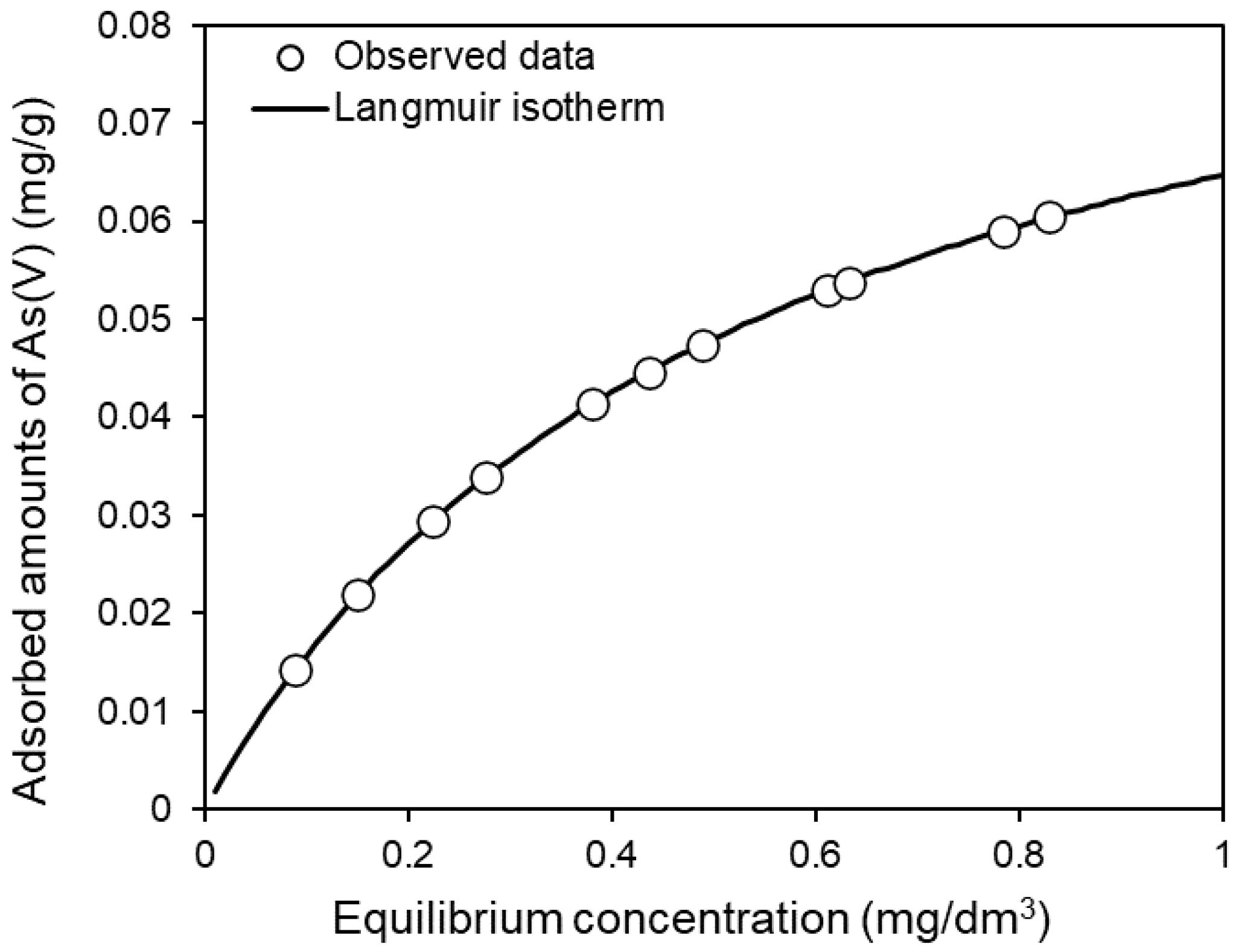
3.2. Batch Adsorption Experiments
3.3. Column Experiments
3.3.1. Changes in Arsenic
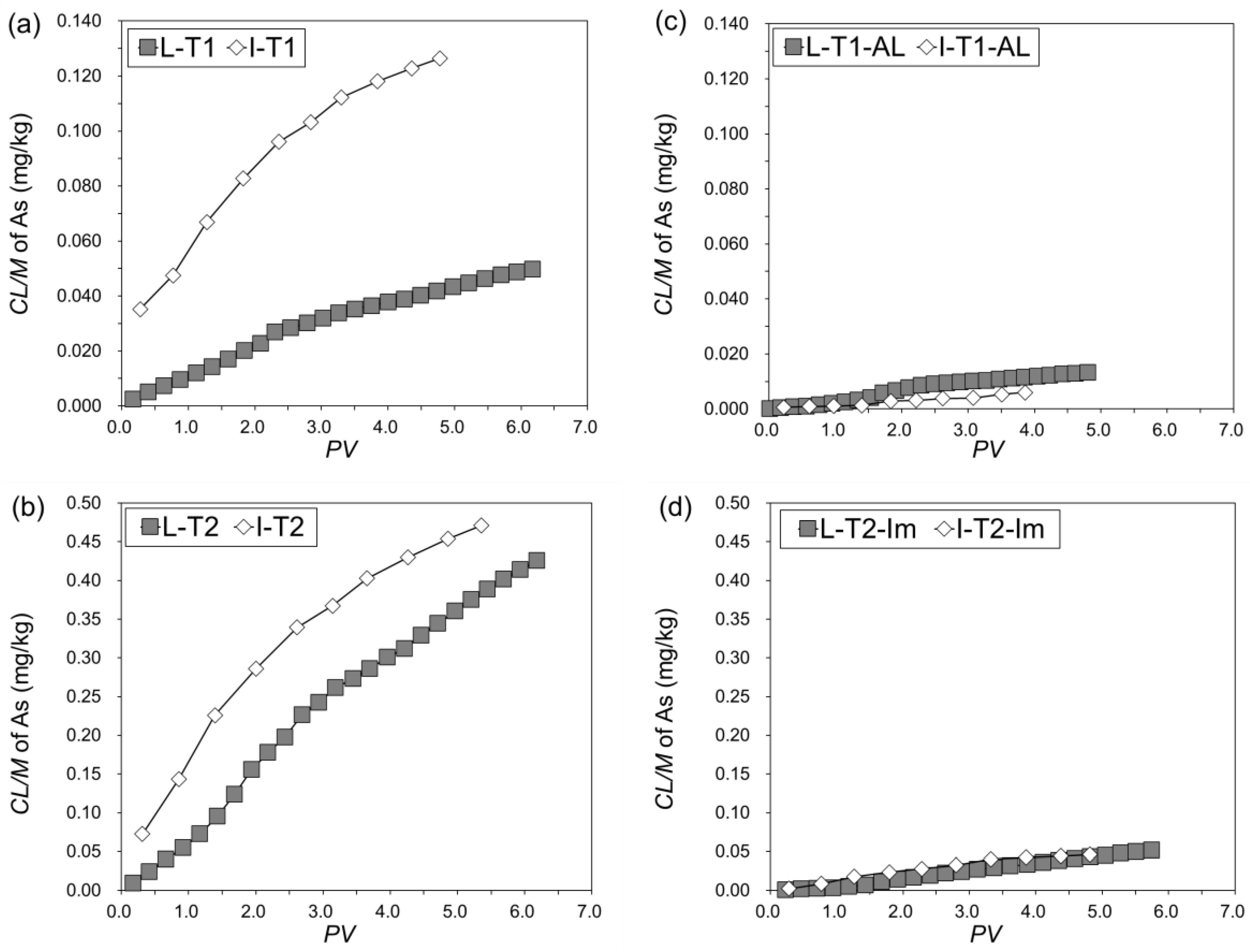
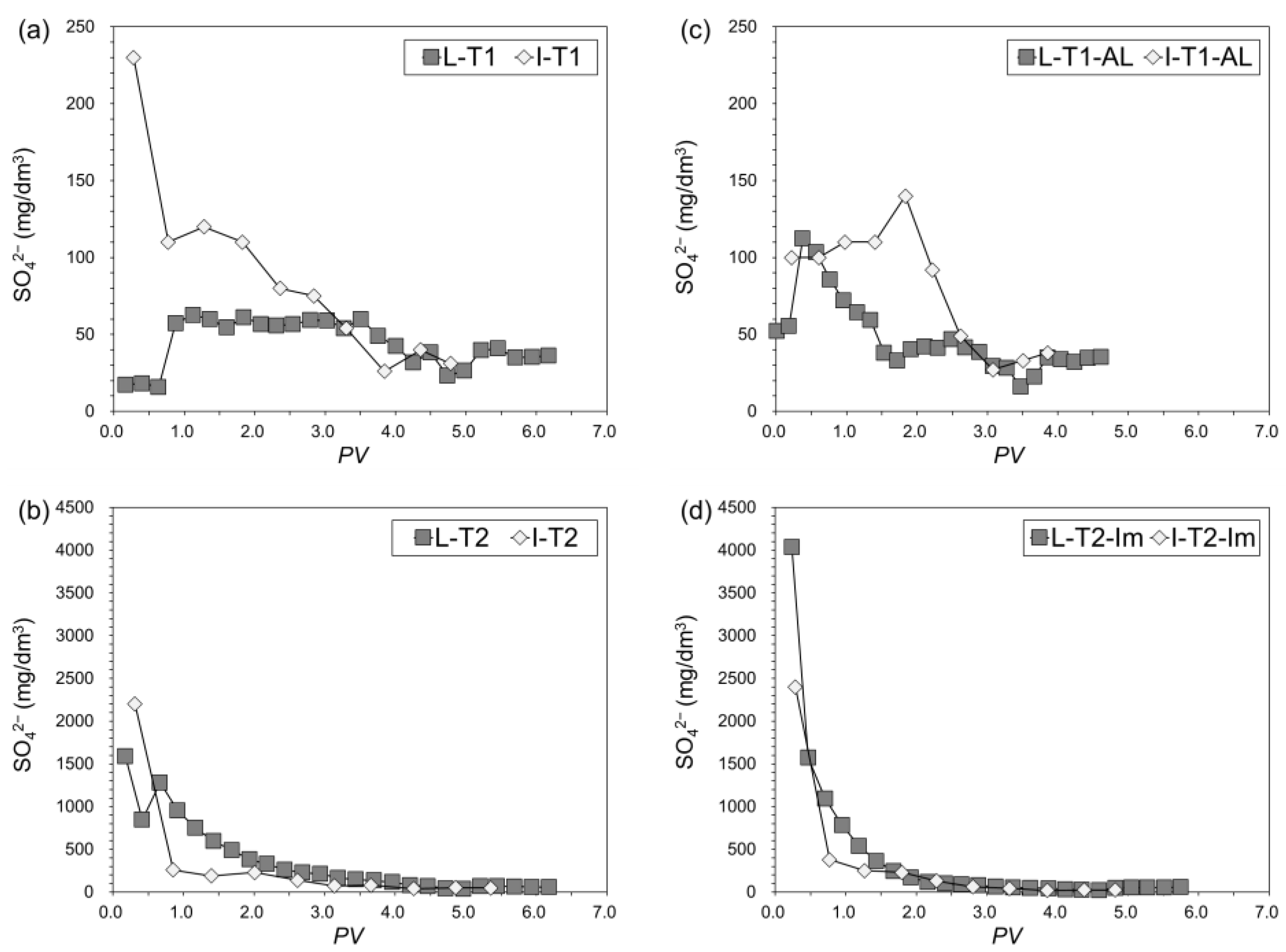
3.3.2. Cumulative Leachability of As
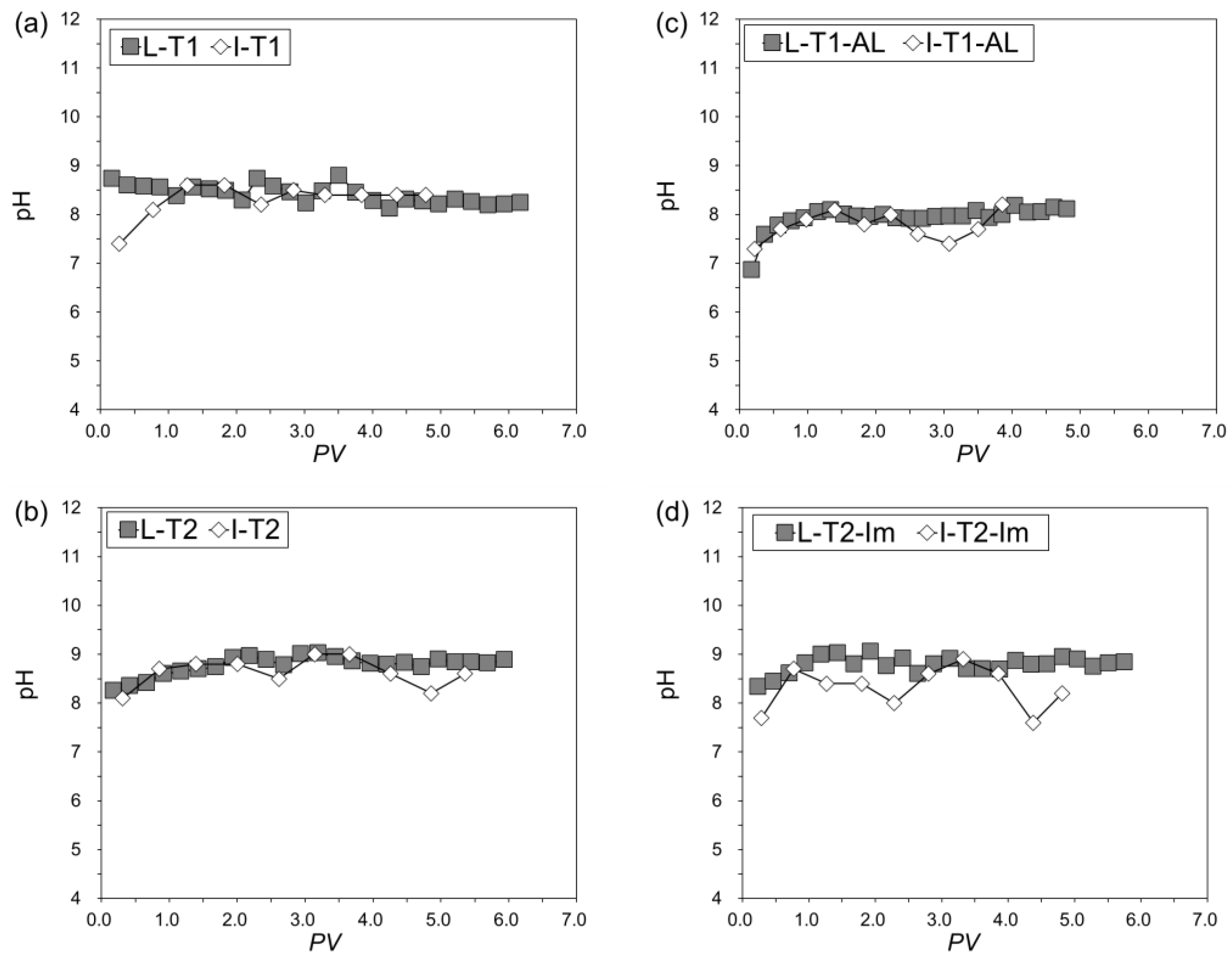
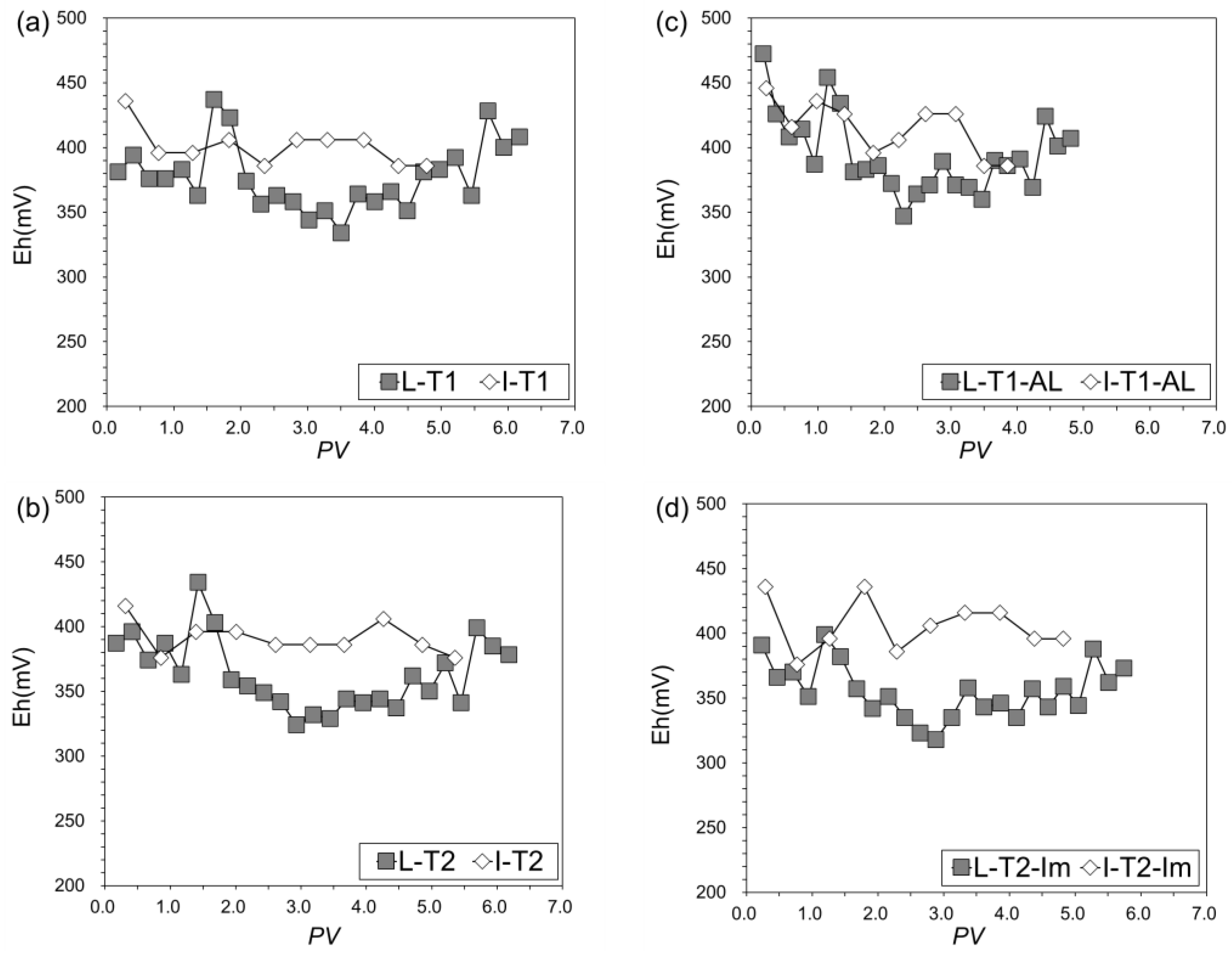
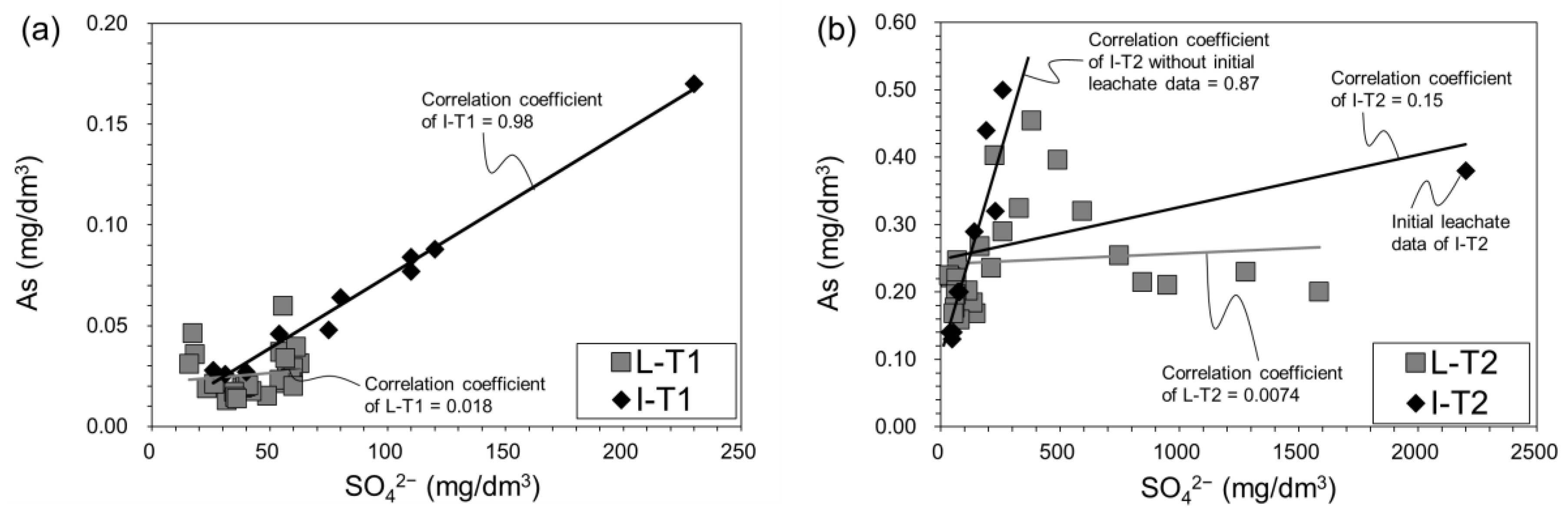
3.3.3. Changes of pH, Eh, and SO42−
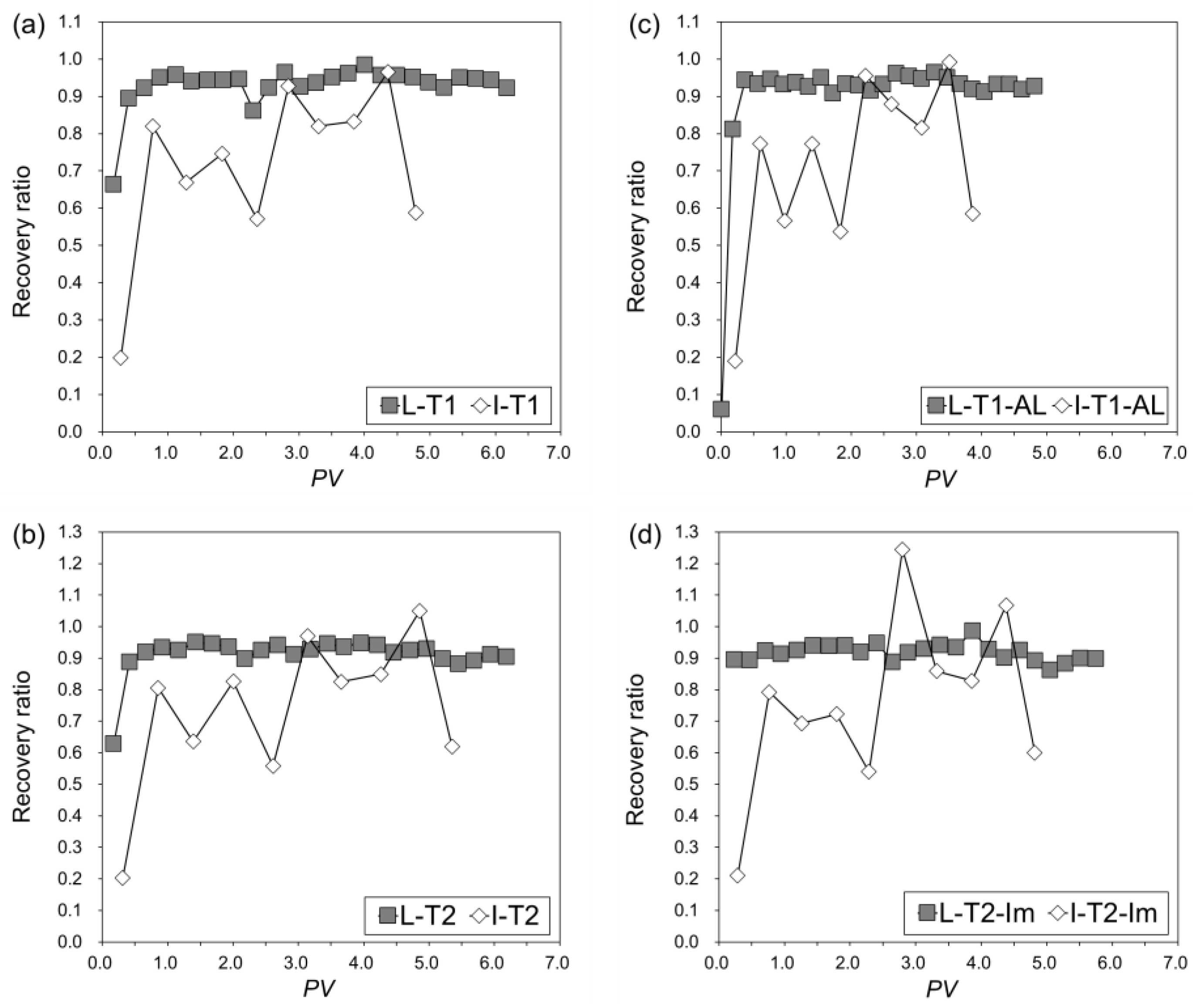
3.3.4. Recovery Ratio
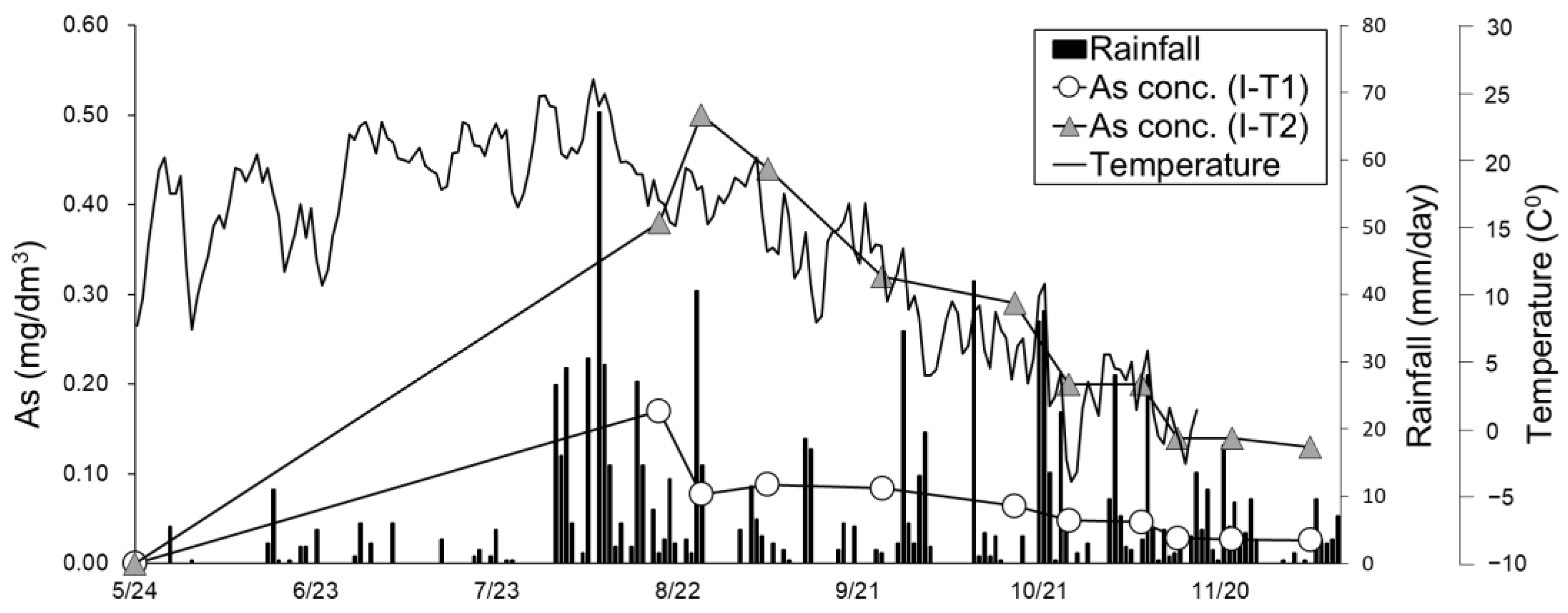
3.3.5. Temperature and Rainfall under In Situ Conditions
3.3.6. Significance of Environmental Conditions for As Behavior
4. Conclusions
- Arsenic leaching concentration from rock samples conducted in situ were slightly higher than those conducted in the laboratory. However, the significant adsorption of As by the RS was observed in both the laboratory and in situ column experiments.
- The pH of in situ column was slightly lower compared to the laboratory columns, whereas Eh and SO42− leaching concentration of the in situ columns were slightly higher compared to those in the laboratory.
- The lower water content and higher temperature of in situ columns enhanced the oxidation of sulfide minerals in the rock, which induced higher leaching of As.
- Although adsorption of As by the RS was influenced by pH in the leachate, the difference of the leachate pH released from the sedimentary rock samples used in this study between laboratory and in situ column experiments was insignificant due to their nearly equivalent pH.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tabelin, C.B.; Basri, A.H.M.; Igarashi, T.; Yoneda, T. Removal of arsenic, boron, and selenium from excavated rocks by consecutive washing. Water Air Soil Pollut. 2012, 223, 4153–4167. [Google Scholar] [CrossRef] [Green Version]
- Katsumi, T. Soil excavation and reclamation in civil engineering: Environmental aspects. Soil Sci. Plant Nutr. 2015, 61, 21–29. [Google Scholar] [CrossRef]
- Suzuki, S.; Katoh, M. Estimation of potential arsenic leaching from its phases in excavated sedimentary and metamorphic rocks. Environ. Geochem. Health 2019, 42, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Hadam, S.; Moriguchi, S.; Akashi, Y.; Katoh, M. Suppression of arsenic release from alkaline excavated rock by calcium dissolved from steel slag. Environ. Geochem. Health 2020, 42, 3983–3993. [Google Scholar] [CrossRef]
- Ure, A.; Berrow, M. The elemental constituents of soils. In Environmental Chemistry; Bowen, H.J.M., Ed.; Royal Society of Chemistry: London, UK, 1982; Chapter 3; pp. 94–203. [Google Scholar]
- Webster, J.G. Arsenic. In Encyclopedia of Geochemistry; Marshall, C.P., Fairbridge, R.W., Eds.; Chapman Hall: London, UK, 1999; pp. 21–22. [Google Scholar]
- Boyle, R.W.; Jonasson, I.R. The geochemistry of As and its use as an indicator element in geochemical prospecting. J. Geochem. Explor. 1973, 2, 251–296. [Google Scholar] [CrossRef]
- Akter, K.Z.; Owens, G.; Davey, D.E.; Naidu, R. Arsenic speciation and toxicity in biological systems. Rev. Rnviron. Contam. Toxicol. 2005, 184, 97–149. [Google Scholar]
- Ho, G.D.; Tabelin, C.B.; Tangviroon, P.; Tamamura, S.; Igarashi, T. Effects of cement addition on arsenic leaching from soils excavated from projects employing shield-tunneling method. Geoderma 2021, 385, 114896. [Google Scholar] [CrossRef]
- Katsumi, T.; Benson, C.H.; Kamon, M. Performance based design of landfill liners. Eng. Geol. 2001, 60, 139–148. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resources availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recy. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Q.; Ren, T. Coal Formation and Metamorphism. In Coal Mechanics; Springer: Singapore, 2021; pp. 41–76. [Google Scholar]
- Huyen, D.T.; Tabelin, C.B.; Thuan, H.M.; Dang, D.H.; Truong, P.T.; Vongphuthone, B.; Kobayashi, M.; Igarashi, T. The solid-phase partitioning of arsenic in unconsolidated sediments of the Mekong Delta, Vietnam and its modes of release under various conditions. Chemosphere 2019, 233, 512–523. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Igarashi, T.; Salgado, P.H.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Ii, H. Acid mine drainage sources and hydrogeochemistry at the Yatani mine, Yamagata, Japan: A geochemical and isotopic study. J. Contam. Hydrol. 2019, 225, 103502. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Silwamba, M.; Paglinawan, F.C.; Mondejar, A.J.S.; Duc, H.G.; Resabal, V.J.; Opiso, E.M.; Igarashi, T.; Tomiyama, S.; Ito, M.; et al. Solid-phase partitioning and release-retention mechanisms of copper, lead, zinc and arsenic in soils impacted by artisanal and small-scale gold mining (ASGM) activities. Chemosphere 2020, 260, 127574. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Inano, H.; Ito, M.; Hiroyoshi, N. Carrier-microencapsulation of arsenopyrite using Al-catecholate complex: Nature of oxidation products, effects on anodic and cathodic reactions, and coating stability under simulated weathering conditions. Heliyon 2020, 6, e03189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamoto, S.; Tabelin, C.B.; Igarashi, T.; Ito, M.; Hiroyoshi, N. Short and long term release mechanisms of arsenic, selenium and boron from a tunnel-excavated sedimentary rock under in situ conditions. J. Contam. Hydrol. 2015, 175–176, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Arima, T.; Sato, D.; Igarashi, T.; Tamoto, S.; Tatsuhara, T. Reduction and retardation of arsenic and boron leached from excavated rocks by volcanic ash adsorption layer. J. Jpn. Soc. Eng. Geol. 2011, 52, 88–96, (In Japanese with English Abstract). [Google Scholar] [CrossRef] [Green Version]
- Tatsuhara, T.; Arima, T.; Igarashi, T.; Tabelin, C.B. Combined neutralization-adsorption system for the disposal of hydrothermally altered excavated rock producing acidic leachate with hazardous elements. Eng. Geol. 2012, 139–140, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Tabelin, C.B.; Igarashi, T.; Yoneda, T.; Tamamura, S. Utilization of natural and artificial adsorbents in the mitigation of arsenic leached from hydrothermally altered rock. Eng. Geol. 2013, 156, 58–67. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Arima, T.; Sato, D.; Tatsuhara, T.; Tamoto, S. Characterization and evaluation of arsenic and boron adsorption onto natural geologic materials, and their application in the disposal of excavated altered rock. Geoderma 2014, 213, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.; Flores, G.; Inui, T.; Katsumi, T. Hydraulic and sorption performances of soil amended with calcium-magnesium composite powder against natural arsenic contamination. Soils Found. 2020, 60, 1084–1096. [Google Scholar] [CrossRef]
- Kato, T.; Lincoln, W.G.; Okada, T.; Takai, A.; Katsumi, T.; Imoto, Y.; Morimoto, K.; Nishikata, M.; Yasutaka, T. Sorption-desorption column tests to evaluate the attenuation layer using soil amended with a stabilizing agent. Soils Found. 2021, 61, 1112–1122. [Google Scholar] [CrossRef]
- Osono, A.; Katoh, M. Characteristics of the immobilization process of arsenic depending on the Size fraction released from excavated rock/sediment after the addition of immobilization materials. J. Environ. Manag. 2021, 298, 113534. [Google Scholar] [CrossRef] [PubMed]
- Tabelin, C.B.; Hashimoto, A.; Igarashi, T.; Yoneda, T. Leaching of boron, arsenic and selenium from sedimentary rocks: I. Effects of contact time, mixing speed and liquid to solid ratio. Sci. Total Environ. 2014, 472, 620–629. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Hashimoto, A.; Igarashi, T.; Yoneda, T. Leaching of boron, arsenic and selenium from sedimentary rocks: II. pH dependence, speciation, and mechanisms of release. Sci. Total Environ. 2014, 473–474, 244–253. [Google Scholar] [CrossRef]
- So, H.U.; Postma, D.; Jakobsen, R.; Larsen, F. Sorption and desorption of arsenate and arsenite on calcite. Geochimi. Cosmochimi. Acta 2008, 72, 5871–5884. [Google Scholar] [CrossRef]
- Toscano, G.; Caristi, C.; Cimino, G. Sorption of heavy metal from aqueous solution by volcanic ash. Comptes Rendus Chim. 2008, 11, 765–771. [Google Scholar] [CrossRef]
- Guo, H.; Stuben, D.; Berner, Z.; Yu, Q. Characteristics of arsenic adsorption from aqueous solution: Effect of species and natural adsorbents. Appl. Geochem. 2009, 24, 657–663. [Google Scholar] [CrossRef]
- Opiso, E.; Sato, T.; Yoned, T. Adsorption and co-precipitation behavior of arsenate, chromate, selenate and boric acid with synthetic allophone-like materials. J. Hazard. Mater. 2009, 170, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Nicomel, N.R.; Leus, K.; Folens, K.; Voort, P.V.D.; Laing, G.D. Technologies for arsenic removal from water: Current status and future perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Laing, G.D. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic removal from water by adsorption onto iron oxide/nano-porous carbon magnetic composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Qu, J. Review on the heterogeneous oxidation and adsorption for arsenic removal from drinking water. J. Environ. Sci. 2021, 110, 178–188. [Google Scholar] [CrossRef]
- Balint, R.; Bartoli, M.; Jagdale, P.; Tagliaferro, A.; Memon, A.S.; Rovere, M.; Martin, M. Defective bismuth oxide as effective adsorbent for arsenic removal from water and wastewater. Toxics 2021, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Salameh, Y.; Albadarin, A.B.; Allen, S.; Walker, G.; Ahmad, M.N.M. Arsenic (III, V) adsorption onto charred dolomite: Charring optimization and batch studies. Chem. Eng. J. 2015, 259, 663–671. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Sasaki, R.; Igarashi, T.; Park, I.; Tamoto, S.; Arima, T.; Ito, M.; Hiroyoshi, N. Simultaneous leaching of arsenite, arsenate, selenite and selenate, and their migration in tunnel-excavated sedimentary rocks: I. Column experiments under intermittent and unsaturated flow. Chemosphere 2017, 186, 558–569. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Sasaki, R.; Igarashi, T.; Park, I.; Tamoto, S.; Arima, T.; Ito, M.; Hiroyoshi, N. Simultaneous leaching of arsenite, arsenate, selenite and selenate, and their migration in tunnel-excavated sedimentary rocks: II. Kinetic and reactive transport modeling. Chemosphere 2017, 188, 444–454. [Google Scholar] [CrossRef]
- Yasutaka, T.; Naka, A.; Sakanakura, H.; Kurosawa, A.; Inui, T.; Takeo, M.; Inoba, S.; Watanabe, Y.; Fujikawa, T.; Miura, T.; et al. Reproducibility of up-flow column percolation tests for contaminated soils. PLoS ONE 2017, 12, e0178979. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.T.H.; Nguyen, B.Q.; Duong, T.T.; Bui, A.T.K.; Nguyen, H.T.A.; Cao, H.T.; Mai, N.T.; Nguyen, K.M.; Pham, T.T.; Kim, K.W. Pilot scale removal of arsenic and heavy metals from mining wastewater using adsorption combined with constructed wetland. Minerals 2019, 9, 379. [Google Scholar] [CrossRef] [Green Version]
- Sosa, A.; Armienta, M.A.; Aguayo, A.; Cruzb, O. Evaluation of the influence of main groundwater ions on arsenic removal by limestones through column experiments. Sci. Total Environ. 2020, 727, 138459. [Google Scholar] [CrossRef]
- Tran, T.H.H.; Kim, S.H.; Jo, H.Y.; Chung, J.; Lee, S. Transient behavior of arsenic in vadose zone under alternating wet and dry conditions: A comparative soil column study. J. Hazard. Mater. 2021, 422, 126957. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Tamoto, S. Factors affecting arsenic mobility from hydrothermally altered rock in impoundment-type in situ experiments. Miner. Eng. 2010, 23, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Takashima, R.; Kawabe, F.; Nishi, H.; Moriya, K.; Wani, R.; Ando, H. Geology and stratigraphy of the forearc basin sediments in Hokkaido, Japan: Cretaceous environmental events on the north-west Pacific margin. Cretac. Res. 2004, 25, 365–390. [Google Scholar] [CrossRef] [Green Version]
- Arima, T.; Sasaki, R.; Tabelin, C.B.; Tamoto, S.; Yamamoto, T.; Tangviroon, P.; Igarashi, T. Leaching and adsorption behavior of arsenic and selenium from excavated mudstones considering their chemical species. J. Min. Mater. Process. Inst. Jpn. 2020, 136, 64–76, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Igarashi, T.; Sasaki, R.; Tabelin, C.B. Chemical forms of arsenic and selenium leached from mudstones. Procedia Earth Planet. Sci. 2013, 6, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Pierce, M.L.; Moore, C.B. Adsorption of arsenite on amorphous iron hydroxide from dilute aqueous solutions. Environ. Sci. Technol. 1980, 14, 214–216. [Google Scholar] [CrossRef]
- Pierce, M.L.; Moore, C.B. Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Res. 1982, 16, 1247–1253. [Google Scholar] [CrossRef]
- Dixit, S.; Hering, G.J. Comparison of arsenic (V) and arsenic (III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Thechnol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef]
- Giménez, J.; Martínez, M.; de Pablo, J.; Rovira, M.; Duro, L. Arsenic sorption onto natural hematite, magnetite, and goethite. J. Hazard. Mater. 2007, 141, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, J.D. Arsenic. Elements 2006, 2, 71–75. [Google Scholar] [CrossRef]
- Takahashi, Y.; Minami, Y.; Ambe, S.; Makide, Y.; Ambe, F. Comparison of adsorption behavior of multiple inorganic ions on kaolinite and silica in the presence of humic acid using the multitracer technique. Geochim. Cosmochim. Acta 1999, 63, 815–836. [Google Scholar] [CrossRef]
- Te, B.; Wichitsathian, B.; Yossapol, C. Adsorptive behavior of low-cost modified natural clay adsorbents for arsenate removal from water. Int. J. Geomate 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Maiti, A.; Thakur, B.K.; Basu, J.K.; De, S. Comparison of treated laterite as arsenic adsorbent from different locations and performance of best filter under field conditions. J. Hazard. Mater. 2013, 262, 1176–1186. [Google Scholar] [CrossRef]
- Li, J.; Kosugi, T.; Riya, S.; Hashimoto, Y.; Hou, H.; Terada, A.; Hosomi, M. Potential for leaching of arsenic from excavated rock after different drying treatments. Chemosphere 2016, 154, 276–282. [Google Scholar] [CrossRef]
- Bowel, R.J. Sorption of arsenic by iron oxides and oxyhydroxides in soils. Appl. Geochem. 1994, 9, 279–286. [Google Scholar] [CrossRef]
- Johnstone, R.; Heijnen, H.; Wurzel, P. Safe water technology. In United Nations Synthesis Report on Arsenic in Drinking Water; WHO: Geneva, Switzerland, 2001; Chapter 6; pp. 1–98. [Google Scholar]
- Kocourková, E.; Sracek, O.; Houzar, S.; Cempírek, J.; Losos, Z.; Filip, J.; Hršelová, P. Geochemical and mineralogical control on the mobility of arsenic in a waste rock pile at Dlouhá Ves, Czech Republic. J. Geochem. Explor. 2011, 110, 61–73. [Google Scholar] [CrossRef]
- Moncur, M.C.; Ptacek, C.J.; Blowes, D.W.; Jambor, J.L. Release, transport and attenuation of metals from an old tailings impoundment. Appl. Geochem. 2005, 20, 639–659. [Google Scholar] [CrossRef]
- Yadav, M.K.; Saidulu, D.; Gupta, A.S.K.; Ghosal, P.S.; Mukherjee, A. Status and management of arsenic pollution in groundwater: A comprehensive appraisal of recent global scenario, Human health impacts, sustainable field-scale treatment technologies. J. Environ. Chem. Eng. 2021, 9, 105203. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T. Mechanisms of arsenic and lead release from hydrothermally altered rock. J. Hazard. Mater. 2009, 169, 980–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamata, A.; Katoh, M. Arsenic release from marine sedimentary rock after excavation from urbanized coastal areas: Oxidation of framboidal pyrite and subsequent natural suppression of arsenic release. Sci. Total Environ. 2019, 670, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Aredes, S.; Klein, B.; Pawlik, M. The removal of arsenic from water using natural iron oxide minerals. J Clean. Prod. 2013, 60, 71–76. [Google Scholar] [CrossRef]
- Igarashi, T.; Saito, R.; Sarashina, M.; Asakura, K. Impoundment of excavated pyrite-bearing rock using silty covering soil. Clay Sci. 2006, 12 (Suppl. 2), 143–148. [Google Scholar]

| Case | L-T1 | L-T1-AL | I-T1 | I-T1-AL | L-T2 | L-T2-Im | I-T2 | I-T2-Im | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Laboratory | IN Situ | Laboratory | In Situ | ||||||
| Used Rock Sample | T1 Sample | T2 Sample | ||||||||
| Usage of RS | – | AL * | – | AL * | – | Im ** | – | Im ** | ||
| Rock layer | Thickness | cm | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | – | 20.0 | – |
| Weight | g | 620 | 620 | 2288 | 2288 | 663 | – | 2540 | – | |
| Soil density | g/cm3 | 2.71 | 2.71 | 2.71 | 2.71 | 2.76 | – | 2.76 | – | |
| Column density | g/cm3 | 1.46 | 1.46 | 1.35 | 1.35 | 1.56 | – | 1.50 | – | |
| Porosity | % | 46.1 | 46.1 | 50.2 | 50.2 | 43.4 | – | 45.8 | – | |
| Pore volume | cm3 | 196 | 196 | 853 | 853 | 184 | – | 778 | – | |
| Adsorption layer | Thickness | cm | – | 4.00 | – | 4.00 | – | – | – | – |
| Weight | g | – | 103 | – | 428 | – | – | – | – | |
| Soil density | g/cm3 | – | 2.70 | – | 2.70 | – | – | – | – | |
| Column density | g/cm3 | – | 1.21 | – | 1.26 | – | – | – | – | |
| Porosity | % | – | 55.2 | – | 53.3 | – | – | – | – | |
| Pore volume | cm3 | – | 46.8 | – | 181 | – | – | – | – | |
| Total | Thickness | cm | – | 24.0 | – | 24.0 | 20.0 | 20.0 | 20.0 | 26.0 |
| Weight | g | – | 723 | – | 2716 | 663 | 638 (T2: 447 g, RS: 191 g) | 2540 | 3630 (T2: 2540 g, RS: 1090 g) | |
| Soil density | g/cm3 | – | – | – | – | 2.76 | 2.74 | 2.76 | 2.74 | |
| Column density | g/cm3 | – | – | – | – | 1.56 | 1.50 | 1.50 | 1.64 | |
| Porosity | % | – | 47.6 | – | 50.7 | 43.4 | 45.1 | 45.8 | 40.0 | |
| Pore volume | cm3 | – | 242 | – | 1034 | 184 | 192 | 778 | 883 | |
| Sample | T1 | T2 | RS | |
|---|---|---|---|---|
| SiO2 | wt.% | 68.2 | 64.3 | 62.0 |
| TiO2 | wt.% | 0.7 | 0.6 | 1.0 |
| Al2O3 | wt.% | 16.5 | 18.8 | 20.9 |
| Fe2O3 | wt.% | 6.3 | 5.3 | 7.7 |
| MnO | wt.% | <0.1 | 0.1 | 0.1 |
| MgO | wt.% | 2.6 | 2.3 | 2.9 |
| CaO | wt.% | 0.9 | 2.4 | 1.8 |
| Na2O | wt.% | <0.1 | 3.0 | <0.1 |
| K2O | wt.% | 2.9 | 2.7 | 1.9 |
| P2O5 | wt.% | 1.6 | <0.1 | 1.5 |
| SO3 | wt.% | 0.2 | 0.4 | <0.1 |
| Total | wt.% | 99.9 | 99.9 | 99.8 |
| As | mg/kg | 6.8 | 20.5 | 11.2 |
| LOI | wt.% | 4.8 | 7.2 | 4.4 |
| TOC | wt.% | 0.22 | 0.20 | 0.20 |
| IC | wt.% | 0.11 | 0.52 | <0.01 |
| Amorphous Al | mg/g | 0.6 | 2.3 | 1.4 |
| Amorphous Fe | mg/g | 13.4 | 4.8 | 3.9 |
| Sample | T1 | T2 | RS |
|---|---|---|---|
| Quartz | +++ | +++ | +++ |
| Albite | ++ | ++ | ++ |
| Siderite | + | ||
| Muscovite | + | + | |
| Kaolinite | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arima, T.; Sasaki, R.; Yamamoto, T.; Tabelin, C.B.; Tamoto, S.; Igarashi, T. Effects of Environmental Factors on the Leaching and Immobilization Behavior of Arsenic from Mudstone by Laboratory and In Situ Column Experiments. Minerals 2021, 11, 1220. https://doi.org/10.3390/min11111220
Arima T, Sasaki R, Yamamoto T, Tabelin CB, Tamoto S, Igarashi T. Effects of Environmental Factors on the Leaching and Immobilization Behavior of Arsenic from Mudstone by Laboratory and In Situ Column Experiments. Minerals. 2021; 11(11):1220. https://doi.org/10.3390/min11111220
Chicago/Turabian StyleArima, Takahiko, Ryosuke Sasaki, Takahiro Yamamoto, Carlito Baltazar Tabelin, Shuichi Tamoto, and Toshifumi Igarashi. 2021. "Effects of Environmental Factors on the Leaching and Immobilization Behavior of Arsenic from Mudstone by Laboratory and In Situ Column Experiments" Minerals 11, no. 11: 1220. https://doi.org/10.3390/min11111220
APA StyleArima, T., Sasaki, R., Yamamoto, T., Tabelin, C. B., Tamoto, S., & Igarashi, T. (2021). Effects of Environmental Factors on the Leaching and Immobilization Behavior of Arsenic from Mudstone by Laboratory and In Situ Column Experiments. Minerals, 11(11), 1220. https://doi.org/10.3390/min11111220








