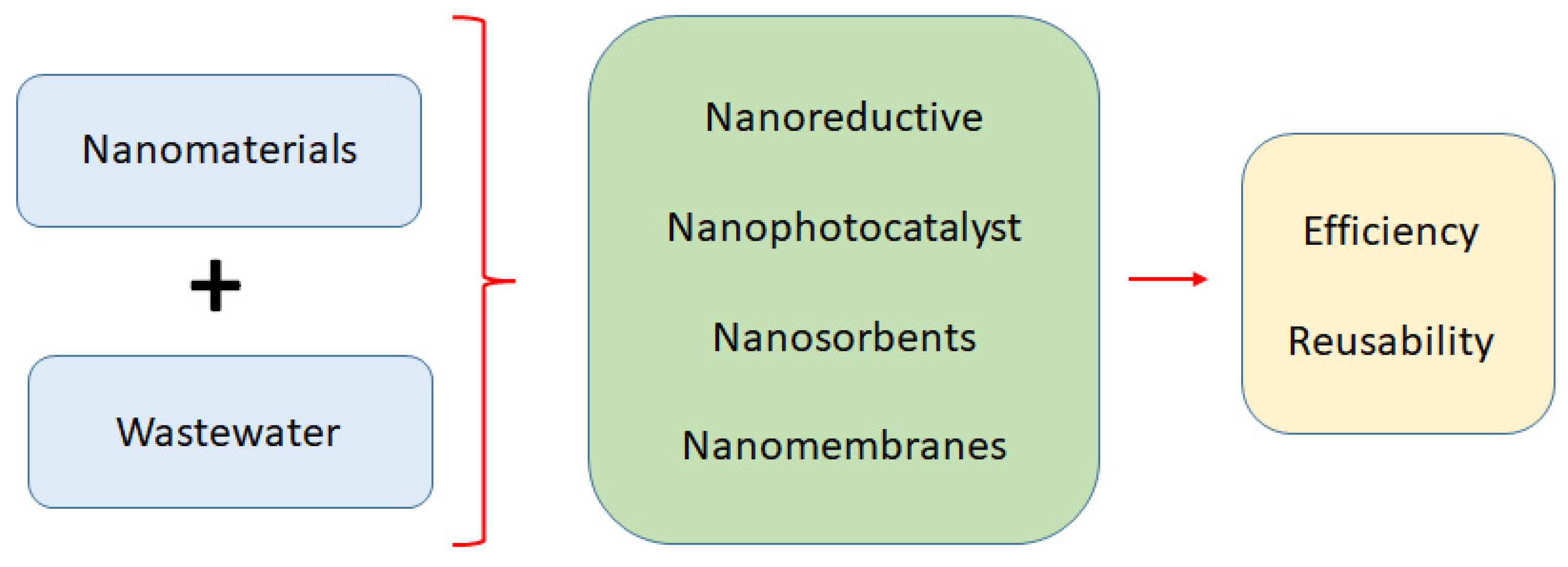
Nanomaterials as a Sustainable Choice for Treating Wastewater: A Review
Abstract
1. Introduction
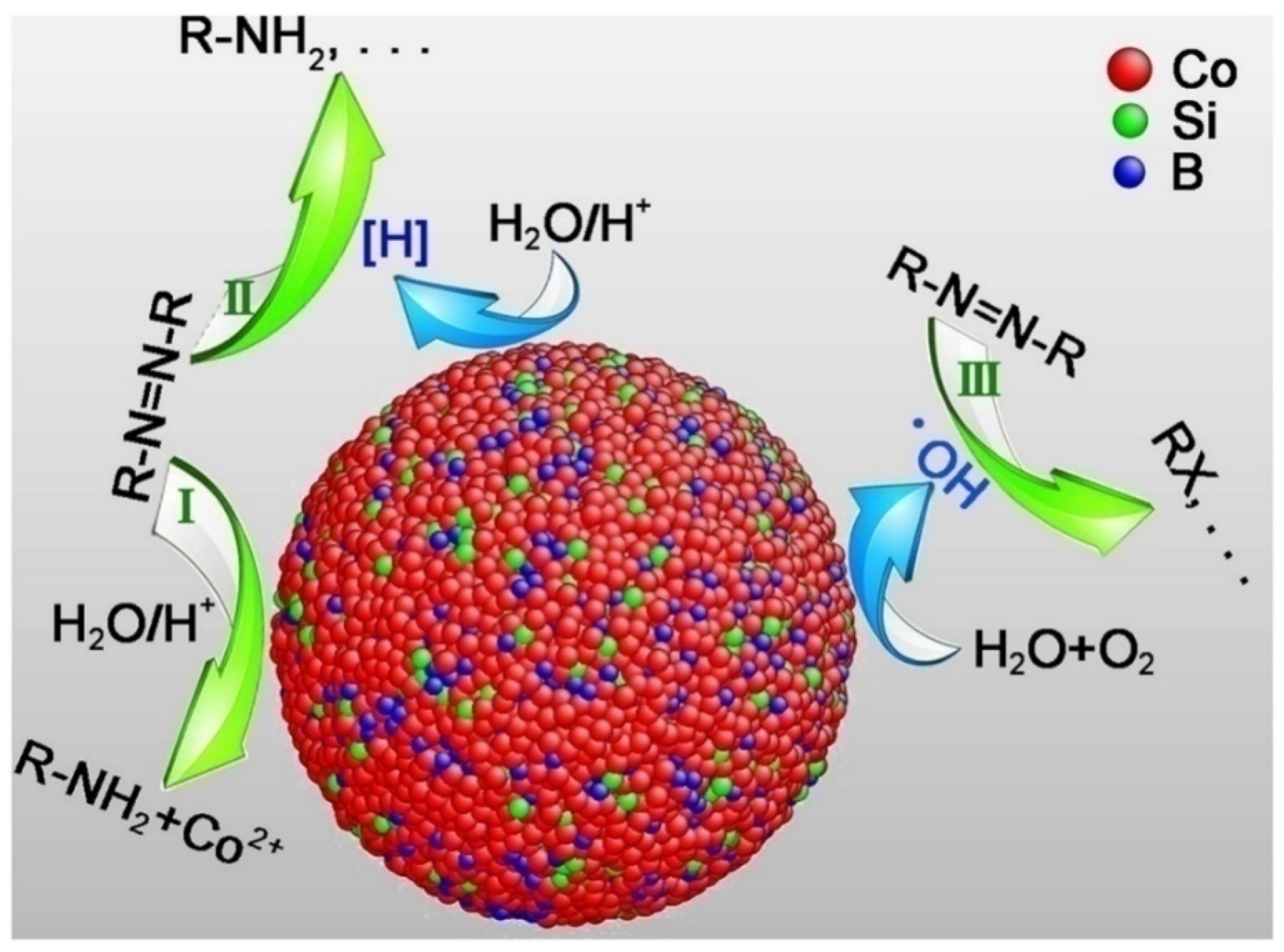
2. Nanoreductive Processes
3. Nanophotocatalysis
3.1. Nanocatalysis
3.1.1. Nanomaterials as Photocatalysts
- Catalyst + hν → Catalyst* + e− + h+
- H2O + h+ → OH* + H+
- O2 + e− → O2−*
- e− + 2H+ + H2O* → H2O2
- H2O2 + e− → OH(e−) + OH*
- Organic pollutants + O2−* + OH* → CO2 + H2O + other small products
3.1.2. Nanomaterials as Electrocatalysts
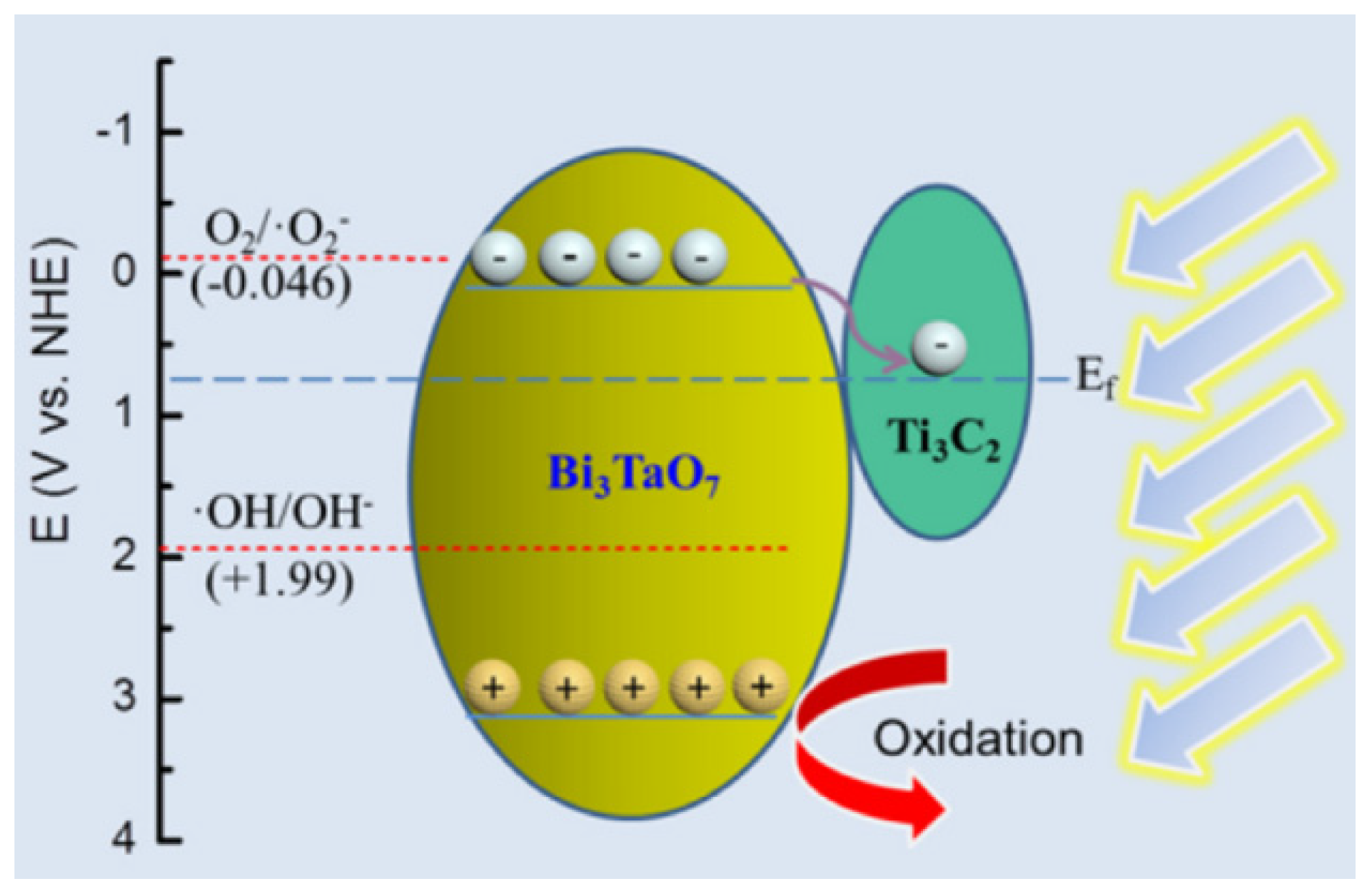
3.1.3. Heterojunction Photocatalytic Material
3.1.4. Nanomaterial-Based Fenton Catalysis
| Alloy | Route of Synthesis | Organic Pollutants | Source of Light | Pollutant Concentration | Catalyst Dose | pH | Time | Removal | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CuO nanosheets | Room temperature | Alura red Ac | UV | 5 mg/L | 5 mg | Neutral | 6 min | 96.65% | [113] |
| MFe2WO6 (M = Co,Ni, Cu,Zn) | Coprecipitation–oxidation method | Methyl red methyl orange Methylene blue bromo green | UV | 10 mg/L | 100 mg | Neutral | 50 min 50 min 50 min 50 min | 78% 92% 89% 93% | [114] |
| Cds/CuS | Hydrothermal method | Methyl orange | UV | 10 mg/L | 30 mg | Neutral | 150 min | 93% | [115] |
| α-Bi4V2O11 | Combustion method | Rhodamine B | UV | 5.106 mol/L | 0.5 g/L | Neutral | 6 min | 100% | [116] |
| TiO2 graphene | Chemical Synthesis | Reactive black 5 | UV | 42 ppm | 3 g | Neutral | 40 min | 96% | [117] |
| Al2O3-NP/SnO2 | Sol-gel technique | Methyl orange | UV | 20 mg/L | Electrode area 4.5 cm2 | 7 | 50 min | 93.95% | [118] |
| CuO-Go/TiO2 | Hydrother-mal method | 2-chlorophenol | UV | 50 mg/L | 0.05 g/L | 5 | 210 min | 86% | [119] |
| CuO nanorods | Chemical synthesis | Reactive black 5 | UV | 20 µM | 20 mg | Neutral | 300 min | 98% | [120] |
| Cu/Cu(OH)2 | Coprecipitation | Rhodamine B | UV | 100 ppm | 20 mg/L | Neutral | 120 min | 99.99% | [121] |
| Copper nanoparticles | Hydrothermal method | Phenyl red | UV | 10 mg/L | 30 mg | Neutral | 15 min | 99.62% | [122] |
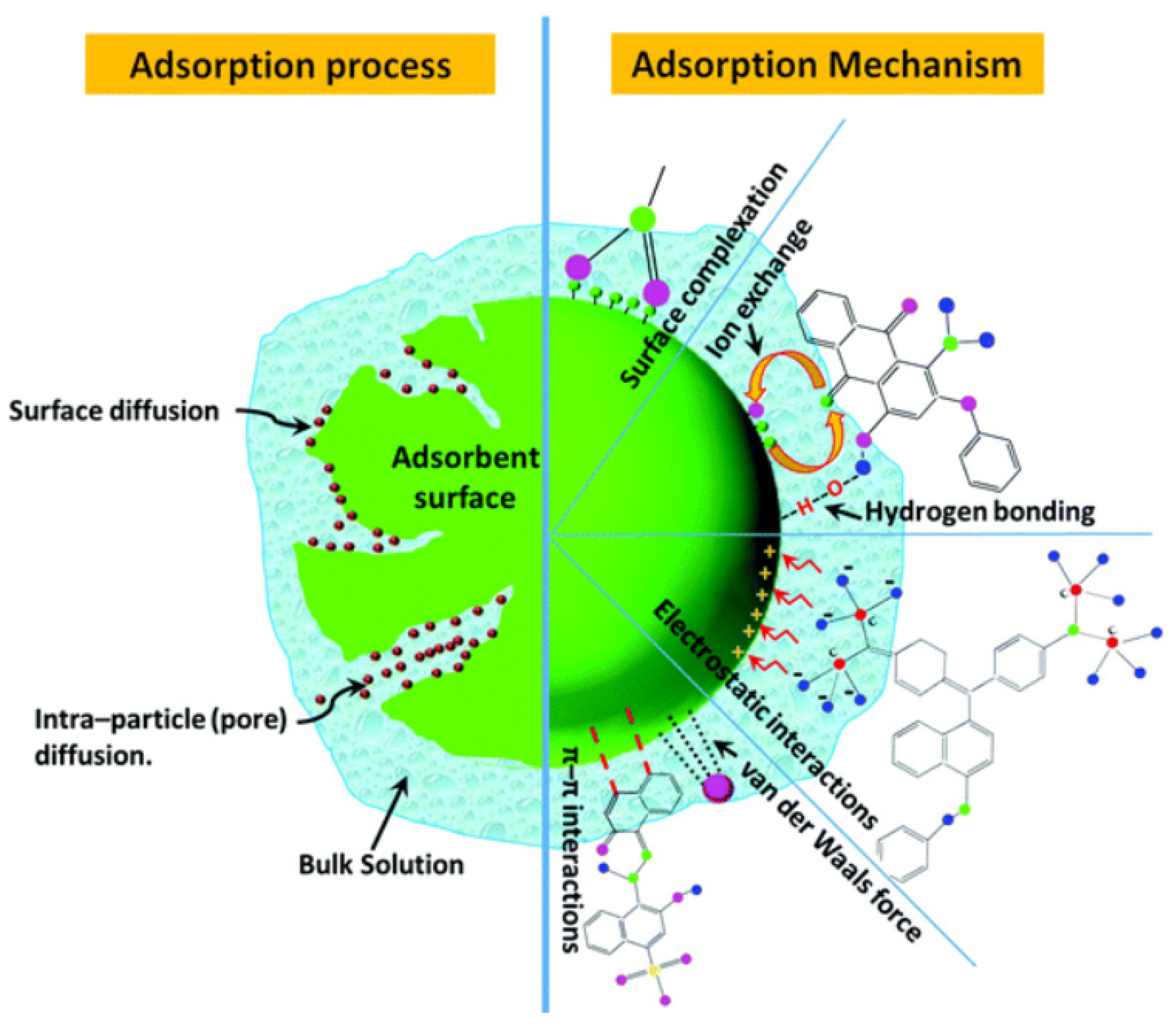
4. Nanoadsorbents
4.1. Metal Oxide-Based Nanoadsorbents
| Alloy Name | Organic Pollutants | Pollutant Concentration | Nanoadsorbent Dosage | pH | Time | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| TiO2/MgO | Methyl orange alizarin red S | 5 ppm | 0.5 g | Neutral | 90 min | 83.2% 43.8% | [131] |
| CeO2 | Methylene blue | 30 mg/L | 200 ppm | Neutral | 60 min | 98% | [132] |
| ZnO/CuO | Congo red | 50 ppm | 50 mg/L | 5.6 | 30 min | 93% | [133] |
| CuO/γ-Al2O3 | Brilliant red X-3B | 0.30 g/L | 5.50 g/L | 8 | 2.50 h | 90.72% | [134] |
| Iron oxide nanoparticles | Metanil yellow Orange II | 20 ppm | 8 mg/L | Neutral | 7 h | 95% 67% | [135] |
| MnO2/SnO2 | Calcon dye | 15 mg/L | Film | 3 | 24 h | 93.5% | [136] |
| α-MnO2/TiO2 | Coomassie brilliant blue R-250 | 13 mg/L | 1 g/L | 3 | 30 min | 98.35% | [137] |
| Bi2WO6/ MnO2 | Methylene blue | 10 mg/L | 100 mg/L | 7 | 100 min | 100% | [138] |
4.2. Carbon-Based Nanoadsorbents
| Alloy Name | Organic Pollutants | Pollutant Concentration | Nano-Sorbent Dosage | pH | Time | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Multiwall carbon nanotubes (MWCNTs) | Methyl blue | 25 mg/L | 10 mg/L | 6 | 10 min | 99% | [146] |
| Carbon nanotubes grown on carbon fiber | Methylene blue | 5 mg/L | Electrode | 1.83 | 180 min | 100% | [147] |
| ZnO/NiO with multiwall carbon nanotubes | Methyl orange | 50 ppm | 3% NiO:92% ZnO:5% CNTS | 7 | 360 min | 71% | [148] |
| Multiwall carbon nanotubes | Reactive Black 5 | 15 mg/L | 3 g/L | 7 | 60 min | 100% | [149] |
| Single-wall carbon nanotube Ru nanoparticle | Congo red | 0.05 mM | 0.3 mg | 5 | 4 min | 97.5% | [150] |
| Single-wall carbon nanotubes | Reactive yellow dye 15 | 50 mg/L | 0.2 g/L | 3 | 5 min | 179.9mg/g | [151] |
| Carbon nanotubes/alumina | Congo red | 10 mg/L | 15 mg | 2 | 90 min | 96.4% | [152] |
| Alloy Name | Route of Synthesis | Organic Pollutants | Pollutant Concentration | Nanoadsorbent Dosage | pH | Time | Adsorption Capacity | Ref. |
|---|---|---|---|---|---|---|---|---|
| Graphene oxide | Modified Hummers method | Acid orange 8 (AO8) and direct red 23 (DR23) | 50 mg/L | 40 mg | 7 | 60 min | AO8 = 25 mg/g and DR23 = 14 mg/g | [162] |
| Graphene oxide and magnetic chitosan | Modified Hummers method | Methyl blue (MB) | 200 mg/L | 0.015 g | 5.3 | 60 min | 95.16 mg/L | [163] |
| Graphene oxide-supported manganese oxide | Modified Hummers method | Reactive black 5 | 60 mg/L | 0.01 g | 3 | 24 h | 87 mg /L | [164] |
4.3. Silica-Based Nanoadsorbents
| Alloy Name | Organic Pollutants | Pollutant Concentration | Nanoadsorbent Dosage | pH | Time | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Nanosilica particles | Methyl orange | 10 mg/L | 10g/3L | 2.5 | 30 min | 100% | [167] |
| Silica nanoparticles | Methyl red dye | 0.05 mM | 10g/L | 7 | 120 min | 95% | [168] |
| Mesoporous silica nanomaterial | Rhodamine B | 20 mg/L | 1g/L | 5.8 | 120 min | 98.92% | [169] |
| Mesoporous silica modified with L-arginine | Crystal violet | 100 ppm | 10 mg | 11 | 30 min | 100% | [170] |
| Porous silicon supporte porous ruthenium nanoparticle system | Condo red | 1 mM | 40 µl | 5 | 60 min | 96% | [171] |
| Titania-coated silica nanocomposite | Safranin-O dye | 17.61 mg/L | 89.80 mg/g | 6.2 | 60 min | 93.29% | [172] |
5. Nanomembranes
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Bacterial Decolorization and Degradation of Azo Dyes: A Review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Marañón, E.; Castrillón, L.; Fernández-Nava, Y.; Fernández-Méndez, A.; Fernández-Sánchez, A. Colour, Turbidity and COD Removal from Old Landfill Leachate by Coagulation-Flocculation Treatment. Waste Manag. Res. 2010, 28, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Van Pham, T.; Phuoc, V.M.; Van Nguyen, D.; Koyama, J. Treatment Efficiency of a Combination of Alternative Technologies in Removing Pollutants from Pesticide Containing Wastewater. Environ. Eng. Res. 2021, 26, 63. [Google Scholar] [CrossRef]
- Huang, F.; Chen, L.; Wang, H.; Feng, T.; Yan, Z. Degradation of Methyl Orange by Atmospheric DBD Plasma: Analysis of the Degradation Effects and Degradation Path. J. Electrostat. 2012, 70, 43–47. [Google Scholar] [CrossRef]
- Bali, U.; Çatalkaya, E.Ç.; Şengül, F. Photochemical Degradation and Mineralization of Phenol: A Comparative Study. J. Environ. Sci. Health Part A 2003, 38, 2259–2275. [Google Scholar] [CrossRef] [PubMed]
- Utari, A.W.; Herdiansyah, H. Filtration as a Water Treatment Method: Used to Remove TSS and COD in Household Wastewater. AIP Conf. Proc. 2020, 2245, 060004. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Seremet, O. Advanced Treatment of Biotreated Textile Industry Wastewater with Ozone, Virgin/Ozonated Granular Activated Carbon and Their Combination. J. Environ. Sci. Health Part A 2004, 39, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Shuvo, H.R.; Sultana, B.; Islam, F.; Rus’d, A.A.; Begum, S.; Hossain, M.N. The Degradation of Textile Industry Dyes Using the Effective Bacterial Consortium. Heliyon 2021, 7, e08102. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and Applications in Theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Q.; Liu, Z.; Pan, K.; Dong, Y.; Li, Y. Removal of Cu(II) by Loofah Fibers as a Natural and Low-Cost Adsorbent from Aqueous Solutions. J. Mol. Liq. 2014, 199, 401–407. [Google Scholar] [CrossRef]
- Wu, S.; Pan, Y.; Wang, N.; Lu, T.; Dai, W. Azo Dye Degradation Behavior of AlFeMnTiM (M = Cr, Co, Ni) High-Entropy Alloys. Int. J. Miner. Metall. Mater. 2019, 26, 124–132. [Google Scholar] [CrossRef]
- Mir, N.; Khan, A.; Umar, K.; Muneer, M. Photocatalytic Study of a Xanthene Dye Derivative, Phloxine B in Aqueous Suspension of TiO2: Adsorption Isotherm and Decolourization Kinetics. Energy Environ. Focus 2013, 2, 208–216. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Li, X.; Pan, S.; Zhang, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Internal Pore Decoration with Polydopamine Nanoparticle on Polymeric Ultrafiltration Membrane for Enhanced Heavy Metal Removal. Chem. Eng. J. 2017, 314, 38–49. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of Nanoparticles in Biofuels: An Overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Sun, R.; Yang, J.; Huang, R.; Wang, C. Controlled Carbonization of Microplastics Loaded Nano Zero-Valent Iron for Catalytic Degradation of Tetracycline. Chemosphere 2022, 303, 135123. [Google Scholar] [CrossRef]
- Hassan, S.E.D.; Fouda, A.; Saied, E.; Farag, M.M.S.; Eid, A.M.; Barghoth, M.G.; Awad, M.A.; Hamza, M.F.; Awad, M.F. Rhizopus Oryzae-Mediated Green Synthesis of Magnesium Oxide Nanoparticles (MgO-NPs): A Promising Tool for Antimicrobial, Mosquitocidal Action, and Tanning Effluent Treatment. J. Fungi 2021, 7, 372. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Zwiehoff, S.; Johny, J.; Behrends, C.; Landmann, A.; Mentzel, F.; Bäumer, C.; Kröninger, K.; Rehbock, C.; Timmermann, B.; Barcikowski, S.; et al. Enhancement of Proton Therapy Efficiency by Noble Metal Nanoparticles Is Driven by the Number and Chemical Activity of Surface Atoms. Small 2022, 18, 2106383. [Google Scholar] [CrossRef] [PubMed]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A. Legal and Practical Challenges in Classifying Nanomaterials According to Regulatory Definitions. Nat. Nanotechnol. 2019, 14, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ben Mbarek, W.; Pineda, E.; Escoda, L.; Suñol, J.J.; Khitouni, M. High Efficiency Decolorization of Azo Dye Reactive Black 5 by Ca-Al Particles. J. Environ. Chem. Eng. 2017, 5, 6107–6113. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Eljamal, O.; Amen, T.W.M.; Sugihara, Y.; Matsunaga, N. Optimized Nano-Scale Zero-Valent Iron Supported on Treated Activated Carbon for Enhanced Nitrate and Phosphate Removal from Water. Chem. Eng. J. 2017, 309, 349–365. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Ji, M.; Kong, X. Treatment of Mixed Chemical Wastewater and the Agglomeration Mechanism via an Internal Electrolysis Filter. Chem. Eng. J. 2013, 215–216, 50–56. [Google Scholar] [CrossRef]
- Xiong, Z.; Lai, B.; Yang, P.; Zhou, Y.; Wang, J.; Fang, S. Comparative Study on the Reactivity of Fe/Cu Bimetallic Particles and Zero Valent Iron (ZVI) under Different Conditions of N2, Air or without Aeration. J. Hazard. Mater. 2015, 297, 261–268. [Google Scholar] [CrossRef]
- Bigg, T.; Judd, S.J. Kinetics of Reductive Degradation of Azo Dye by Zero-Valent Iron. Process Saf. Environ. Prot. 2001, 79, 297–303. [Google Scholar] [CrossRef]
- Elliott, D.W.; Zhang, W.X. Field Assessment of Nanoscale Bimetallic Particles for Groundwater Treatment. Environ. Sci. Technol. 2001, 35, 4922–4926. [Google Scholar] [CrossRef]
- Zhang, W.X.; Wang, C.B.; Lien, H.L. Treatment of Chlorinated Organic Contaminants with Nanoscale Bimetallic Particles. Catal. Today 1998, 40, 387–395. [Google Scholar] [CrossRef]
- Lien, H.-L.; Zhang, W. Transformation of Chlorinated Methanes by Nanoscale Iron Particles. J. Environ. Eng. 1999, 125, 1042–1047. [Google Scholar] [CrossRef]
- Grittini, C.; Malcomson, M.; Fernando, Q.; Korte, N. Rapid Dechlorination of Polychlorinated Biphenyls on the Surface of a Pd/Fe Bimetallic System. Environ. Sci. Technol. 2002, 29, 2898–2900. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.; Zhang, W. Hydrodechlorination of Chlorinated Ethanes by Nanoscale Pd/Fe Bimetallic Particles. ASCE 2005, 131, 4–10. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.X. Subcolloidal Fe/Ag Particles for Reductive Dehalogenation of Chlorinated Benzenes. Ind. Eng. Chem. Res. 2000, 39, 2238–2244. [Google Scholar] [CrossRef]
- Tee, Y.H.; Bachas, L.; Bhattacharyya, D. Degradation of Trichloroethylene and Dichlorobiphenyls by Iron-Based Bimetallic Nanoparticles. J. Phys. Chem. C 2009, 113, 9454–9464. [Google Scholar] [CrossRef]
- Barnes, R.J.; Riba, O.; Gardner, M.N.; Scott, T.B.; Jackman, S.A.; Thompson, I.P. Optimization of Nano-Scale Nickel/Iron Particles for the Reduction of High Concentration Chlorinated Aliphatic Hydrocarbon Solutions. Chemosphere 2010, 79, 448–454. [Google Scholar] [CrossRef]
- Barnes, R.J.; Riba, O.; Gardner, M.N.; Singer, A.C.; Jackman, S.A.; Thompson, I.P. Inhibition of Biological TCE and Sulphate Reduction in the Presence of Iron Nanoparticles. Chemosphere 2010, 80, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Schrick, B.; Blough, J.L.; Jones, A.D.; Mallouk, T.E. Hydrodechlorination of Trichloroethylene to Hydrocarbons Using Bimetallic Nickel-Iron Nanoparticles. Chem. Mater. 2002, 14, 5140–5147. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Wu, S.; Pan, Y.; Lu, J.; Wang, N.; Dai, W.; Lu, T. Effect of the Addition of Mg, Ti, Ni on the Decoloration Performance of AlCrFeMn High Entropy Alloy. J. Mater. Sci. Technol. 2019, 35, 1629–1635. [Google Scholar] [CrossRef]
- Qin, P.; Yang, Y.; Zhang, X.; Niu, J.; Yang, H.; Tian, S.; Zhu, J.; Lu, M. Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent. Nanomaterials 2017, 8, 4. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, H.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Mechanical Properties Improvement of AlCrFeNi2Ti0.5 High Entropy Alloy through Annealing Design and Its Relationship with Its Particle-Reinforced Microstructures. J. Mater. Sci. Technol. 2015, 31, 397–402. [Google Scholar] [CrossRef]
- Dolique, V.; Thomann, A.L.; Brault, P.; Tessier, Y.; Gillon, P. Thermal Stability of AlCoCrCuFeNi High Entropy Alloy Thin Films Studied by In-Situ XRD Analysis. Surf. Coat. Technol. 2010, 204, 1989–1992. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of Al XCoCrFeNi High-Entropy Alloys: Al-Content and Potential Scan-Rate Dependent Pitting Behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Chou, Y.L.; Wang, Y.C.; Yeh, J.W.; Shih, H.C. Pitting Corrosion of the High-Entropy Alloy Co1.5CrFeNi1.5Ti0.5Mo0.1 in Chloride-Containing Sulphate Solutions. Corros. Sci. 2010, 52, 3481–3491. [Google Scholar] [CrossRef]
- Luo, X.; Li, R.; Zong, J.; Zhang, Y.; Li, H.; Zhang, T. Enhanced Degradation of Azo Dye by Nanoporous-Copper-Decorated Mg-Cu-Y Metallic Glass Powder through Dealloying Pretreatment. Appl. Surf. Sci. 2014, 305, 314–320. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, Z.; Zhang, C.; Deng, J.; Hou, K.; Cheng, Y.; Zhang, L.; Zeng, G. Removal of Tetracycline by Fe/Ni Bimetallic Nanoparticles in Aqueous Solution. J. Colloid Interface Sci. 2018, 513, 117–125. [Google Scholar] [CrossRef]
- Safavi, A.; Momeni, S. Highly Efficient Degradation of Azo Dyes by Palladium/Hydroxyapatite/Fe3O4 Nanocatalyst. J. Hazard. Mater. 2012, 201–202, 125–131. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale Zero-Valent Iron: Future Prospects for an Emerging Water Treatment Technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef]
- Ben Mbarek, W.; Azabou, M.; Pineda, E.; Fiol, N.; Escoda, L.; Suñol, J.J.; Khitouni, M. Rapid Degradation of Azo-Dye Using Mn-Al Powders Produced by Ball-Milling. RSC Adv. 2017, 7, 12620–12628. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.Q.; Li, H.; Yang, H.; Huo, J.; Wang, J.; Chang, C.; Wang, X.; Li, R.W.; Wang, G. Fast Decolorization of Azo Dyes in Both Alkaline and Acidic Solutions by Al-Based Metallic Glasses. J. Alloys Compd. 2017, 701, 759–767. [Google Scholar] [CrossRef]
- Mao, A.; Ding, P.; Quan, F.; Zhang, T.; Ran, X.; Li, Y.; Jin, X.; Gu, X. Effect of Aluminum Element on Microstructure Evolution and Properties of Multicomponent Al-Co-Cr-Cu-Fe-Ni Nanoparticles. J. Alloys Compd. 2018, 735, 1167–1175. [Google Scholar] [CrossRef]
- Sha, Y.; Mathew, I.; Cui, Q.; Clay, M.; Gao, F.; Zhang, X.J.; Gu, Z. Rapid Degradation of Azo Dye Methyl Orange Using Hollow Cobalt Nanoparticles. Chemosphere 2016, 144, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- AboliGhasemabadi, M.; Mbarek, W.B.; Casabella, O.; Roca-Bisbe, H.; Pineda, E.; Escoda, L.; Suñol, J.J. Application of Mechanically Alloyed MnAl Particles to De-Colorization of Azo Dyes. J. Alloys Compd. 2018, 741, 240–245. [Google Scholar] [CrossRef]
- AboliGhasemabadi, M.; Ben Mbarek, W.; Cerrillo-Gil, A.; Roca-Bisbe, H.; Casabella, O.; Blánquez, P.; Pineda, E.; Escoda, L.; Suñol, J.J. Azo-Dye Degradation by Mn–Al Powders. J. Environ. Manag. 2020, 258, 110012. [Google Scholar] [CrossRef] [PubMed]
- Mbarek, W.B.; Saurina, J.; Escoda, L.; Pineda, E.; Khitouni, M.; Suñol, J.-J. Effects of the Addition of Fe, Co on the Azo Dye Degradation Ability of Mn-Al Mechanically Alloyed Powders. Metals 2020, 10, 1578. [Google Scholar] [CrossRef]
- Fan, J.; Guo, Y.; Wang, J.; Fan, M. Rapid Decolorization of Azo Dye Methyl Orange in Aqueous Solution by Nanoscale Zerovalent Iron Particles. J. Hazard. Mater. 2009, 166, 904–910. [Google Scholar] [CrossRef]
- Shu, H.Y.; Chang, M.C.; Yu, H.H.; Chen, W.H. Reduction of an Azo Dye Acid Black 24 Solution Using Synthesized Nanoscale Zerovalent Iron Particles. J. Colloid Interface Sci. 2007, 314, 89–97. [Google Scholar] [CrossRef]
- Hamdy, A.; Mostafa, M.K.; Nasr, M. Zero-Valent Iron Nanoparticles for Methylene Blue Removal from Aqueous Solutions and Textile Wastewater Treatment, with Cost Estimation. Water Sci. Technol. 2018, 78, 367–378. [Google Scholar] [CrossRef]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. The Mechanism for Degrading Orange II Based on Adsorption and Reduction by Ion-Based Nanoparticles Synthesized by Grape Leaf Extract. J. Hazard. Mater. 2015, 296, 37–45. [Google Scholar] [CrossRef]
- Poursaberi, T.; Hassanisadi, M.; Nourmohammadian, F. Application of Synthesized Nanoscale Zero-Valent Iron in the Treatment of Dye Solution Containing Basic Yellow 28. Prog. Color. Color. Coat. 2012, 5, 35–40. [Google Scholar] [CrossRef]
- Satapanajaru, T.; Chompuchan, C.; Suntornchot, P.; Pengthamkeerati, P. Enhancing Decolorization of Reactive Black 5 and Reactive Red 198 during Nano Zerovalent Iron Treatment. Desalination 2011, 266, 218–230. [Google Scholar] [CrossRef]
- Li, P.; Song, Y.; Wang, S.; Tao, Z.; Yu, S.L.; Liu, Y.A. Enhanced decolorization of methyl orange using zero-valent copper nanoparticles under assistance of hydrodynamic cavitation. Ultrason. Chem. 2015, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, C.R.; Puiatti, G.A.; Nascimento, M.A.; Oliveira, A.F.; Lopes, R.P. Degradation of the Reactive Blue 4 Dye in Aqueous Solution Using Zero-Valent Copper Nanoparticles. J. Nanomater. 2018, 2018, 4642038. [Google Scholar] [CrossRef]
- Schroers, J. Bulk Metallic Glasses. Phys. Today 2013, 66, 32. [Google Scholar] [CrossRef]
- Sun, S.P.; Wang, K.Y.; Rajarathnam, D.; Hatton, T.A.; Chung, T.S. Polyamide-Imide Nanofiltration Hollow Fiber Membranes with Elongation-Induced Nano-Pore Evolution. AIChE J. 2010, 56, 1481–1494. [Google Scholar] [CrossRef]
- Qin, X.D.; Zhu, Z.W.; Liu, G.; Fu, H.M.; Zhang, H.W.; Wang, A.M.; Li, H.; Zhang, H.F. Ultrafast Degradation of Azo Dyes Catalyzed by Cobalt-Based Metallic Glass. Sci. Rep. 2015, 5, 18226. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Zhu, Z.; Wu, J. Efficient Degradation of Rhodamine B Using Fe-Based Metallic Glass Catalyst by Fenton-like Process. Chemosphere 2014, 117, 638–643. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, X.H.; Chan, K.C.; Liu, L.; Li, T. Fe-Based Metallic Glass Catalyst with Nanoporous Surface for Azo Dye Degradation. Chemosphere 2017, 174, 76–81. [Google Scholar] [CrossRef]
- Jia, Z.; Liang, S.X.; Zhang, W.C.; Wang, W.M.; Yang, C.; Zhang, L.C. Heterogeneous Photo Fenton-like Degradation of Cibacron Brilliant Red 3B-A Dye Using Amorphous Fe78Si9B13 and Fe73.5Si13.5B9Cu1Nb3 Alloys: The Influence of Adsorption. J. Taiwan Inst. Chem. Eng. 2017, 71, 128–136. [Google Scholar] [CrossRef]
- Xie, S.; Huang, P.; Kruzic, J.J.; Zeng, X.; Qian, H. A Highly Efficient Degradation Mechanism of Methyl Orange Using Fe-Based Metallic Glass Powders. Sci. Rep. 2016, 6, 21947. [Google Scholar] [CrossRef]
- Ramya, M.; Karthika, M.; Selvakumar, R.; Raj, B.; Ravi, K.R. A Facile and Efficient Single Step Ball Milling Process for Synthesis of Partially Amorphous Mg-Zn-Ca Alloy Powders for Dye Degradation. J. Alloys Compd. 2017, 696, 185–192. [Google Scholar] [CrossRef]
- Ma, H.; Wang, H.; Na, C. Microwave-Assisted Optimization of Platinum-Nickel Nanoalloys for Catalytic Water Treatment. Appl. Catal. B Environ. 2015, 163, 198–204. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N.; Shah, N.K. Applications of Nano-Catalyst in New Era. J. Saudi Chem. Soc. 2012, 16, 307–325. [Google Scholar] [CrossRef]
- Dutta, A.K.; Maji, S.K.; Adhikary, B. γ-Fe2O3 Nanoparticles: An Easily Recoverable Effective Photo-Catalyst for the Degradation of Rose Bengal and Methylene Blue Dyes in the Waste-Water Treatment Plant. Mater. Res. Bull. 2014, 49, 28–34. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Hua, F.; Xue, M.; Xie, X.; Xie, Y.; Yu, Y.; Shouhan, Z.; Longshuai, Y.; Zuozhu, X.; et al. Adsorption and photodegradation of reactive red 120 with nickel-iron-layered double hydroxide/biochar composites. J. Hazard. Mater. 2023, 443, 130300. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Kurian, M.; Nair, D.S. Heterogeneous Fenton Behavior of Nano Nickel Zinc Ferrite Catalysts in the Degradation of 4-Chlorophenol from Water under Neutral Conditions. J. Water Process Eng. 2015, 8, e37–e49. [Google Scholar] [CrossRef]
- Lin, S.T.; Thirumavalavan, M.; Jiang, T.Y.; Lee, J.F. Synthesis of ZnO/Zn Nano Photocatalyst Using Modified Polysaccharides for Photodegradation of Dyes. Carbohydr. Polym. 2014, 105, 1–9. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered Nanomaterials for Water Treatment and Remediation: Costs, Benefits, and Applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous Removal of Antibiotics and Inactivation of Antibiotic-Resistant Bacteria by Photocatalysis: A Review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Reyes, C.; Fernandez, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and inactivation of tetracycline by TiO 2 photocatalysis. J. Photochem. Photobiol. A Chem. 2006, 184, 141–146. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Zhang, W.; Xia, L.; Zhou, S.; Russell, C.K.; Fan, M.; Feng, J.; Sun, J. Double-shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater. Chem. Eng. J. 2020, 384, 123279. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, D.; Han, J.; Liu, C.; Ding, Y.; Wang, Z.; Wang, A. Adsorption enhanced photocatalytic degradation sulfadiazine antibiotic using porous carbon nitride nanosheets with carbon vacancies. Chem. Eng. J. 2020, 382, 123017. [Google Scholar] [CrossRef]
- Kumar, R.; Barakat, M.A.; Al-Mur, B.A.; Alseroury, F.A.; Eniola, J.O. Photocatalytic degradation of cefoxitin sodium antibiotic using novel BN/CdAl2O4 composite. J. Clean. Prod. 2020, 246, 119076. [Google Scholar] [CrossRef]
- Musial, J.; Mlynarczyk, D.T.; Stanisz, B.J. Photocatalytic Degradation of Sulfamethoxazole Using TiO2-Based Materials–Perspectives for the Development of a Sustainable Water Treatment Technology. Sci. Total Environ. 2023, 856, 159122. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver Nanoparticles: Green Synthesis and Their Antimicrobial Activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Durgalakshmi, D.; Rajendran, S.; Naushad, M. Current Role of Nanomaterials in Environmental Remediation. In Advanced Nanostructured Materials for Environmental Remediation. Environmental Chemistry for a Sustainable World; Naushad, M., Rajendran, S., Gracia, F., Eds.; Springer: Cham, Switzerland, 2019; Volume 25. [Google Scholar] [CrossRef]
- Singh, T.; Jayaprakash, A.; Alsuwaidi, M.; Madhavan, A.A. Green Synthesized Gold Nanoparticles with Enhanced Photocatalytic Activity. Mater. Today Proc. 2021, 42, 1166–1169. [Google Scholar] [CrossRef]
- Raza, W.; Haque, M.M.; Muneer, M.; Fleisch, M.; Hakki, A.; Bahnemann, D. Photocatalytic Degradation of Different Chromophoric Dyes in Aqueous Phase Using La and Mo Doped TiO2 Hybrid Carbon Spheres. J. Alloys Compd. 2015, 632, 837–844. [Google Scholar] [CrossRef]
- Rajkumar, K.; Muthukumar, M.; Mangalaraja, R.V. Electrochemical Degradation of C.I. Reactive Orange 107 Using Gadolinium (Gd3+), Neodymium (Nd3+) and Samarium (Sm3+) Doped Cerium Oxide Nanoparticles. Int. J. Ind. Chem. 2015, 6, 285–295. [Google Scholar] [CrossRef]
- Chang, J.H.; Cheng, S.F. The Remediation Performance of a Specific Electrokinetics Integrated with Zero-Valent Metals for Perchloroethylene Contaminated Soils. J. Hazard. Mater. 2006, 131, 153–162. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, K.; Guo, B.; Liu, Q.; Fang, L.; Gong, J.R. Graphene-Based Materials for Hydrogen Generation from Light-Driven Water Splitting. Adv. Mater. 2013, 25, 3820–3839. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2008, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Huo, J.; Xu, W.; Wang, X.; Wang, G.; Wang, P.P.; Wang, J.Q.; Huo, J.T.; Xu, W.; et al. Fast Degradation of Azo Dye by Nanocrystallized Fe-Based Alloys. Sci. China Phys. Mech. Astron. 2017, 60, 076112. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-Photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Xuan, J.; Xiao, W.J. Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828–6838. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic Conversion of CO2 into Renewable Hydrocarbon Fuels: State-of-the-Art Accomplishment, Challenges, and Prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef]
- Yang, G.; Yan, W.; Zhang, Q.; Shen, S.; Ding, S. One-Dimensional CdS/ZnO Core/Shell Nanofibers via Single-Spinneret Electrospinning: Tunable Morphology and Efficient Photocatalytic Hydrogen Production. Nanoscale 2013, 5, 12432–12439. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chaiwichain, S.; Inceesungvorn, B.; Pingmuang, K.; Phanichphant, S.; Minett, A.I.; Chen, J. BiVO4/CeO2 Nanocomposites with High Visible-Light-Induced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 3718–3723. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, F.; Wang, L.; Zhang, J. Preparation of Cu2O/CeO2 Heterojunction Photocatalyst for the Degradation of Acid Orange 7 under Visible Light Irradiation. Catal. Commun. 2011, 12, 794–797. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Zhai, Y.; Wang, E. Nafion–Graphene Nanocomposite Film as Enhanced Sensing Platform for Ultrasensitive Determination of Cadmium. Electrochem. Commun. 2009, 11, 1085–1088. [Google Scholar] [CrossRef]
- Li, C.; Chen, R.; Zhang, X.; Shu, S.; Xiong, J.; Zheng, Y.; Dong, W. Electrospinning of CeO2–ZnO Composite Nanofibers and Their Photocatalytic Property. Mater. Lett. 2011, 65, 1327–1330. [Google Scholar] [CrossRef]
- Li, K.; Lu, X.; Zhang, Y.; Liu, K.; Huang, Y.; Liu, H. Bi3TaO7/Ti3C2 Heterojunctions for Enhanced Photocatalytic Removal of Water-Borne Contaminants. Environ. Res. 2020, 185, 109409. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, W.; Long, B.; Li, H.; Zhao, F.; Liu, Z.; Tong, Y.; Ji, H. Visible light Bi2S3/Bi2O3/Bi2O2CO3 photocatalyst for effective degradation of organic pollutions. Appl. Catal. B Environ. 2016, 185, 68–76. [Google Scholar] [CrossRef]
- Navalon, S.; Alvaro, M.; Garcia, H. Heterogeneous Fenton Catalysts Based on Clays, Silicas and Zeolites. Appl. Catal. B Environ. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Baldrian, P. Wood-Inhabiting Ligninolytic Basidiomycetes in Soils: Ecology and Constraints for Applicability in Bioremediation. Fungal Ecol. 2008, 1, 4–12. [Google Scholar] [CrossRef]
- Corma, A.; García, H. Lewis Acids: From Conventional Homogeneous to Green Homogeneous and Heterogeneous Catalysis. Chem. Rev. 2003, 103, 4307–4365. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of Chlorophenols by Means of Advanced Oxidation Processes: A General Review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Nazim, M.; Khan, A.A.P.; Asiri, A.M.; Kim, J.H. Exploring Rapid Photocatalytic Degradation of Organic Pollutants with Porous CuO Nanosheets: Synthesis, Dye Removal, and Kinetic Studies at Room Temperature. ACS Omega 2021, 6, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Ghaffari, Y.; Kim, S.; Bae, J.; Kim, K.S.; Saifuddin, M. Photocatalytic Degradation of Organic Pollutants over MFe2O4 (M=Co, Ni, Cu, Zn) Nanoparticles at Neutral PH. Sci. Rep. 2020, 10, 4942. [Google Scholar] [CrossRef] [PubMed]
- Kirankumar, V.S.; Sumathi, S. Structural, Optical, Magnetic and Photocatalytic Properties of Bismuth Doped Copper Aluminate Nanoparticles. Mater. Chem. Phys. 2017, 197, 17–26. [Google Scholar] [CrossRef]
- Kumar, S.; Sahare, P.D. Photocatalytic activity of bismuth vanadate for the degradation of organic compounds. Nano 2013, 8, 1350007. [Google Scholar] [CrossRef]
- Saygi, B.; Tekin, D. Photocatalytic Degradation Kinetics of Reactive Black 5 (RB5) Dyestuff on TiO2 Modified by Pretreatment with Ultrasound Energy. React. Kinet. Mech. Catal. 2013, 110, 251–258. [Google Scholar] [CrossRef]
- Ateş, S.; Baran, E.; Yazıcı, B. Fabrication of Al2O3 Nanopores/SnO2 and Its Application in Photocatalytic Degradation under UV Irradiation. Mater. Chem. Phys. 2018, 214, 17–27. [Google Scholar] [CrossRef]
- Alafif, Z.O.; Anjum, M.; Ansari, M.O.; Kumar, R.; Rashid, J.; Madkour, M.; Barakat, M.A. Synthesis and Characterization of S-Doped-RGO/ZnS Nanocomposite for the Photocatalytic Degradation of 2-Chlorophenol and Disinfection of Real Dairy Wastewater. J. Photochem. Photobiol. A Chem. 2019, 377, 190–197. [Google Scholar] [CrossRef]
- Rao, M.P.C.; Kulandaivelu, K.; Ponnusamy, V.K.; Wu, J.J.; Sambandam, A. Surfactant-Assisted Synthesis of Copper Oxide Nanorods for the Enhanced Photocatalytic Degradation of Reactive Black 5 Dye in Wastewater. Environ. Sci. Pollut. Res. 2020, 27, 17438–17445. [Google Scholar] [CrossRef]
- Akram, N.; Guo, J.; Ma, W.; Guo, Y.; Hassan, A.; Wang, J. Synergistic Catalysis of Co(OH)2/CuO for the Degradation of Organic Pollutant Under Visible Light Irradiation. Sci. Rep. 2020, 10, 1939. [Google Scholar] [CrossRef]
- Deng, X.; Wang, C.; Yang, H.; Shao, M.; Zhang, S.; Wang, X.; Ding, M.; Huang, J.; Xu, X. One-Pot Hydrothermal Synthesis of CdS Decorated CuS Microflower-like Structures for Enhanced Photocatalytic Properties. Sci. Rep. 2017, 7, 3877. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent Advances on the Removal of Dyes from Wastewater Using Various Adsorbents: A Critical Review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Nadeem Baig; Irshad Kammakakam; Wail Falath Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [CrossRef]
- Thirunavukkarasu, A.; Muthukumaran, K.; Nithya, R. Adsorption of Acid Yellow 36 onto Green Nanoceria and Amine Functionalized Green Nanoceria: Comparative Studies on Kinetics, Isotherm, Thermodynamics, and Diffusion Analysis. J. Taiwan Inst. Chem. Eng. 2018, 93, 211–225. [Google Scholar] [CrossRef]
- Rajarathinam, N.; Arunachalam, T.; Raja, S.; Selvasembian, R. Fenalan Yellow G Adsorption Using Surface-Functionalized Green Nanoceria: An Insight into Mechanism and Statistical Modelling. Environ. Res. 2020, 181, 108920. [Google Scholar] [CrossRef]
- Mirjavadi, E.S.; Tehrani, R.M.A.; Khadir, A. Effective Adsorption of Zinc on Magnetic Nanocomposite of Fe3O4/Zeolite/Cellulose Nanofibers: Kinetic, Equilibrium, and Thermodynamic Study. Environ. Sci. Pollut. Res. 2019, 26, 33478–33493. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Suganya, E.; Lity, A.V.; Selvaraju, N. Equilibrium and Kinetics Studies of Hexavalent Chromium Biosorption on a Novel Green Macroalgae enteromorpha sp. Res. Chem. Intermed. 2015, 42, 1275–1294. [Google Scholar] [CrossRef]
- Zach-Maor, A.; Semiat, R.; Shemer, H. Removal of Heavy Metals by Immobilized Magnetite Nano-Particles. New Pub Balaban 2012, 31, 64–70. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Muralidhara, H.B.; Nayaka, Y.A.; Balasubramanyam, J.; Hanumanthappa, H. Low-Cost Synthesis of Metal Oxide Nanoparticles and Their Application in Adsorption of Commercial Dye and Heavy Metal Ion in Aqueous Solution. Powder Technol. 2013, 246, 125–136. [Google Scholar] [CrossRef]
- Arikal, D.; Kallingal, A. Photocatalytic Degradation of Azo and Anthraquinone Dye Using TiO2/MgO Nanocomposite Immobilized Chitosan Hydrogels. Environ. Technol. 2021, 42, 2278–2291. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, J.; Zhang, Y. Effects of Calcination Temperature on Morphology and Structure of CeO2 Nanofibers and Their Photocatalytic Activity. Mater. Lett. 2019, 241, 76–79. [Google Scholar] [CrossRef]
- Hitkari, G.; Chowdhary, P.; Kumar, V.; Singh, S.; Motghare, A. Potential of Copper-Zinc Oxide Nanocomposite for Photocatalytic Degradation of Congo Red Dye. Clean. Chem. Eng. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, Y.; Guo, Y.; Guo, R.; Tian, Y.; Zhao, W. Preparation of CuO/ΓAl2O3 Catalyst for Degradation of Azo Dyes (Reactive Brilliant Red X–3B): An Optimization Study. J. Clean. Prod. 2021, 328, 129624. [Google Scholar] [CrossRef]
- Rizvi, M.; Tiwari, N.; Mishra, A.; Gupta, R. Kinetic and Computational Study of Degradation of Two Azo Dyes, Metanil Yellow and Orange II, by Iron Oxide Nanoparticles Synthesized Using Hylocereus Undatus. ACS Omega 2022, 7, 31667–31681. [Google Scholar] [CrossRef]
- Ait Himi, M.; El Ghachtouli, S.; Amarray, A.; Zaroual, Z.; Bonnaillie, P.; Azzi, M. Nanostructured Manganese Oxide as an Efficient Eco-Friendly Catalyst for Removing Azo Dye Calcon from Water. Mater. Sci. 2020, 37, 3905–3912. [Google Scholar] [CrossRef]
- Ullah, A.; Rahman, L.; Hussain, S.Z.; Abbas, W.; Tawab, A.; Jilani, A.; Bajwa, S.Z.; Khan, W.S.; Riaz, R.; Hussain, I.; et al. Mechanistic Insight of Dye Degradation Using TiO2 Anchored α-MnO2 Nanorods as Promising Sunlight Driven Photocatalyst. Mater. Sci. Eng. B 2021, 271, 115257. [Google Scholar] [CrossRef]
- Salari, H.; Yaghmaei, H. Z-Scheme 3D Bi2WO6/MnO2 Heterojunction for Increased Photoinduced Charge Separation and Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2020, 532, 147413. [Google Scholar] [CrossRef]
- Madima, N.; Mishra, S.B.; Inamuddin, I.; Mishra, A.K. Carbon-Based Nanomaterials for Remediation of Organic and Inorganic Pollutants from Wastewater. A Review. Environ. Chem. Lett. 2020, 18, 1169–1191. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Inamuddin Carbon Nanotube-Based Adsorbents for the Removal of Dyes from Waters: A Review. Environ. Chem. Lett. 2020, 18, 605–629. [Google Scholar] [CrossRef]
- Visakh, P.M. Introduction for Nanomaterials and Nanocomposites: State of Art, New Challenges, and Opportunities. In Nanomaterials and Nanocomposites: Zero to Three-Dimensional Materials and Their Composites; Wiley: Hoboken, NJ, USA, 2016; pp. 1–20. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon Nanotubes as Adsorbents in Environmental Pollution Management: A Review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of Organic Compounds by Carbon Nanomaterials in Aqueous Phase: Polanyi Theory and Its Application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Duan, L.; Zhu, D. Adsorption of Polar and Nonpolar Organic Chemicals to Carbon Nanotubes. Environ. Sci. Technol. 2007, 41, 8295–8300. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, W.; Jing, Q.; Zhu, L. Aqueous Adsorption of Aniline, Phenol, and Their Substitutes by Multi-Walled Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 7931–7936. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, D.N.; Kim, H. Electronic Structure Modulation of Multi-Walled Carbon Nanotubes Using Azo Dye for Inducing Non-Radical Reaction: Effect of Graphitic Nitrogen and Structural Defect. Chemosphere 2022, 307, 136023. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.H.; Yang, Y.T.; Chang, C.F. Electrochemical Degradation of Methylene Blue Accompanied with the Reduction of CO2 by Using Carbon Nanotubes Grown on Carbon Fiber Electrodes. Sustain. Environ. Res. 2022, 32, 13. [Google Scholar] [CrossRef]
- Khan, J.; Ilyas, S.; Akram, B.; Ahmad, K.; Hafeez, M.; Siddiq, M.; Ashraf, M.A. Zno/NiO Coated Multi-Walled Carbon Nanotubes for Textile Dyes Degradation. Arab. J. Chem. 2018, 11, 880–896. [Google Scholar] [CrossRef]
- De Luca, P.; Nagy, J.B. Treatment of Water Contaminated with Reactive Black-5 Dye by Carbon Nanotubes. Materials 2020, 13, 5508. [Google Scholar] [CrossRef]
- Hemraj-Benny, T.; Pimentel, L.; Emeran, G. Formation of Single-Walled Carbon Nanotube-Ruthenium Nanoparticles in Ethanol upon Microwave Radiation. Inorg. Chem. Commun. 2020, 112, 107707. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Karimi, A.; Derakhshani, E.; Esform, A. Single-Walled Carbon Nanotubes (SWCNTs) as an Efficient Adsorbent for Removal of Reactive Dyes from Water Solution: Equilibrium, Kinetic, and Thermodynamic. Environ. Qual. Manag. 2022, 31, 133–140. [Google Scholar] [CrossRef]
- Agarwal, M.; Kumari, P.; Dubey, S.; Gupta, R.; Dohare, R.K. Adsorption Behavior of Azo Dyes on Carbon Nanotubes Grown on Alumina: Process Optimization, Kinetics, and Equilibrium Study. J. Environ. Eng. 2018, 145, 04018134. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Zhang, Q.; Li, C.; Bao, C.; Liu, X.; Xiao, P. Adsorption of Au(III), Pd(II), and Pt(IV) from Aqueous Solution onto Graphene Oxide. J. Chem. Eng. Data 2013, 58, 209–216. [Google Scholar] [CrossRef]
- Machida, M.; Mochimaru, T.; Tatsumoto, H. Lead(II) Adsorption onto the Graphene Layer of Carbonaceous Materials in Aqueous Solution. Carbon 2006, 44, 2681–2688. [Google Scholar] [CrossRef]
- Yang, S.T.; Chang, Y.; Wang, H.; Liu, G.; Chen, S.; Wang, Y.; Liu, Y.; Cao, A. Folding/Aggregation of Graphene Oxide and Its Application in Cu2+ Removal. J. Colloid Interface Sci. 2010, 351, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption Characteristics and Behaviors of Graphene Oxide for Zn(II) Removal from Aqueous Solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Bian, Y.; Bian, Z.Y.; Zhang, J.X.; Ding, A.Z.; Liu, S.L.; Wang, H. Effect of the Oxygen-Containing Functional Group of Graphene Oxide on the Aqueous Cadmium Ions Removal. Appl. Surf. Sci. 2015, 329, 269–275. [Google Scholar] [CrossRef]
- Gao, W.; Majumder, M.; Alemany, L.B.; Narayanan, T.N.; Ibarra, M.A.; Pradhan, B.K.; Ajayan, P.M. Engineered Graphite Oxide Materials for Application in Water Purification. ACS Appl. Mater. Interfaces 2011, 3, 1821–1826. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Choi, Y.-L.; Chang, Y.-Y.; Yang, J.-K. Studies on Removal of Pb(II) and Cr(III) Using Graphene Oxide Based Inverse Spinel Nickel Ferrite Nano-Composite as Sorbent. Hydrometallurgy 2016, 165, 64–72. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of Graphene/Metal Oxide Composites as Photocatalysts, Adsorbents and Disinfectants in Water Treatment: A Review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Jayanthi, S.; Krishnarao Eswar, N.; Singh, S.A.; Chatterjee, K.; Madras, G.; Sood, A.K. Macroporous Three-Dimensional Graphene Oxide Foams for Dye Adsorption and Antibacterial Applications. RSC Adv. 2016, 6, 1231–1242. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Moszyński, D.; Mijowska, E. Adsorption of Anionic Azo-Dyes from Aqueous Solutions onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies. J. Colloid Interface Sci. 2017, 496, 188–200. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of Novel Magnetic Chitosan Grafted with Graphene Oxide to Enhance Adsorption Properties for Methyl Blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Saroyan, H.; Kyzas, G.Z.; Deliyanni, E.A. Effective Dye Degradation by Graphene Oxide Supported Manganese Oxide. Processes 2019, 7, 40. [Google Scholar] [CrossRef]
- Idoia Martín-de-Lucía, C.; Campos-Mañas, M.; Agüera, A.; Rodea-Palomares, I.; Pulido-Reyes, G.; Leganés, F.; Fernández-Piñas, F. Roberto Rosal Reverse Trojan-Horse Effect Decreased Wastewater Toxicity in the Presence of Inorganic Nanoparticles. Environ. Sci. Nano 2017, 4, 1273–1282. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Maghsoodi, A. Kinetics and Isotherm of Cationic Dye Removal from Multicomponent System Using the Synthesized Silica Nanoparticle. Desalination Water Treat. 2014, 54, 562–571. [Google Scholar] [CrossRef]
- Da’na, E. Nano-Silica Modified with Diamine for Capturing Azo Dye from Aqueous Solutions. Molecule 2022, 27, 3366. [Google Scholar] [CrossRef]
- Vinoda, B.M.; Vinuth, M. Photocatalytic Degradation of Toxic Methyl Red Dye Using Silica Nanoparticles Synthesized from Rice Husk Ash. J. Environ. Anal. Toxicol. 2015, 5, 1000336. [Google Scholar] [CrossRef]
- Chen, J.; Sheng, Y.; Song, Y.; Chang, M.; Zhang, X.; Cui, L.; Meng, D.; Zhu, H.; Shi, Z.; Zou, H. Multimorphology Mesoporous Silica Nanoparticles for Dye Adsorption and Multicolor Luminescence Applications. ACS Sustain. Chem. Eng. 2018, 6, 3533–3545. [Google Scholar] [CrossRef]
- Alswieleh, A.M. Efficient Removal of Dyes from Aqueous Solution by Adsorption on L-Arginine-Modified Mesoporous Silica Nanoparticles. Processes 2022, 10, 1079. [Google Scholar] [CrossRef]
- Sahoo, A.; Patra, S. A Combined Process for the Degradation of Azo-Dyes and Efficient Removal of Aromatic Amines Using Porous Silicon Supported Porous Ruthenium Nanocatalyst. ACS Appl. Nano Mater. 2018, 1, 5169–5178. [Google Scholar] [CrossRef]
- Ekka, B.; Sahu, M.K.; Patel, R.K.; Dash, P. Titania Coated Silica Nanocomposite Prepared via Encapsulation Method for the Degradation of Safranin-O Dye from Aqueous Solution: Optimization Using Statistical Design. Water Resour. Ind. 2019, 22, 100071. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A Comprehensive Review on Anti-Fouling Nanocomposite Membranes for Pressure Driven Membrane Separation Processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Reddy, A.S.; Kalla, S.; Murthy, Z.V.P. Nano-Particles Enhanced Hydrophobic Membranes: High-Performance Study for Dye Wastewater Treatment Using Membrane Distillation. J. Water Process Eng. 2022, 46, 102610. [Google Scholar] [CrossRef]
- Yang, B.; Geng, P.; Chen, G. One-Dimensional Structured IrO2 Nanorods Modified Membrane for Electrochemical Anti-Fouling in Filtration of Oily Wastewater. Sep. Purif. Technol. 2015, 156, 931–941. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A Review of Water Treatment Membrane Nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of Synthetic Dyes from Wastewaters: A Review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Singh, K.; Arora, S. Removal of Synthetic Textile Dyes from Wastewaters: A Critical Review on Present Treatment Technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807–878. [Google Scholar] [CrossRef]
- Aliabadi, M.; Irani, M.; Ismaeili, J.; Piri, H.; Parnian, M.J. Electrospun Nanofiber Membrane of PEO/Chitosan for the Adsorption of Nickel, Cadmium, Lead and Copper Ions from Aqueous Solution. Chem. Eng. J. 2013, 220, 237–243. [Google Scholar] [CrossRef]
- Deng, J.; Kang, X.; Chen, L.; Wang, Y.; Gu, Z.; Lu, Z. A Nanofiber Functionalized with Dithizone by Co-Electrospinning for Lead (II) Adsorption from Aqueous Media. J. Hazard. Mater. 2011, 196, 187–193. [Google Scholar] [CrossRef]
- Wu, S.; Li, F.; Wang, H.; Fu, L.; Zhang, B.; Li, G. Effects of Poly (Vinyl Alcohol) (PVA) Content on Preparation of Novel Thiol-Functionalized Mesoporous PVA/SiO2 Composite Nanofiber Membranes and Their Application for Adsorption of Heavy Metal Ions from Aqueous Solution. Polymer 2010, 51, 6203–6211. [Google Scholar] [CrossRef]
- Asouhidou, D.D.; Triantafyllidis, K.S.; Lazaridis, N.K.; Matis, K.A. Adsorption of Remazol Red 3BS from Aqueous Solutions Using APTES- and Cyclodextrin-Modified HMS-Type Mesoporous Silicas. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 346, 83–90. [Google Scholar] [CrossRef]
- Akduman, C.; Akçakoca Kumbasar, E.P.; Morsunbul, S. Electrospun Nanofiber Membranes for Adsorption of Dye Molecules from Textile Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 102001. [Google Scholar] [CrossRef]
- Long, Q.; Zhang, Z.; Qi, G.; Wang, Z.; Chen, Y.; Liu, Z.-Q. Fabrication of Chitosan Nanofiltration Membranes by the Film Casting Strategy for Effective Removal of Dyes/Salts in Textile Wastewater. ACS Sustain. Chem. Eng. 2020, 8, 2512–2522. [Google Scholar] [CrossRef]
- Hassan, A.R.; Rozali, S.; Safari, N.H.M.; Besar, B.H. The Roles of Polyethersulfone and Polyethylene Glycol Additive on Nanofiltration of Dyes and Membrane Morphologies. Environ. Eng. Res. 2018, 23, 316–322. [Google Scholar] [CrossRef]
- Gunawan, F.M.; Mangindaan, D.; Khoiruddin, K.; Wenten, I.G. Nanofiltration Membrane Cross-Linked by m-Phenylenediamine for Dye Removal from Textile Wastewater. Polym. Adv. Technol. 2019, 30, 360–367. [Google Scholar] [CrossRef]
- Buscio, V.; García-Jiménez, M.; Vilaseca, M.; López-Grimau, V.; Crespi, M.; Gutiérrez-Bouzán, C. Reuse of Textile Dyeing Effluents Treated with Coupled Nanofiltration and Electrochemical Processes. Materials 2016, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Askari, N.; Farhadian, M.; Razmjou, A.; Hashtroodi, H. Nanofiltration Performance in the Removal of Dye from Binary Mixtures Containing Anthraquinone Dyes. Desalination Water Treat. 2015, 57, 18194–18201. [Google Scholar] [CrossRef]
- Zhang, M.; Field, R.W.; Zhang, K. Biogenic Silver Nanocomposite Polyethersulfone UF Membranes with Antifouling Properties. J. Memb. Sci. 2014, 471, 274–284. [Google Scholar] [CrossRef]



| Alloy Name | Route of Synthesis | Organic Pollutants | Pollutant Concentration | Alloy Dose | pH | Time | Removal | Ref. |
|---|---|---|---|---|---|---|---|---|
| Zerovalent iron | Liquid phase reduction method | Methyl orange | 100 mg/L | 0.5 g/L | 4 | 60 min | 100% | [56] |
| Nanozerovalent iron | Chemically synthesized | Acid black 24 | 100 mg/L | 0.1647 g/L | 6.7 | 80 min | 80% | [57] |
| Nanoscale zerovalent iron | Chemically synthesized | Methylene blue | 70 mg/L | 10 g/L | 7 | 30 min | 72.1% | [58] |
| Iron-based nanoparticles | Green synthesized | Orange II | 5 mg/L | 10 mg/L | 3 | 60 min | 91.12% | [59] |
| Nanoscale zerovalent iron | Liquid phase reduction method | Basic yellow 28 | 100 mg/L | 2 g/L | 2 | 15 min | 99.2% | [60] |
| Zerovalent iron powder | Commercial | Reactive black 5 | 100 mg/L | 0.5 g/L | 3 | 120 min | 100% | [61] |
| Copper nanoparticles | Hydrodynamic cavitation | Methyl orange | 10 mg/L | 40 mg/L | 3 | 20 min | 83% | [62] |
| Zerovalent copper nanoparticles | Chemical reduction | Reactive blue 4 | 15 mg/L | 1 g/L | 3 | 10 min | 90% | [63] |
| Zerovalent cobalt nanoparticles | Galvanic replacement method | Methyl orange | 100 mg/L | 0.5 g/L | 2.5 | 4 min | 99% | [52] |
| AlFeMnTiCr | Ball milled | Direct blue 6 | 200 mg/L | 1 g/L | 7 | 11 min | 100% | [13] |
| AlCrFeMnMg | Ball milled | Direct blue 6 | 200 mg/L | 0.5 g/L | 7 | 6 min | 100% | [39] |
| Mn–Al | Ball milled | Reactive black 5 | 40 mg/L | 2.5 g/L | 3 | 20 min | 100% | [49] |
| Mn–Al | Ball milled | Orange II | 40 mg/L | 2.5 g/L | 3 | 30 min | 100% | [53] |
| Mn–Al | Ball milled | Brilliant green | 150 mg/L | 2.5 g/L | 3 | 75 min | 100% | [54] |
| MnAlFe | Ball milled | Reactive black 5 | 40 mg/L | 2.5 g/L | 3 | 5 min | 100% | [55] |
| Ca–Al | Ball milled | Reactive black 5 | 40 mg | 1 g/L | 6 | 1 min | 100% | [23] |
| Alloy Name | Route of Synthesis | Organic Pollutants | Pollutant Concentration | Alloy Dose | pH | Time | Removal | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fe–Si–B amorphous ribbon | Melt spinning | Rhodamine B | 20 mg/L | 0.5 g/L | 3 | 10 min | 100% | [67] |
| Fe-Si-B-Nb amourphousribbon | Melt spinning | Direct blue 15 | 100 mg/L | 0.03 g/L | - | 60 min | 100% | [68] |
| Fe–Si–B–Cu–Nb amourphousribbon | Melt spinning | Brilliant red 3B-A | 20 ppm | 2 g/L | 2 | 10 min | 90% | [69] |
| Fe–B–Si–Y | Melt spinning | Methyl orange | 20 mg/L | 4 g/L | 2 | 10 min | 92% | [70] |
| Mg–Cu–Y | Melt spinning + mechanical milling | Direct blue 6 | 0.02 g/L | 1.2 g/L | - | 8 min | 100% | [45] |
| Mg–Zn–Ca | Mechanical milling | Congo red | 200 ppm | 4 g/L | 6.7 | 120 min | 100% | [71] |
| Co–Si–B | Melt spinning + mechanical milling | Acid orange II | 0.2 g/L | 6 g/L | 3 | 2 min | 100% | [66] |
| Nanomembranes | Organic Pollutants | References |
|---|---|---|
| Chitosan | Methyl viologen, methylene blue, methyl orange, orange G, rose bengal, brilliant blue and methyl red | [184] |
| Nanofiltration surfactant (NFS) | Methyl violet, methyl blue and acid orange 74 | [185] |
| Polyetherimide (PEI)-based nanofiltration (NF) | Reactive red | [186] |
| Hydracore 10 and hydracore 50 | Cibacron yellow S-3R | [187] |
| Polyamide NF | Anthraquinone dyes | [188] |
| ZrO2 | Dimethyl formamide | [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbarek, W.B.; Escoda, L.; Saurina, J.; Pineda, E.; Alminderej, F.M.; Khitouni, M.; Suñol, J.-J. Nanomaterials as a Sustainable Choice for Treating Wastewater: A Review. Materials 2022, 15, 8576. https://doi.org/10.3390/ma15238576
Mbarek WB, Escoda L, Saurina J, Pineda E, Alminderej FM, Khitouni M, Suñol J-J. Nanomaterials as a Sustainable Choice for Treating Wastewater: A Review. Materials. 2022; 15(23):8576. https://doi.org/10.3390/ma15238576
Chicago/Turabian StyleMbarek, Wael Ben, Lluisa Escoda, Joan Saurina, Eloi Pineda, Fahad M. Alminderej, Mohamed Khitouni, and Joan-Josep Suñol. 2022. "Nanomaterials as a Sustainable Choice for Treating Wastewater: A Review" Materials 15, no. 23: 8576. https://doi.org/10.3390/ma15238576
APA StyleMbarek, W. B., Escoda, L., Saurina, J., Pineda, E., Alminderej, F. M., Khitouni, M., & Suñol, J.-J. (2022). Nanomaterials as a Sustainable Choice for Treating Wastewater: A Review. Materials, 15(23), 8576. https://doi.org/10.3390/ma15238576











