Statistical Study of Nonthermal Plasma-Assisted ZnO Coating of Cotton Fabric through Ultrasonic-Assisted Green Synthesis for Improved Self-Cleaning and Antimicrobial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Complete Composite Design for Statistical Optimization
2.3. Configuration of Nonthermal Plasma
2.4. Guava Plant Extract and Synthesis of ZnO Nanoparticles
2.5. Characterization of Samples
2.6. Photocatalytic Activity of Nanocoated Samples
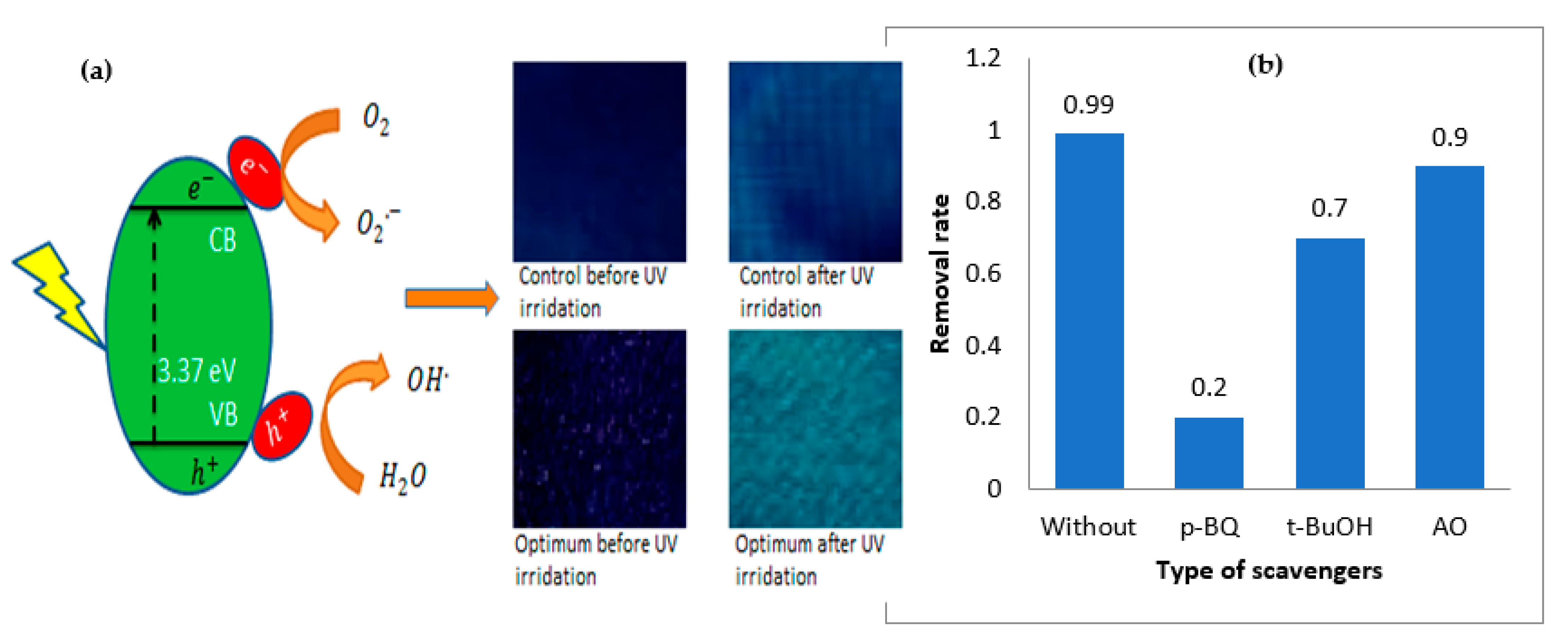
2.7. Radical Scavenger Analysis
2.8. Antimicrobial and UV Protection Activity
3. Results and Discussion
3.1. Mechanism of ZnO Coating of Cotton
3.2. Photocatalytic Activity of ZnO-Coated Cotton
3.3. Statistical Optimization and Analysis of Variance
3.4. XRD Analysis
3.5. SEM Analysis
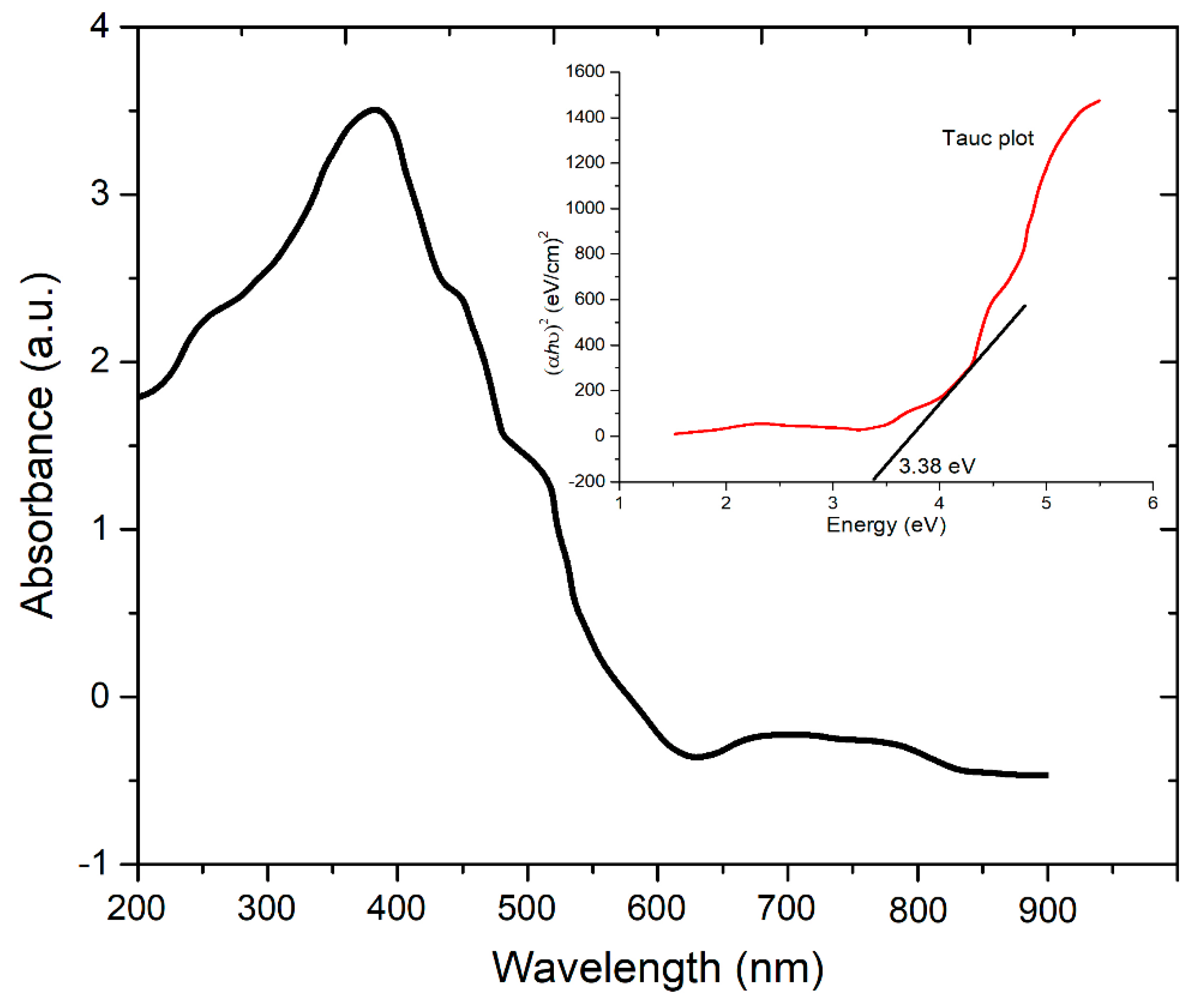
3.6. UV-Vis Analysis
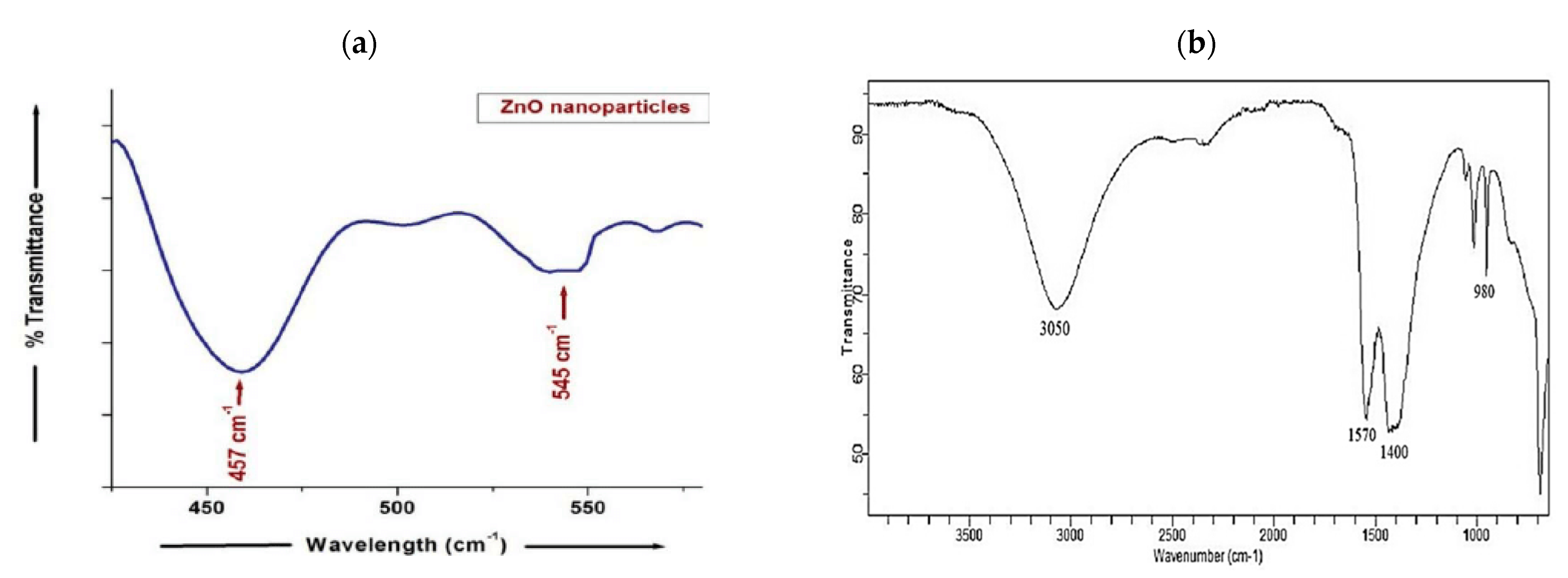
3.7. FTIR Analysis
3.8. Antimicrobial Activity of ZnO-Coated Cotton
3.9. Ultraviolet Protection and Zeta Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Saleem, M.; Naz, M.Y.; Shukrullah, S.; Ali, S.; Hamdani, S.T.A. Ultrasonic biosynthesis of TiO2 nanoparticles for improved self-cleaning and wettability coating of DBD plasma pre-treated cotton fabric. Appl. Phys. A 2021, 127, 608. [Google Scholar] [CrossRef]
- Saleem, M.; Naz, M.Y.; Shukrullah, S.; Shujah, M.A.; Akhtar, M.; Ullah, S.; Ali, S. One-pot sonochemical preparation of carbon dots, influence of process parameters and potential applications: A review. Carbon Lett. 2021, 1–17. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. -Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Ngene, A.C.; Aguiyi, J.C.; Chibuike, C.J.; Ifeanyi, V.O.; Ukaegbu-Obi, K.M.; Kim, E.G.; Ohaeri, U.C.; Onyemegbulem, B.O. Antibacterial Activity of Psidium guajava Leaf Extract against Selected Pathogenic Bacteria. Adv. Microbiol. 2019, 9, 1012–1022. [Google Scholar] [CrossRef]
- Fernandes, M.; Dias, A.; Carvalho, R.; Souza, C.; Oliveira, W. Antioxidant and antimicrobial activities of Psidium guajava L. spray dried extracts. Ind. Crop. Prod. 2014, 60, 39–44. [Google Scholar] [CrossRef]
- Saleem, M.; Naz, M.Y.; Shoukat, B.; Shukrullah, S.; Hussain, Z. Functionality and applications of non-thermal plasma activated textiles: A review. Mater. Today Proc. 2021, 47, S74–S82. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Rajendra, R.; Balakumar, C.; Ahammed, H.A.M.; Jayakumar, S.; Vaideki, K.; Rajesh, E. Use of zinc oxide nano particles for production of antimicrobial textiles. Int. J. Eng. Sci. Technol. 2010, 2, 202–208. [Google Scholar] [CrossRef]
- Gokarneshan, N.; Gopalakrishnan, P.; Jeyanthi, B. Influence of nanofinishes on the antimicrobial properties of fabrics. Int. Sch. Res. Not. 2012, 2012, 1–8. [Google Scholar] [CrossRef][Green Version]
- Vigneshwaran, N.; Kumar, S.; Kathe, A.; Varadarajan, P.; Prasad, V. Functional finishing of cotton fabrics using zinc oxide–soluble starch nanocomposites. Nanotechnology 2006, 17, 5087. [Google Scholar] [CrossRef]
- Chinnammal, S.K.; Arunkumar, K. Effect of plasma treatment on plain woven cotton fabric. Int. J. Sci. Res. 2014, 15, 83–88. [Google Scholar]
- Vu, N.K.; Zille, A.; Oliveira, F.R.; Carneiro, N.; Souto, A.P. Effect of particle size on silver nanoparticle deposition onto dielectric barrier discharge (DBD) plasma functionalized polyamide fabric. Plasma Process. Polym. 2013, 10, 285–296. [Google Scholar] [CrossRef]
- Shahidi, S.; Rezaee, H.; Rashidi, A.; Ghoranneviss, M. In situ synthesis of ZnO Nanoparticles on plasma treated cotton fabric utilizing durable antibacterial activity. J. Nat. Fibers 2018, 15, 639–647. [Google Scholar] [CrossRef]
- Fito, J.; Abrham, S.; Angassa, K. Adsorption of methylene blue from textile industrial wastewater onto activated carbon of Parthenium hysterophorus. Int. J. Environ. Res. 2020, 14, 501–511. [Google Scholar] [CrossRef]
- Raho, G.B.; Abouni, B. Escherichia coli and Staphylococcus aureus most common source of infection. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Formatex Research Center: Badajoz, Spain, 2015. [Google Scholar]
- Saleem, M.; Naz, M.Y.; Shukrullah, S.; Shujah, M.A.; Ullah, S.; Al-Sehemi, A.G. Statistical optimization of open air dielectric barrier discharge plasma effect on self-cleaning activity of ultrasonically Cu2O/TiO2-coated cotton fabric. Appl. Phys. A 2021, 127, 776. [Google Scholar] [CrossRef]
- Uduak, E.U.; Timbuak, J.A.; Musa, S.A.; Ikyembe, D.T.; Abdurrashid, S.; Hamman, W.O. Ulceroprotective effect of methanol extract of psidium guajava leaves on ethanol induced gastric ulcer in adult wistar rats. Asian J. Med Sci. 2012, 4, 75–78. [Google Scholar]
- Santhoshkumar, T.; Rahuman, A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976. [Google Scholar] [CrossRef]
- Saha, R.; Subramani, K.; Raju, S.A.K.P.M.; Rangaraj, S.; Venkatachalam, R. Psidium guajava leaf extract-mediated synthesis of ZnO nanoparticles under different processing parameters for hydrophobic and antibacterial finishing over cotton fabrics. Prog. Org. Coat. 2015, 124, 80–91. [Google Scholar] [CrossRef]
- Rashidi, A.; Shahidi, S.; Ghoranneviss, M.; Dalalsharifi, S.; Wiener, J. Effect of plasma on the zeta potential of cotton fabrics. Plasma Sci. Technol. 2013, 15, 455. [Google Scholar] [CrossRef]
- Singh, G.; Joyce, E.M.; Beddow, J.; Mason, T.J. Evaluation of antibacterial activity of ZnO nanoparticles coated sonochemically onto textile fabrics. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 106–120. [Google Scholar]
- Jazbec, K.; Šala, M.; Mozetič, M.; Vesel, A.; Gorjanc, M. Functionalization of cellulose fibres with oxygen plasma and ZnO nanoparticles for achieving UV protective properties. J. Nanomater. 2015, 16, 25. [Google Scholar] [CrossRef]
- Huang, N.; Shu, J.; Wang, Z.; Chen, M.; Ren, C.; Zhang, W. One-step pyrolytic synthesis of ZnO nanorods with enhanced photocatalytic activity and high photostability under visible light and UV light irradiation. J. Alloys Compd. 2015, 648, 919–929. [Google Scholar] [CrossRef]
- Saleem, M.; Naz, M.Y.; Shukrullah, S.; Farooq, M.U.; Zahid, M.; Hussain, Z.; Makhlouf, M.M. Experimental and statistical analysis of dielectric barrier discharge plasma effect on sonochemically TiO2 coated cotton fabric using complete composite design. Curr. Appl. Phys. 2021, 31, 158–170. [Google Scholar] [CrossRef]
- Moholkar, V.S.; Warmoeskerken, M.M.C.G. Investigations in mass transfer enhancement in textiles with ultrasound. Chem. Eng. Sci. 2004, 59, 299–311. [Google Scholar] [CrossRef]
- Guo, L.; Campagne, C.; Perwuelz, A.; Leroux, F. Zeta potential and surface physico-chemical properties of atmospheric air-plasma-treated polyester fabrics. Text. Res. J. 2009, 79, 1371–1377. [Google Scholar] [CrossRef]
- Regmi, C.; Joshi, B.; Ray, S.K.; Gyawali, G.; Pandey, R.P. Understanding mechanism of photocatalytic microbial decontamination of environmental wastewater. Front. Chem. 2018, 6, 33. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Rajeswari, V.D. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. Open Nano 2018, 3, 48–55. [Google Scholar] [CrossRef]

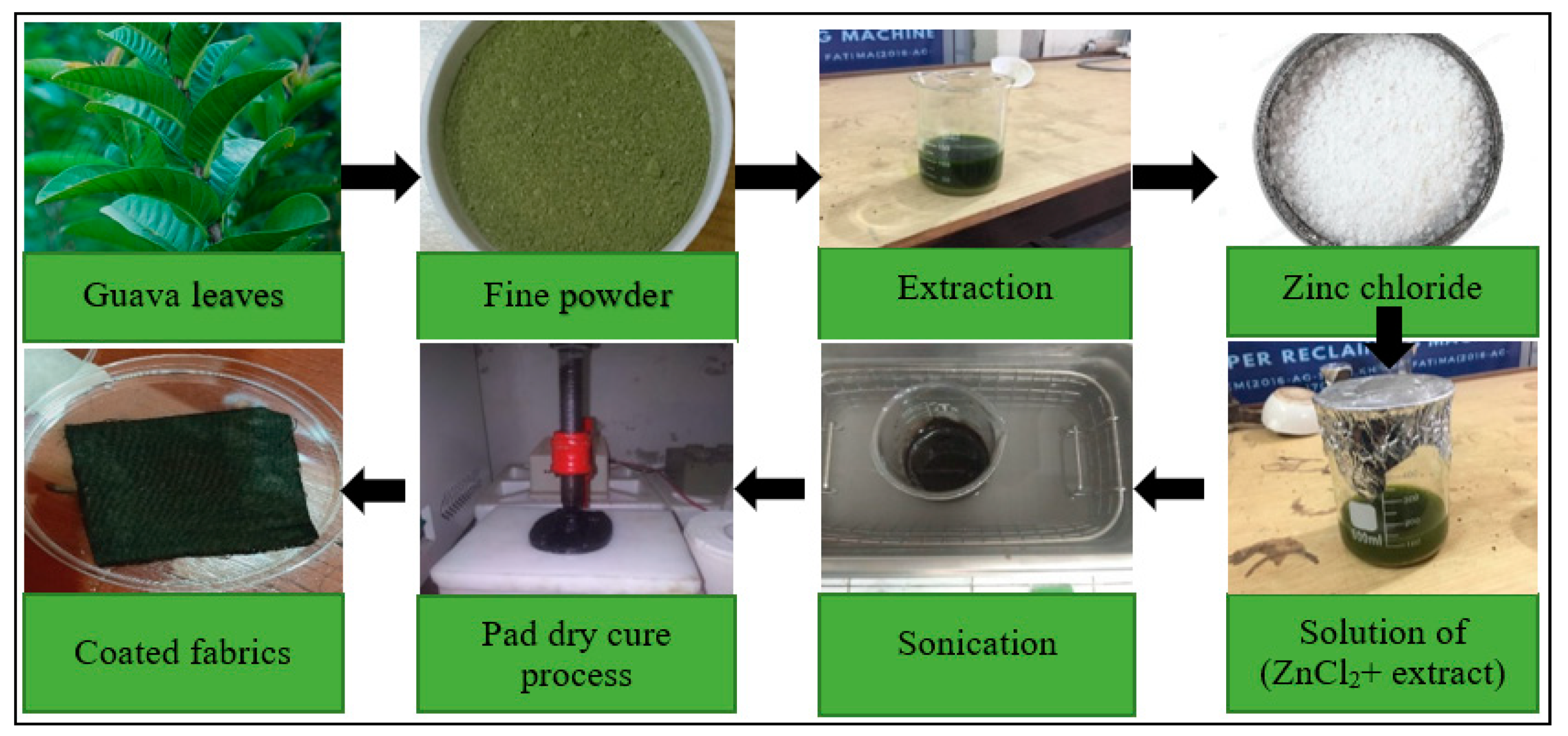
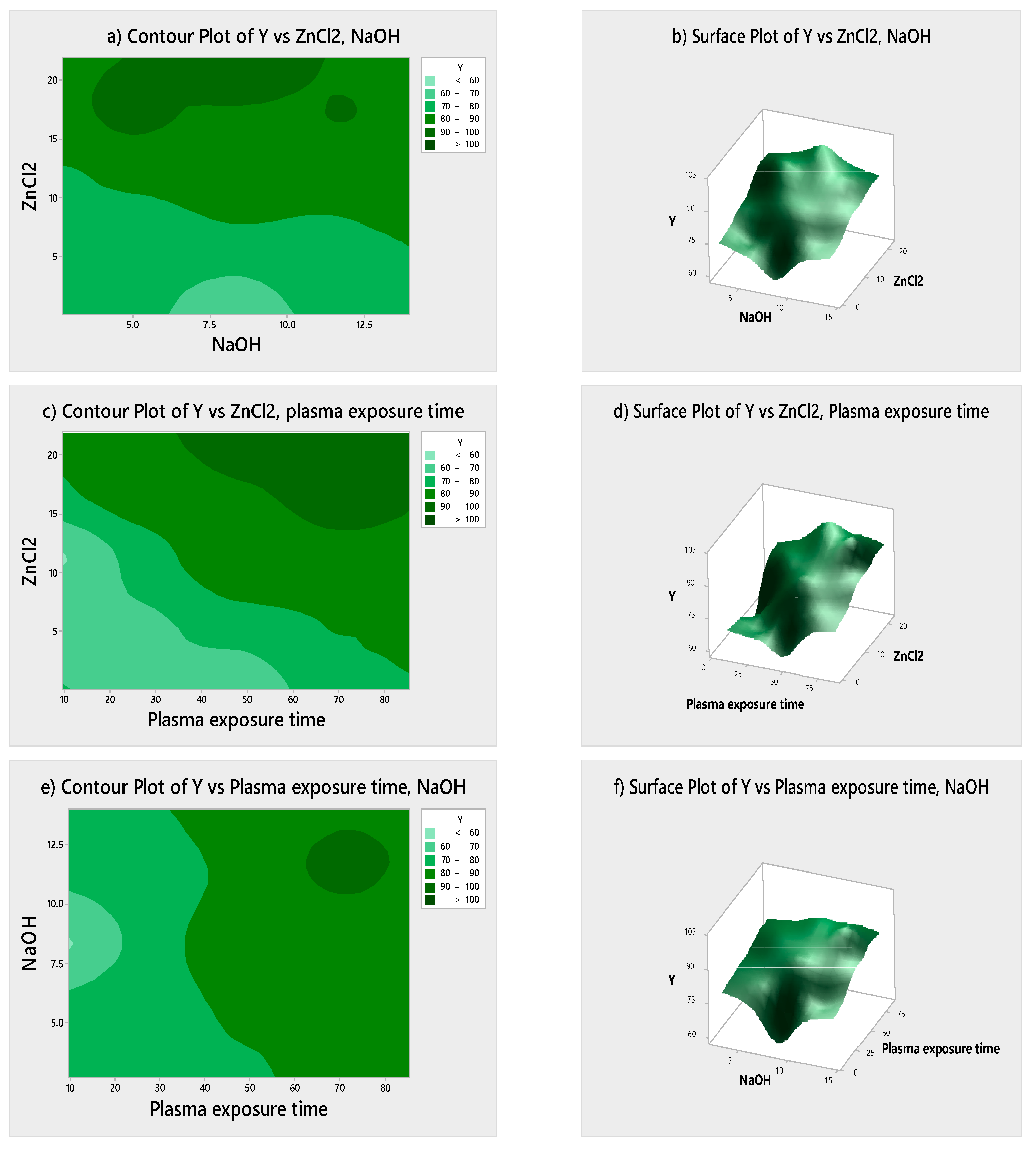




| Sr. No | ZnCl2 (g) | NaOH (g) | DBD Plasma Time (s) | Self-Cleaning Value (ΔRGB) |
|---|---|---|---|---|
| 1 | 17.5 | 5.0 | 70.2 | 95.6 |
| 2 | 11.0 | 8.4 | 47.6 | 87.6 |
| 3 | 17.5 | 5.0 | 25.0 | 90.2 |
| 4 | 4.5 | 5.0 | 70.2 | 74.4 |
| 5 | 17.5 | 11.7 | 70.2 | 100.4 |
| 6 | 11.0 | 2.7 | 47.6 | 79.1 |
| 7 | 4.5 | 11.7 | 25.0 | 65.7 |
| 8 | 11.0 | 8.4 | 47.6 | 82.3 |
| 9 | 21.9 | 8.4 | 47.6 | 96.8 |
| 10 | 11.0 | 8.4 | 47.6 | 84.2 |
| 11 | 11.0 | 8.4 | 9.6 | 59.7 |
| 12 | 11.0 | 8.4 | 85.6 | 89.1 |
| 13 | 17.5 | 11.7 | 25.0 | 80.3 |
| 14 | 11.0 | 8.4 | 47.6 | 88.6 |
| 15 | 11.0 | 13.9 | 47.6 | 85.3 |
| 16 | 4.5 | 11.7 | 70.2 | 85.6 |
| 17 | 4.5 | 5.0 | 25.0 | 66.1 |
| 18 | 11.0 | 8.4 | 47.6 | 85.5 |
| 19 | 0.1 | 8.4 | 47.6 | 62.4 |
| 20 | 11.0 | 8.4 | 47.6 | 86.1 |
| M | 17.5 | 11.7 | 0.0 | 94.2 |
| Source | DF | Adj. SS | Adj. MS | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 9 | 2362.48 | 262.50 | 26.72 | 0.001 | Significant |
| Linear | 3 | 2084.62 | 694.87 | 70.72 | 0.000 | Significant |
| ZnCl2 | 1 | 1286.57 | 1286.57 | 130.94 | 0.000 | Significant |
| NaOH | 1 | 19.04 | 19.04 | 1.94 | 0.194 | Not Significant |
| Plasma exposure time | 1 | 779.01 | 779.01 | 79.29 | 0.000 | Significant |
| Square | 3 | 158.88 | 52.96 | 5.39 | 0.018 | Significant |
| (ZnCl2) 2 | 1 | 25.23 | 25.23 | 2.57 | 0.040 | Significant |
| (NaOH) 2 | 1 | 2.35 | 2.35 | 0.24 | 0.635 | Not Significant |
| (Plasma exposure time) | 1 | 144.05 | 144.05 | 14.66 | 0.003 | Significant |
| 2-Way Interaction | 3 | 118.97 | 39.66 | 4.04 | 0.040 | Significant |
| ZnCl2 × NaOH | 1 | 31.60 | 31.60 | 3.22 | 0.103 | Not Significant |
| ZnCl2 × Plasma time | 1 | 0.91 | 0.91 | 0.9 | 0.767 | Not Significant |
| NaOH × Plasma time | 1 | 86.46 | 86.46 | 8.80 | 0.014 | Significant |
| Error | 10 | 98.25 | 9.83 | |||
| Lack of Fit | 5 | 72.23 | 14.45 | 2.77 | 0.144 | |
| Pure Error | 5 | 26.03 | 5.21 | |||
| Model Summary | R-sq = 96.01%, R-sq (adj) = 92.41% | |||||
| 2θ | h k l | d-Spacing (Å) | FWHM |
|---|---|---|---|
| 31.54° | (100) | 2.814 | 0.39 |
| 34.40° | (002) | 2.601 | 0.17 |
| 36.71° | (101) | 2.471 | 0.46 |
| 47.45° | (102) | 1.911 | 0.40 |
| 56.36° | (110) | 1.624 | 0.60 |
| 62.82° | (103) | 1.477 | 0.36 |
| 67.67° | (112) | 1.377 | 0.50 |
| 70.13° | (201) | 1.342 | 0.30 |
| 71.3° | (004) | 1.312 | 0.29 |
| Zone of Inhabitation against Gram-Positive Bacteria | Zone of Inhabitation against Gram-Negative Bacteria | |||
|---|---|---|---|---|
| Control Sample | Optimum Sample | Control Sample | Optimum Sample | |
| Coating Cycles | Inhabitation Zone | Inhabitation Zone | Inhabitation Zone | Inhabitation Zone |
| 1 | 1.90 mm | 3 mm | 1.95 mm | 3.1 mm |
| 3 | 2 mm | 6 mm | 2.1 mm | 6.3 mm |
| 5 | 3 mm | 10 mm | 2.98 mm | 11 mm |
| Samples | UPF | UV Transmittance (%) | UPF Rating | |
|---|---|---|---|---|
| UV-A (315–400 nm) | UV-B (280–315 nm) | |||
| Five time coated (control) | 5.7 | 29.27 | 26.84 | Unrelatable |
| One time coated (optimum) | 33.56 | 7.43 | 2.36 | 30 |
| Three time coated (optimum) | 58.22 | 6.71 | 1.93 | 50+ |
| Five time coated (optimum) | 69.87 | 6.41 | 1.76 | 50+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irfan, M.; Naz, M.Y.; Saleem, M.; Tanawush, M.; Głowacz, A.; Glowacz, W.; Rahman, S.; Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; et al. Statistical Study of Nonthermal Plasma-Assisted ZnO Coating of Cotton Fabric through Ultrasonic-Assisted Green Synthesis for Improved Self-Cleaning and Antimicrobial Properties. Materials 2021, 14, 6998. https://doi.org/10.3390/ma14226998
Irfan M, Naz MY, Saleem M, Tanawush M, Głowacz A, Glowacz W, Rahman S, Mahnashi MH, Alqahtani YS, Alyami BA, et al. Statistical Study of Nonthermal Plasma-Assisted ZnO Coating of Cotton Fabric through Ultrasonic-Assisted Green Synthesis for Improved Self-Cleaning and Antimicrobial Properties. Materials. 2021; 14(22):6998. https://doi.org/10.3390/ma14226998
Chicago/Turabian StyleIrfan, Muhammad, Muhammad Y. Naz, Muhammad Saleem, Malik Tanawush, Adam Głowacz, Witold Glowacz, Saifur Rahman, Mater H. Mahnashi, Yahya S. Alqahtani, Bandar A. Alyami, and et al. 2021. "Statistical Study of Nonthermal Plasma-Assisted ZnO Coating of Cotton Fabric through Ultrasonic-Assisted Green Synthesis for Improved Self-Cleaning and Antimicrobial Properties" Materials 14, no. 22: 6998. https://doi.org/10.3390/ma14226998
APA StyleIrfan, M., Naz, M. Y., Saleem, M., Tanawush, M., Głowacz, A., Glowacz, W., Rahman, S., Mahnashi, M. H., Alqahtani, Y. S., Alyami, B. A., Alqarni, A. O., & Alsaiari, M. A. (2021). Statistical Study of Nonthermal Plasma-Assisted ZnO Coating of Cotton Fabric through Ultrasonic-Assisted Green Synthesis for Improved Self-Cleaning and Antimicrobial Properties. Materials, 14(22), 6998. https://doi.org/10.3390/ma14226998






