Real Time Breath Analysis Using Portable Gas Chromatography for Adult Asthma Phenotypes
Abstract
:1. Introduction
2. Results
2.1. Clinical Cohort
2.2. Biomarkers to Distinguish Asthma and Non-Asthma
2.3. Biomarkers to Distinguish Atopic Subjects
2.4. Biomarkers for Other Asthma Sub-Categories
3. Discussion
4. Materials and Methods
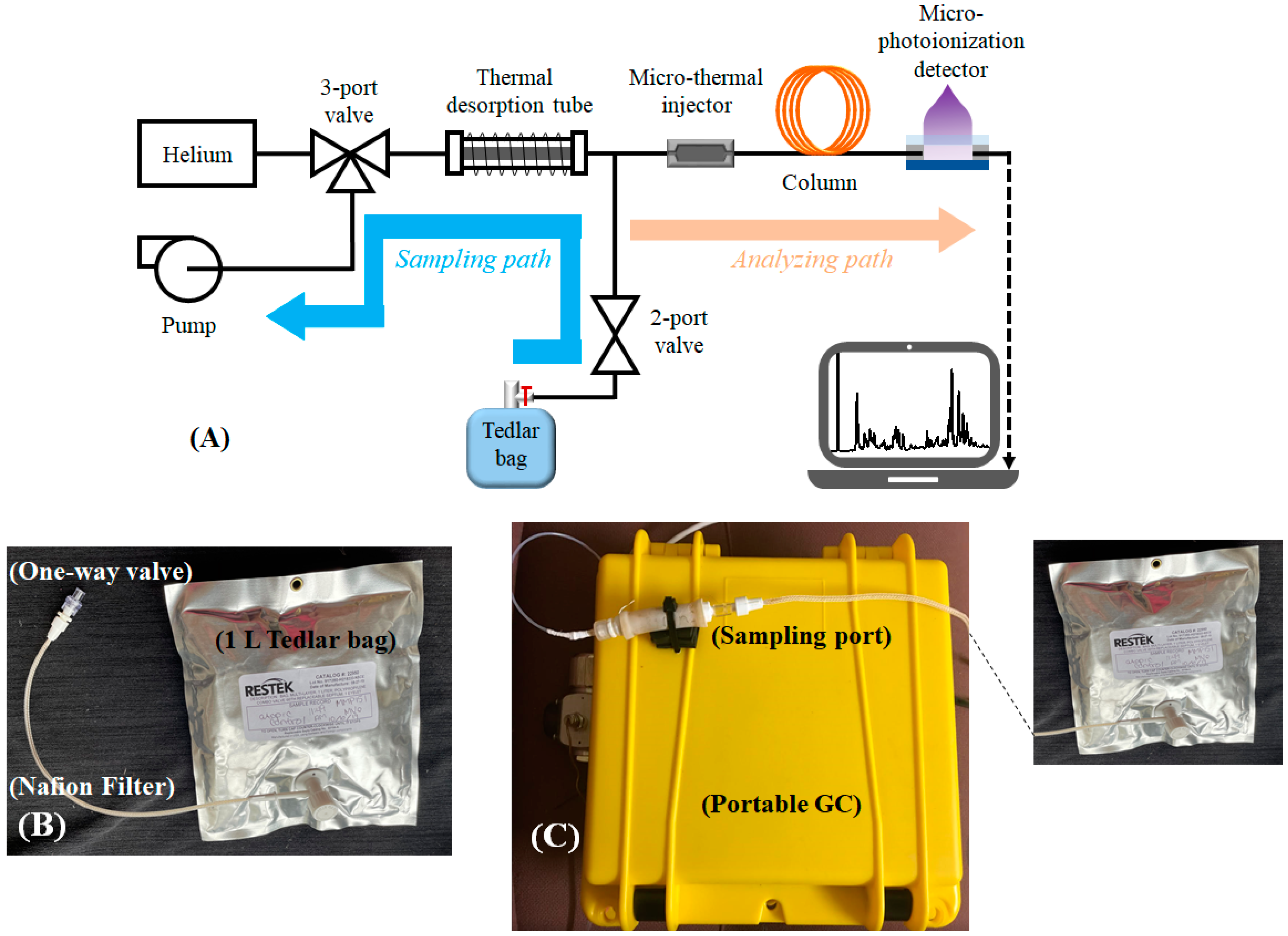
4.1. Description of the Portable GC Device
4.2. Exhaled Breath Collection and Analysis
4.3. Chromatogram Processing and Statistical Analysis
4.4. Identification of VOCs Using Mass Spectrometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, K.F. Asthma phenotyping: A necessity for improved therapeutic precision and new targeted therapies. J. Intern. Med. 2016, 279, 192–204. [Google Scholar] [CrossRef]
- Douwes, J.; Gibson, P.; Pekkanen, J.; Pearce, N. Non-eosinophilic asthma: Importance and possible mechanisms. Thorax 2002, 57, 643–648. [Google Scholar] [CrossRef] [Green Version]
- McGrath, K.W.; Icitovic, N.; Boushey, H.A.; Lazarus, S.C.; Sutherland, E.R.; Chinchilli, V.M.; Fahy, J.V. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am. J. Respir. Crit. Care Med. 2012, 185, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Santonico, M.; Mondino, C.; Pennazza, G.; Mantini, G.; Martinelli, E.; Capuano, R.; Ciabattoni, G.; Paolesse, R.; Di Natale, C.; et al. Diagnostic performance of an electronic nose, fractional exhaled nitric oxide, and lung function testing in asthma. Chest 2010, 137, 790–796. [Google Scholar] [CrossRef]
- Santini, G.; Mores, N.; Penas, A.; Capuano, R.; Mondino, C.; Trové, A.; Macagno, F.; Zini, G.; Cattani, P.; Martinelli, E.; et al. Electronic nose and exhaled breath NMR-based metabolomics applications in airways disease. Curr. Top. Med. Chem. 2016, 16, 1610–1630. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dweik, R.A.; Sorkness, R.L.; Wenzel, S.; Hammel, J.; Curran-Everett, D.; Comhair, S.A.A.; Bleecker, E.; Busse, W.; Calhoun, W.J.; Castro, M.; et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aziz, M.I.; Brinkman, P.; Vijverberg, S.J.; Neerincx, A.H.; de Vries, R.; Dagelet, Y.W.; Riley, J.H.; Hashimoto, S.; Montuschi, P.; Chung, K.F.; et al. eNose breath prints as a surrogate biomarker for classifying patients with asthma by atopy. J. Allergy Clin. Immunol. 2020, 146, 1045–1055. [Google Scholar] [CrossRef]
- Brinkman, P.; Wagener, A.H.; Hekking, P.P.; Bansal, A.T.; Maitland-van der Zee, A.H.; Wang, Y.; Weda, H.; Knobel, H.H.; Vink, T.J.; Rattray, N.J.; et al. Identification and prospective stability of electronic nose (eNose)-derived inflammatory phenotypes in patients with severe asthma. J. Allergy Clin. Immunol. 2019, 143, 1811–1820.e7. [Google Scholar] [CrossRef] [Green Version]
- Brinkman, P.; Van De Pol, M.A.; Gerritsen, M.G.; Bos, L.D.; Dekker, T.; Smids, B.S.; Sinha, A.; Majoor, C.J.; Sneeboer, M.M.; Knobel, H.H.; et al. Exhaled breath profiles in the monitoring of loss of control and clinical recovery in asthma. Clin. Exp. Allergy 2017, 47, 1159–1169. [Google Scholar] [CrossRef]
- Schleich, F.N.; Zanella, D.; Stefanuto, P.-H.; Bessonov, K.; Smolinska, A.; Dallinga, J.W.; Henket, M.; Paulus, V.; Guissard, F.; Graff, S.; et al. Exhaled volatile organic compounds are able to discriminate between neutrophilic and eosinophilic asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 444–453. [Google Scholar] [CrossRef]
- Begley, L.; Madapoosi, S.; Opron, K.; Ndum, O.; Baptist, A.; Rysso, K.; Erb-Downward, J.R.; Huang, Y.J. Gut microbiota relationships to lung function and adult asthma phenotype: A pilot study. BMJ Open Respir. Res. 2018, 5, e000324. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update. An official american thoracic society and european respiratory society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.L.; Wanger, J.; Cockcroft, D.W.; Culver, B.H.; Carlsen, K.-H.; Diamant, Z.; Gauvreau, G.; Hall, G.L.; Hallstrand, T.S.; Horvath, I.; et al. ERS technical standard on bronchial challenge testing: General considerations and performance of methacholine challenge tests. Eur. Respir. J. 2017, 49, 1601526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchet, L.; Smolinska, A.; Baranska, A.; Tigchelaar, E.; Swertz, M.; Zhernakova, A.; Dallinga, J.W.; Wijmenga, C.; Van Schooten, F.J. Factors that influence the volatile organic compound content in human breath. J. Breath Res. 2017, 11, 016013. [Google Scholar] [CrossRef]
- Ibrahim, B.; Basanta, M.; Cadden, P.; Singh, D.; Douce, D.; Woodcock, A.; Fowler, S.J. Non-invasive phenotyping using exhaled volatile organic compounds in asthma. Thorax 2011, 66, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Dragonieri, S.; Schot, R.; Mertens, B.J.; Le Cessie, S.; Gauw, S.A.; Spanevello, A.; Resta, O.; Willard, N.P.; Vink, T.J.; Rabe, K.F.; et al. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clin. Immunol. 2007, 120, 856–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaleiro Rufo, J.; Madureira, J.; Oliveira Fernandes, E.; Moreira, A. Volatile organic compounds in asthma diagnosis: A systematic review and meta-analysis. Allergy 2016, 71, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [Green Version]
- Lärstad, M.A.E.; Torén, K.; Bake, B.; Olin, A.-C. Determination of ethane, pentane and isoprene in exhaled air ? effects of breath-holding, flow rate and purified air. Acta Physiol. 2007, 189, 87–98. [Google Scholar] [CrossRef]
- Azim, A.; Barber, C.; Dennison, P.; Riley, J.; Howarth, P. Exhaled volatile organic compounds in adult asthma: A systematic review. Eur. Respir. J. 2019, 54, 1900056. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Klaassen, E.M.M.; Dallinga, J.W.; Van De Kant, K.D.G.; Jobsis, Q.; Moonen, E.J.C.; Van Schayck, O.C.P.; Dompeling, E.; Van Schooten, F.J. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PLoS ONE 2014, 9, e95668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldeira, M.; Barros, A.S.; Bilelo, M.J.; Parada, A.; Câmara, J.D.S.; Rocha, S.M. Profiling allergic asthma volatile metabolic patterns using a headspace-solid phase microextraction/gas chromatography based methodology. J. Chromatogr. A 2011, 1218, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- García-Marcos, L.; Edwards, J.; Kennington, E.; Aurora, P.; Baraldi, E.; Carraro, S.; Gappa, M.; Louis, R.; Moreno-Galdó, A.; Peroni, D.G.; et al. Priorities for future research into asthma diagnostic tools: A PAN-EU consensus exercise from the European asthma research innovation partnership (EARIP). Clin. Exp. Allergy 2017, 48, 104–120. [Google Scholar] [CrossRef]
- Dallinga, J.W.; Robroeks, C.M.H.H.T.; Van Berkel, J.J.B.N.; Moonen, E.J.C.; Godschalk, R.W.L.; Jöbsis, Q.; Dompeling, E.; Wouters, E.F.M.; Van Schooten, F.J. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin. Exp. Allergy 2009, 40, 68–76. [Google Scholar] [PubMed]
- Lugogo, N.; Green, C.L.; Agada, N.; Zhang, S.; Meghdadpour, S.; Zhou, R.; Yang, S.; Anstrom, K.J.; Israel, E.; Martin, R. Obesity’s effect on asthma extends to diagnostic criteria. J. Allergy Clin. Immunol. 2018, 141, 1096–1104. [Google Scholar] [CrossRef] [Green Version]
- Alkhouri, N.; Eng, K.; Cikach, F.; Patel, N.; Yan, C.; Brindle, A.; Rome, E.; Hanouneh, I.; Grove, D.; Lopez, R.; et al. Breathprints of childhood obesity: Changes in volatile organic compounds in obese children compared with lean controls. Pediatr. Obes. 2015, 10, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Van Gossum, A.; Decuyper, J. Breath alkanes as an index of lipid peroxidation. Eur. Respir J. 1989, 2, 787–791. [Google Scholar]
- Wood, L.; Gibson, P.; Garg, M. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur. Respir. J. 2003, 21, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Maniscalco, M.; Paris, D.; Melck, D.J.; D’Amato, M.; Zedda, A.; Sofia, M.; Stellato, C.; Motta, A. Coexistence of obesity and asthma determines a distinct respiratory metabolic phenotype. J. Allergy Clin. Immunol. 2017, 139, 1536–1547.e5. [Google Scholar] [CrossRef] [Green Version]
- Boulet, L.-P. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010, 65, 374–376. [Google Scholar] [CrossRef] [Green Version]
- Salerno-Kennedy, R.; Cashman, K.D. Potential applications of breath isoprene as a biomarker in modern medicine: A concise overview. Wien. Klin. Wochenschr. 2005, 117, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Nidetz, R.; Zhou, M.; Lee, J.; Buggaveeti, S.; Kurabayashi, K.; Fan, X. Flow-through microfluidic photoionization detectors for rapid and highly sensitive vapor detection. Lab Chip 2015, 15, 3021–3029. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Paris, D.; Melck, D.; De Laurentiis, G.; Maniscalco, M.; Sofia, M.; Montuschi, P. Nuclear magnetic resonance-based metabolomics of exhaled breath condensate: Methodological aspects. Eur. Respir. J. 2012, 39, 498–500. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Sharma, R.; Zhu, H.; Li, Z.; Li, J.; Wang, S.; Bisco, E.; Massey, J.; Pennington, A.; Sjoding, M.; et al. Rapid breath analysis for acute respiratory distress syndrome diagnostics using a portable two-dimensional gas chromatography device. Anal. Bioanal. Chem. 2019, 411, 6435–6447. [Google Scholar] [CrossRef]
- Sharma, R.; Zhou, M.; Hunter, M.D.; Fan, X. Rapid in situ analysis of plant emission for disease diagnosis using a portable gas chromatography device. J. Agric. Food Chem. 2019, 67, 7530–7537. [Google Scholar] [CrossRef] [PubMed]

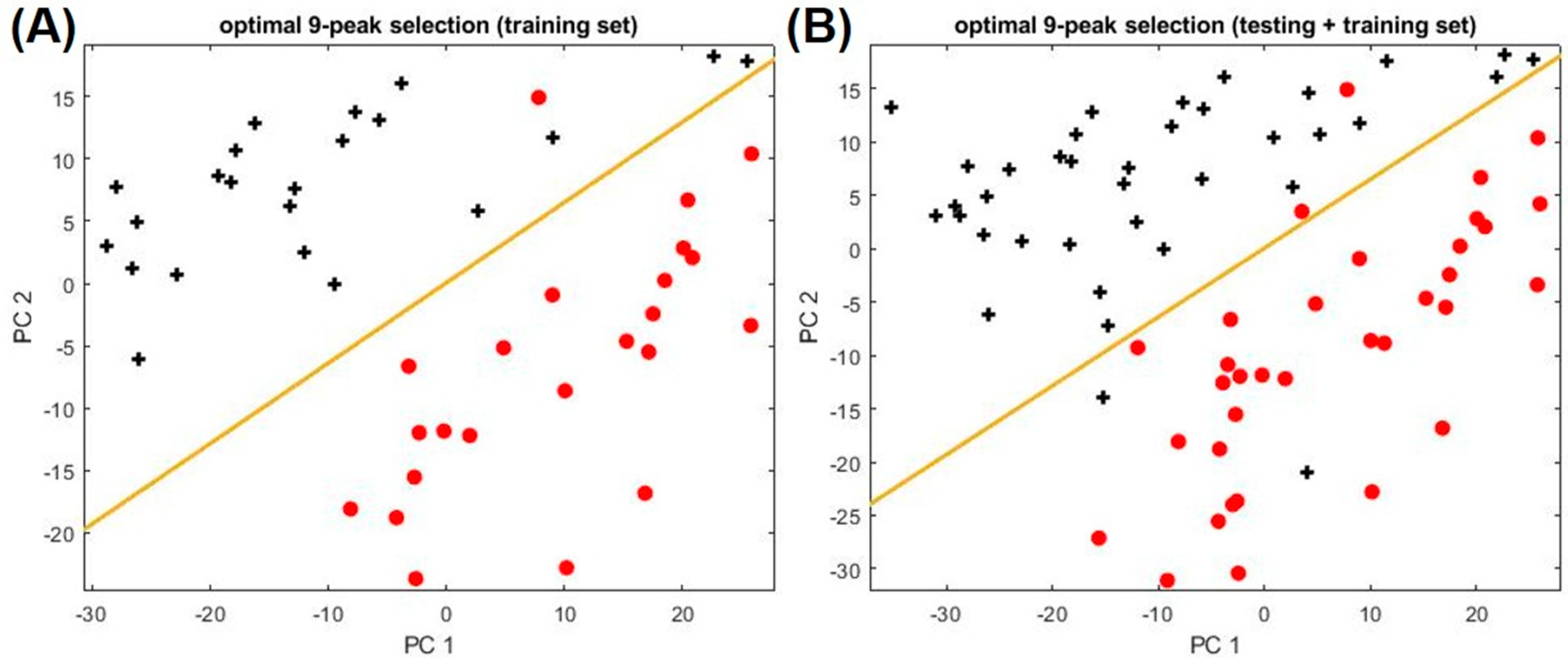
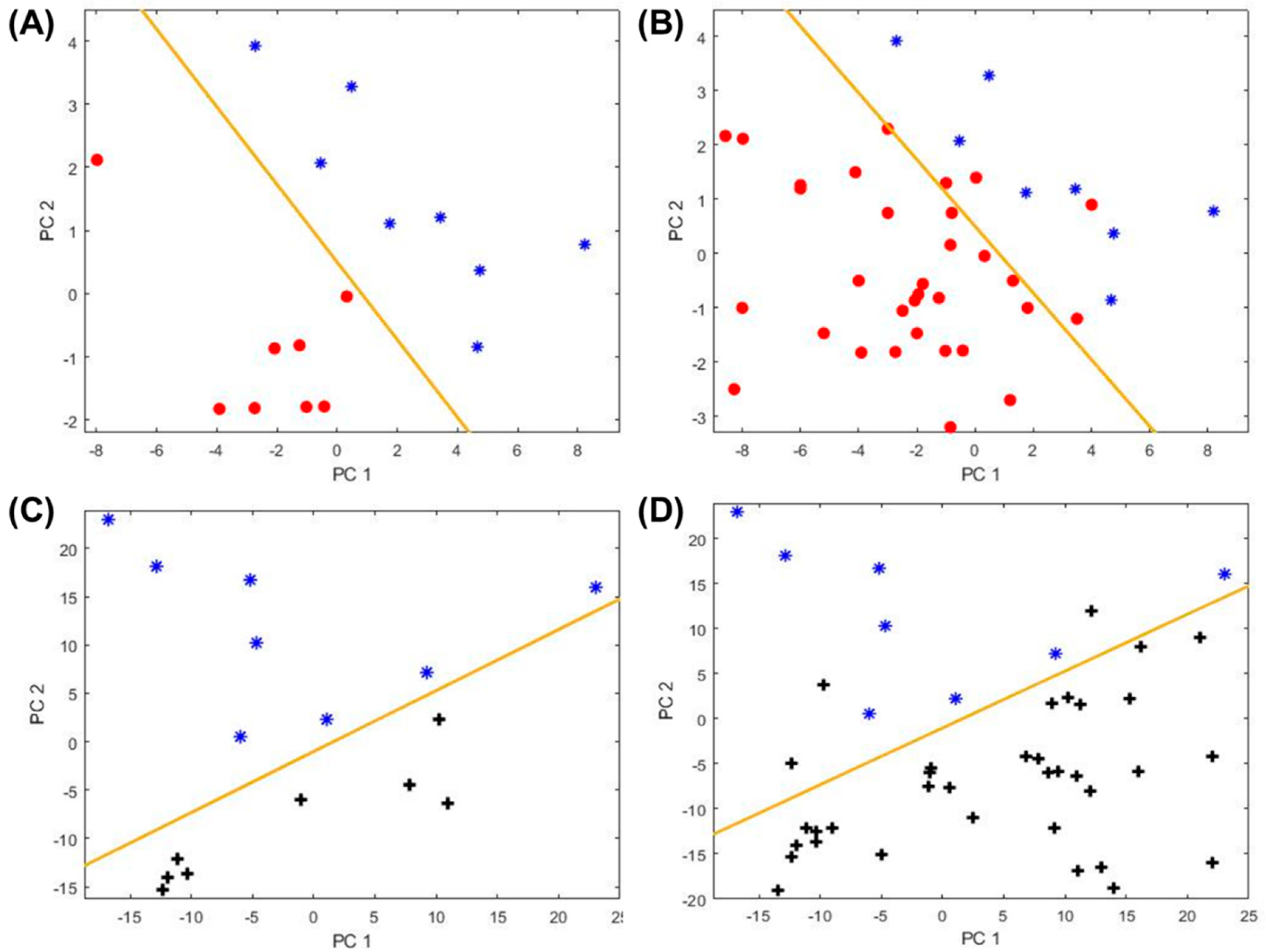
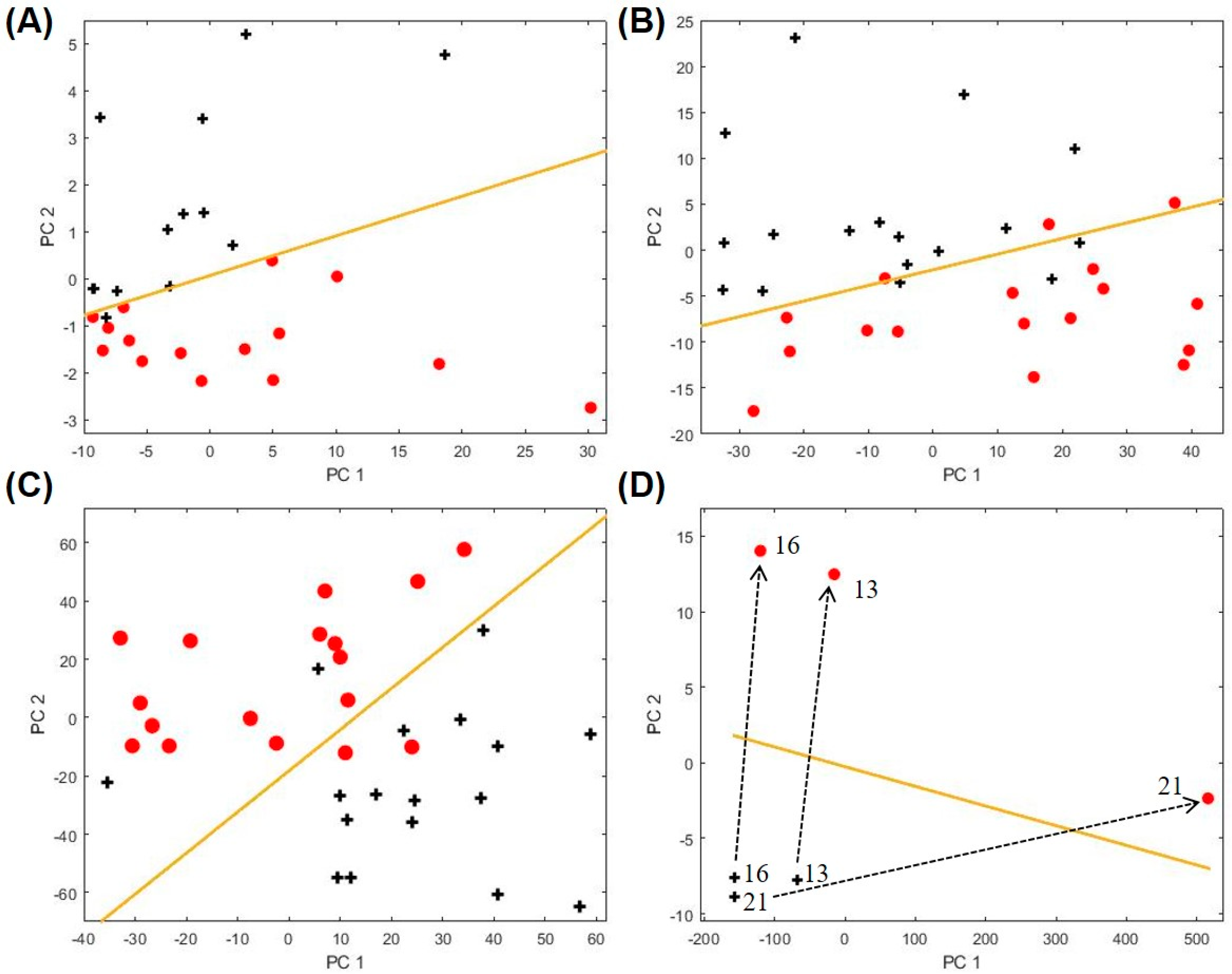
| Category | Non-Asthma, Non-Atopic | Non-Asthma, Atopic | Asthma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | FEV1/FVC # | FEV1(% pred) # | ICS Treatment | No ICS Treatment | Obese (BMI ≥ 30) | Non-Obese (BMI < 30) | Blood Eosinophils ≥ 0.3 × 109/L | Blood Eosinophils < 0.3 × 109/L | Upper Respiratory Illness | No Upper Respiratory Illness | |||
| Number of subjects | 35 | 8 | 30 | 0.78 ± 0.12 | 97.7 ± 18.7 | 20 | 13 | 17 | 17 | 17 | 17 | 3 | Same 3 subjects as those with upper respiratory illness |
| Number of breath samples analyzed | 37 | 8 | 34 | 21 | 13 | 17 | 17 | 17 | 17 | 3 | 3 | ||
| Age, yrs (range) | 40.9 (23–71) | 29.4 (19–43) | 40.2 (18–72) | ||||||||||
| Sex, % female | 75 | 50 | 69 | ||||||||||
| Study Group | Biomarker Peak ID | |
|---|---|---|
| Asthma vs. non-asthma/atopic | 6, 67 | |
| Non-asthma/non-atopic vs. non-asthma/atopic | 7, 32, 50, 54 | |
| Asthma clinical variables | Inhaled corticosteroid treatment | 59, 62, 86, 91 |
| Obesity | 8, 28, 37, 54, 58 | |
| Blood eosinophils level | 34, 51, 60, 91 | |
| Upper respiratory illness | 1, 2, 4, 5, 8 | |
| Peak ID | Compound Name | Peak ID | Compound Name |
|---|---|---|---|
| 1 | 2-Methylbutane, | 54 | 2,3,6-Trimethylheptane |
| 2 | Isoprene | 58 | 2,6-Dimethyl (S,E)-4-octene, |
| 4 | 1-Cyclopropaneethanol | 59 | 1-Butyl-1-methyl-2-propyl- cyclopropane |
| 5 | 4-Methyl-1-pentene | 60 | 1-Fluorododecane |
| 6 | 2-Methylpentane | 62 | Chloroacetic acid dodecyl ester |
| 7 | 2-methyl-1-pentene | 67 | 2,5,9-Trimethyldecane |
| 8 | n-Hexane | 69 | 2,3,5-Trimethylheptane |
| 28 | 2,3,4-Trimethylpentane | 73 | 2,4,6-Trimethyldecane |
| 32 | 2,4-Dimethylheptane | 80 | 2,6,10,14-Tetramethylheptadecane |
| 34 | 2-Octene | 85 | 2,8-Dimethylundecane |
| 37 | 2-Methyloctane | 86 | 3,6-Dimethylundecane |
| 50 | 2,2,4-Trimethylheptane | 91 | 2,6,6-Trimethyldecane |
| 51 | 3,3-Dimethyloctane | 93 | 5-Methyl-5-propylnonane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, R.; Zang, W.; Zhou, M.; Schafer, N.; Begley, L.A.; Huang, Y.J.; Fan, X. Real Time Breath Analysis Using Portable Gas Chromatography for Adult Asthma Phenotypes. Metabolites 2021, 11, 265. https://doi.org/10.3390/metabo11050265
Sharma R, Zang W, Zhou M, Schafer N, Begley LA, Huang YJ, Fan X. Real Time Breath Analysis Using Portable Gas Chromatography for Adult Asthma Phenotypes. Metabolites. 2021; 11(5):265. https://doi.org/10.3390/metabo11050265
Chicago/Turabian StyleSharma, Ruchi, Wenzhe Zang, Menglian Zhou, Nicole Schafer, Lesa A. Begley, Yvonne J. Huang, and Xudong Fan. 2021. "Real Time Breath Analysis Using Portable Gas Chromatography for Adult Asthma Phenotypes" Metabolites 11, no. 5: 265. https://doi.org/10.3390/metabo11050265





