Electrophoresis-Aided Biomimetic Mineralization System Using Graphene Oxide for Regeneration of Hydroxyapatite on Dentin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Lesion Formation and pH Cycling
2.3. Synthesis of GO
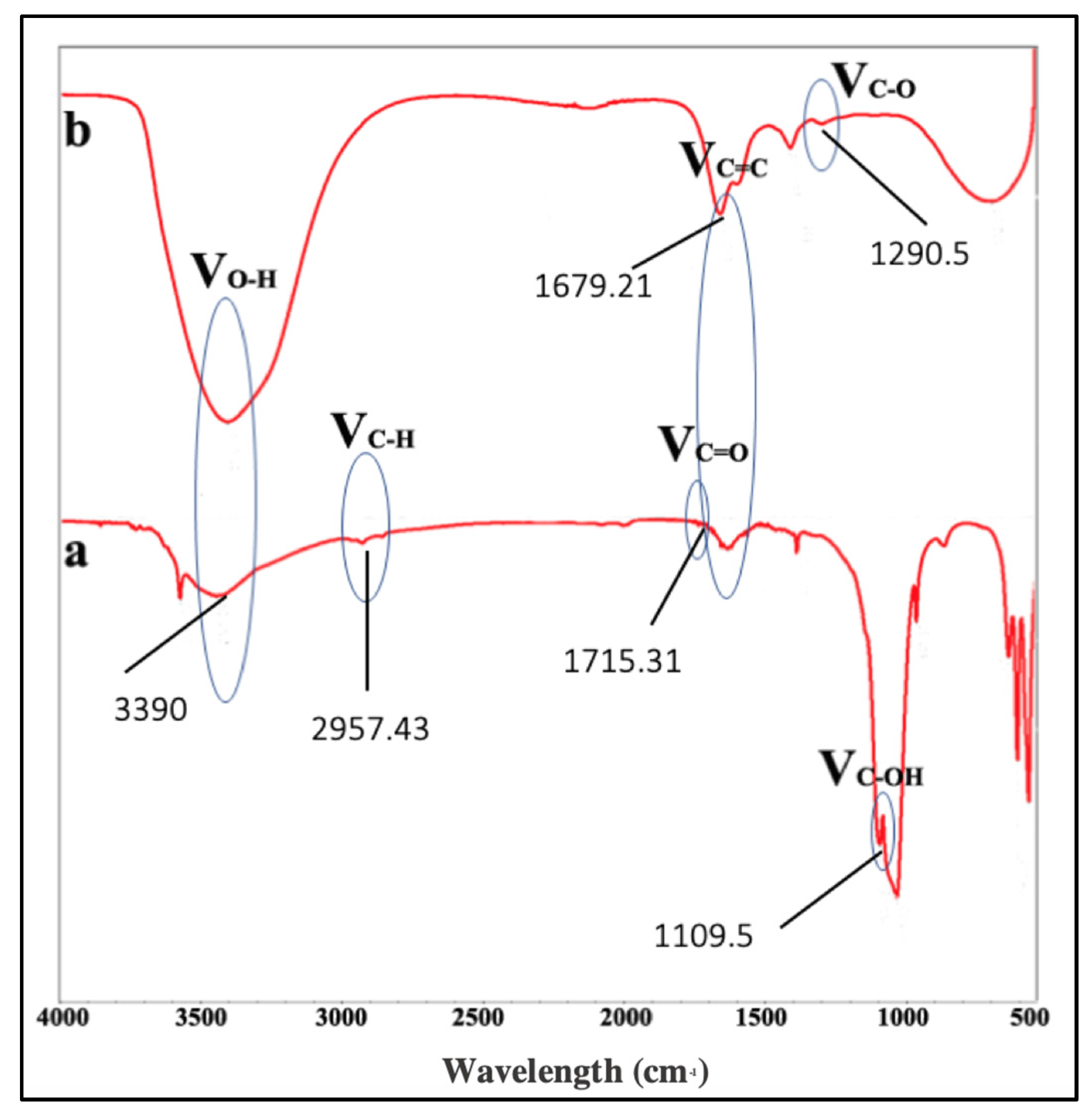
2.4. Characterization of GO
2.5. Preparation of the Mineralizing Medium in Agarose Hydrogel
2.6. Regeneration of Hydroxyapatite in Agarose Hydrogel Aided by Electrophoresis
2.7. Characterization and Evaluation of New Crystal
3. Results
3.1. The Synthesis of GO
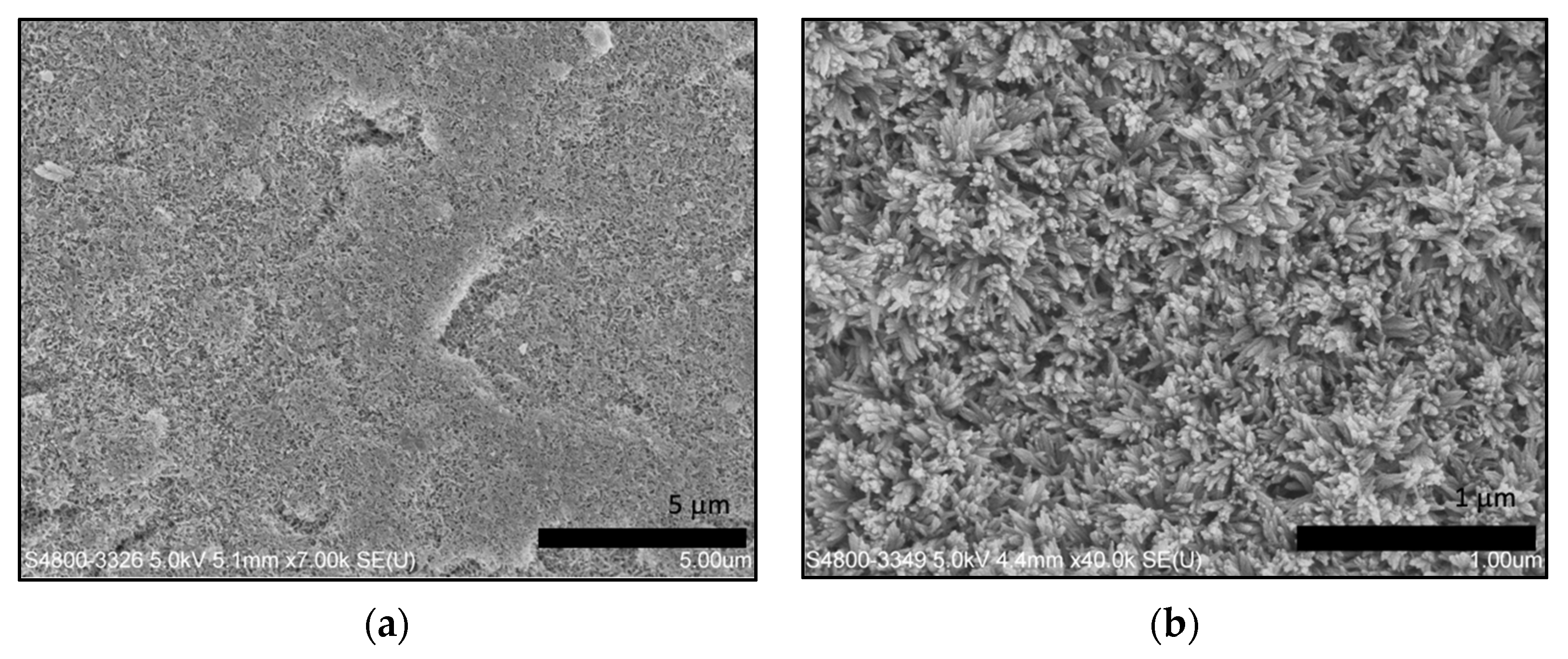
3.2. Characterization of Remineralized Dentin Dlices
3.3. Mechanical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Tang, Z.; Liu, J.; Sun, K.; Chang, S.-R.; Peters, M.C.; Mansfield, J.F.; Czajka-Jakubowska, A.; Clarkson, B.H. Acellular Synthesis of a Human Enamel-like Microstructure. Adv. Mater. 2006, 18, 1846–1851. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Wong, H.M.; McGrath, C.P.J.; Li, Q.L. Repair of dentine-related lesions without a drill or injection. RSC Adv. 2019, 9, 15099–15107. [Google Scholar] [CrossRef] [Green Version]
- Crespi, R.; Capparè, P.; Gherlone, E. Comparison of Magnesium-Enriched Hydroxyapatite and Porcine Bone in Human Extraction Socket Healing: A Histologic and Histomorphometric Evaluation. Int. J. Oral Maxillofac. Implants 2011, 26, 1057–1062. [Google Scholar]
- Enax, J.; Fabritius, H.O.; Fabritius-Vilpoux, K.; Amaechi, B.T.; Meyer, F. Modes of action and clinical efficacy of particulate hydroxyapatite in preventive oral health care—State of the art. Open Dent. J. 2019, 13, 274–287. [Google Scholar] [CrossRef]
- Hu, M.-L.; Zheng, G.; Zhang, Y.-D.; Yan, X.; Li, X.-C.; Lin, H. Effect of desensitizing toothpastes on dentine hypersensitivity: A systematic review and meta-analysis. J. Dent. 2018, 75, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, W.; Ning, T.; Mei, M.L.; Mei, M.L.; Li, Q.-L.; Lo, E.C.M.; Chu, C.H. A novel oligopeptide simulating dentine matrix protein 1 for biomimetic mineralization of dentine. Clin. Oral Investig. 2014, 18, 873–881. [Google Scholar] [CrossRef]
- Yamagishi, K.; Onuma, K.; Suzuki, T.; Okada, F.; Tagami, J.; Otsuki, M.; Senawangse, P. Materials chemistry: A synthetic enamel for rapid tooth repair. Nature 2005, 433, 819. [Google Scholar] [CrossRef] [PubMed]
- Forsback, A.P.; Areva, S.; Salonen, J.I. Mineralization of dentin induced by treatment with bioactive glass S53P4 in vitro. Acta Odontol. Scand. 2004, 62, 14–20. [Google Scholar] [CrossRef]
- Chen, H.; Clarkson, B.H.; Sun, K.; Mansfield, J.F. Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J. Colloid. Interface Sci. 2005, 288, 97–103. [Google Scholar] [CrossRef]
- Li, L.; Mao, C.; Wang, J.; Xu, X.; Pan, H.; Deng, Y.; Gu, X.; Tang, R. Bio-inspired enamel repair via Glu-directed assembly of apatite nanoparticles: An approach to biomaterials with optimal characteristics. Adv. Mater. 2011, 23, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Zhang, Y.; Yang, X.; Nutt, S.; Moradian-Oldak, J. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013, 9, 7289–7297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Wen, Z.T.; Liao, S.; Lallier, T.; Hagan, J.L.; Twomley, J.T.; Zhang, J.F.; Sun, Z.; Xu, X. Novel amelogenin-releasing hydrogel for remineralization of enamel artificial caries. J. Bioact. Compat. Polym. 2012, 27, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Busch, S. Regeneration of human tooth enamel. Angew. Chem. Int. Ed. Engl. 2004, 43, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, J.; Li, J.; Chen, L.; Tang, B.; Chen, X.; Wu, W.; Li, J. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 2013, 34, 5036–5047. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Feng, Z.; Li, S.; Xu, B. EDTA-Assisted Self-Assembly of Fluoride-Substituted Hydroxyapatite Coating on Enamel Substrate. Cryst. Growth Des. 2011, 11, 5206–5214. [Google Scholar] [CrossRef]
- Liu, S.; Yin, Y.; Chen, H. PEO-assisted precipitation of human enamel-like fluorapatite films for tooth whitening. Cryst. Eng. Comm. 2013, 15, 5853–5859. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Cao, Y.; Liu, W.; Chu, C.H.; Li, Q.L. Polydopamine-induced tooth remineralization. ACS Appl. Mater. Interfaces 2012, 4, 6901–6910. [Google Scholar] [CrossRef]
- Tetè, G.; D’Orto, B.; Nagni, M.; Agostinacchio, M.; Polizzi, E.; Agliardi, E. Role of induced pluripotent stem cells (IPSCS) in bone tissue regeneration in dentistry: A narrative review. J. Biol. Regul. Homeost. Agents 2020, 34, 1–10. [Google Scholar]
- Ramírez-Rodríguez, G.B.; Pereira, A.R.; Herrmann, M.; Hansmann, J.; Delgado-López, J.M.; Sprio, S.; Tampieri, A.; Sandri, M. Biomimetic Mineralization Promotes Viability and Differentiation of Human Mesenchymal Stem Cells in a Perfusion Bioreactor. Int. J. Mol. Sci. 2021, 22, 1447. [Google Scholar] [CrossRef]
- Wu, X.-T.; Cao, Y.; Mei, M.L.; Chen, J.-L.; Li, Q.-L.; Chu, C.H. An Electrophoresis-Aided Biomineralization System for Regenerating Dentin- and Enamel-Like Microstructures for the Self-Healing of Tooth Defects. Cryst. Growth Des. 2014, 14, 5537–5548. [Google Scholar] [CrossRef]
- Watanabe, J.; Akashi, M. Novel Biomineralization for Hydrogels: Electrophoresis Approach Accelerates Hydroxyapatite Formation in Hydrogels. Biomacromolecules 2006, 7, 3008–3011. [Google Scholar] [CrossRef]
- Wu, X.T.; Mei, M.L.; Li, Q.L.; Cao, C.Y.; Chen, J.L.; Xia, R.; Zhang, Z.H.; Chu, C.H. A Direct Electric Field-Aided Biomimetic Mineralization System for Inducing the Remineralization of Dentin Collagen Matrix. Materials 2015, 8, 7889–7899. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Wong, H.M.; McGrath, C.P.J.; Li, Q.L. In vitro and in vivo evaluation of electrophoresis-aided casein phosphopeptide-amorphous calcium phosphate remineralisation system on pH-cycling and acid-etching demineralised enamel. Sci. Rep. 2018, 8, 8904. [Google Scholar] [CrossRef]
- Nosrati, H.; Sarraf-Mamoory, R.; Le, D.Q.S.; Zolfaghari Emameh, R.; Canillas Perez, M.; Bünger, C.E. Improving the mechanical behavior of reduced graphene oxide/hydroxyapatite nanocomposites using gas injection into powders synthesis autoclave. Sci. Rep. 2020, 10, 8552. [Google Scholar] [CrossRef]
- Wei, G.; Gong, C.; Hu, K.; Wang, Y.; Zhang, Y. Biomimetic Hydroxyapatite on Graphene Supports for Biomedical Applications: A Review. Nanomaterials 2019, 9, 1435. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef]
- Gao, F.; Xu, C.; Hu, H.; Wang, Q.; Gao, Y.; Chen, H.; Guo, Q.; Chen, D.; Eder, D. Biomimetic synthesis and characterization of hydroxyapatite/graphene oxide hybrid coating on Mg alloy with enhanced corrosion resistance. Mater. Lett. 2015, 138, 25–28. [Google Scholar] [CrossRef]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.L.; Lo, E.C.; Chu, C.H. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J. Dent. 2013, 41, 809–817. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Wong, H.M.; Zhang, Y.Y.; Li, Q.L. An enamel-inspired bioactive material with multiscale structure and antibacterial adhesion property. Bioact. Mater. 2022, 7, 491–503. [Google Scholar] [CrossRef]
- Khan, S.; Khan, S.; Khan, L.; Farooq, A.; Akhtar, K.; Asiri, A.M. Fourier Transform Infrared Spectroscopy: Fundamentals and Application in Functional Groups and Nanomaterials Characterization. In Handbook of Materials Characterization; Springer: Berlin/Heidelberg, Germany, 2018; pp. 317–344. [Google Scholar]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Liu, H.; Xi, P.; Xie, G.; Shi, Y.; Hou, F.; Huang, L.; Chen, F.; Zeng, Z.; Shao, C.; Wang, J. Simultaneous Reduction and Surface Functionalization of Graphene Oxide for Hydroxyapatite Mineralization. J. Phys. Chem. C 2012, 116, 3334–3341. [Google Scholar] [CrossRef]
- Adetayo, A.; Runsewe, D. Synthesis and Fabrication of Graphene and Graphene Oxide: A Review. Open J. Compos. Mater. 2019, 9, 207–229. [Google Scholar] [CrossRef] [Green Version]
- Eda, G.; Lin, Y.Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.A.; Chen, I.S.; Chen, C.W.; Chhowalla, M. Blue photoluminescence from chemically derived graphene oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of Enhanced Stem Cell Growth and Differentiation on Graphene and Graphene Oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, Y.; Somers, L.A.; Johnson, A.T.C. High Yield Preparation of Macroscopic Graphene Oxide Membranes. J. Am. Chem. Soc. 2009, 131, 898–899. [Google Scholar] [CrossRef]
- Zeng, Y.; Pei, X.; Yang, S.; Qin, H.; Cai, H.; Hu, S.; Sui, L.; Wan, Q.; Wang, J. Graphene oxide/hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf. Coat. Technol. 2016, 286, 72–79. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Khalili, D. Graphene oxide: A promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. New J. Chem. 2016, 40, 2547–2553. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Nishina, Y.; Yamamoto, T.; Shinoda-Ito, Y.; Takashiba, S. Functionalized Graphene Oxide Shields Tooth Dentin from Decalcification. J. Dent. Res. 2020, 99, 182–188. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhang, L.; Du, C.; Fang, T.; Hu, J. Direct Reduction of Graphene Oxide/Nanofibrillated Cellulose Composite Film and its Electrical Conductivity Research. Sci. Rep. 2020, 10, 3124. [Google Scholar] [CrossRef] [Green Version]
- Ambrosio, G.; Drera, G.; Di Santo, G.; Petaccia, L.; Daukiya, L.; Brown, A.; Hirsch, B.; De Feyter, S.; Sangaletti, L.; Pagliara, S. Interface Chemistry of Graphene/Cu Grafted By 3,4,5-Tri-Methoxyphenyl. Sci. Rep. 2020, 10, 4114. [Google Scholar] [CrossRef]
- Nazri, S.R.B.; Liu, W.-W.; Khe, C.-S.; Hidayah, N.M.S.; Teoh, Y.-P.; Voon, C.H.; Lee, H.C.; Adelyn, P.Y.P. Synthesis, characterization and study of graphene oxide. AIP Conf. Proc. 2018, 2045, 020033. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef]
- Rosso, M.; Blasi, G.; Gherlone, E.; Rosso, R. Effect of granulocyte-macrophage colony-stimulating factor on prevention of mucositis in head and neck cancer patients treated with chemo-radiotherapy. J. Chemother. 1997, 9, 382–385. [Google Scholar] [CrossRef]
- Muñoz, R.; Singh, D.P.; Kumar, R.; Matsuda, A. Chapter 22—Graphene Oxide for Drug Delivery and Cancer Therapy. In Nanostructured Polymer Composites for Biomedical Applications; Swain, S.K., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–488. [Google Scholar]
- Garg, K.; Papponen, P.; Johansson, A.; Puttaraksa, N.; Gilbert, L. Preparation of graphene nanocomposites from aqueous silver nitrate using graphene oxide’s peroxidase-like and carbocatalytic properties. Sci. Rep. 2020, 10, 5126. [Google Scholar] [CrossRef] [Green Version]
- Adamowicz, J.; Pasternak, I.; Kloskowski, T.; Gniadek, M.; Van Breda, S.V.; Buhl, M.; Balcerczyk, D.; Gagat, M.; Grzanka, D.; Strupinski, W.; et al. Development of a conductive biocomposite combining graphene and amniotic membrane for replacement of the neuronal network of tissue-engineered urinary bladder. Sci. Rep. 2020, 10, 5824. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Huang, P. Chapter 9—Graphene-Based Nanomaterials in Bioimaging. In Biomedical Applications of Functionalized Nanomaterials; Sarmento, B., das Neves, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 247–287. [Google Scholar]
- Chen, G.Y.; Pang, D.W.; Hwang, S.M.; Tuan, H.Y.; Hu, Y.C. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials 2012, 33, 418–427. [Google Scholar] [CrossRef]
- Baradaran, S.; Moghaddam, E.; Basirun, W.J.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Moghaddam, M.R.N.; Alias, Y. Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon 2014, 69, 32–45. [Google Scholar] [CrossRef]
- Zhou, Z.; Ge, X.; Bian, M.; Xu, T.; Li, N.; Lu, J.; Yu, J. Remineralization of dentin slices using casein phosphopeptide-amorphous calcium phosphate combined with sodium tripolyphosphate. Biomed. Eng. Online 2020, 19, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Neoh, K.G.; Lin, C.C.; Kishen, A. Remineralization of partially demineralized dentine substrate based on a biomimetic strategy. J. Mater. Sci. Mater. Med. 2012, 23, 733–742. [Google Scholar] [CrossRef] [PubMed]





| Concentration (wt.%) | Control Group—Without the Aid of Electrophoresis | Experimental Group—With the Aid of Electrophoresis and Addition of GO |
|---|---|---|
| C | 6.198 | 8.842 |
| Ca | 42.524 | 37.644 |
| P | 19.604 | 18.726 |
| F | 4.792 | 5.223 |
| O | 26.883 | 29.565 |
| Ca/P ratio | 1.628 | 1.533 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosalim, I.P.; Zhang, Y.Y.; Yiu, C.K.Y.; Wong, H.M. Electrophoresis-Aided Biomimetic Mineralization System Using Graphene Oxide for Regeneration of Hydroxyapatite on Dentin. Materials 2022, 15, 199. https://doi.org/10.3390/ma15010199
Khosalim IP, Zhang YY, Yiu CKY, Wong HM. Electrophoresis-Aided Biomimetic Mineralization System Using Graphene Oxide for Regeneration of Hydroxyapatite on Dentin. Materials. 2022; 15(1):199. https://doi.org/10.3390/ma15010199
Chicago/Turabian StyleKhosalim, Ingrid Patricia, Yu Yuan Zhang, Cynthia Kar Yung Yiu, and Hai Ming Wong. 2022. "Electrophoresis-Aided Biomimetic Mineralization System Using Graphene Oxide for Regeneration of Hydroxyapatite on Dentin" Materials 15, no. 1: 199. https://doi.org/10.3390/ma15010199






