Versatile Applications of Cyanobacteria in Biotechnology
Abstract
1. Introduction
2. Cyanobacterial Metabolites
2.1. Phenolic Acids
2.2. Vitamins
2.3. Peptides
2.4. Terpenoids
3. Biotechnological Applications of Cyanobacteria
3.1. Cyanobacteria as Food Supplements
3.2. Cyanobacteria in Medical and Pharmaceutical Biotechnology
3.3. Cyanobacteria in Bioplastic and Biofuel Production
3.4. Cyanobacteria in Bioremediation
3.5. Applications of Cyanobacteria Species in Biocatalytic Processes
4. Conclusions and Future Perspective
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Stomp, M.; Huisman, J.; Vörös, L.; Pick, F.R.; Laamanen, M.; Haverkamp, T.; Stal, L.J. Colourful Coexistence of Red and Green Picocyanobacteria in Lakes and Seas. Ecol. Lett. 2007, 10, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2012; pp. 1–13. [Google Scholar]
- Rasmussen, B.; Fletcher, I.R.; Brocks, J.J.; Kilburn, M.R. Reassessing the First Appearance of Eukaryotes and Cyanobacteria. Nature 2008, 455, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Catherine, Q.; Susanna, W.; Isidora, E.S.; Mark, H.; Aurélie, V.; Jean-François, H. A Review of Current Knowledge on Toxic Benthic Freshwater Cyanobacteria--Ecology, Toxin Production and Risk Management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony Formation in the Cyanobacterium Microcystis. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1399–1420. [Google Scholar] [CrossRef]
- Amadu, A.A.; de Graft-Johnson, K.A.A.; Ameka, G.K. Industrial Applications of Cyanobacteria. Cyanobacteria Recent Adv. Taxon. Appl. 2021, 106, 1–12. [Google Scholar]
- Costa, J.A.V.; Moreira, J.B.; Lucas, B.F.; Da Silva Braga, V.; Cassuriaga, A.P.A.; De Morais, M.G. Recent Advances and Future Perspectives of PHB Production by Cyanobacteria. Ind. Biotechnol. 2018, 14, 249–256. [Google Scholar] [CrossRef]
- Sukenik, A.; Hadas, O.; Kaplan, A.; Quesada, A. Invasion of Nostocales (Cyanobacteria) to Subtropical and Temperate Freshwater Lakes—Physiological, Regional, and Global Driving Forces. Front. Microbiol. 2012, 3, 86. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate Change: Links to Global Expansion of Harmful Cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Berg, M.; Sutula, M. Factors Affecting Growth of Cyanobacteria With Special Emphasis on the Sacramento-San Joaquin Delta. South. Calif. Coast. Water Res. Proj. Tech. Rep. 2015, 869, 100. [Google Scholar]
- Koller, M. Advances in Polyhydroxyalkanoate (PHA) Production, Volume 3. Bioengineering 2022, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Afreen, R.; Tyagi, S.; Singh, G.P.; Singh, M. Challenges and Perspectives of Polyhydroxyalkanoate Production From Microalgae/Cyanobacteria and Bacteria as Microbial Factories: An Assessment of Hybrid Biological System. Front. Bioeng. Biotechnol. 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Kurmayer, R.; Deng, L.; Entfellner, E. Role of Toxic and Bioactive Secondary Metabolites in Colonization and Bloom Formation by Filamentous Cyanobacteria Planktothrix. Harmful Algae 2016, 54, 69–86. [Google Scholar] [CrossRef]
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny Microbes with a Big Impact: The Role of Cyanobacteria and Their Metabolites in Shaping Our Future. Mar. Drugs 2016, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.-S.; Chew, L.-L. Valuable Compounds Produced by Microalgae. Handb. Biorefinery Res. Technol. 2022, 1–23. [Google Scholar] [CrossRef]
- Orona-Navar, A.; Aguilar-Hernández, I.; Nigam, K.D.P.; Cerdán-Pasarán, A.; Ornelas-Soto, N. Alternative Sources of Natural Pigments for Dye-Sensitized Solar Cells: Algae, Cyanobacteria, Bacteria, Archaea and Fungi. J. Biotechnol. 2021, 332, 29–53. [Google Scholar] [CrossRef]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P. Cyanobacteria as a Source of Biofertilizers for Sustainable Agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Nicoletti, M. The Nutraceutical Potential of Cyanobacteria. Pharmacol. Potential Cyanobacteria 2022, 287–330. [Google Scholar] [CrossRef]
- Agarwal, P.; Soni, R.; Kaur, P.; Madan, A.; Mishra, R.; Pandey, J.; Singh, S.; Singh, G. Cyanobacteria as a Promising Alternative for Sustainable Environment: Synthesis of Biofuel and Biodegradable Plastics. Front. Microbiol. 2022, 13, 939347. [Google Scholar] [CrossRef]
- Böttger, A.; Vothknecht, U.; Bolle, C.; Wolf, A. Plant Secondary Metabolites and Their General Function in Plants. In Lessons on Caffeine, Cannabis & Co; Springer: Cham, Switzerland, 2018; pp. 3–17. [Google Scholar]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant Secondary Metabolites: A Key Driver of Litter Decomposition and Soil Nutrient Cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Baselga-Cervera, B.; Balboa, C.G.; Costas, E.; Lopez-Rodas, V. Why Cyanobacteria Produce Toxins? Evolutionary Game Theory Suggests the Key. Int. J. Biol. 2014, 7, 64. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Verma, S.; Meena, K.K.; Yandigeri, M. Antioxidant Properties and Polyphenolic Content in Terrestrial Cyanobacteria. 3 Biotech 2017, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Formighieri, C.; Melis, A. Cyanobacterial Production of Plant Essential Oils. Planta 2018, 248, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Knoot, C.J.; Khatri, Y.; Hohlman, R.M.; Sherman, D.H.; Pakrasi, H.B. Engineered Production of Hapalindole Alkaloids in the Cyanobacterium Synechococcus sp. UTEX 2973. ACS Synth. Biol. 2019, 8, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef]
- Dong, Q.; Dong, R.; Xing, X.; Li, Y. A new antibiotic produced by the cyanobacterium-symbiotic fungus Simplicillium lanosoniveum. Nat. Prod. Res. 2018, 32, 1348–1352. [Google Scholar] [CrossRef]
- Sunday, O.S.; Emmanuel, E.; Cornelius, A.O. In-Vitro Assessment of Antibacterial Activity of Crude Methanolic and Aqueous Extracts of Mitracarpus Villosus. Afr. J. Microbiol. Res. 2021, 15, 62–68. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Rachedi, R.; Foglino, M.; Latifi, A. Stress Signaling in Cyanobacteria: A Mechanistic Overview. Life 2020, 10, 312. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Mehmood, M.A.; Chang, J.S.; Bai, F.; Zhao, X. Manipulating Environmental Stresses and Stress Tolerance of Microalgae for Enhanced Production of Lipids and Value-Added Products—A Review. Bioresour. Technol. 2017, 244, 1198–1206. [Google Scholar] [CrossRef]
- Ge, S.; Champagne, P.; Plaxton, W.C.; Leite, G.B.; Marazzi, F. Microalgal Cultivation with Waste Streams and Metabolic Constraints to Triacylglycerides Accumulation for Biofuel Production. Biofuels Bioprod. Biorefining 2017, 11, 325–343. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering Potential Applications of Cyanobacteria and Algal Metabolites in Biology, Agriculture and Medicine: Current Status and Future Prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [PubMed]
- El-Baz, F.K.; El-Senousy, W.M.; El-Sayed, A.B.; Kamel, M.M. In vitro antiviral and antimicrobial activities of algal extract of Spirulina platensis. J. Appl. Pharm. Sci. 2013, 3, 52–56. [Google Scholar]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial Strategies Centered around Reactive Oxygen Species–Bactericidal Antibiotics, Photodynamic Therapy and Beyond. FEMS Microbiol. Rev. 2013, 37, 955. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Moh, S.H.; Lee, T.K.; Kottuparambil, S.; Kim, Y.J.; Rhee, J.S.; Choi, E.M.; Brown, M.T.; Häder, D.P.; et al. Ultraviolet Radiation and Cyanobacteria. J. Photochem. Photobiol. B Biol. 2014, 141, 154–169. [Google Scholar] [CrossRef]
- Mahfooz, S.; Shamim, A.; Husain, A.; Hasan, Z.; Farooqui, A. Oxidative Stress Biomarkers in Cyanobacteria Exposed to Heavy Metals. Contam. Water Health Risk Assess. Treat. Strateg. 2021, 385–403. [Google Scholar] [CrossRef]
- Badri, H.; Monsieurs, P.; Coninx, I.; Wattiez, R.; Leys, N. Molecular investigation of the radiation resistance of edible cyanobacterium Arthrospira sp. PCC 8005. Microbiologyopen 2015, 4, 187–207. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Meena, K.K.; Sharma, L.; Sharma, A.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Sharma, L.; Sharma, A.K. Induced Accumulation of Polyphenolics and Flavonoids in Cyanobacteria under Salt Stress Protects Organisms through Enhanced Antioxidant Activity. Am. J. Plant Sci. 2014, 5, 726–735. [Google Scholar] [CrossRef]
- Patipong, T.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. Induction of Antioxidative Activity and Antioxidant Molecules in the Halotolerant Cyanobacterium Halothece sp. PCC7418 by Temperature Shift. Nat. Prod. Commun. 2019, 14, 1934578X19865680. [Google Scholar]
- Park, H.J.; Cho, J.H.; Hong, S.H.; Kim, D.H.; Jung, H.Y.; Kang, I.K.; Cho, Y.J. Whitening and Anti-Wrinkle Activities of Ferulic Acid Isolated from Tetragonia Tetragonioides in B16F10 Melanoma and CCD-986sk Fibroblast Cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef]
- Monteiro e Silva, S.A.; Calixto, G.M.F.; Cajado, J.; de Carvalho, P.C.A.; Rodero, C.F.; Chorilli, M.; Leonardi, G.R. Gallic Acid-Loaded Gel Formulation Combats Skin Oxidative Stress: Development, Characterization and Ex Vivo Biological Assays. Polymers 2017, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Kee, H.J.; Ryu, Y.; Choi, S.Y.; Kim, G.R.; Kim, H.S.; Kee, S.J.; Jeong, M.H. Gentisic Acid Prevents the Transition from Pressure Overload-Induced Cardiac Hypertrophy to Heart Failure. Sci. Rep. 2019, 9, 3018. [Google Scholar] [CrossRef] [PubMed]
- Frazzini, S.; Scaglia, E.; Dell’anno, M.; Reggi, S.; Panseri, S.; Giromini, C.; Lanzoni, D.; Rossi, C.A.S.; Rossi, L. Antioxidant and Antimicrobial Activity of Algal and Cyanobacterial Extracts: An In Vitro Study. Antioxidants 2022, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.B.B.; Easley, A.D.; Grant, K.; Fletcher, G.K.; Boyer, C.; Maitland, D.J. Multifunctional Shape-Memory Polymer Foams with Bio-Inspired Antimicrobials. ChemPhysChem 2018, 19, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Narita, R.; Nishimura, H.; Marumoto, S.; Yamamoto, S.P.; Ouda, R.; Yatagai, M.; Fujita, T.; Watanabe, T. Antiviral Activity of Phenolic Derivatives in Pyroligneous Acid from Hardwood, Softwood, and Bamboo. ACS Sustain. Chem. Eng. 2018, 6, 119–126. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb Cell Fact. 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Lawrence, A.D.; Holzer, A.; Kudahl, U.J.; Sasso, S.; Kräutler, B.; Scanlan, D.J.; Warren, M.J.; Smith, A.G. Cyanobacteria and Eukaryotic Algae Use Different Chemical Variants of Vitamin B12. Curr. Biol. 2016, 26, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in Plants: Occurrence, Biosynthesis and Antioxidant Function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Dawoud, A.; ElTahtawy, O.; Farag, M.A. Fat-souluble vitamins: Updated review of their role and orchestration in human nutrition throughout life cycle with sex differences. Nutr. Metab. 2022, 19, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Golriz, F.; Donnelly, L.F.; Devaraj, S.; Krishnamurthy, R. Modern American Scurvy—Experience with Vitamin C Deficiency at a Large Children’s Hospital. Pediatr. Radiol. 2017, 47, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C Content in Fruits: Biosynthesis and Regulation. Front. Plant Sci. 2018, 9, 2006. [Google Scholar] [CrossRef] [PubMed]
- Al Saleh, Y.; Beshyah, S.A.; Hussein, W.; Almadani, A.; Hassoun, A.; Al Mamari, A.; Ba-Essa, E.; Al-Dhafiri, E.; Hassanein, M.; Fouda, M.A.; et al. Diagnosis and Management of Vitamin D Deficiency in the Gulf Cooperative Council (GCC) Countries: An Expert Consensus Summary Statement from the GCC Vitamin D Advisory Board. Arch. Osteoporos. 2020, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.E.; Phillips, T.W.; Udani, J. Nutritional Supplementation with Chlorella Pyrenoidosa Lowers Serum Methylmalonic Acid in Vegans and Vegetarians with a Suspected Vitamin B12 Deficiency. J. Med. Food 2015, 18, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Kraemer, K.; Somoza, V. The True Value of Spirulina. J. Agric. Food Chem. 2020, 68, 4109–4115. [Google Scholar] [CrossRef]
- Watanabe, F.; Katsura, H.; Takenaka, S.; Fujita, T.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Pseudovitamin B12 Is the Predominant Cobamide of an Algal Health Food, Spirulina Tablets. J. Agric. Food Chem. 1999, 47, 4736–4741. [Google Scholar] [CrossRef]
- Agostoni, C.; Canani, R.B.; Fairweather-Tait, S.; Heinonen, M.; Korhonen, H.; La Vieille, S.; Marchelli, R.; Martin, A.; Naska, A.; Neuhäuser-Berthold, M.; et al. Scientific Opinion on Dietary Reference Values for Cobalamin (Vitamin B12). EFSA J. 2015, 13, 4150. [Google Scholar]
- Muys, M.; Sui, Y.; Schwaiger, B.; Lesueur, C.; Vandenheuvel, D.; Vermeir, P.; Vlaeminck, S.E. High Variability in Nutritional Value and Safety of Commercially Available Chlorella and Spirulina Biomass Indicates the Need for Smart Production Strategies. Bioresour. Technol. 2019, 275, 247–257. [Google Scholar] [CrossRef]
- Anantharajappa, K.; Dharmesh, S.M.; Ravi, S. Gastro-Protective Potentials of Spirulina: Role of Vitamin B12. J. Food Sci. Technol. 2020, 57, 745–753. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Chong, P.N.; Chan, H.K.; Mohamed, N.; Froemming, G.R.A.; Okechukwu, P.N. Spirulina Supplementation Improves Bone Structural Strength and Stiffness in Streptozocin-Induced Diabetic Rats. J. Tradit. Complement. Med. 2022, 12, 225–234. [Google Scholar] [CrossRef]
- Carcea, M.; Sorto, M.; Batello, C.; Narducci, V.; Aguzzi, A.; Azzini, E.; Fantauzzi, P.; Finotti, E.; Gabrielli, P.; Galli, V.; et al. Nutritional Characterization of Traditional and Improved Dihé, Alimentary Blue-Green Algae from the Lake Chad Region in Africa. LWT Food Sci. Technol. 2015, 62, 753–763. [Google Scholar] [CrossRef]
- Santiago-Morales, I.S.; Trujillo-Valle, L.; Márquez-Rocha, F.J.; Hernández, J.F.L. Tocopherols, Phycocyanin and Superoxide Dismutase from Microalgae: As Potential Food Antioxidants. Appl. Food Biotechnol. 2018, 5, 19–27. [Google Scholar]
- Aaronson, S.; Dhawale, S.W.; Patni, N.J.; Deangelis, B.; Frank, O.; Baker, H. The Cell Content and Secretion of Water-Soluble Vitamins by Several Freshwater Algae. Arch. Microbiol. 1977, 112, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Ljubic, A.; Jacobsen, C.; Holdt, S.L.; Jakobsen, J. Microalgae Nannochloropsis Oceanica as a Future New Natural Source of Vitamin D3. Food Chem. 2020, 320, 126627. [Google Scholar] [CrossRef] [PubMed]
- Fais, G.; Manca, A.; Bolognesi, F.; Borselli, M.; Concas, A.; Busutti, M.; Broggi, G.; Sanna, P.; Castillo-Aleman, Y.M.; Rivero-Jiménez, R.A.; et al. Wide Range Applications of Spirulina: From Earth to Space Missions. Mar. Drugs 2022, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Tarento, T.D.C.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a Source of Vitamin K1. Algal Res. 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Scheidler, C.M.; Kick, L.M.; Schneider, S. Ribosomal Peptides and Small Proteins on the Rise. ChemBioChem 2019, 20, 1479–1486. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Zhou, Z.; Yan, X. Diversity of Gene Clusters for Polyketide and Nonribosomal Peptide Biosynthesis Revealed by Metagenomic Analysis of the Yellow Sea Sediment. Front. Microbiol. 2018, 9, 295. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Welker, M.; Von Döhren, H. Cyanobacterial Peptides—Nature’s Own Combinatorial Biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef]
- Maurya, S.K.; Niveshika; Mishra, R. Importance of Bioinformatics in Genome Mining of Cyanobacteria for Production of Bioactive Compounds. Cyanobacteria Basic Sci. Appl. 2019, 477–506. [Google Scholar] [CrossRef]
- Wang, H.; Fewer, D.P.; Holm, L.; Rouhiainen, L.; Sivonen, K. Atlas of Nonribosomal Peptide and Polyketide Biosynthetic Pathways Reveals Common Occurrence of Nonmodular Enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, 9259–9264. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J.C.; Picchi, D.G.; Dittmann, E. Natural Product Biosyntheses in Cyanobacteria: A Treasure Trove of Unique Enzymes. Beilstein J. Org. Chem. 2011, 7, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sher, D.; Kelly, L.; Shi, Y.; Huang, K.; Knerr, P.J.; Joewono, I.; Rusch, D.; Chisholm, S.W.; Van Der Donk, W.A. Catalytic Promiscuity in the Biosynthesis of Cyclic Peptide Secondary Metabolites in Planktonic Marine Cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 10430–10435. [Google Scholar] [CrossRef] [PubMed]
- Knerr, P.J.; Van Der Donk, W.A. Discovery, Biosynthesis, and Engineering of Lantipeptides. Annu. Rev. Biochem. 2012, 81, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, A.; Berta-Thompson, J.W.; Becker, J.W.; Van Der Donk, W.A.; Chisholm, S.W. Evolutionary Radiation of Lanthipeptides in Marine Cyanobacteria. Proc. Natl. Acad. Sci. USA 2017, 114, E5424–E5433. [Google Scholar] [CrossRef]
- Breitmaier: Terpenes: Importance, General Structure. Available online: https://scholar.google.com/scholar_lookup?title=Terpenes:+Importance,+general+structure,+and+biosynthesis&author=Breitmaier,+E.&publication_year=2006&journal=Terpenes+Flavors+Fragr.+Pharmaca+Pheromones&volume=1&pages=1–3 (accessed on 22 September 2022).
- Kandi, S.; Godishala, V.; Rao, P.; Biomedicine, K.R. Biomedical Significance of Terpenes: An Insight. Citeseer 2015, 3, 8–10. [Google Scholar]
- Jahnke, L.L.; Embaye, T.; Hope, J.; Turk, K.A.; van Zuilen, M.; des Marais, D.J.; Farmer, J.D.; Summons, R.E. Lipid Biomarker and Carbon Isotopic Signatures for Stromatolite-Forming, Microbial Mat Communities and Phormidium Cultures from Yellowstone National Park. Geobiology 2004, 2, 31–47. [Google Scholar] [CrossRef]
- Summons, R.E.; Jahnke, L.L.; Hope, J.M.; Logan, G.A. 2-Methylhopanoids as Biomarkers for Cyanobacterial Oxygenic Photosynthesis. Nature 1999, 400, 554–557. [Google Scholar] [CrossRef]
- Agger, S.A.; Lopez-Gallego, F.; Hoye, T.R.; Schmidt-Dannert, C. Identification of Sesquiterpene Synthases from Nostoc Punctiforme PCC 73102 and Nostoc sp. Strain PCC 7120. J. Bacteriol. 2008, 190, 6084–6096. [Google Scholar] [CrossRef]
- Vítek, P.; Ascaso, C.; Artieda, O.; Casero, M.C.; Wierzchos, J. Discovery of Carotenoid Red-Shift in Endolithic Cyanobacteria from the Atacama Desert. Sci. Rep. 2017, 7, 11116. [Google Scholar] [CrossRef]
- Boucar, M.C.M.; Shen, L.Q.; Wang, K.; Zhang, Z.C.; Qiu, B.S. UV-B Irradiation Enhances the Production of Unique Mycosporine-like Amino Acids and Carotenoids in the Subaerial Cyanobacterium Pseudanabaena sp. CCNU1. Eur. J. Phycol. 2021, 56, 316–323. [Google Scholar] [CrossRef]
- Kusama, Y.; Inoue, S.; Jimbo, H.; Takaichi, S.; Sonoike, K.; Hihara, Y.; Nishiyama, Y. Zeaxanthin and Echinenone Protect the Repair of Photosystem II from Inhibition by Singlet Oxygen in Synechocystis sp. PCC 6803. Plant Cell Physiol. 2015, 56, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Dienst, D.; Wichmann, J.; Mantovani, O.; Rodrigues, J.S.; Lindberg, P. High Density Cultivation for Efficient Sesquiterpenoid Biosynthesis in Synechocystis sp. PCC 6803. Sci. Rep. 2020, 10, 5932. [Google Scholar] [CrossRef] [PubMed]
- Höckelmann, C.; Becher, P.G.; Von Reuß, S.H.; Jüttner, F. Sesquiterpenes of the Geosmin-Producing Cyanobacterium Calothrix PCC 7507 and Their Toxicity to Invertebrates. Z. Fur Naturforsch. Sect. C J. Biosci. 2009, 64, 49–55. [Google Scholar] [CrossRef]
- Lopes, G.; Clarinha, D.; Vasconcelos, V. Carotenoids from Cyanobacteria: A Biotechnological Approach for the Topical Treatment of Psoriasis. Microorganisms 2020, 8, 302. [Google Scholar] [CrossRef]
- Cabanillas, A.H.; Tena Pérez, V.; Maderuelo Corral, S.; Rosero Valencia, D.F.; Martel Quintana, A.; Ortega Doménech, M.; Rumbero Sánchez, Á. Cybastacines A and B: Antibiotic Sesterterpenes from a Nostoc sp. Cyanobacterium. J. Nat. Prod. 2018, 81, 410–413. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Pegan, S.D.; Franzblau, S.G.; Orjala, J. An Antimicrobial Guanidine-Bearing Sesterterpene from the Cultured Cyanobacterium Scytonema sp. J. Nat. Prod. 2009, 72, 2043–2045. [Google Scholar] [CrossRef]
- Kulasooriya, S.A.; Magana-Arachchi, D.N. Nitrogen Fixing Cyanobacteria: Their Diversity, Ecology and Utilization with Special Reference to Rice Cultivation. J. Natl. Sci. Found. Sri Lanka 2016, 44, 111–128. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of Cyanobacteria for Nano-Marine Drugs: Relevance of Nanoformulations in Cancer Therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef]
- Deviram, G.; Mathimani, T.; Anto, S.; Ahamed, T.S.; Ananth, D.A.; Pugazhendhi, A. Applications of Microalgal and Cyanobacterial Biomass on a Way to Safe, Cleaner and a Sustainable Environment. J. Clean. Prod. 2020, 253, 119770. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Bertucco, A. Bioethanol from Microalgae and Cyanobacteria: A Review and Technological Outlook. Process Biochem. 2016, 51, 1833–1842. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable Products from Biotechnology of Microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Angermayr, S.A.; Hellingwerf, K.J.; Lindblad, P.; Teixeira de Mattos, M.J. Energy Biotechnology with Cyanobacteria. Curr. Opin. Biotechnol. 2009, 20, 257–263. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of Cyanobacteria in Biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Markou, G.; Chentir, I.; Tzovenis, I. Microalgae and Cyanobacteria as Food: Legislative and Safety Aspects. Cult. Microalgae Food Ind. Curr. Potential Appl. 2021, 249–264. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P. Nutritional and Therapeutic Potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef]
- Campanella, L.; Russo, M.; Chim, P.A.-A. Free and Total Amino Acid Composition in Blue-Green Algae. Ann. Chim 2002, 92, 343–352. [Google Scholar]
- Marsan, D.W.; Conrad, S.M.; Stutts, W.L.; Parker, C.H.; Deeds, J.R. Evaluation of Microcystin Contamination in Blue-Green Algal Dietary Supplements Using a Protein Phosphatase Inhibition-Based Test Kit. Heliyon 2018, 4, e00573. [Google Scholar] [CrossRef]
- Omatola, C.A.; Okolo, M.L.O.; Adaji, D.M.; Mofolorunsho, C.K.; Oyiguh, J.A.; Zige, D.V.; Akpala, N.S.; Samson, S.O. Coinfection of Human Immunodeficiency Virus-Infected Patients with Hepatitis B Virus in Lokoja, North Central Nigeria. Viral Immunol. 2020, 33, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, E.; Pirotte, B.F.; Moerman, F.; Altdorfer, A.; Gaspard, L.; Firre, E.; Moonen, M.; Darcis, G. Mycobacterium Avium Complex and Cryptococcus Neoformans Co-Infection in a Patient with Acquired Immunodeficiency Syndrome: A Case Report. Acta Clin. Belgica Int. J. Clin. Lab. Med. 2022, 77, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Qamar, H.; Hussain, K.; Soni, A.; Khan, A.; Hussain, T.; Chénais, B. Cyanobacteria as Natural Therapeutics and Pharmaceutical Potential: Role in Antitumor Activity and as Nanovectors. Molecules 2021, 26, 247. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Hamann, M.T. Marine Pharmacology in 2005-6: Marine Compounds with Anthelmintic, Antibacterial, Anticoagulant, Antifungal, Anti-Inflammatory, Antimalarial, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Cardiovascular, Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar] [PubMed]
- Wase, N.V.; Wright, P.C. Systems Biology of Cyanobacterial Secondary Metabolite Production and Its Role in Drug Discovery. Expert Opin. Drug Discov. 2008, 3, 903–929. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Neilan, B.; Börner, T. Molecular Biology of Peptide and Polyketide Biosynthesis in Cyanobacteria. Appl. Microbiol. Biotechnol. 2001, 57, 467–473. [Google Scholar]
- Volk, R.B. Screening of Microalgae for Species Excreting Norharmane, a Manifold Biologically Active Indole Alkaloid. Microbiol. Res. 2008, 163, 307–313. [Google Scholar] [CrossRef]
- Sato, S.I.; Murata, A.; Orihara, T.; Shirakawa, T.; Suenaga, K.; Kigoshi, H.; Uesugi, M. Marine Natural Product Aurilide Activates the OPA1-Mediated Apoptosis by Binding to Prohibitin. Chem. Biol. 2011, 18, 131–139. [Google Scholar] [CrossRef]
- Han, B.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Aurilides B and C, Cancer Cell Toxins from a Papua New Guinea Collection of the Marine Cyanobacterium Lyngbya Majuscula. J. Nat. Prod. 2006, 69, 572–575. [Google Scholar] [CrossRef]
- Watanabe, A.; Ohno, O.; Morita, M.; Inuzuka, T.; Suenaga, K. Structures and Biological Activities of Novel Biselyngbyaside Analogs Isolated from the Marine Cyanobacterium Lyngbya sp. Bull. Chem. Soc. Jpn. 2015, 88, 1256–1264. [Google Scholar] [CrossRef]
- Teruya, T.; Sasaki, H.; Kitamura, K.; Nakayama, T.; Suenaga, K. Biselyngbyaside, a Macrolide Glycoside from the Marine Cyanobacterium Lyngbya sp. Org. Lett. 2009, 11, 2421–2424. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Choi, M.C.; Seo, C.H.; Park, Y. Therapeutic Properties and Biological Benefits of Marine-Derived Anticancer Peptides. Int. J. Mol. Sci. 2018, 19, 919. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.; Figueras, E.; Martins, A.; Esposito, S.; Auciello, G.; Monteagudo, E.; Di Marco, A.; Summa, V.; Cordella, P.; Perego, R.; et al. Synthesis and Biological Evaluation of RGD−Cryptophycin Conjugates for Targeted Drug Delivery. Pharmaceutics 2019, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.; Armant, M.; Brown, E.; Rubio, M.; Ishihara, H.; Ulrich, D.; Caspary, R.G.; Lindberg, F.P.; Armitage, R.; Maliszewski, C.; et al. The Vitronectin Receptor and Its Associated CD47 Molecule Mediates Proinflammatory Cytokine Synthesis in Human Monocytes by Interaction with Soluble CD23. J. Cell Biol. 1999, 144, 767–775. [Google Scholar] [CrossRef]
- Robles-Bañuelos, B.; Durán-Riveroll, L.M.; Rangel-López, E.; Pérez-López, H.I.; González-Maya, L. Marine Cyanobacteria as Sources of Lead Anticancer Compounds: A Review of Families of Metabolites with Cytotoxic, Antiproliferative, and Antineoplastic Effects. Molecules 2022, 27, 4814. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Cowley, E.S.; Sikorska, J.; Shaala, L.A.; Ishmael, J.E.; Youssef, D.T.A.; McPhail, K.L. Apratoxin H and Apratoxin A Sulfoxide from the Red Sea Cyanobacterium Moorea Producens. J. Nat. Prod. 2013, 76, 1781. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Tiwari, B.S. Cyanotherapeutics: An emerging field for future drug discovery. Appl. Phycol. 2020, 1, 44–57. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Menakha, M. Pharmaceutical Applications of Cyanobacteria—A Review. J. Acute Med. 2015, 5, 15–23. [Google Scholar] [CrossRef]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria Secondary Metabolites as Biotechnological Ingredients in Natural Anti-Aging Cosmetics: Potential to Overcome Hyperpigmentation, Loss of Skin Density and UV Radiation-Deleterious Effects. Mar. Drugs 2022, 20, 183. [Google Scholar] [CrossRef]
- Morone, J.; Lopes, G.; Oliveira, B.; Vasconcelos, V.; Martins, R. Cyanobacteria in Cosmetics: A Natural Alternative for Anti-Aging Ingredients. Pharmacol. Potential Cyanobacteria 2022, 257–286. [Google Scholar] [CrossRef]
- Samadhiya, K.; Sangtani, R.; Nogueira, R.; Bala, K. Insightful Advancement and Opportunities for Microbial Bioplastic Production. Front. Microbiol. 2022, 12, 3755. [Google Scholar] [CrossRef] [PubMed]
- Savakis, P.; Hellingwerf, K.J. Engineering Cyanobacteria for Direct Biofuel Production from CO2. Curr. Opin. Biotechnol. 2015, 33, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Klanchui, A.; Raethong, N.; Prommeenate, P.; Vongsangnak, W.; Meechai, A. Cyanobacterial Biofuels: Strategies and Developments on Network and Modeling. Adv. Biochem. Eng. Biotechnol. 2017, 160, 75–102. [Google Scholar] [PubMed]
- Kiran, B.; Kumar, R.; Deshmukh, D. Perspectives of Microalgal Biofuels as a Renewable Source of Energy. Energy Convers. Manag. 2014, 88, 1228–1244. [Google Scholar] [CrossRef]
- Durall, C.; Kukil, K.; Hawkes, J.A.; Albergati, A.; Lindblad, P.; Lindberg, P. Production of Succinate by Engineered Strains of Synechocystis PCC 6803 Overexpressing Phosphoenolpyruvate Carboxylase and a Glyoxylate Shunt. Microb. Cell Fact. 2021, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Successful Method Yielding High Rate of D-Lactate Using Cyanobacteria Could Revolutionize Bioplastic Production | Research at Kobe. Available online: https://www.kobe-u.ac.jp/research_at_kobe_en/NEWS/news/2020_03_10_02.html (accessed on 26 September 2022).
- Hidese, R.; Matsuda, M.; Osanai, T.; Hasunuma, T.; Kondo, A. Malic Enzyme Facilitates d -Lactate Production through Increased Pyruvate Supply during Anoxic Dark Fermentation in Synechocystis sp. PCC 6803. ACS Synth. Biol. 2020, 9, 260–268. [Google Scholar] [CrossRef]
- Coverage of Greenhouse Gas Emissions from Petroleum Use under Climate Policy—Center for Climate and Energy SolutionsCenter for Climate and Energy Solutions. Available online: https://www.c2es.org/document/coverage-of-greenhouse-gas-emissions-from-petroleum-use-under-climate-policy/ (accessed on 26 September 2022).
- What Are Greenhouse Gases | GHG Emissions & Canada’s Carbon Footprint. Available online: https://www.capp.ca/explore/greenhouse-gas-emissions/ (accessed on 26 September 2022).
- Doamekpor, L.K.; Onwona-Agyeman, R.; Ameka, G.K. Bioenergy: Biodiesel from Freshwater Green Microalgae and a Cyanobacterium Occurring in Ghana. West Afr. J. Appl. Ecol. 2020, 27, 51–60. [Google Scholar]
- Nozzi, N.E.; Oliver, J.W.K.; Atsumi, S. Cyanobacteria as a Platform for Biofuel Production. Front. Bioeng. Biotechnol. 2013, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Cyanobacteria: Recent Advances in Taxonomy and Applications–Google Books. Available online: https://books.google.pl/books?id=sbZaEAAAQBAJ&pg=PA30&lpg=PA30&dq=cyanobacteria+capable+of+growing+in+brackish+or+saltwater+to+produce+a+range+of+biofuels+such+as+ethanol,+biodiesel,+gasoline+and+jet+fuel+in+addition+to+other+valuable+chemicals&source=bl&ots=ZrzLnyti-S&sig=ACfU3U0LletVZ4G5VWJpux2zCP8AojcWOQ&hl=en&sa=X&ved=2ahUKEwirhNDE27L6AhXzhP0HHc-uA7kQ6AF6BAgCEAM#v=onepage&q=cyanobacteria capable of growing in brackish or saltwater to produce a range of biofuels such as ethanol%2C biodiesel%2C gasoline and jet fuel in addition to other valuable chemicals&f=false (accessed on 26 September 2022).
- Joule Unlimited | Solving the Energy Crisis with Affordable, Renewable Clean Fuel. Available online: https://www.jouleunlimited.com/ (accessed on 26 September 2022).
- The Algenol Advantage | Algenol Biotech. Available online: https://www.algenol.com/ (accessed on 26 September 2022).
- Tom, A.P.; Jayakumar, J.S.; Biju, M.; Somarajan, J. Aquaculture wastewater treatment technologies and their sustainability: A review. Energy Nexus 2021, 4, 100022. [Google Scholar] [CrossRef]
- Srimongkola, P.; Thongchul, N.; Phunpruchd, S.; Karnchanatat, A. Ability of marine cyanobacterium Synechococcus sp. VDW to remove ammonium from brackish aquaculture wastewater. Agric. Water Manag. 2019, 212, 155–161. [Google Scholar] [CrossRef]
- Callieri, C. Synechococcus plasticity under environmental changes. FEMS Microbiol. Lett. 2017, 364, fnx229. [Google Scholar] [CrossRef] [PubMed]
- Potnis, A.A.; Raghavan, P.S.; Rajaram, H. Overview on cyanobacterial exopolysaccharides and biofilms: Role in bioremediation. Rev. Environ. Sci. Biotechnol. 2021, 20, 781–794. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Dailianis, S.; Charalampous, N.; Stefanidou, N.; Moustaka-Gouni, M.; Tekerlekopoulou, A.G.; Vayenasa, D.V. Brewery wastewater treatment using cyanobacterial-bacterial settleable aggregates. Algal. Res. 2020, 49, 101957. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Tekerlekopoulou, A.G.; Vayenasa, D.V. Two-step treatment of brewery wastewater using electrocoagulation and cyanobacteria-based cultivation aggregates. J. Environ. Manag. 2020, 265, 110543. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Economou, C.N.; Tekerlekopoulou, A.G.; Vayenasa, D.V. A Cyanobacteria-Based Biofilm System for Advanced Brewery Wastewater Treatment. Appl. Sci. 2021, 11, 174. [Google Scholar] [CrossRef]
- Sen, S.; Bhardwaj, K.; Thakurta, S.G.; Chakrabarty, J.; Ghanta, K.C.; Dutta, S. Phycoremediation of cyanide from coke–oven wastewater using cyanobacterial consortium. Int. J. Environ. Sci. Technol. 2018, 15, 2151–2164. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García, J. Assessing the potential of soil cyanobacteria for simultaneous wastewater treatment and carbohydrate-enriched biomass production. Algal. Res. 2020, 51, 102042. [Google Scholar] [CrossRef]
- El-Bestawy, E.A.; Abd El-Salam, A.Z.; Mansy, A.E. Potential use of environmental cyanobacterial species in bioremediation of lindane-contaminated effluents. Int. Biodeterior. Biodegrad. 2007, 59, 180–192. [Google Scholar] [CrossRef]
- Baptista, M.; Vasconcelos, T. Cyanobacteria metal interactions: Requirements, toxicity, and ecological implications. Crit. Rev. Microbiol. 2006, 32, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xie, Y.; Sun, T.; Chen, L.; Zhang, W. Deciphering and engineering photosynthetic cyanobacteria for heavy metal bioremediation. Sci. Total Environ. 2021, 761, 144111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dwivedi, S.K. A review on accessible techniques for removal of hexavalent Chromium and divalent Nickel from industrial wastewater: Recent research and future outlook. J. Clean. Prod. 2021, 295, 126229. [Google Scholar] [CrossRef]
- Sena, S.; Dutta, S.; Guhathakurata, S.; Chakrabarty, J.; Nandi, S.; Dutta, A. Removal of Cr(VI) using a cyanobacterial consortium and assessment of biofuel production. Int. Biodeterior. Biodegrad. 2017, 119, 211–224. [Google Scholar] [CrossRef]
- Shukla, D.; Vankar, P.S.; Srivastava, S.K. Bioremediation of hexavalent chromium by a cyanobacterial mat. Appl. Water Sci. 2012, 2, 245–251. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Raghavana, P.S.; Potnis, A.A.; Bhattacharyya, K.; Salaskar, D.A.; Rajaram, H. Axenic cyanobacterial (Nostoc muscorum) biofilm as a platform for Cd(II) sequestration from aqueous solutions. Algal. Res. 2020, 46, 101778. [Google Scholar] [CrossRef]
- Ghorbani, E.; Nowruzi, B.; Nezhadali, M.; Hekmat, M. Metal removal capability of two cyanobacterial species in autotrophic and mixotrophic mode of nutrition. BMC Microbiol. 2022, 22, 58. [Google Scholar] [CrossRef]
- Bon, I.C.; Salvatierra, L.C.; Lario, L.D.; Morató, J.; Pérez, L.M. Prospects in Cadmium-ContaminatedWater Management Using Free-Living Cyanobacteria (Oscillatoria sp.). Water 2021, 13, 542. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Mohamed, A.K.S.H. Bioremediation of heavy metals from municipal sewage by cyanobacteria and its effects on growth and some metabolites of Beta vulgaris. J. Plant. Nutr. 2017, 40, 2550–2561. [Google Scholar] [CrossRef]
- Akoijam, C.; Langpoklakpam, J.S.; Chettri, B.; Singh, K.M. Cyanobacterial diversity in hydrocarbon-polluted sediments and their possible role in bioremediation. Int. Biodeterior. Biodegrad. 2015, 103, 97–104. [Google Scholar] [CrossRef]
- Forlani, G.; Prearo, V.; Wieczorek, D.; Kafarski, P.; Lipok, J. Phosphonate degradation by Spirulina strains: Cyanobacterial biofilters for the removal of anticorrosive polyphosphonates from wastewater. Enzyme Microb. Technol. 2011, 48, 299–305. [Google Scholar] [CrossRef]
- Thakurta, S.G.; Aakula, M.; Chakrabarty, J.; Dutta, S. Bioremediation of phenol from synthetic and real wastewater using Leptolyngbya sp.: A comparison and assessment of lipid production. 3 Biotech 2018, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, I.B.M. Biodegradability of hydrocarbons by cyanobacteria. J. Phycol. 2010, 46, 818–824. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Gharieb, M.M.; Abou-El-Souod, G.W. Biodegradation of dyes by some green algae and cyanobacteria. Int. Biodeterior. Biodegrad. 2009, 63, 699–704. [Google Scholar] [CrossRef]
- Dellamatrice, P.M.; Silva-Stenico, M.E.; Beraldo de Moraes, L.A.; Fiore, M.F.; Rosim Monteiro, R.T. Degradation of textile dyes by cyanobacteria. Braz. J. Microbiol. 2017, 48, 25–31. [Google Scholar] [CrossRef]
- Bonini, C.; Righi, G. Enantio-and stereo-selective route to the taxol side chain via asymmetric epoxidation of trans-cinnamyl alcohol and subsequent expoxide ring opening. J. Chem. Soc. Chem. Commun. 1994, 24, 2767–2768. [Google Scholar] [CrossRef]
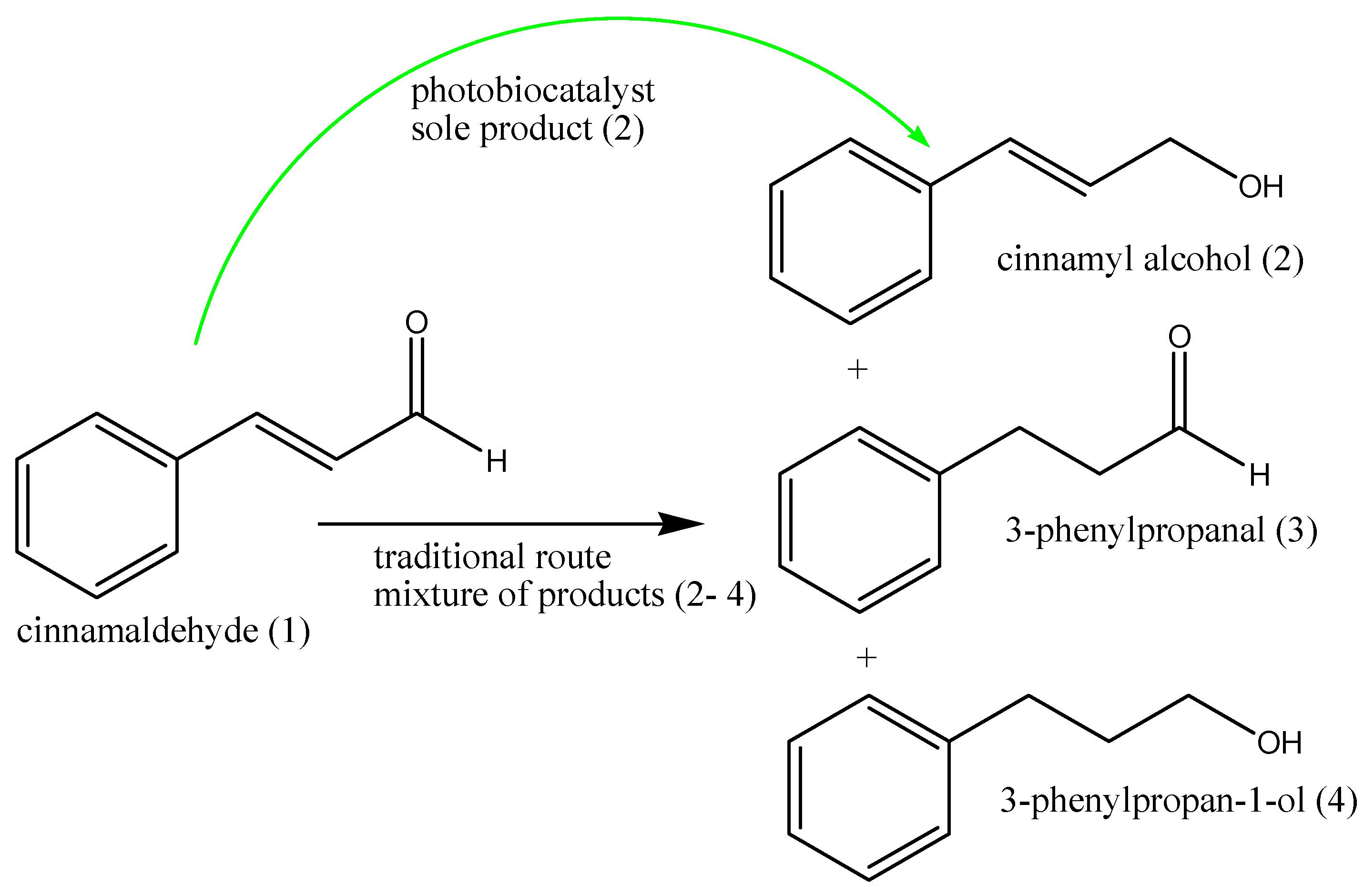
- Yamanaka, R.; Nakamura, K.; Murakami, M.; Murakami, A. Selective synthesis of cinnamyl alcohol by cyanobacterial photobiocatalysts. Tetrahedron Lett. 2015, 56, 1089–1091. [Google Scholar] [CrossRef]
- Molinari, F.; Gandolfi, R.; Villa, R.; Occhiato, E.G. Lyophilised yeasts: Easy-to-handle biocatalysts for stereoselective reduction of ketones. Tetrahedron Asymmetr. 1999, 10, 3515–3520. [Google Scholar] [CrossRef]
- Głąb, A.; Szmigiel-Merena, B.; Brzezińska-Rodak, M.; Żymańczyk-Duda, E. Biotransformation of 1- and 2-phenylethanol to products of high value via redox reactions. BioTechnologia 2016, 97, 203–210. [Google Scholar] [CrossRef]
- Chang, X.; Yang, Z.; Zeng, R.; Yang, G.; Yan, J. Production of chiral aromatic alcohol by asymmetric reduction with vegetable catalyst. Chin. J. Chem. Eng. 2010, 18, 1029–1033. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Luo, L.; Chang, X.; Zhou, W.; Chen, G.-H.; Zhao, Y.; Wang, Y.-J. Production of chiral alcohols from prochiral ketones by microalgal photo-biocatalytic asymmetric reduction reaction. J. Ind. Microbiol. Biotechnol. 2012, 39, 835–841. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Głąb, A.; Górak, M.; Klimek-Ochab, M.; Brzezińska-Rodak, M.; Strub, D.; Śliżewska, A. Reductive capabilities of different cyanobacterial strains towards acetophenone as a model substrate—Prospect of applications for chiral building blocks synthesis. Bioorg. Chem. 2019, 93, 102810. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.V.; Rielly-Gauvin, K.; Ryono, D.E.; Free, C.A.; Rogers, W.L.; Smith, S.A.; DeForrest, J.M.; Oehl, R.S.; Petrillo, E.W., Jr. Alpha-Hydroxy phosphinyl-based inhibitors of human renin. J. Med. Chem. 1995, 38, 4557–4569. [Google Scholar] [CrossRef]
- Stowasser, B.; Budt, K.-H.; Jian-Qi, L.; Peyman, A. New hybrid transition state analog inhibitors of HIV protease with peripheric C2-symmetry. Tetrahedron Lett. 1992, 33, 6625. [Google Scholar] [CrossRef]
- Yokomatsu, T.; Murano, T.; Akiyama, T.; Koizumi, J.; Shimeno, H.; Tsuji, Y.; Soeda, S.; Shimeno, H. Improved Synthesis of 1,3-Propanediol Derivatives Having a Diethoxyphosphoryldifluoroethyl Functional Group at the 2-Position: Application to Chemoenzymatic Synthesis of Novel Acyclic Nucleotide Analogues of Adenosine Bisphosphates. Bioorg. Med. Chem. Lett. 2003, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Żymańczyk-Duda, E.; Kafarski, P.; Lejczak, B. Reductive biotransformation of diethyl beta-, gamma- and delta-oxoalkylphosphonates by cells of baker’s yeast. Enzyme Microb. Technol. 2000, 26, 265. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Latajka, R.; Kafarski, P.; Lejczak, B. Chiral O-phosphorylated derivative of 2-hydroxy-phenylethylphosphonate as a valuable product of microbial biotransformation of diethyl 2-oxo-2-phenylethylphosphonate. J. Mol. Catal. B Enzym. 2008, 52, 74. [Google Scholar] [CrossRef]
- Górak, M.; Żymańczyk-Duda, E. Application of cyanobacteria for chiral phosphonate synthesis. Green Chem. 2015, 17, 4570. [Google Scholar] [CrossRef]
- Górak, M.; Żymańczyk-Duda, E. Reductive activity of free and immobilized cells of cyanobacteria towar oxophosphonates—Comparative study. J. Appl. Phycol. 2017, 29, 245–253. [Google Scholar] [CrossRef][Green Version]
- Harrison, W.; Huang, X.; Zhao, H. Photobiocatalysis for abiological transformations. Acc. Chem. Res. 2022, 55, 1087–1096. [Google Scholar] [CrossRef]
- Emmanuel, M.A.; Greenberg, N.R.; Oblinsky, D.G.; Hyster, T.K. Accessing non-natural reactivity by irradiating nicotinamide dependent enzymes with light. Nature 2016, 540, 414–417. [Google Scholar] [CrossRef]
- Biegasiewicz, K.F.; Cooper, S.J.; Gao, X.; Oblinsky, D.G.; Kim, J.H.; Garfinkle, S.E.; Joyce, L.A.; Sandoval, B.A.; Scholes, G.D.; Hyster, T.K. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 2019, 364, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Turek-Herman, J.R.; Joo Choi, Y.; Cohen, R.; Hyster, T. Photoenzymatic synthesis of α-tertiary amines by engineered flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 2021, 143, 19643–19647. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, B.A.; Clayman, P.D.; Oblinsky, D.G.; Oh, S.; Nakano, Y.; Bird, M.; Scholes, G.D.; Hyster, T.K. Photoenzymatic reductions enabled by direct excitation of flavin-dependent “ene”- reductases. J. Am. Chem. Soc. 2021, 143, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
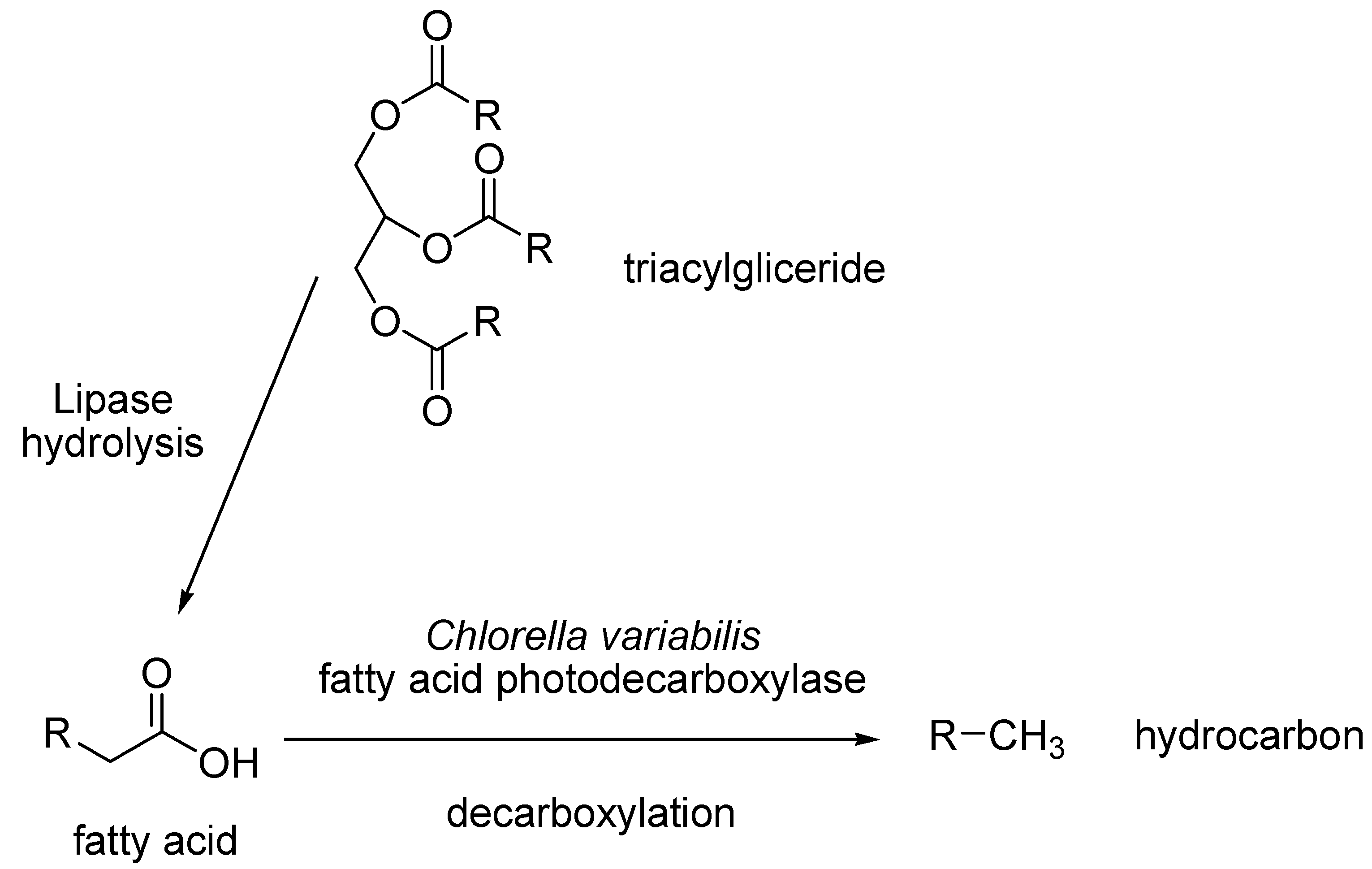
- Sorigue, D.; Legeret, B.; Cuine, S.; Blangy, S.; Moulin, S.; Billon, E.; Richaud, P.; Brugiere, S.; Coute, Y.; Nurizzo, D.; et al. An algal photoenzyme converts fatty acids to hydrocarbons. Science 2017, 357, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, M.M.E.; Zhang, W.; Tonin, F.; Hollman, F. Light-Driven Enzymatic Decarboxylation of Fatty Acids. Angew. Chem. Int. Ed. 2018, 57, 13648–13651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, M.; Huijbers, M.M.E.; Filonenko, G.A.; Pidko, E.A.; van Schie, M.; de Boer, S.; Burek, B.O.; Bloh, J.Z.; van Berkel, W.J.H.; et al. Hydrocarbon Synthesis via Photoenzymatic Decarboxylation of Carboxylic Acids. J. Am. Chem. Soc. 2019, 141, 3116–3120. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, X.; Zhang, W.; Li, P.; Hollmann, F.; Wang, Y. Photoenzymatic production of next generation biofuels from natural triglycerides combining a hydrolase and a photodecarboxylase. ChemPhotoChem 2020, 4, 39–44. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żymańczyk-Duda, E.; Samson, S.O.; Brzezińska-Rodak, M.; Klimek-Ochab, M. Versatile Applications of Cyanobacteria in Biotechnology. Microorganisms 2022, 10, 2318. https://doi.org/10.3390/microorganisms10122318
Żymańczyk-Duda E, Samson SO, Brzezińska-Rodak M, Klimek-Ochab M. Versatile Applications of Cyanobacteria in Biotechnology. Microorganisms. 2022; 10(12):2318. https://doi.org/10.3390/microorganisms10122318
Chicago/Turabian StyleŻymańczyk-Duda, Ewa, Sunday Ocholi Samson, Małgorzata Brzezińska-Rodak, and Magdalena Klimek-Ochab. 2022. "Versatile Applications of Cyanobacteria in Biotechnology" Microorganisms 10, no. 12: 2318. https://doi.org/10.3390/microorganisms10122318
APA StyleŻymańczyk-Duda, E., Samson, S. O., Brzezińska-Rodak, M., & Klimek-Ochab, M. (2022). Versatile Applications of Cyanobacteria in Biotechnology. Microorganisms, 10(12), 2318. https://doi.org/10.3390/microorganisms10122318








