Abstract
Arsenic contamination of the environment is a serious problem threatening the health of millions of people exposed to arsenic (As) via drinking water and crops grown in contaminated areas. The remediation of As-contaminated soil and water bodies needs to be sustainable, low-cost and feasible to apply in the most affected low-to-middle income countries, like India and Bangladesh. Phytoremediation is an aesthetically appreciable and successful approach that can be used for As decontamination with use of the best approach(es) and the most promising plant(s). However, phytoremediation lacks the required speed and sometimes the stress caused by As could diminish plants’ potential for remediation. To tackle these demerits, we need augment plants’ potential with appropriate technological methods including microbial and nanoparticles applications and genetic modification of plants to alleviate the As stress and enhance As accumulation in phytoremediator plants. The present review discusses the As phytoremediation prospects of soil and water bodies and the usefulness of various plant systems in terms of high biomass, high As accumulation, bioenergy potential, and economic utility. The potential and prospects of assisted phytoremediation approaches are also presented.
Keywords:
arsenic; hyperaccumulator; nanoparticles; microorganisms; phytoremediation; Pteris vittata 1. Introduction
Arsenic (As) contamination of the soil and water is a serious problem in several parts of the world, especially in South and Southeast Asian countries. It is an issue of concern owing to the toxic impacts of As on plants and humans and due to the span of the affected areas being very large [1]. The contamination of As has been caused mainly by biogeochemical processes in countries in South and Southeast Asia and by industrial and agricultural processes in European and North American countries [2,3,4]. The severely affected countries of South and Southeast Asia are renowned for intensive rice cultivation along with the dense population [5]. Thus, if even a single well or hand pump is contaminated with As in an area, a large number of people are affected. Further, rice cultivation is performed for two seasons or even three seasons in a year with the use of groundwater plus rainwater for irrigation. Therefore, when the groundwater in the area has As contamination, its use for irrigation adds a huge amount of As to the soil every year [6,7]. Another important point to consider is the fact that rice is the best-known accumulator of As among crop plants [8].
The availability, solubility and toxicity of different forms of As depend on the pH, ionic conditions, phosphorous and other elemental contents in the environment, whereas differences in uptake rates contribute to the degree of cellular exposure to arsenic. A majority of As released into the environment is inorganic and is accumulated by binding to organic soil matter. In an aerobic environment, mostly the arsenate [As(V)] form predominates, whereas the arsenite [As(III)] form is predominant in anaerobic conditions. A higher As(III) contamination in paddy fields due to water logged conditions and the presence of a potential As(III) accumulator plant, rice, are both of serious concern [9,10].
The problem of As contamination is the need for use of sustainable and low-cost solutions for the remediation of groundwater and soil [5,11]. There are several physical and chemical methods for the treatment of contaminated water and soil [12]. The natural microbial or plant-based approaches are known as bioremediation and phytoremediation, respectively. These methods are dependent on natural resources (minerals, water and solar energy) and therefore cost less and do not add any xenobiotics [13]. However, both methods have merits and limitations. The treatment of huge amounts of water/soil under in situ conditions by physico-chemical methods would be extremely costly [14], while the use of plants for this purpose would make the process very slow. In this regard, any method should have feasibility for application at the site itself, low-cost and be sustainable. Therefore, future research endeavors will require an optimum integration of physico-chemical and biological methods for effective sustainable remediation of contaminated areas.
Plants enhance soil fertility and enrich microorganisms of the soil during the course of remediation. In addition, the application of economically useful plants in phytoremediation makes it feasible for farmers to adopt it [15]. Plants with a faster growth rate, high biomass, and high shoot As accumulation are desirable for phytoremediation [16]. However, it has been difficult to find all three qualities in one plant. The plants with high As accumulation in shoots and a short life cycle have been found to have low biomass, while there are other plants which have high biomass but accumulate As with low efficiency [17]. Further, some high biomass economically useful plants suffer from As toxicity and cannot grow at their full potential. To overcome such difficulties, microbial association as a sustainable strategy has been utilized to enhance the growth and biomass of plants and to enhance their As accumulation efficiency [18,19]. Currently, the application of nanoparticles has become an acceptable approach for the reclamation of polluted ecosystems [20,21,22]. The concept of nano-phytoremediation technology has been emerging for the removal of contaminants from soil/water, which involves the application of both nanotechnology and phytoremediation [23,24,25,26]. However, the main challenge in using nanoparticles for the remediation of pollutants is the lack of an adequate number of reports proving its efficacy.
2. Phytoremediation: A Sustainable Approach
There are various approaches of As phytoremediation that can be utilized judiciously for remediation of contaminated sites. Various approaches are summarized in Figure 1 and are discussed below. Recent studies demonstrating the potential of various approaches have been presented in Table 1.
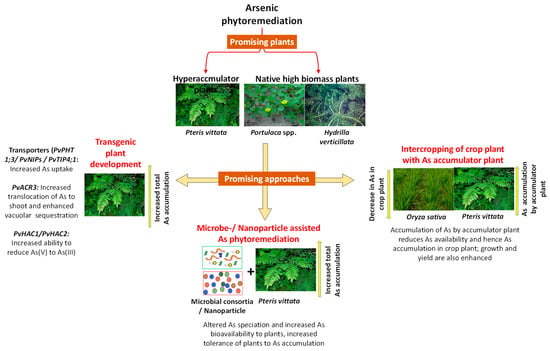
Figure 1.
Various approaches to arsenic phytoremediation: use of hyperaccumulator plants or native high biomass and bioenergy plants; intercropping of arsenic accumulator plant with a crop plant for reduced arsenic toxicity to crop plant; microbe-or nanoparticle-assisted arsenic phytoremediation and the use of genetic engineering approaches to enhance phytoremediation potential of plants.

Table 1.
A summary of recent studies on various phytoremediation approaches.
2.1. Selection of Plants for Arsenic Phytoremediation
2.1.1. Arsenic Hyperaccumulators
Hyperaccumulator plants can accumulate metal in their shoots beyond a certain threshold limit, which is 1000 mg/kg for As [39]. Further, the bioaccumulation factor (BF; indicative of soil to plant metal transfer) and translocation factor (TF; indicative of root to shoot metal transfer) are also considered while categorizing a plant as a hyperaccumulator [40]. Both BF and TF should be more than one (>1) for an As hyperaccumulator plant. Hyperaccumulation of As has been observed mostly in fern plants of the Pteris genus like P. vittata [40], P. longifolia [41], P. quadriaurita, P. cretica, P. ryiunkensis [42], etc. and Pityrogramma calomelanos [43]. One of the plants from the Brassicaceae family, Isatis cappadocica, shows As hyperaccumulation [44]. P. vittata has worldwide distribution from North America to Europe and Asia and can grow in a wide range of environmental conditions ranging from temperate to tropical [45].
Arsenic can make up to about 2% of the biomass of P. vittata [40]. P. vittata is a perennial plant and, therefore, plantation of a field does not need replantation, and harvesting and collection of fronds is needed at regular intervals. Several studies have focused on the use of P. vittata for the remediation of As-contaminated soil in laboratory, pot and field studies [46]. Liao et al. [47] found that from soil containing 64 mg/kg As, P. vittata removed 7.8% of the As in seven months. P. vittata plants showed higher As accumulation when grown in soil with added phosphate rock than in soil without phosphate rock amendment [48]. Phosphorus addition in the form of phosphate rock induces mobilization of As to some extent that, in turn, helps to induce As removal by Pteris plants [49,50].
In a pilot-scale study [51], P. vittata was used to minimize As concentration from drinking water through a continuous phytofiltration system. During the 3 month experimental period, up to 1900 L/day water with an initial As concentration of 10.2 μg/L was remediated and was found to contain As concentrations as low as 2 μg/L. The fronds of P. vittata accumulated 66–407 mg/kg As [51]. Groundwater remediation has also been demonstrated with the use of P. vittata [52]. The authors tested the efficiency of one to four Pteris plants per container of 30 L and with variable nitrogen and phosphorus supply to remediate groundwater containing 130 μg/L As. The As concentration was reduced to less than 10 μg/L in 3 weeks with 4 plants while in 4–6 weeks with 1–2 plants. When fully grown plants with a high root density were reused, one plant per container gave good results. In a recent study, P. vittata was used in a hydroponic system without any mechanical aeration. The method used was simple in that the plants were grown with rhizomes over the water surface and nutrients were given in a low amount for achieving root proliferation (500 mm root length in four months). From a variable initial water As concentration of 50 μg/L, 500 μg/L, and 1000 μg/L, Pteris plants could bring down the concentration to 10 to 0.1 μg/L in 1–5 days, 4–6 days and 8–10 days, respectively [53]. The results suggest the potential of P. vittata for phytoremediation purposes; however, the use of P. vittata has been mostly in hydroponics limited to pilot-scale studies. Extension of the approach to field conditions will necessarily require higher biomass development of large scale hydroponic systems, large amounts of water for treatment, and maintenance with optimum nutrient supply and regular cleaning.
2.1.2. High Biomass Plants for Arsenic Cleanup
The remediation of a site in a short time warrants the need of high biomass plants with moderate to high As accumulation and a short life cycle enabling harvesting followed by the use of the field for subsequent cropping of the same or other appropriate plants. This would enable cultivation of phytoremediator crops in a contaminated field throughout the year in changing weather conditions. Some of the high biomass plants with good potential for As accumulation include Jatropha curcas [54], shrub willow (Salix spp.), sunflower (Helianthus annuus) [55] and Indian mustard (Brassica juncea) [56]. In a small field study, sunflower plants were exposed to different As levels in three soil types (sandy, loamy, and clayey) and As accumulation was found to vary from 270 mg/kg to 408 mg/kg in roots, 13 mg/kg to 28 mg/kg in stem and 35 mg/kg to 68 mg/kg in leaves in different soil [57]. The application of Salix in phytoremediation has been demonstrated [58]. Invasive plants like Parthenium hysterophorus can also be successfully used in remediation strategies as they can grow and cover an area at rapid rates in a wide range of environments and accumulate metals in high amounts [59]. Favas et al. [60] found Callitriche lusitanica to be a potential As accumulator with As concentrations reaching up to 2346 mg/kg DW. Other potential accumulators in higher plants have been identified in lab and field studies, e.g., Isatis cappadocica [44], Sesuvium portulacastrum [61], and Eclipta alba [62]. Sesuvium is a halophytic plant with a high tolerance not only to salt but also to a number of metals and showed As accumulation 155 µg/g dw upon exposure to 1000 µM As(V) in 30 d [61].
The contaminated water bodies may be remediated with the help of high biomass aquatic plants like Ceratophyllum demersum [63], Hydrilla verticillata [64], Lemna gibba [65], Lemna minor [66], Azolla caroliniana [67], Pistia stratiotes [68], Salvinia natans [69] and Eichhornia crassipes [70]. Lemna gibba has been demonstrated to accumulate As up to 1022 mg/kg dry biomass in 21 d from contaminated surface water containing 41.37–47 µg/L As. The biomass accumulation and As removal potential of L. gibba were found to be as high as 73.6 t/ha/y and 752 kg As/ha/y, respectively [65]. In another study, E. crassipes was found to accumulate about 498 mg As/kg dry weight from a solution of 0.5 mg/L As in 10 d with a reduction of initial As concentration by 83% [71]. H. verticillata plants were found to remove up to 72% of As from 8 L As (1500 ug/L) medium in 45 d with the maximum As concentration of 388 μg/g dry weight [72]. These plants show fast growth and high biomass accumulation, can be easily harvested and can reestablish themselves. Aquatic plants also need very little input for growth and have high tolerance to waste water. The use of water fern, Mircanthemum umbrosum, in As and Cd remediation was studied by Islam et al. [73]. The use of emergent aquatic plants like Cyperus vaginatus and Vetiveria zizanioides has also been demonstrated in phytoremediation studies [74]. With the use of a high biomass moderate As accumulator, the effective removal of As per year can be higher than that achieved with a low biomass hyperaccumulator. For example, the calculation of yearly As removal by Sesuvium was found to be as high as 1955 g As/ha/yr at 500 µM As, which was higher than the calculated As removal by Pteris (525–1470 g As/ha/yr) [61].
2.1.3. Plants with Bioenergy Potential and Economic Utility
Besides plant biomass, the economic value of the plant system such as high value metabolites, biofuel generation, compost formation, etc. is now considered as one of the prime criteria for selecting plants for phytoremediation. With such an approach, farmers can move from normal cropping patterns to phytoremediator plants [17,75]. Plant-based waste material can also be successfully reutilized in remediation projects. This approach not only handles the problem of plant waste utilization at one end but also remediates the contaminated site on the other. Rice husk, mustard husk, coconut coir waste, crop straw, etc. are some of the examples of materials derived from plant materials that can act as biosorbents and remediators of As and can sustain soil fertility and reduce As accumulation in crop plants [76]. The potential of aquatic plants can also be used with judicious controlled and proper management of generated biomass with biodiesel, biogas, biochar, or compost preparation [77,78]. Biochar has emerged as one of the most potential plant based materials that have a number of functional groups (hydroxyl, carboxyl, etc.), making it an excellent binder of metals and therefore its application in soil reduces As stress to crop plants. Further, the use of biochar has also been demonstrated in water filtration [79]. Zhu et al. [80] designed a biochar plus periphyton-based system for the removal of As from the wastewater. The first phase of the column contained biochar that removed up to 60% of As(III) from wastewater (containing 2 mg/L As(III); flow rate 1 mL/min) while subsequent a periphyton bioreactor enhanced As removal efficiency up to 90–95%.
2.2. Promising Approaches for Augmenting Arsenic Remediation by Plants
2.2.1. Microbe-Assisted Arsenic Phytoremediation
Even with the selection of an appropriate hyperaccumulator plant or a high biomass economically useful plant depending on the features of the site for As phytoremediation, it is desirable to further augment plants’ remediation potential and growth so as to make remediation more lucrative and feasible. Plant associated microbiota and their synergetic interaction can be an effective strategy and is referred to as phytobial remediation [81]. There are successful examples of microbe-assisted enhanced phytoremediation efficiency for As [82,83]. There are certain crucial considerations like root colonization, survival, growth and competition with other pathogenic microbes and stimulation of plant growth. Microbial communities through their mutualistic association, either as free living, root symbiont or endophyte [84,85], produce certain metabolites which augment plant growth, alleviate stress and participate in As remediation [82,86]. Plant growth is promoted through the production of plant growth hormones and nutrient absorption is improved by siderophores [87,88,89,90]. In a study on isolation of As-resistant plant growth promoting microbes (PGPMs), Microbacterium sp. strain SZ1 from As-bearing gold ores was shown to be useful for phytobial remediation as the bacterial genome had the necessary genes responsible for siderophore production [91]. From a contaminated site in Spain, Moens et al. [92] reported reduced As toxicity on plant growth with concomitant lower As accumulation in rice plants by inoculating Ochrobactrum tritici As5 to the plant’s rhizosphere. The As-resistant bacteria (Pseudomonas gessardii and Brevundimonas intermedia) and As-resistant fungi (Fimetariella rabenhortii and Hormonem aviticola) isolated from the Puchuncaví valley in Chile exhibited higher plant growth-promoting properties and good As remediation properties in soil cultivated with wheat [93].
In an interesting three year field study, Yang et al. [94] demonstrated that rhizobacteria (Pseudomonas vancouverensis strain m318) mediated As(III) to As(V) conversion, and efficient As phytoextraction. Treatment with rhizobacteria enhanced fern biomass, As accumulation, and As removal (<10 mg kg−1) in the soil, suggesting that in a span of three cycles of fern growth, a clean field could be achieved. Awasthi et al. [86] studied the prospects of using a consortium of rhizobacteria (Pseudomonas putida) and alga (Chlorella vulgaris) for ameliorating As toxicity through measurements of growth and As uptake. An estimated 79–82% drop in As accumulation in rice was shown, suggesting the usefulness of the approach. Using isolates from the mangrove rhizosphere of Sundarban, Mallick et al. [95] applied two As-resistant halophilic bacteria, Kocuria flava AB402 and Bacillus vietnamensis AB403, for growth promotion and As remediation. Both bacteria showed up to a 52% reduction in As accumulation in the roots and shoots of rice seedlings.
Arbuscular mycorrhiza (AM), belonging to symbiotic fungi, have the remarkable feature of positively influencing plant growth and stress tolerance [96]. Plant-AM association has been studied and several reports have demonstrated AM application for alleviating heavy metal contamination [97,98] via mechanisms, which include converting inorganic As to less toxic forms and enhancing plant biomass [99,100], increased uptake of metals through metal transporters and activation of genes related to signaling and detoxification pathways [101,102]. AM has been shown to have good potential for reclamation of abandoned fly-ash containing heavy metals such as As, lead, cadmium and mercury [103]. In a study with the application of Glomus mosseae BEG167, Xu et al. [104] found higher phosphorous (P) accumulation and reduced As in Medicago truncatula grown in soils supplemented with As (10–200 mg/kg). AM-mediated As toxicity alleviation has also been demonstrated in tomato [105], ryegrass and clover [106].
2.2.2. Intercropping and Co-Cultivation Methods
Intercropping is a common agricultural practice in which two different crops are grown together to improve soil conditions for plant growth, improved nutrient availability and soil enzyme activity [107]. The intercropping of As hyperaccumulator P. vittata with As sensitive and non-accumulator plants has been tested in order to reduce As contamination of the field and to mitigate As stress on the other plants. The intercropping of P. vittata and Panax notoginseng, two economically useful plants, was studied by Lin et al. [108]. It was observed that As concentrations in the rhizosphere of Panax plants were reduced. The intercropping of P. vittata with Morus alba was also found to reduce As levels in Morus alba plants due to significant As removal by Pteris plants [109]. The intercropping of P. vittata with maize (Zea mays) plants has also been studied [110] and the two plants were grown in both coordinate and malposed intercropping. It was found that level of Fe-hydroxides-associated As were lower in soil layers (10–20 cm and 20–30 cm) while As accumulation in P. vittata was higher in malposed intercropping than in coordinate intercropping. The rate of As removal was 2.4-fold higher in malposed than in coordinate intercropping. Maize grains showed lower As concentration in grains, within the suggested maximum contaminant limit, during malposed intercropping [110].
The roots of different intercropped plants may concentrate in different zones from the top layer to a few centimeters’ deep. Correspondingly, As distribution also varies sharply in different layers of soil by a few centimeters (0–40 cm) [111]. Therefore, intercropping of Pteris with other cash crops/economically important crops can give interesting results. However, it has been considered as the best approach to remediate and use the field for economic gain at the same time [109,112,113]. If the harvesting of P. vittata can be managed in a timely manner along with management of fallen leaves and shoot tissues (not to be used) of intercropped cash crops, this strategy can effectively remediate the As-contaminated sites along with economic gains to the landowner [110]. Ye et al. [114] studied co-cultivation of P. vittata with rice and found that As removal by Pteris reduced the As level in rice with a significant decline in DMA content.
The combination or sequential use of aquatic plants has been found to enhance As removal from a medium in a given time frame as compared to that of a single plant. The successive application of three aquatic plants, Lemna, Hydrilla, and Ceratophyllum, for As removal was tested. The medium used contained 2500 µg/L As, and plants were used in succession for a total of 21 days with 7 days allocated for each plant. The study found reported the maximum As removal (27% in 21 d) when Hydrilla-Cerotophyllum-Lemna succession was used [115]. In a combination approach used by Srivastava et al. [116], the combination of Ceratophyllum demersum and Lemna minor achieved the maximum As removal (4365 µg) in 30 d from an As supplemented medium (2500 µg/L).
2.2.3. Nanotechnological Approaches to Enhance Phytoremediation
Nano-phytoremediation is an emerging strategy that has shown the potential to enhance plants’ ability to grow in a polluted stressful environment and accumulate As in plant tissues. Fabrication of effective and eco-friendly nanoparticles for successful application in managing widespread contamination of hazardous metalloids has received much attention [117]. Nanoparticles (NPs) may increase the plant’s stress tolerance to increase phytoremediation as well as help in the alleviation of toxicity [118,119]. Nano-phytoremediation can effectively remediate the polluted soils/water using those plants that possess high efficiency for NPs/metal uptake [26,120,121], and can be used as an alternative solution for As phytoremediation (Figure 2).

Figure 2.
The use of nanoparticles through foliar spray and via roots can effectively enhance tolerance of plants to arsenic stress, improve their growth and biomass and also increase total arsenic accumulation.
Application of nanoparticles for the management of contaminated agricultural lands and improvement of plant growth and developments has shown significant prospects [26]. In this context, it was shown that nanostructured silicon dioxide can act as a potential agent that can improve the phytoremediation process to attain the desired outcomes [24,122]. Similarly, the NPs of aluminum oxide (nAl2O3) can be used in phytoremediation as they did not exert any toxicity consequences in Arabidopsis thaliana up to 4000 mg/L [123].
It was noted that the nanoscale zero-valent iron was widely used to facilitate the phytoremediation process [124]. It was found that the use of salicylic acid-based NPs enhances As remediation by Isatis cappadocica [125] while the use of nano-Zn improved As stabilization by Helianthus annuus [126]. A review summarized that the composites of nano titanium (Ti) such as Zr-TiO2 and TiO2-αFe2O3Ce-Ti oxide are frequently used to treat As-contaminated water [127]. The application of TiO2, Si NPs and Au NPs has been found to counteract the toxic effects of different metals in Zea mays [128], Glycine max [129] and Oryza sativa [130], respectively. The application of fullerene nanoparticles could stimulate the phytoavailability of soil contaminants [124].
The application of NPs not only enhances the phytoremediation capability of As, but also reduces the bioaccumulation of As in crops. Recent research showed that the application of 1000 mg/L nano-TiO2 reduced As accumulation in rice by 40–90% [131], and in Vigna radiata nano-TiO2 reduced As phytotoxicity at the rate of 4000 mg/L [132]. The amendment of ZnO increased the growth of rice seedlings, reduced accumulation of As in roots and shoots, and saw a rise in phytochelatin level [133]. Noteworthy advances in nano-phytoremediation could form a basis for the development of non-toxic, cost-effective, and environmentally sustainable technologies for phytoremediation of As from various environmental matrices.
2.2.4. Genetic Engineering for Improving Arsenic Phytoremediation
The potential mitigation strategies for reducing the As burden involve As efflux and its sequestration in intracellular compartment [134]. Strategies for developing genetically engineered plants for As phytoremediation encompass increased uptake of As by roots, enhanced translocation of As from root to shoot including xylem loading, arsenate reduction, vacuolar sequestration and enhanced tolerance to As [135,136].
As(V) and As(III) uptake and transport are mediated by phosphate transporters (PHTs) and members of membrane intrinsic proteins (MIPs), respectively [137,138]. Thus, for designing a phytoremediation strategy, a high biomass crop can be genetically engineered by overexpression of the candidate MIP genes, particularly NIP3;1, NIP7;1, PIPs, Lsi2 and PvTIP4;1, which could increase As uptake and translocation and lead to enhanced As accumulation in genetically engineered plants. P. vittata showed increased As(V) uptake due to the increased expression of PvPHT1;3 (a phosphate transporter) and higher affinity for As(V) over phosphate [139,140]. In P. vittata, As(III) is primarily sequestered into the vacuole by PvACR3 (Arsenic Compound Resistant 3), an arsenite effluxer localized the in plasma membrane of gametophyte, and its homolog is absent in angiosperm [141]. Interestingly, over-expression of PvACR3 in Arabidopsis enhances As translocation in shoots [142], which could be a potential strategy for developing As-hyperaccumulating plants. A. thaliana was converted into an As hyperaccumulator by heterologous expression of PvACR3 in athac1 (arsenate reductase) mutant [143], and the same strategy could be tested in fast growing high biomass crop plants for efficient phytoextraction.
To mitigate As-induced stress to plants so as to enhance their As accumulation, redox transformation of As(V) and As(III), and further methylation of organic As species, can be targeted. The pioneering research on the development of a transgenic Arabidopsis plant for As phytoremediation involved stacking two bacterial genes by overexpression of arsenate reductase (arsC) in shoots and constitutive expression of γ-glutamylcysteinesynthetase (γ-ECS), which resulted in enhanced tolerance and higher As accumulation in the double transgenic plant [144]. Arsenate reductase (AtACR2) knock down lines of Arabidopsis resulted in enhanced translocation of As from roots to shoots [145].
However, transgenic lines generated with heterologous expression of Arabidospsis AtACR2 in tobacco were more tolerant to As, but accumulated reduced As level in shoots [146], which suggested that the identification of the ACR2 gene from high biomass crop plants is a potential candidate, and its knock down/knock out by a gene editing approach can be a promising tool for developing genetically engineered plants for phytoremediation. Recently, two novel arsenate reductases (PvHAC1 and PvHAC2) from P. vittata were isolated, where PvHAC1 was expressed in the rhizomes, while PvHAC2 was expressed in the fronds and played a crucial role in As hyperaccumulation [147]. Therefore, heterologous expression of the phosphate transporter (PvPHT1;3) and arsenate reductase (PvHAC1/2) in a high biomass crop plant can be utilized as a potential strategy for efficient phytoextraction of As. In a recent report regarding As stress, RNA-seq analysis of P. vittata identified three upregulated genes viz. glyceradehyde 3-phosphate dehydrogenase (PvGAPC1), organic cation transporter 4 (PvOCT4), glutathione S-transferase (PvGSTF1) and RNAi demonstrated that the identified genes are essential for As tolerance. PvGAPC1 converts As(V) to 1-arseno-3-phosphoglyerate (1-As-3-PG), PvOCT4 transports 1-As-3-PG into the vesicle and PvGSTF1 acts as arsenate reductase, which sequestered (AsIII) into vesicles and moved it long distances for storage [148]. These genes can be utilized in genetically modified plants for phytoremediation after proper and thorough investigation of the pathways involved.
3. Conclusions and Future Directions
Arsenic contamination in the ecosystem has created serious environmental concerns due to the toxicity and carcinogenicity of this metalloid. In light of this, research and development efforts have been made for As remediation from soil and water sources through sustainable biostrategies which are environmentally friendly and easy to adopt in contaminated sites. The available options include ‘phytoremediation’ involving exploitation of plant species with high As-hyperaccumulating efficiency and a good biomass and bioprospecting potential. Based on the mechanistic view of As uptake, metabolism and transport and identification of novel candidate genes, biotechnological methods have been refined to genetically manipulate plants for enhancing the efficiency of phytoremediation and reducing the As load in crop plants. The application of plant growth promoting microorganisms and nanoparticles has immense potential for managing As contamination in plants and in the ecosystem. Extensive studies should be conducted to realize the prospects of microbe-/nano-assisted phytoremediation for the decontamination of As polluted soils/water. However, various approaches of phytoremediation have some merits and limitations (Table 2) and, therefore, future research must be focused on integration of different methods, suitably at a site so as to enhance the phytoremediation potential and speed up the process in addition to providing economic benefits to the landowner.

Table 2.
Merits and limitations of various phytoremediation approaches.
Author Contributions
Conceptualization, S.S., K.K. and P.S.; writing—original draft preparation, S.S., A.S. (Anurakti Shukla), V.D.R., T.M., S.M., A.S. (Antonina Shmaraeva) and K.K.; writing—review and editing, S.S. and P.S.; supervision, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
S.S. wishes to acknowledge IoE scheme of Banaras Hindu University for the grant (Scheme No. 6031). T.M. and S.M. would like to thanks the financial support from the Ministry of Science and Higher Education of the Russian Federation, project no. 0852-2020-0029. K.K. wishes to thank BITS-Pilani, K.K.Birla Goa Campus, for providing the necessary support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Srivastava, S. Arsenic in Drinking Water and Food; Springer Nature: Singapore, 2020. [Google Scholar]
- Shukla, A.; Awasthi, S.; Chauhan, R.; Srivastava, S. The Status of Arsenic Contamination in India. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer Nature: Singapore, 2020; pp. 1–12. [Google Scholar]
- Medunic, G.; Fiket, Z.; Ivanic, M. Arsenic Contamination Status in Europe, Australia, and Other Parts of the World. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer Nature: Singapore, 2020; pp. 183–233. [Google Scholar]
- Jankovic, M.M. Arsenic Contamination Status in North America. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer Nature: Singapore, 2020; pp. 41–69. [Google Scholar]
- Srivastava, S.; Pathak, S.; Ponsin, M.; Hensawang, S.; Chanpiwat, P.; Yoeurn, C.; Phan, K. Sustainable solutions to arsenic ac-cumulation in rice grown in south and southeast Asia. Crop Pasture Sci. 2021, in press. [Google Scholar] [CrossRef]
- Neumann, R.B.; Vincent, A.P.S.; Roberts, L.C.; Badruzzaman, A.B.M.; Ali, M.A.; Harvey, C.F. Rice Field Geochemistry and Hydrology: An Explanation for Why Groundwater Irrigated Fields in Bangladesh are Net Sinks of Arsenic from Groundwater. Environ. Sci. Technol. 2011, 45, 2072–2078. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, M.K.; Majumdar, A.; Kumar, J.S.; Srivastava, S. Arsenic in Rice Agro-Ecosystem: Solutions for Safe and Sustainable Rice Production. Front. Sustain. Food Syst. 2020, 4, 53. [Google Scholar] [CrossRef]
- Awasthi, S.; Chauhan, R.; Srivastava, S.; Tripathi, R.D. The Journey of Arsenic from Soil to Grain in Rice. Front. Plant Sci. 2017, 8, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himeno, S.; Sumi, D.; Fujishiro, H. Toxicometallomics of Cadmium, Manganese and Arsenic with Special Reference to the Roles of Metal Transporters. Toxicol. Res. 2019, 35, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Huang, L.; Xue, S.G.; Shi, L.Z.; Hartley, W.; Cui, M.; Wong, M.H. Arsenic sorption by red mud-modified biochar pro-duced from rice straw. Environ. Sci. Pollut. Res. 2017, 24, 18168–18178. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.D.; Srivastava, S.; Mishra, S.; Singh, N.; Tuli, R.; Gupta, D.K.; Maathuis, F.J. Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 2007, 25, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lei, M.; Chen, T. Review on remediation technologies for arsenic-contaminated soil. Front. Environ. Sci. Eng. 2019, 14, 24. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phy-toremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kumar, A.; Kumar, R. Phytoremediation and Nanoremediation. In New Frontiers of Nanomaterials in Environmental Science; Kumar, R., Kumar, R., Kaur, G., Eds.; Springer Nature: Singapore, 2021; pp. 281–297. [Google Scholar]
- Juwarkar, A.A.; Singh, S.K.; Mudhoo, A. A comprehensive overview of elements in bioremediation. Rev. Environ. Sci. Biotechnol. 2010, 9, 215–288. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Phytoextraction of mine wastes: Opinion and impossibilities. Chem. Erde Geochem. 2005, 65, 29–42. [Google Scholar] [CrossRef]
- Tripathi, P.; Dwivedi, S.; Mishra, A.; Kumar, A.; Dave, R.; Srivastava, S.; Shukla, M.K.; Srivastava, P.K.; Chakrabarty, D.; Trivedi, P.K.; et al. Arsenic accumulation in native plants of West Bengal, India: Prospects for phytoremediation but concerns with the use of medicinal plants. Environ. Monit. Assess. 2011, 184, 2617–2631. [Google Scholar] [CrossRef]
- Mesa, V.; Navazas, A.; González-Gil, R.; González, A.; Weyens, N.; Lauga, B.; Gallego, J.L.R.; Sánchez, J.; Peláez, A.I. Use of Endophytic and Rhizosphere Bacteria to Improve Phytoremediation of Arsenic-Contaminated Industrial Soils by Autochthonous Betula celtiberica. Appl. Environ. Microbiol. 2017, 83, e03411-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchi, E.; Rolli, E.; Marasco, R.; Agazzi, G.; Borin, S.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G. Phytoremediation of a multi contaminated soil: Mercury and arsenic phytoextraction assisted by mobilizing agent and plant growth promoting bacteria. J. Soils Sediments 2016, 17, 1224–1236. [Google Scholar] [CrossRef]
- Ranjan, A.; Rajput, V.D.; Minkina, T.; Bauer, T.; Chauhan, A.; Jindal, T. Nanoparticles induced stress and toxicity in plants. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100457. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2016, 110, 236–264. [Google Scholar] [CrossRef] [Green Version]
- Trujillo-Reyes, J.; Majumdar, S.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure studies of core-shell Fe/Fe(3)O(4) and Cu/CuO NPs to lettuce (Lactuca sativa) plants: Are they a potential physiological and nutritional hazard? J. Hazard. Mater. 2014, 267, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Srivastav, A.; Yadav, K.K.; Yadav, S.; Gupta, N.; Singh, J.K.; Katiyar, R.; Kumar, V. Nano-phytoremediation of Pollutants from Contaminated Soil Environment: Current Scenario and Future Prospects. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 6, pp. 383–401. [Google Scholar]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Alam Cheema, S.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef]
- Canatto, R.A.; De Oliveira, J.A.; Da-Silva, C.J.; Albino, B.S. Tolerance of Landoltia punctata to arsenate: An evaluation of the potential use in phytoremediation programs. Int. J. Phytoremediat. 2020, 23, 102–110. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Tsang, D.C.; Song, L.; Zhang, C.; Yin, M.; Liu, J.; Xiao, T.; Zhang, G.; Wang, J. Hyperaccumulation and transport mechanism of thallium and arsenic in brake ferns (Pteris vittata L.): A case study from mining area. J. Hazard. Mater. 2019, 388, 121756. [Google Scholar] [CrossRef]
- Singh, S.; Fulzele, D.P. Phytoextraction of arsenic using a weed plant Calotropis procera from contaminated water and soil: Growth and biochemical response. Int. J. Phytoremediat. 2021, 1–9. [Google Scholar] [CrossRef]
- Negi, S. Heavy metal accumulation in Portulaca oleracea Linn. J. Pharmacogn. Phytochem. 2018, 7, 2978–2982. [Google Scholar]
- Sahito, Z.A.; Zehra, A.; Tang, L.; Ali, Z.; Hashmi, M.L.R.; Ullah, M.A.; He, Z.; Yang, X. Arsenic and mercury uptake and accumulation in oilseed sunflower accessions selected to mitigate co-contaminated soil coupled with oil and bioenergy pro-duction. J. Clean. Prod. 2021, 291, 125226. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhen, Z.; Wang, Z.; Zeng, L.; Yan, C. Influence of environmental factors on arsenic accumulation and biotransformation using the aquatic plant species Hydrilla verticillata. J. Environ. Sci. 2019, 90, 244–252. [Google Scholar] [CrossRef]
- Guarino, F.; Miranda, A.; Castiglione, S.; Cicatelli, A. Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium. Chemosphere 2020, 251, 126310. [Google Scholar] [CrossRef] [PubMed]
- Debiec-Andrzejewska, K.; Krucon, T.; Piatkowska, K.; Drewniak, L. Enhancing the plants growth and arsenic uptake from soil using arsenite-oxidizing bacteria. Environ. Pollut. 2020, 264, 114692. [Google Scholar] [CrossRef] [PubMed]
- Kamath, V.; Chandra, P.; Jeppu, G.P. Comparative study of using five different leaf extracts in the green synthesis of iron oxide nanoparticles for removal of arsenic from water. Int. J. Phytoremediat. 2020, 22, 1278–1294. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Karimi, N.; Norouzi, L.; Ma, X. Elucidating the physiological mechanisms underlying enhanced arsenic hyperaccu-mulation by glutathione modified superparamagnetic iron oxide nanoparticles in Isatis cappadocica. Ecotox. Environ. Saf. 2020, 53, 111336. [Google Scholar] [CrossRef] [PubMed]
- Deromachi, Y.; Uraguchi, S.; Kiyono, M.; Kuga, K.; Nishimura, K.; Sato, M.H.; Hirano, T. Stable expression of bacterial trans-porter ArsB attached to SNARE molecule enhances arsenic accumulation in Arabidopsis. Plant Signal. Behav. 2020, 15, 1802553. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, H.; Sun, D.; Xu, G.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Heterologous Expression of Pteris vittata Phosphate Transporter PvPht1;3 Enhances Arsenic Translocation to and Accumulation in Tobacco Shoots. Environ. Sci. Technol. 2019, 53, 10636–10644. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Dunham, S.; McGrath, S.P. Arsenic hyperaccumulation by different fern species. New Phytol. 2002, 156, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, M.; Ma, L.Q.; Singh, N.; Singh, S. Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J. Exp. Bot. 2005, 56, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francesconi, K.; Visoottiviseth, P.; Sridokchan, W.; Goessler, W. Arsenic species in an arsenic hyperaccumulating fern, Pityrogramm acalomelanos: A potential phytoremediator of arsenic-contaminated soils. Sci. Total Environ. 2002, 284, 27–35. [Google Scholar] [CrossRef]
- Karimi, N.; Ghaderian, S.M.; Raab, A.; Feldmann, J.; Meharg, A.A. An arsenic-accumulating, hypertolerant brassica, Isatis cap-padocica. New Phytol. 2009, 184, 41–47. [Google Scholar] [CrossRef]
- Vetterlein, D.; Wesenberg, D.; Nathan, P.; Brautigam, A.; Schierhorn, A.; Mattusch, J.; Jahn, R. Pteris vittata—Revisited: Uptake of As and its speciation, impact of P.; role of phytochelatins and S. Environ. Pollut. 2013, 157, 3016–3024. [Google Scholar] [CrossRef]
- Xiyuan, X.; Tongbin, C.; Zhizhuang, A.; Mei, L.; Zechun, H.; Xiaoyong, L.; Yingru, L. Potential of Pteris vittata L. for phytore-mediation of sites co-contaminated with cadmium and arsenic: The tolerance and accumulation. J. Environ. Sci. 2008, 20, 62–67. [Google Scholar]
- Liao, X.Y.; Chen, T.B.; Xie, H.; Xiao, X.Y. Effect of application of P fertilizer on efficiency of As removal in contaminated soil using phytoremediation: Field demonstration. Acta Sci. Circumst. 2004, 24, 455–462. [Google Scholar]
- Fayiga, A.O.; Ma, L.Q. Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci. Total Environ. 2006, 359, 17–25. [Google Scholar] [CrossRef]
- Bolan, N.; Mahimairaja, S.; Kunhikrishnan, A.; Choppala, G. Phosphorus-arsenic interactions in variable-charge soils in relation to arsenic mobility and bioavailability. Sci. Total Environ. 2013, 463–464, 1154–1162. [Google Scholar] [CrossRef]
- Fu, J.W.; Liu, X.; Han, Y.H.; Mei, H.; Cao, Y.; de Oliveira, L.M.; Liu, Y.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arse-nic-hyperaccumulator Pteris vittata efficiently solubilized phosphate rock to sustain plant growth and As uptake. J. Hazard. Mater. 2017, 330, 68–75. [Google Scholar] [CrossRef]
- Elless, M.P.; Poynton, C.Y.; Willms, C.A.; Doyle, M.P.; Lopez, A.C.; Sokkary, D.A.; Ferguson, B.W.; Blaylock, M.J. Pilot-scale demonstration of phytofiltration for treatment of arsenic in New Mexico drinking water. Water Res. 2005, 39, 3863–3872. [Google Scholar] [CrossRef]
- Natarajan, S.; Stamps, R.H.; Saha, U.K.; Ma, L.Q. Phytofiltration of arsenic-contaminated groundwater using Pteris vittata L.: Effect of plant density and nitrogen and phosphorus levels. Int. J. Phytoremediat. 2008, 10, 222–235. [Google Scholar] [CrossRef]
- Huang, Y.; Miyauchi, K.; Inoue, C.; Endo, G. Development of suitable hydroponics system for phytoremediation of arse-nic-contaminated water using an arsenic hyperaccumulator plant Pteris vittata. Biosci. Biotechnol. Biochem. 2016, 80, 614–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.K.; Juwarkar, A.A.; Phani Kumar, G.; Thawale, P.R.; Singh, S.K.; Chakrabarti, T. Bioaccumulation and phy-to-translocation of arsenic, chromium and zinc by Jatropha curcas L.: Impact of dairy sludge and biofertilizer. Bioresour. Technol. 2009, 100, 4616–4622. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lei, M.; Duan, L.; Longhurst, P. Integrating phytoremediation with biomass valorisation and critical element recovery: A UK contaminated land perspective. Biomass Bioenergy 2015, 83, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Srivastava, A.K.; Suprasanna, P.; D’Souza, S.F. Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J. Exp. Bot. 2009, 60, 3419–3431. [Google Scholar] [CrossRef] [Green Version]
- Piracha, M.A.; Ashraf, M.; Niaz, A. Arsenic fractionation and its impact on physiological behavior of sunflower (Helianthus annuus L.) in three texturally different soils under alkaline calcareous conditions. Environ. Sci. Pollut. Res. 2019, 26, 17438–17449. [Google Scholar] [CrossRef]
- Purdy, J.J.; Smart, L.B. Hydroponic Screening of Shrub Willow (Salix spp.) for Arsenic Tolerance and Uptake. Int. J. Phytoremediat. 2008, 10, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Ali, N.; Ahmad, A. Enhanced phytoremediation of cadmium contaminated soil by Parthenium hysterophorus plant: Effect of gibberelic acid (GA3) and synthetic chelator, alone and in combinations. Bioremediat. J. 2014, 18, 46–55. [Google Scholar] [CrossRef]
- Favas, P.J.; Pratas, J.; Prasad, M. Accumulation of arsenic by aquatic plants in large-scale field conditions: Opportunities for phytoremediation and bioindication. Sci. Total Environ. 2012, 433, 390–397. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Srivastava, S.; Patade, V.Y.; Dwivedi, S.; Tripathi, R.; Nikam, T.; Suprasanna, P. Investigation of arsenic accumulation and tolerance potential of Sesuvium portulacastrum (L.) L. Chemosphere 2011, 82, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Srivastava, S.; Mishra, S.; Dixit, B.; Kumar, A.; Tripathi, R. Screening of native plants and algae growing on fly-ash affected areas near National Thermal Power Corporation, Tanda, Uttar Pradesh, India for accumulation of toxic heavy metals. J. Hazard. Mater. 2008, 158, 359–365. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef]
- Nigam, S.; Gopal, K.; Vankar, P.S. Biosorption of arsenic in drinking water by submerged plant: Hydrilla verticilata. Environ. Sci. Pollut. Res. 2012, 20, 4000–4008. [Google Scholar] [CrossRef]
- Mkandawire, M.; Lyubun, Y.V.; Kosterin, P.V.; Dudel, E.G. Toxicity of arsenic species to Lemna gibba L. and the influence of phosphate on arsenic bioavailability. Environ. Toxicol. 2004, 19, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Majumder, A.; Misra, A.K.; Bandyopadhyay, K. Arsenic uptake by Lemna minor in hydroponic system. Int. J. Phytoremediat. 2014, 16, 1221–1227. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, A.-J.; Zhao, F.-J.; Xu, G.-Z.; Duan, G.-L.; Zhu, Y.-G. Arsenic accumulation by the aquatic fern Azolla: Comparison of arsenate uptake, speciation and efflux by A. caroliniana and A. filiculoides. Environ. Pollut. 2008, 156, 1149–1155. [Google Scholar] [CrossRef]
- Farnese, F.; Oliveira, J.; Lima, F.; Leão, G.; Gusman, G.; Silva, L. Evaluation of the potential of Pistia stratiotes L. (water lettuce) for bioindication and phytoremediation of aquatic environments contaminated with arsenic. Braz. J. Biol. 2014, 74, S108–S112. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Hasegawa, H.; Ueda, K.; Maki, T. Influence of phosphate and iron ions in selective uptake of arsenic species by water fern (Salvinia natans L.). Chem. Eng. J. 2008, 145, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Zimmels, Y.; Kirzhner, F.; Malkovskaja, A. Application of Eichhornia crassipes and Pistia stratiotes for treatment of urban sewage in Israel. J. Environ. Manag. 2006, 81, 420–428. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.D.; Borges, A.C.; de Matos, A.T.; Veloso, R.W.; Braga, A.F. Optimization of arsenic phytoremediation using Eic-chornia crassipes. Int. J. Phytoremediat. 2018, 20, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Shrivastava, M.; Suprasanna, P.; D’Souza, S. Phytofiltration of arsenic from simulated contaminated water using Hydrilla verticillata in field conditions. Ecol. Eng. 2011, 37, 1937–1941. [Google Scholar] [CrossRef]
- Islam, M.S.; Saito, T.; Kurasaki, M. Phytofiltration of arsenic and cadmium by using an aquatic plant, Micranthemum umbrosum: Phytotoxicity, uptake kinetics, and mechanism. Ecotoxicol. Environ. Saf. 2015, 112, 193–200. [Google Scholar] [CrossRef]
- Aryal, R.; Nirola, R.; Beecham, S.; Kamruzzaman, M. Impact of elemental uptake in the root chemistry of wetland plants. Int. J. Phytoremediat. 2016, 18, 936–942. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, B.; Korstad, J. Phytoremediation Potential of Bioenergy Plants; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Chaukura, N.; Gwenzi, W.; Tavengwa, N.; Manyuchi, M.M. Biosorbents for the removal of synthetic organics and emerging pollutants: Opportunities and challenges for developing countries. Environ. Dev. 2016, 19, 84–89. [Google Scholar] [CrossRef]
- Bote, M.A.; Naik, V.R.; Jagadeeshgouda, K.B. Review on water hyacinth weed as a potential biofuel crop to meet collective energy needs. Mater. Sci. Energy Technol. 2020, 3, 397–406. [Google Scholar]
- Nahar, K.; Sunny, S.A. Duckweed-based clean energy production dynamics (ethanol and biogas) and phyto-remediation po-tential in Bangladesh. Model. Earth Syst. Environ. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, J.; Tang, J.; Zhu, Y.; Wu, Y. Arsenic removal by periphytic biofilm and its application combined with biochar. Bioresour. Technol. 2018, 248, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Alka, S.; Shahir, S.; Ibrahim, N.; Chai, T.-T.; Bahari, Z.M.; Manan, F.A. The role of plant growth promoting bacteria on arsenic removal: A review of existing perspectives. Environ. Technol. Innov. 2020, 17, 100602. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Zloch, M.; Kowalkowski, T.; Baum, C.; Buszewski, B. Efficiency of microbially assisted phytoremediation of heavy-metal contaminated soils. Environ. Rev. 2018, 26, 316–332. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Yadav, P.; Shukla, A.; Srivastava, S. Utilizing the potential of microorganisms for managing arsenic contam-ination: A feasible and sustainable approach. Front. Environ. Sci. 2018, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an enhanced understanding of plant-microbiome inter-actions to improve phytoremediation: Engineering the metaorganism. Front. Microbiol. 2016, 16, 341. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Genet. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Chauhan, R.; Dwivedi, S.; Srivastava, S.; Srivastava, S.; Tripathi, R.D. A consortium of alga (Chlorella vulgaris) and bacterium (Pseudomonas putida) for amelioration of arsenic toxicity in rice: A promising and feasible approach. Environ. Exp. Bot. 2018, 150, 115–126. [Google Scholar] [CrossRef]
- Asad, S.A.; Farooq, M.; Afzal, A.; West, H. Integrated phytobial heavy metal remediation strategies for a sustainable clean environment—A review. Chemosphere 2018, 217, 925–941. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Hao, T.; Chen, S. Colonization and Maize Growth Promotion Induced by Phosphate Solubilizing Bacterial Isolates. Int. J. Mol. Sci. 2017, 18, 1253. [Google Scholar] [CrossRef] [Green Version]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microor-ganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef] [PubMed]
- Bahari, Z.M.; Ibrahim, Z.; Jaafar, J.; Shahir, S. Draft Genome Sequence of Arsenic-Resistant Microbacterium sp. Strain SZ1 Isolated from Arsenic-Bearing Gold Ores. Genome Announc. 2017, 5, e01183-17. [Google Scholar] [CrossRef] [Green Version]
- Moens, M.; Branco, R.; Morais, P.V. Arsenic accumulation by a rhizosphere bacterial strain Ochrobactrumtritici reduces rice plant arsenic levels. World J. Microbiol. Biotechnol. 2020, 36, 23. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Ortiz, J.; Herrera, H.; Fuentes, A.; Almonacid, L.; Charles, T.C.; Arriagada, C. Enhanced arsenic tolerance in Triticum aestivuminoculated with arsenic-resistant and plant growth promoter microorganisms from a heavy metal-polluted soil. Micro-organisms 2019, 7, 348. [Google Scholar]
- Yang, C.; Ho, Y.-N.; Makita, R.; Inoue, C.; Chien, M.-F. A multifunctional rhizobacterial strain with wide application in different ferns facilitates arsenic phytoremediation. Sci. Total Environ. 2020, 712, 134504. [Google Scholar] [CrossRef] [PubMed]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilmformation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610–611, 1239. [Google Scholar] [CrossRef]
- Cantamessa, S.; Massa, N.; Gamalero, E.; Berta, G. Phytoremediation of a Highly Arsenic Polluted Site, Using Pteris vittata L. and Arbuscular Mycorrhizal Fungi. Plants 2020, 9, 1211. [Google Scholar] [CrossRef]
- He, X.; Lilleskov, E. Arsenic Uptake and Phytoremediation Potential by Arbuscular Mycorrhizal Fungi. In Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration; Solaiman, Z.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 259–273. [Google Scholar]
- Vijaya Kumar, V.; Suprasanna, P. Mycorrhizoremediation: A Novel Tool for Bioremediation. In Rhizomicrobiome Dynamics in Bioremediation; Kumar, V., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–12. [Google Scholar]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular Mycorrhiza Augments Arsenic Tolerance in Wheat (Triticum aestivum L.) by Strengthening Antioxidant Defense System and Thiol Metabolism. Front. Plant Sci. 2017, 8, 906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Chen, X.; Wong, M. Arbuscular mycorrhizal fungi reduced the ratios of inorganic/organic arsenic in rice grains. Chem-osphere 2016, 145, 224–230. [Google Scholar] [CrossRef]
- Pathare, V.; Srivastava, S.; Sonawane, B.V.; Suprasanna, P. Arsenic stress affects the expression profile of genes of 14-3-3 proteins in the shoot of mycorrhiza colonized rice. Physiol. Mol. Biol. Plants 2016, 22, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poonam; Srivastava, S.; Pathare, V.; Suprasanna, P. Physiological and molecular insights into rice-arbuscular mycorrhizal in-teractions under arsenic stress. Plant Gene 2017, 11, 232–237. [Google Scholar] [CrossRef]
- Yin, N.; Zhang, Z.; Wang, L.; Qian, K. Variations in organic carbon, aggregation, and enzyme activities of gangue-fly ash-reconstructed soils with sludge and arbuscular mycorrhizal fungi during 6-year reclamation. Environ. Sci. Pollut. Res. 2016, 23, 17840–17849. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Christie, P.; Liu, Y.; Zhang, J.; Li, X. The arbuscular mycorrhizal fungus Glomus mosseae can enhance arsenic tolerance in Medicago truncatula by increasing plant phosphorus status and restricting arsenate uptake. Environ. Pollut. 2008, 156, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhu, Y.-G.; Chen, B.; Christie, P.; Li, X. Yield and arsenate uptake of arbuscular mycorrhizal tomato colonized by Glomus mosseae BEG167 in As spiked soil under glasshouse conditions. Environ. Int. 2005, 31, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Zhu, Y.-G.; Smith, F.A.; Wang, Y.; Chen, B. Arbuscular mycorrhiza enhanced arsenic resistance of both white clover (Trifolium repens Linn.) and ryegrass (Lolium perenne L.) plants in an arsenic-contaminated soil. Environ. Pollut. 2008, 155, 174–181. [Google Scholar] [CrossRef] [PubMed]
- De Conti, L.; Ceretta, C.A.; Melo, G.W.B.; Tiecher, T.L.; Silva, L.O.S.; Garlet, L.P.; Mimmo, T.; Cesco, S.; Brunetto, G. Inter-cropping of young grapevines with native grasses for phytoremediation of Cu-contaminated soils. Chemosphere 2019, 216, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.Y.; Yan, X.L.; Liao, X.Y.; Zhang, Y.X.; Ma, X. Arsenic Accumulation in Panax notoginseng Monoculture and Intercropping with Pteris vittata. Water Air Soil Pollut. 2015, 226, 1–8. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T.; Yang, J. Intercropped Pteris vittata L. and Morus alba L. presents a safe utilization mode for arse-nic-contaminated soil. Sci. Total Environ 2017, 579, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lei, E.; Lei, M.; Liu, Y.; Chen, T. Remediation of Arsenic contaminated soil using malposed intercropping of Pteris vittata L. and maize. Chemosphere 2018, 194, 737–744. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Kwon, E.E.; Biswas, J.K.; Tack, F.M.G.; Ok, Y.S.; Rinklebe, J. Arsenic, chromium, molybdenum, and selenium: Geochemical fractions and potential mobilization in riverine soil profiles originating from Germany and Egypt. Chemosphere 2017, 180, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lei, M.; Chen, T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total. Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, X.; Lei, M. Application of arsenic hyperaccumulator Pteris vittata L. to contaminated soil in Northern China. J. Geochem. Explor. 2017, 182, 132–137. [Google Scholar] [CrossRef]
- Ye, W.; Khan, M.A.; McGrath, S.; Zhao, F.-J. Phytoremediation of arsenic contaminated paddy soils with Pteris vittata markedly reduces arsenic uptake by rice. Environ. Pollut. 2011, 159, 3739–3743. [Google Scholar] [CrossRef] [PubMed]
- Poonam; Upadhyay, M.K.; Gautam, A.; Mallick, S.; Srivastava, S. A successive application approach for effective utilization of three aquatic plants in arsenic removal. Water Air Soil Pollut. 2017, 228, 54. [Google Scholar] [CrossRef]
- Srivastava, S.; Sounderajan, S.; Udas, A.; Suprasanna, P. Effect of combinations of aquatic plants (Hydrilla, Ceratophyllum, Eichhornia, Lemna and Wolffia) on arsenic removal in field conditions. Ecol. Eng. 2014, 73, 297–301. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.; Ma, Y.; Huang, Y. Can phosphate compounds be used to reduce the plant uptake of Pb and resist the Pb stress in Pb-contaminated soils? J. Environ. Sci. (China) 2009, 21, 360–365. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Chen, S.; Ma, Y. Alleviation of cadmium-induced root growth inhibition in crop seedlings by nanoparticles. Ecotoxicol. Environ. Saf. 2012, 79, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; de la Rosa-Álvarez, M.G. Use of Nan-otechnology for the bioremediation of contaminants: A review. Processes 2020, 8, 826. [Google Scholar] [CrossRef]
- Desai, M.; Haigh, M.; Walkington, H. Phytoremediation: Metal decontamination of soils after the sequential forestation of former opencast coal land. Sci. Total Environ. 2018, 656, 670–680. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Semenkov, I.; Klink, G.; Tarigholizadeh, S.; Sushkova, S. Phylogenetic analysis of hyperaccumulator plant species for heavy metals and polycyclic aromatic hydrocarbons. Environ. Geochem. Health 2020, 43, 1629–1654. [Google Scholar] [CrossRef]
- Bao-Shan, L.; Shao-Qi, D.; Chun-Hui, L.; Li-Jun, F.; Shu-Chun, Q.; Min, Y. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Song, B.; Xu, P.; Chen, M.; Tang, W.; Zeng, G.; Gong, J.; Zhang, P.; Ye, S. Using nanomaterials to facilitate the phytoremediation of contaminated soil. Crit. Rev. Environ. Sci. Technol. 2019, 49, 791–824. [Google Scholar] [CrossRef]
- Souri, Z.; Karimi, N.; Sarmadi, M.; Rostami, E. Salicylic acid nanoparticles (SANPs) improve growth and phytoremediation efficiency of Isatis cappadocica Desv., under As stress. IET Nanobiotechnol. 2017, 11, 650–655. [Google Scholar] [CrossRef]
- Vítková, M.; Puschenreiter, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal(loid) contaminated soils. Chemosphere 2018, 200, 217–226. [Google Scholar] [CrossRef]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2019, 239, 124794. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Liang, X.; Bai, X.; Zheng, L.; Zhao, J.; Li, Y.-F.; Zhang, Z.; Gao, Y. Silica nanoparticles alleviate mercury toxicity via immobilization and inactivation of Hg(ii) in soybean (Glycine max). Environ. Sci. Nano 2020, 7, 1807–1817. [Google Scholar] [CrossRef]
- Jiang, M.; Dai, S.; Wang, B.; Xie, Z.; Li, J.; Wang, L.; Li, S.; Tan, Y.; Tian, B.; Shu, Q.; et al. Gold nanoparticles synthesized using melatonin suppress cadmium uptake and alleviate its toxicity in rice. Environ. Sci. Nano 2021, 8, 1042–1056. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Wu, F.; Zhang, X.; Wang, B.; Yang, Y.; Shen, G.; Liu, J.; Tao, S.; Wang, X. Application of TiO2 nanoparticles to reduce bioaccumulation of arsenic in rice seedlings (Oryza sativa L.): A mechanistic study. J. Hazard. Mater. 2020, 405, 124047. [Google Scholar] [CrossRef]
- Katiyar, P.; Yadu, B.; Korram, J.; Satnami, M.L.; Kumar, M.; Keshavkant, S. Titanium nanoparticles attenuates arsenic toxicity by up-regulating expressions of defensive genes in Vigna radiata L. J. Environ. Sci. 2020, 92, 18–27. [Google Scholar] [CrossRef]
- Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X.; Ye, W. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Ben Fekih, I.; Zhang, C.; Li, Y.P.; Zhao, Y.; Alwathnani, H.A.; Saquib, Q.; Rensing, C.; Cervantes, C. Distribution of Arsenic Resistance Genes in Prokaryotes. Front. Microbiol. 2018, 9, 2473. [Google Scholar] [CrossRef]
- Mosa, K.A.; Saadoun, I.; Kumar, K.; Helmy, M.; Dhankher, O.P. Potential Biotechnological Strategies for the Cleanup of Heavy Metals and Metalloids. Front. Plant Sci. 2016, 7, 303. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Choudhary, S.; Majeed, A.; Singh, A.; Bhardwaj, P. Insights into the molecular mechanism of arsenic phytoreme-diation. J. Plant Growth Regul. 2020, 39, 532–543. [Google Scholar] [CrossRef]
- Lindsay, E.; Maathuis, F.J.M. Arabidopsis thalianaNIP7;1 is involved in tissue arsenic distribution and tolerance in response to arsenate. FEBS Lett. 2016, 590, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, P.; Rastogi, A.; Shukla, A.; Srivastava, S.; Yadav, S. Prospects of genetic engineering utilizing potential genes for reg-ulating arsenic accumulation in plants. Chemosphere 2018, 211, 397–406. [Google Scholar] [CrossRef] [PubMed]
- DiTusa, S.F.; Fontenot, E.B.; Wallace, R.W.; Silvers, M.; Steele, T.N.; Elnagar, A.H.; Dearman, K.M.; Smith, A.P. A member of the Phosphate transporter 1 (Pht1) family from the arsenic-hyperaccumulating fern Pteris vittata is a high-affinity arsenate transporter. New Phytol. 2015, 209, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Feng, H.-Y.; Li, X.-Y.; Ai, H.; Sun, S.; Chen, Y.; Xu, G.; Rathinasabapathi, B.; Cao, Y.; Ma, L.Q. Expression of New Pteris vittata Phosphate Transporter PvPht1;4 Reduces Arsenic Translocation from the Roots to Shoots in Tobacco Plants. Environ. Sci. Technol. 2019, 54, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Indriolo, E.; Na, G.; Ellis, D.; Salt, D.E.; Banks, J.A. A Vacuolar Arsenite Transporter Necessary for Arsenic Tolerance in the Arsenic Hyperaccumulating Fern Pteris vittata Is Missing in Flowering Plants. Plant Cell 2010, 22, 2045–2057. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, W.; Shen, H.; Yan, H.; Xu, W.; He, Z.; Ma, M. Engineering Arsenic Tolerance and Hyperaccumulation in Plants for Phytoremediation by a PvACR3 Transgenic Approach. Environ. Sci. Technol. 2013, 47, 9355–9362. [Google Scholar] [CrossRef]
- Wang, C.; Na, G.; Bermejo, E.S.; Chen, Y.; Banks, J.A.; Salt, D.E.; Zhao, F. Dissecting the components controlling root-to-shoot arsenic translocation in Arabidopsis thaliana. New Phytol. 2017, 217, 206–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhankher, O.P.; Li, Y.; Rosen, B.P.; Shi, J.; Salt, D.; Senecoff, J.F.; Sashti, N.A.; Meagher, R.B. Engineering tolerance and hy-peraccumulation of arsenic in plants by combining arsenate reductase and gamma-glutamyl cysteine synthetase expression. Nat. Biotechnol. 2002, 20, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Rosen, B.P.; McKinney, E.C.; Meagher, R.B. Hyperaccumulation of arsenic in the shoots of Arabidopsis silenced for arsenate reductase (ACR2). Proc. Natl. Acad. Sci. USA 2006, 103, 5413–5418. [Google Scholar] [CrossRef] [Green Version]
- Nahar, N.; Rahman, A.; Nawani, N.N.; Ghosh, S.; Mandal, A. Phytoremediation of arsenic from the contaminated soil using transgenic tobacco plants expressing ACR2 gene of Arabidopsis thaliana. J. Plant Physiol. 2017, 218, 121–126. [Google Scholar] [CrossRef]
- Li, X.; Sun, D.; Feng, H.; Chen, J.; Chen, Y.; Li, H.; Cao, Y.; Ma, L.Q. Efficient arsenate reduction in As-hyperaccumulator Pteris vittata are mediated by novel arsenate reductases PvHAC1 and PvHAC2. J. Hazard. Mater. 2020, 399, 122895. [Google Scholar] [CrossRef]
- Cai, C.; Lanman, N.A.; Withers, K.A.; DeLeon, A.M.; Wu, Q.; Gribskov, M.; Salt, D.E.; Banks, J.A. Three genes define a bac-terial-like arsenic tolerance mechanism in the arsenic hyperaccumulating fern Pteris vittata. Curr. Biol. 2019, 29, 1625–1633. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).