Resistance of Aluminide Coatings on Austenitic Stainless Steel in a Nitriding Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
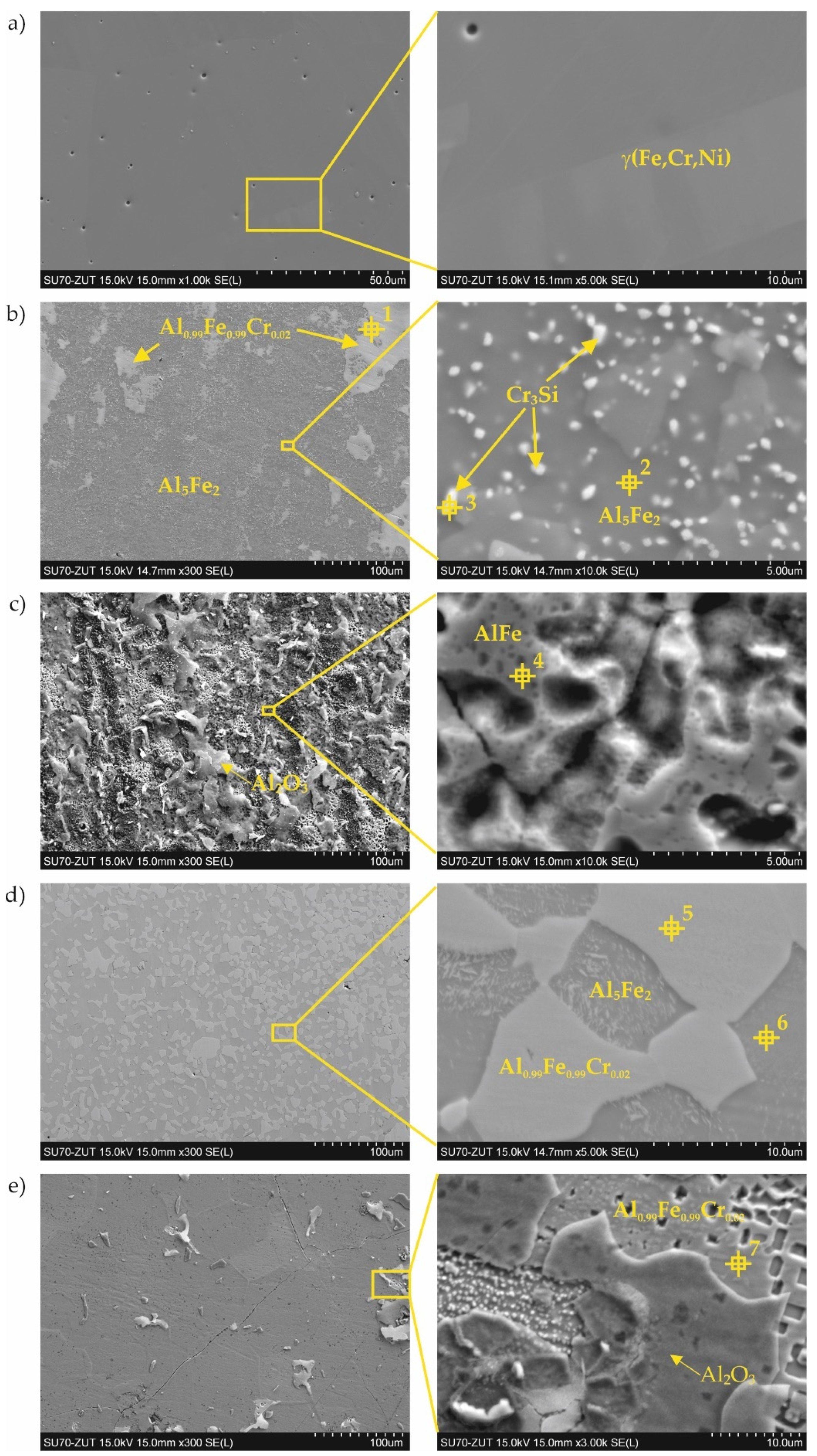
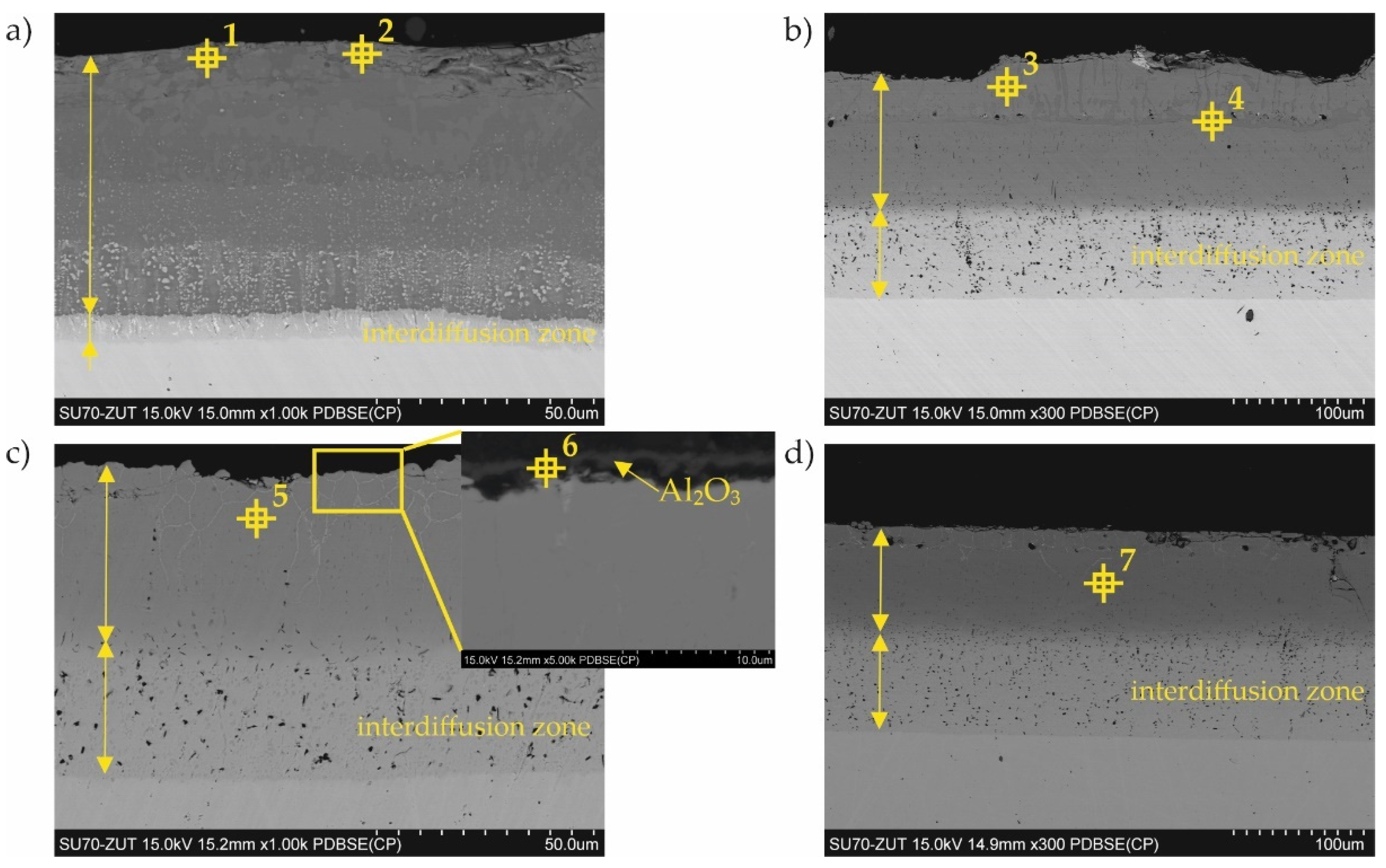
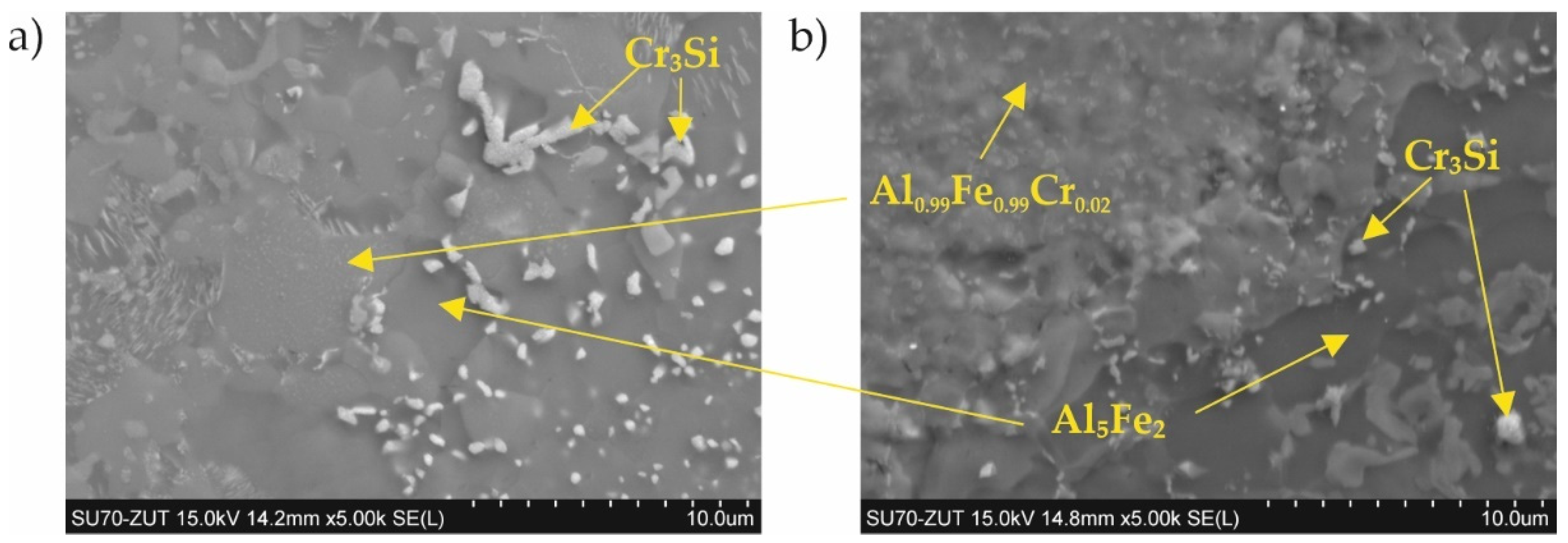
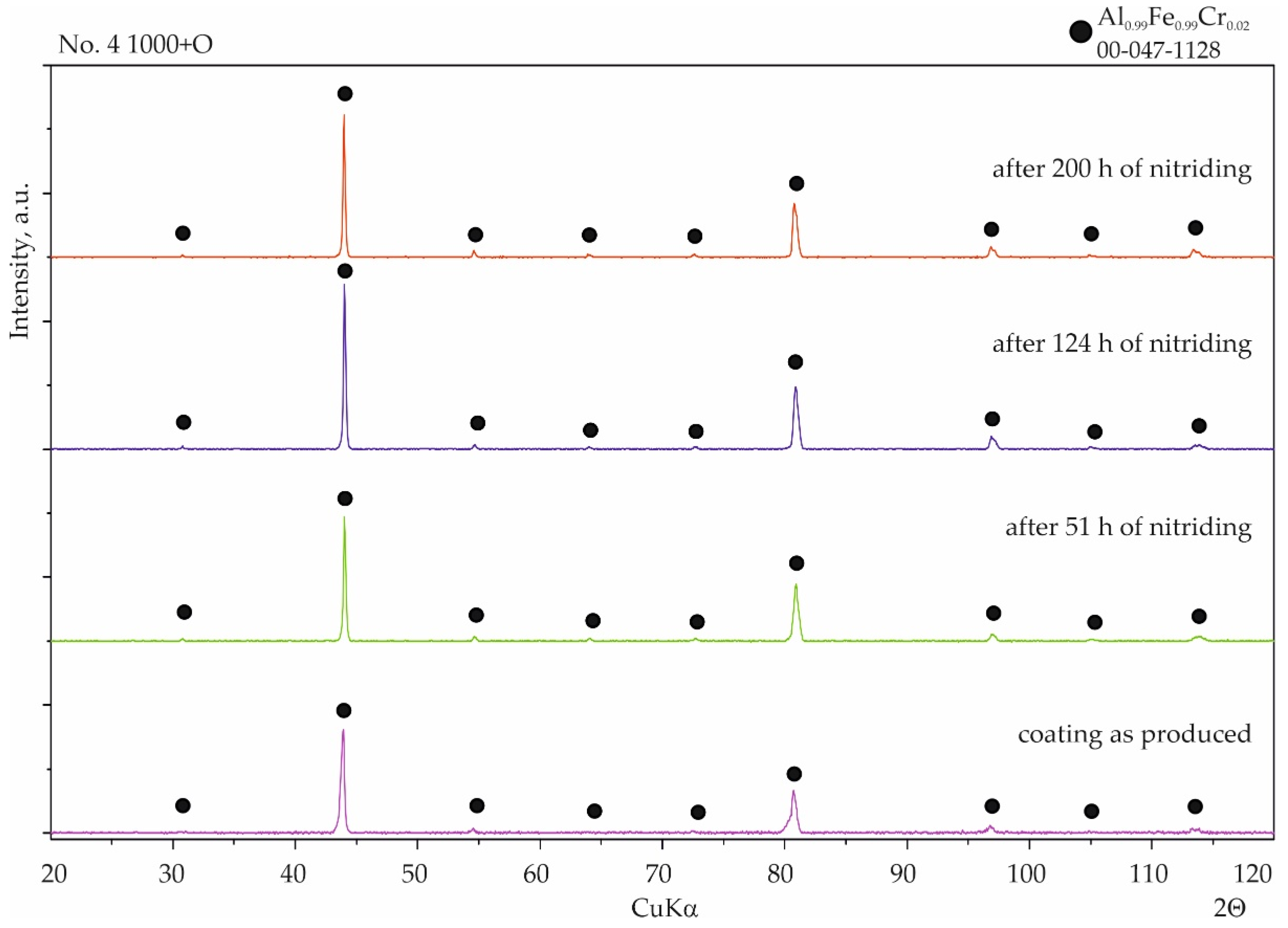
3.1. Coatings as Produced
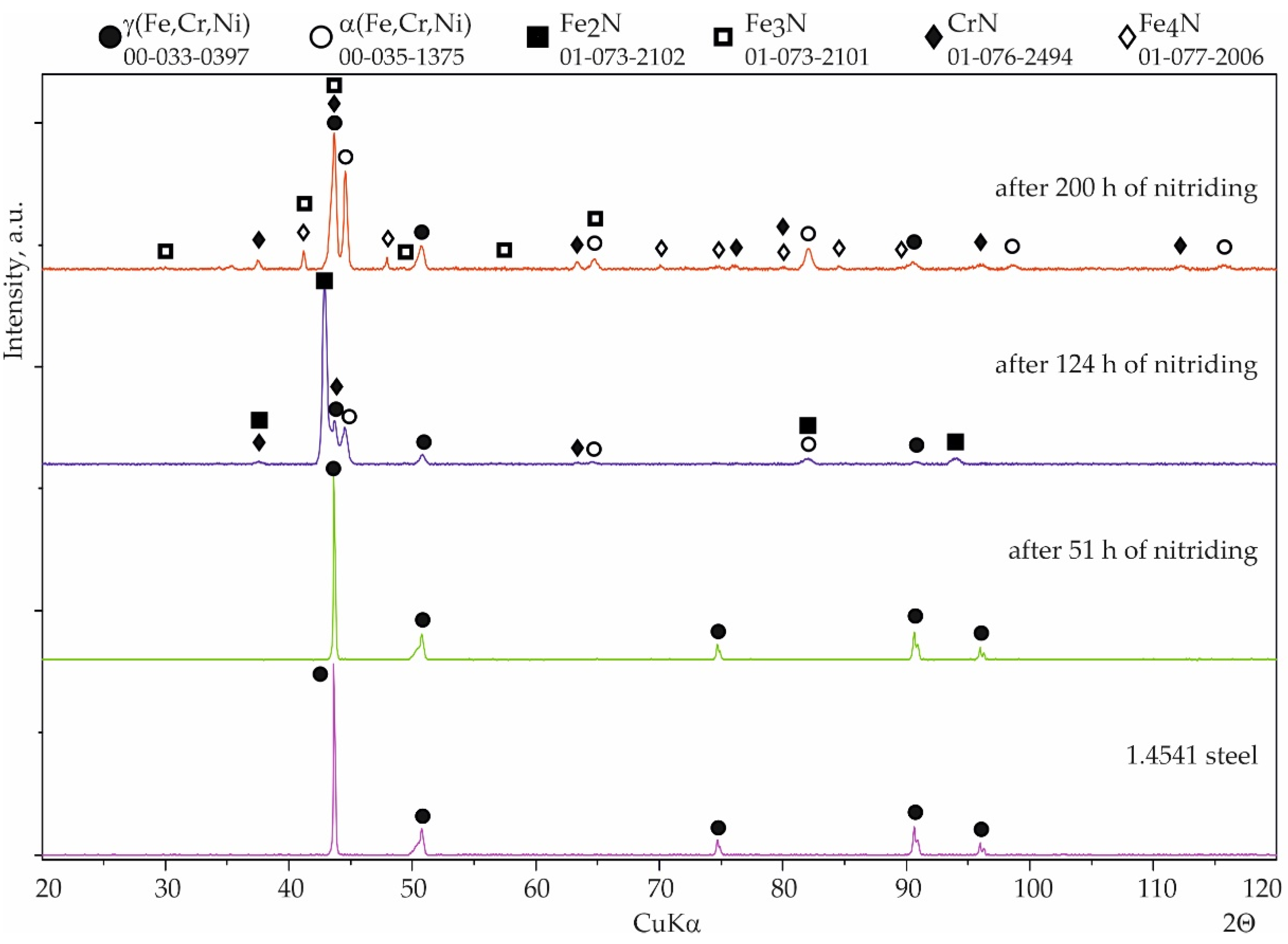
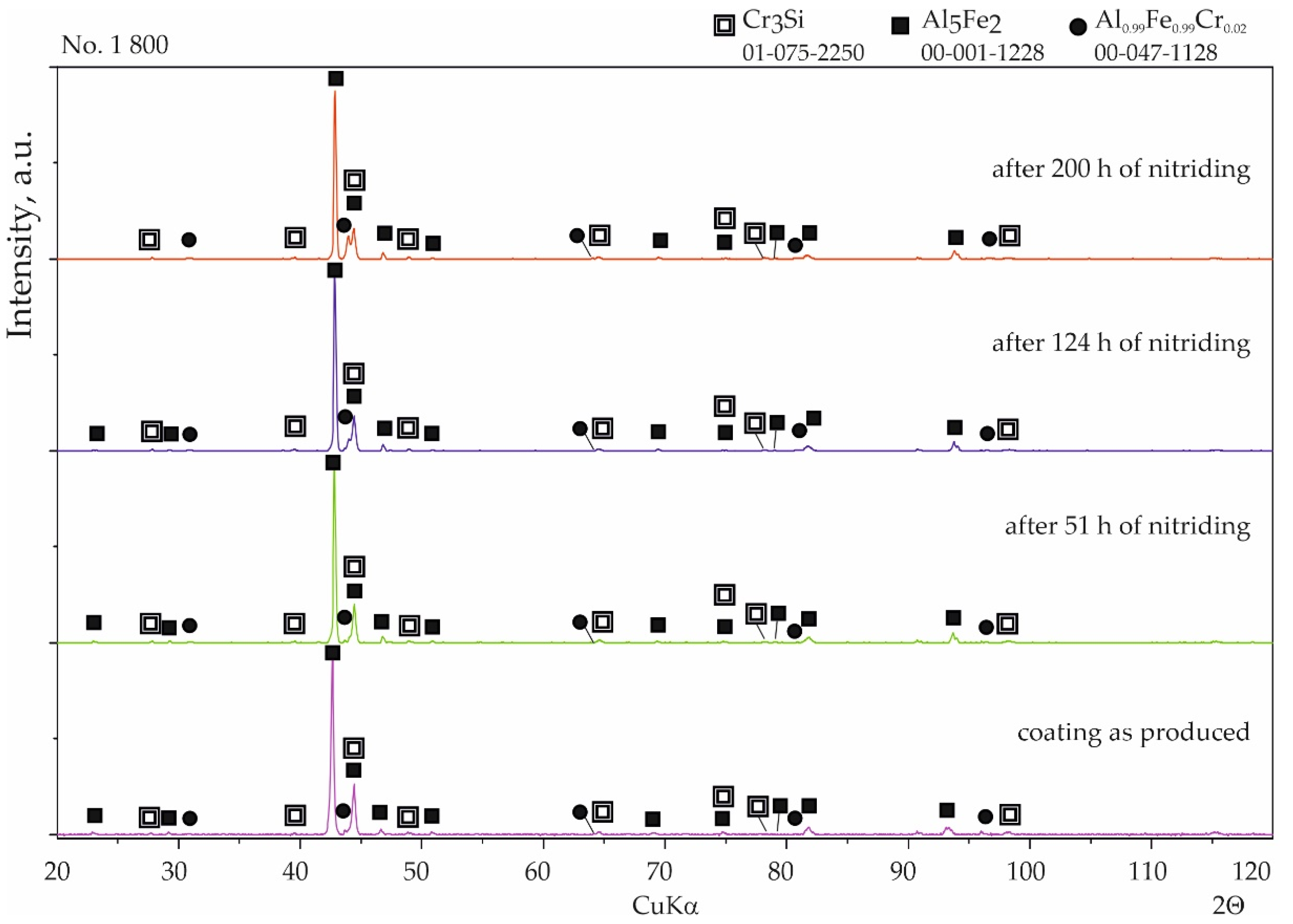
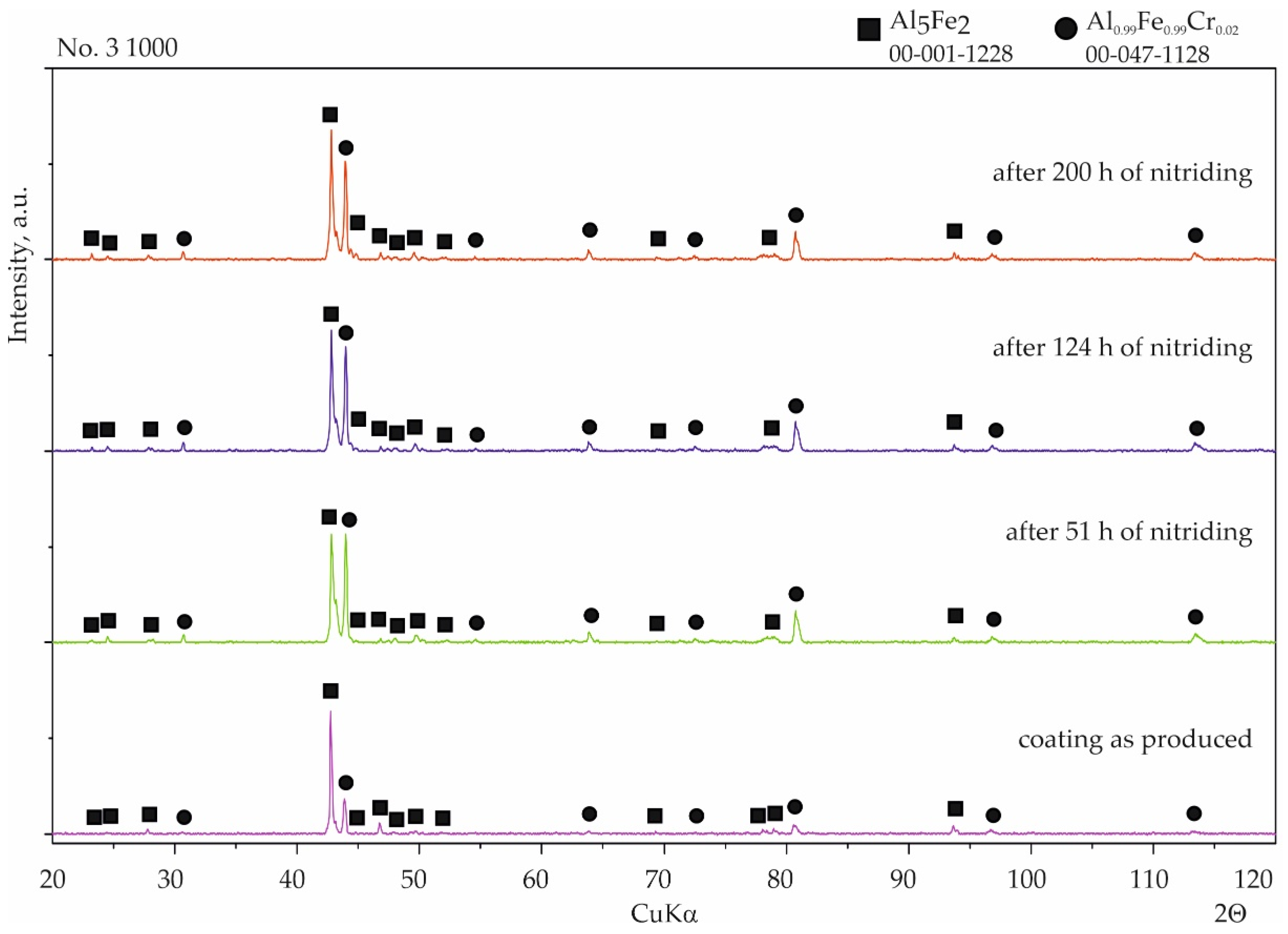
3.2. Nitriding Test
4. Discussion
5. Conclusions
- Austenitic stainless steel 1.4541 is not resistant to nitridation; this was confirmed by examinations after 51 h of nitriding.
- The production of a diffusion coating on austenitic stainless steel 1.4541 is possible by the method of slurry cementation at temperatures of 800 and 1000 °C.
- The technological parameters of the production process have a significant impact on the structure of silicide-aluminide coatings: the higher the coating formation temperature, the more homogeneous the structure and the greater its stability.
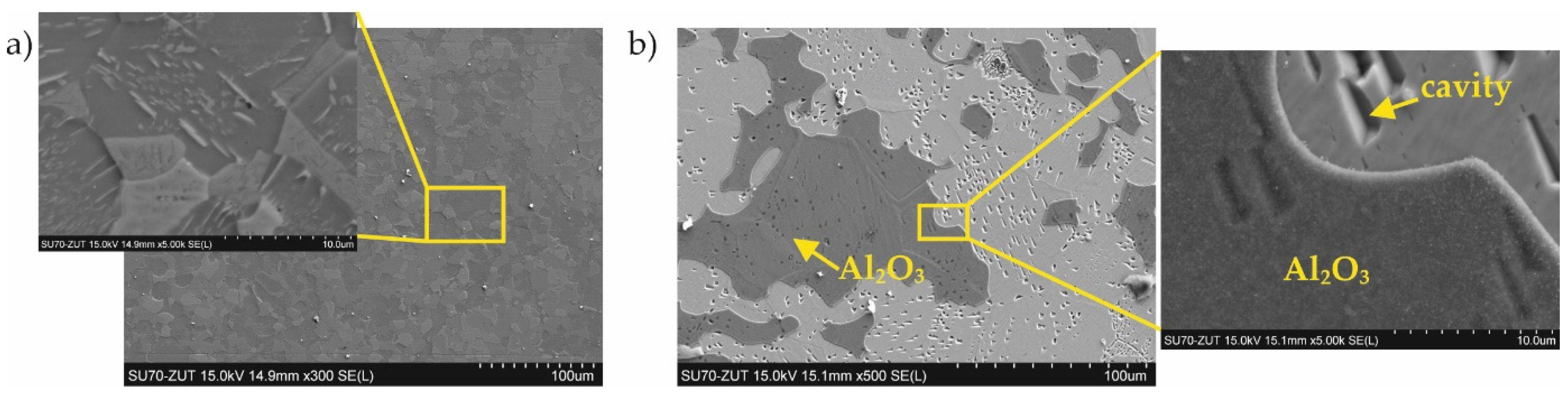
- The four designed coating production processes resulted in the formation of four different phase compositions of the coatings. The coating produced at 800 °C was composed of Al0.99Fe0.99Cr0.02, Al5Fe2, and Cr3Si. The coating produced at 800 °C with additional oxidation at 1000 °C was composed of AlFe and a passive layer of Al2O3. The coating produced at 1000 °C was composed of Al0.99Fe0.99Cr0.02 and Al5Fe2. The coating produced at 1000 °C with additional oxidation at 1000 °C was composed of Al0.99Fe0.99Cr0.02 and a passive layer of Al2O3.
- The diffusion coatings obtained enhanced the resistance of steel to high-temperature corrosion in a nitriding atmosphere. Besides the sample with the coating produced at 800 °C with additional oxidation at 1000 °C, silicon-aluminide coatings were resistant to aggressive ammonia during a 200 h treatment.
- The phases Al0.99Fe0.99Cr0.02 and Al5Fe2 are resistant to high-temperature corrosion under a nitriding atmosphere, in contrast to the AlFe phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venkataraman, S.; Jakobi, D. Review on the Heat Resistant Stainless Steel Alloys Used for the Steam Methane Reformer Outlet Systems. In Proceedings of the NACE International Corrosion Conference Proceedings, New Orleans, LA, USA, 26–30 March 2017; NACE International: Houston, TX, USA, 2017; pp. 1–15. [Google Scholar]
- Kondrat’ev, S.Y.; Kraposhin, V.S.; Anastasiadi, G.P.; Talis, A.L. Experimental observation and crystallographic description of M7C3 carbide transformation in Fe-Cr-Ni-C HP type alloy. Acta Mater. 2015, 100, 275–281. [Google Scholar] [CrossRef]
- Kondrat’ev, S.Y.; Anastasiadi, G.P.; Ptashnik, A.V.; Petrov, S.N. Kinetics of the High-Temperature Oxidation of Heat-Resistant Statically and Centrifugally Cast HP40NbTi Alloys. Oxid. Met. 2019, 91, 33–53. [Google Scholar] [CrossRef]
- Picasso, A.C.; Lanz, C.A.; Lissarrague, M.S.; Garófoli, A.D. Microstructure Evolution of a Nickel-Base Alloy Resistant to High Temperature during Aging. J. Miner. Mater. Charact. Eng. 2016, 4, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Tillack, D.J.; Guthrie, J.E. Wrought and cast heat-resistant stainless steels and nickel alloys for the refining and petrochemical industries. Nickel Dev. Inst. 1998, 10071, 1–16. [Google Scholar]
- Col, A.; Parry, V.; Pascal, C. Oxidation of a Fe-18Cr-8Ni austenitic stainless steel at 850 °C in O2: Microstructure evolution during breakaway oxidation. Corros. Sci. 2017, 114, 17–27. [Google Scholar] [CrossRef]
- Gheno, T.; Monceau, D.; Young, D.J. Kinetics of breakaway oxidation of Fe-Cr and Fe-Cr-Ni alloys in dry and wet carbon dioxide. Corros. Sci. 2013, 77, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Agüero, A.; Baráibar, I.; Muelas, R.; Oskay, C.; Galetz, M.; Korner, E. Analysis of an aluminide coating on austenitic steel 800HT exposed to metal dusting conditions: Lessons from an industrial hydrogen production plant. Int. J. Press. Vessel. Pip. 2020, 186, 104129. [Google Scholar] [CrossRef]
- Sequeira, C. High Temperature Corrosion, Fundamentals and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 9781119474425. [Google Scholar]
- Roberge, P.R. Handbook of Corrosion Engineering, 3rd ed.; McGraw Hill Education: New York, NY, USA, 2019; ISBN 9781260116977. [Google Scholar]
- Kung, S.C. High-temperature coating for titanium aluminides using the pack-cementation technique. Oxid. Met. 1990, 34, 217–228. [Google Scholar] [CrossRef]
- Grabke, H. Metal dusting. In Corrosion by Carbon and Nitrogen; Woodhead Publishing: Sawston, UK, 2007. [Google Scholar]
- Sakidja, R.; Perepezko, J.H.; Calhoun, P. Synthesis, Thermodynamic Stability and Diffusion Mechanism of Al5Fe2-Based Coatings. Oxid. Met. 2014, 81, 167–177. [Google Scholar] [CrossRef]
- Wang, J.; Wu, D.J.; Zhu, C.Y.; Xiang, Z.D. Low temperature pack aluminising kinetics of nickel electroplated on creep resistant ferritic steel. Surf. Coat. Technol. 2013, 236, 135–141. [Google Scholar] [CrossRef]
- Duminica, F.D.; Vanden Eynde, X.; Mandy, M.; Nabi, B.; Georges, C.; Sturel, T.; Drillet, P.; Grigorieva, R. Investigation of PVD thin films as hydrogen barriers in aluminized press hardened steels (PHS). Surf. Coat. Technol. 2020, 397, 125940. [Google Scholar] [CrossRef]
- Fryska, S.; Baranowska, J.; Przekop, J.; Suszko, T. The properties of hard coating composed of S-phase obtained by PVD method. Adv. Manuf. Sci. Technol. 2009, 33, 59–69. [Google Scholar]
- Bhuvaneswaran, N.; Kamachi Mudali, U.; Shankar, P. Characterization of aluminide coatings formed by diffusion alloying on nitrogen-containing type 316L stainless steels. Scr. Mater. 2003, 49, 1133–1138. [Google Scholar] [CrossRef]
- Kochmańska, A. Hot corrosion resistance properties of Al-Si coatings obtained by slurry method. Defect Diffus. Forum 2012, 326–328, 273–278. [Google Scholar] [CrossRef]
- Kochmańska, A.E. Microstructure of Al-Si Slurry Coatings on Austenitic High-Temperature Creep Resisting Cast Steel. Adv. Mater. Sci. Eng. 2018, 2018, 5473079. [Google Scholar] [CrossRef] [Green Version]
- Deevi, S.C. Advanced intermetallic iron aluminide coatings for high temperature applications. Prog. Mater. Sci. 2021, 118, 100769. [Google Scholar] [CrossRef]
- Tortorelli, P.F.; Natesan, K. Critical factors affecting the high-temperature corrosion performance of iron aluminides. Mater. Sci. Eng. A 1998, 258, 115–125. [Google Scholar] [CrossRef]
- Mortazavi, A.N.; Esmaily, M.; Geers, C.; Birbilis, N.; Svensson, J.E.; Halvarsson, M.; Chandrasekaran, D.; Johansson, L.G. Exploring failure modes of alumina scales on FeCrAl and FeNiCrAl alloys in a nitriding environment. Acta Mater. 2020, 201, 131–146. [Google Scholar] [CrossRef]
- Meka, S.R.; Bischoff, E.; Schacherl, R.E.; Mittemeijer, E.J. Unusual nucleation and growth of γ iron nitride upon nitriding Fe-4.75 at. % Al alloy. Philos. Mag. 2012, 92, 1083–1105. [Google Scholar] [CrossRef]
- Le, N.M.; Schimpf, C.; Biermann, H.; Dalke, A. Effect of Nitriding Potential KN on the Formation and Growth of a “White Layer” on Iron Aluminide Alloy. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2021, 52, 414–424. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Dong, H. Plasma-nitriding and characterization of FeAl40 iron aluminide. Acta Mater. 2015, 86, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Berrached, I.; Rabahi, L.; Gallouze, M.; Kellou, A. Nitriding effect on structural stability and magnetic properties of FeAl alloys: DFT study. J. Magn. Magn. Mater. 2019, 480, 79–86. [Google Scholar] [CrossRef]
- Tamarin, Y. Protective Coatings for Turbine Blades; ASM International: Almere, The Netherlands, 2002; 300p. [Google Scholar]
- Kepa, T.; Pedraza, F.; Rouillard, F. Intermetallic formation of Al-Fe and Al-Ni phases by ultrafast slurry aluminization (flash aluminizing). Surf. Coat. Technol. 2020, 397, 126011. [Google Scholar] [CrossRef]
- Kochmański, P.; Baranowska, J.; Fryska, S. Microstructure of low-temperature gas-carbonitrided layers on austenitic stainless steel. Metals 2019, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Yakou, T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater. Sci. Eng. A 2002, 338, 44–53. [Google Scholar] [CrossRef]
- Shahverdi, H.R.; Ghomashchi, M.R.; Shabestari, S.; Hejazi, J. Microstructural analysis of interfacial reaction between molten aluminium and solid iron. J. Mater. Process. Technol. 2002, 124, 345–352. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Gesmundo, F.; Hou, P.Y.; Niu, Y. Criteria for the formation of protective Al2O3 scales on Fe-Al and Fe-Cr-Al alloys. Corros. Sci. 2006, 48, 741–765. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K. Nickel and iron aluminides: An overview on properties, processing, and applications. Intermetallics 1996, 4, 357–375. [Google Scholar] [CrossRef]
- Pint, B.A.; Leibowitz, J.; Devan, J.H. Effect of an oxide dispersion on the critical Al content in Fe-Al alloys. Oxid. Met. 1999, 51, 181–197. [Google Scholar] [CrossRef]
- Liu, C.T.; Stringer, J.; Mundy, J.N.; Horton, L.L.; Angelini, P. Ordered intermetallic alloys: An assessment. Intermetallics 1997, 5, 579–596. [Google Scholar] [CrossRef]
- Ortega, Y.; de Diego, N.; Plazaola, F.; Jiménez, J.A.; del Río, J. Influence of Cr addition on the defect structure of Fe-Al alloys. Intermetallics 2007, 15, 177–180. [Google Scholar] [CrossRef]
- Klein, O.; Baker, I. Effect of chromium on the environmental sensitivity of FeA1 at room temperature. Scr. Metall. Mater. 1992, 27, 1823–1828. [Google Scholar] [CrossRef]
- Agüero, A.; Gutiérrez, M.; Korcakova, L.; Nguyen, T.T.M.; Hinnemann, B.; Saadi, S. Metal dusting protective coatings. A literature review. Oxid. Met. 2011, 76, 23–42. [Google Scholar] [CrossRef]
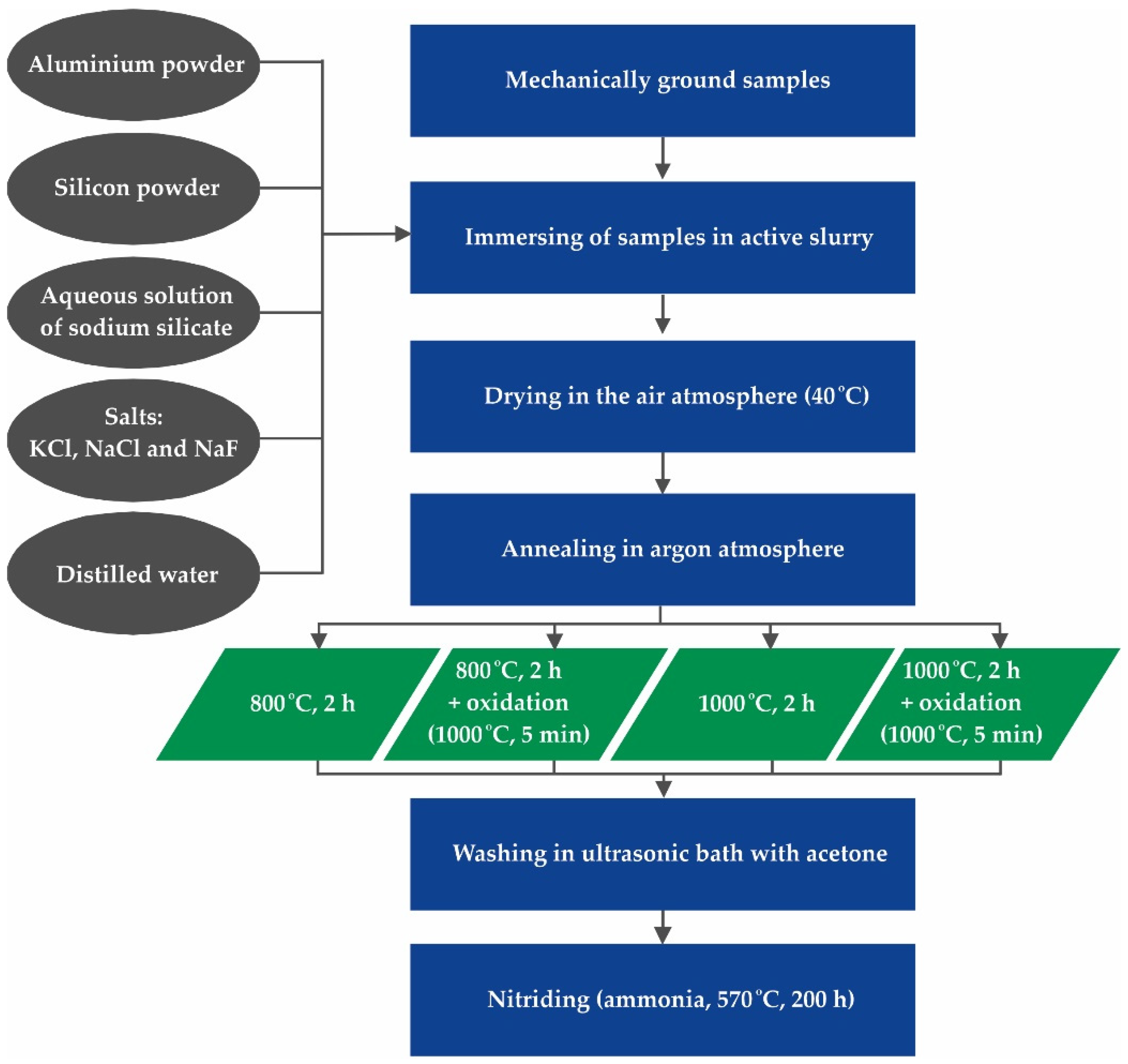




| Number of Coating Process | Annealing Temperature [°C] | Annealing Duration [h] | Oxidation: 5 min at 1000 °C (Heating Up to 1000 °C during 50 min) |
|---|---|---|---|
| No. 1 | 800 | 2 | No |
| No. 2 | Yes | ||
| No. 3 | 1000 | No | |
| No. 4 | Yes |
| Point of Analysis | Chemical Composition, at.% | Probable Phase | ||||
|---|---|---|---|---|---|---|
| Al | Si | Cr | Fe | Ni | ||
| 1 | 47.4 | 0.4 | 6.3 | 43.8 | 2.1 | Al0.99Fe0.99Cr0.02 |
| 2 | 72.5 | 0.3 | 0.6 | 26.2 | 0.3 | Al5Fe2 |
| 3 | 16.8 | 22.4 | 56.1 | 4.6 | 0.1 | Cr3Si |
| 4 | 45.6 | 1.0 | 10.6 | 40.0 | 2.7 | AlFe |
| 5 | 49.5 | 0.5 | 6.2 | 37.4 | 6.4 | Al0.99Fe0.99Cr0.02 |
| 6 | 74.8 | 1.3 | 7.4 | 16.4 | 0.8 | Al5Fe2 |
| 7 | 49.5 | 1.6 | 8.7 | 37.1 | 2.9 | Al0.99Fe0.99Cr0.02 |
| Point of Analysis | Chemical Composition, at.% | Probable Phase | |||||
|---|---|---|---|---|---|---|---|
| O | Al | Si | Cr | Fe | Ni | ||
| 1 | 72.0 | 4.2 | 2.8 | 17.2 | 3.1 | Al5Fe2 | |
| 2 | 51.1 | 0.3 | 8.2 | 33.6 | 6.8 | Al0.99Fe0.99Cr0.02 | |
| 3 | 48.0 | 6.4 | 9.6 | 31.0 | 4.4 | Al0.99Fe0.99Cr0.02 | |
| 4 | 64.2 | 6.5 | 3.1 | 21.5 | 4.4 | Al5Fe2 | |
| 5 | 31.1 | 2.1 | 10.0 | 49.2 | 0.6 | AlFe | |
| 6 | 52.0 | 47.2 | 0.0 | 0.3 | 0.5 | 0.0 | Al2O3 |
| 7 | 43.3 | 5.5 | 9.0 | 37.4 | 4.9 | Al0.99Fe0.99Cr0.02 | |
| Point of Analysis | Chemical Composition, at.% | ||||||
|---|---|---|---|---|---|---|---|
| C(WDS) | N (WDS) | O (WDS) | Si | Cr | Fe | Ni | |
| 1 | 1.0 | 8.9 | 7.6 | 1.9 | 18 | 57.7 | 4.9 |
| 2 | 1.1 | 10.9 | 17.3 | 0.6 | 14.6 | 51.9 | 3.6 |
| 3 | 1.0 | 13.8 | 3.6 | 0.2 | 1.7 | 75.0 | 4.7 |
| 4 | 1.1 | 7.0 | 13.9 | 1.5 | 16.5 | 53.9 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbowska, K.; Kochmańska, A.E.; Kochmański, P. Resistance of Aluminide Coatings on Austenitic Stainless Steel in a Nitriding Atmosphere. Materials 2022, 15, 162. https://doi.org/10.3390/ma15010162
Wierzbowska K, Kochmańska AE, Kochmański P. Resistance of Aluminide Coatings on Austenitic Stainless Steel in a Nitriding Atmosphere. Materials. 2022; 15(1):162. https://doi.org/10.3390/ma15010162
Chicago/Turabian StyleWierzbowska, Karolina, Agnieszka Elżbieta Kochmańska, and Paweł Kochmański. 2022. "Resistance of Aluminide Coatings on Austenitic Stainless Steel in a Nitriding Atmosphere" Materials 15, no. 1: 162. https://doi.org/10.3390/ma15010162
APA StyleWierzbowska, K., Kochmańska, A. E., & Kochmański, P. (2022). Resistance of Aluminide Coatings on Austenitic Stainless Steel in a Nitriding Atmosphere. Materials, 15(1), 162. https://doi.org/10.3390/ma15010162






