Effect of a Nanostructured Titanium Surface on Gingival Cell Adhesion, Viability and Properties against P. gingivalis
Abstract
:1. Introduction
2. Materials & Methods
2.1. Preparation of Nanostructured Titanium Specimen
2.2. Characterization of the Surface Structure
2.3. Interaction between Oral Bacteria and Specimen (ND, MD)
2.4. Interaction between Gingival Fibroblasts and Specimen (ND, MD)
2.4.1. Cell Culture
2.4.2. MTT Assay
2.4.3. Immunofluorescence Microscopy
2.4.4. Flow Cytometry
2.4.5. Cell Morphology by Scanning Electron Microscopy (SEM)
2.4.6. Statistical Analysis
3. Results
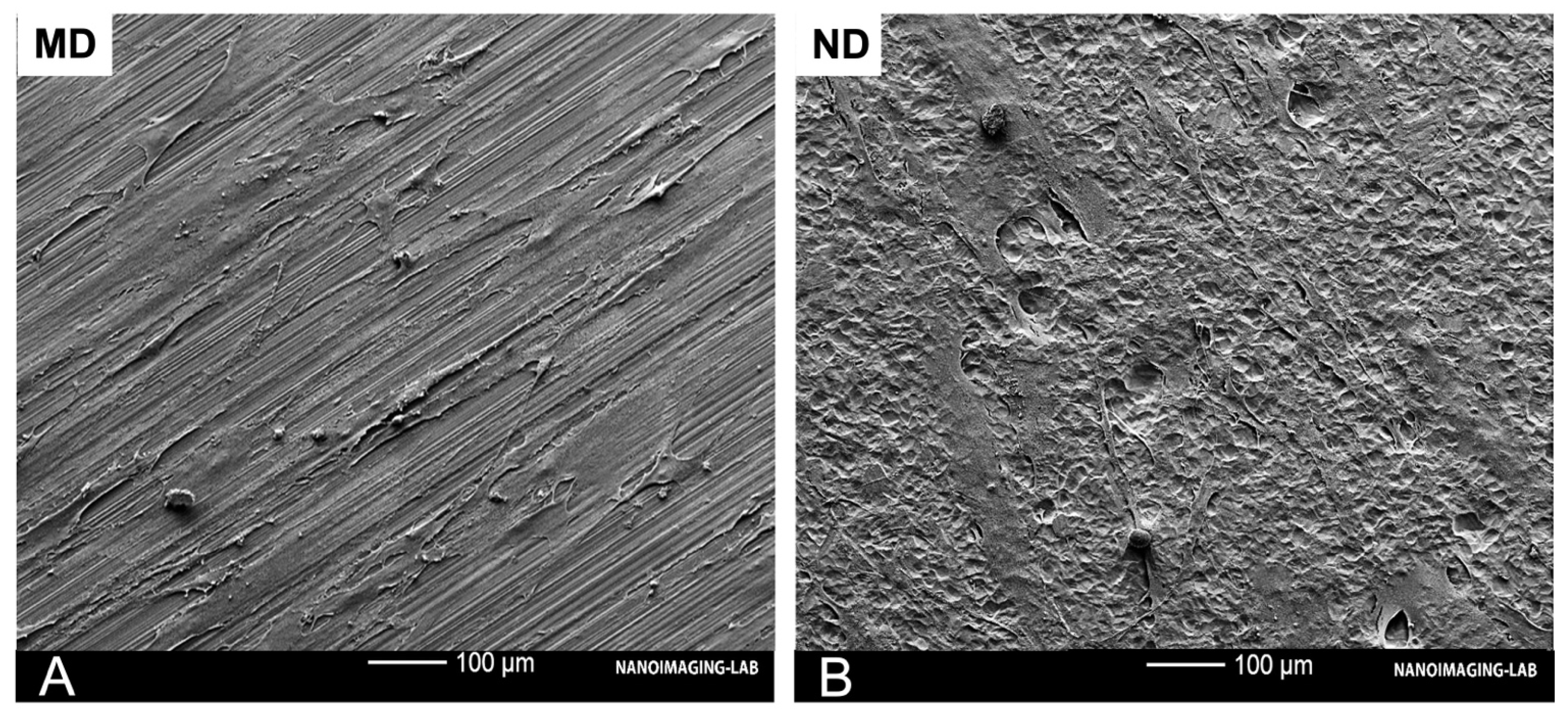
3.1. Characteristics/Morphology of the Nanostructured Titanium Sample Discs
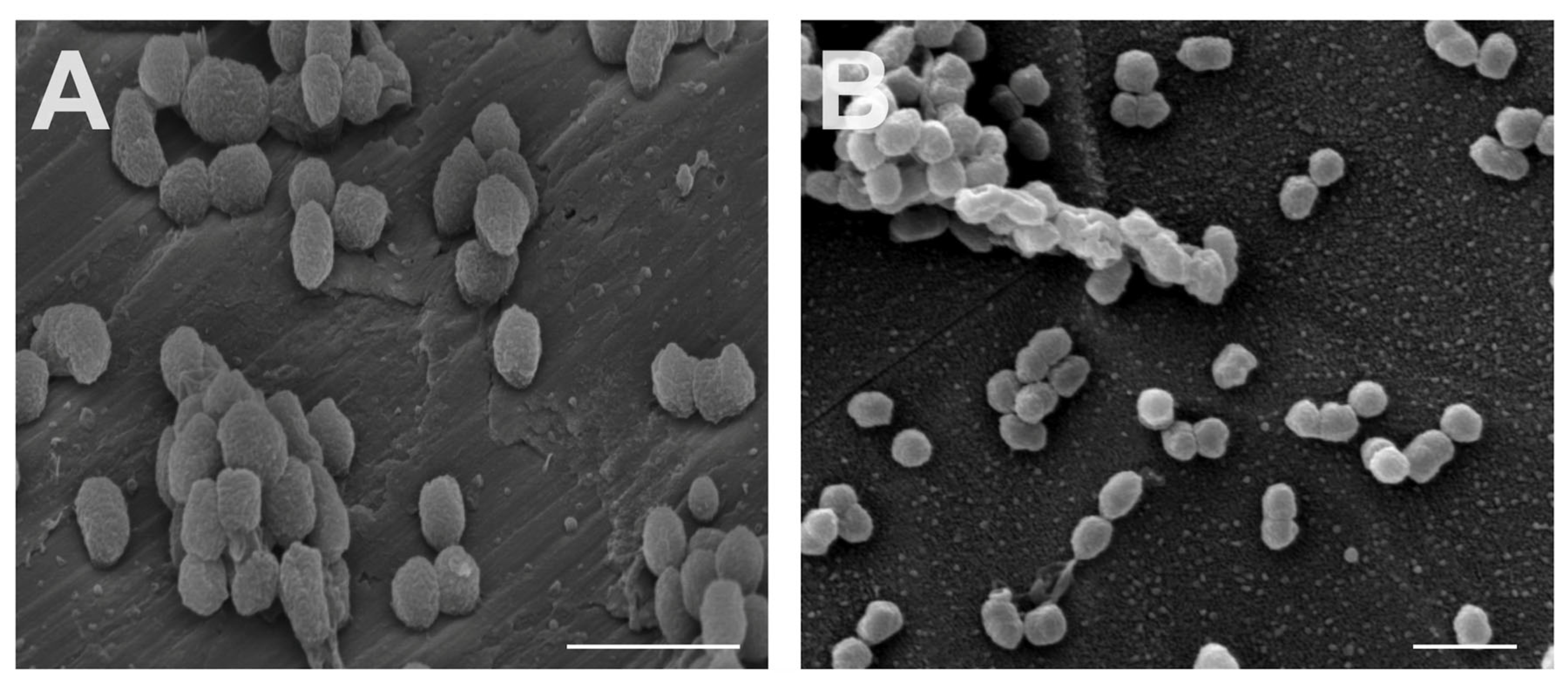
3.2. Interaction between Oral Bacteria and Specimen
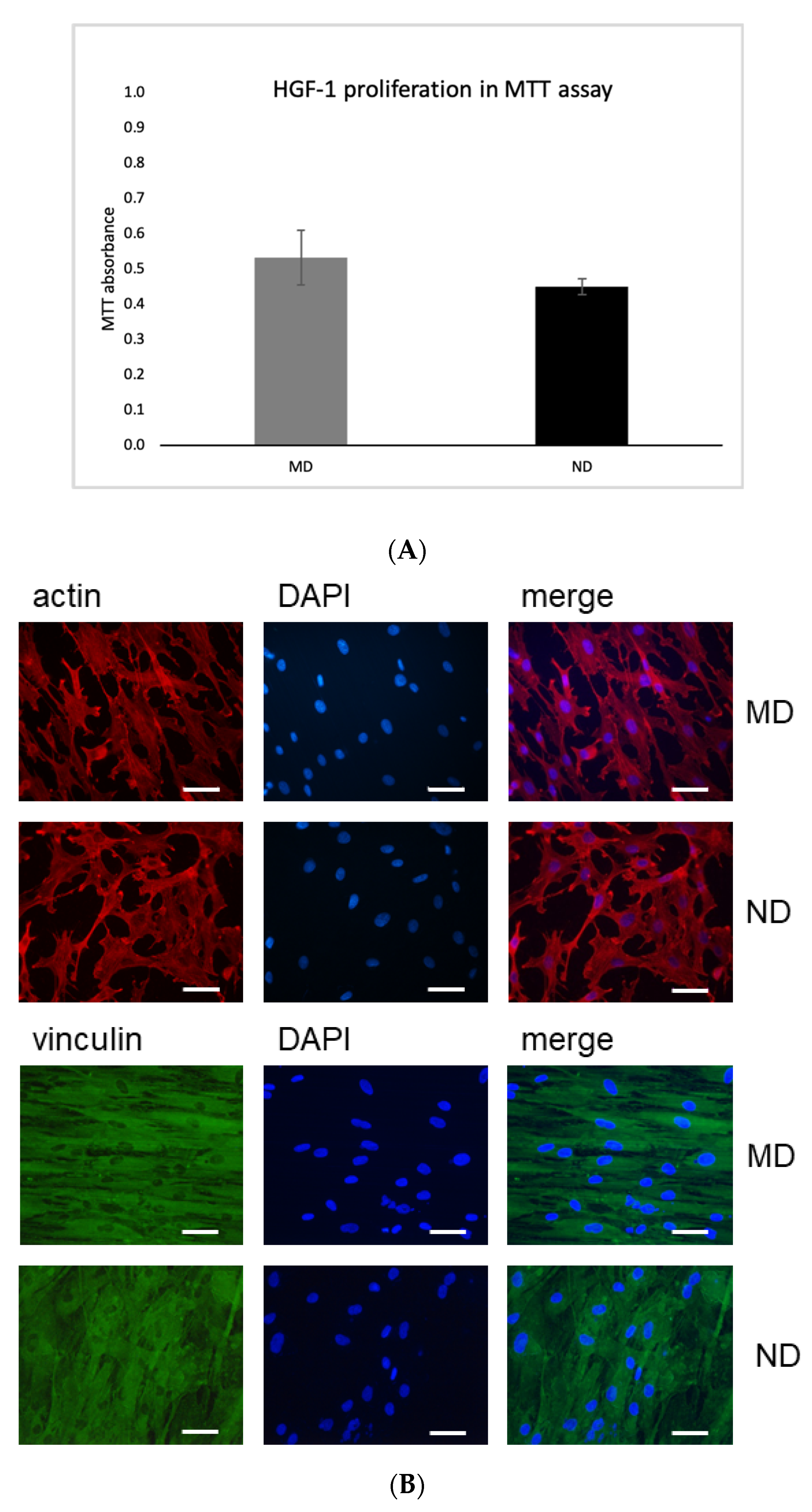
3.3. Interaction between Gingival Fibroblasts (HGF-1) and Specimen
MTT Assay
3.4. Expression of Actin and Vinculin in HGF Cells Incubated on MD and ND Determined by Immunofluorescence Microscopy
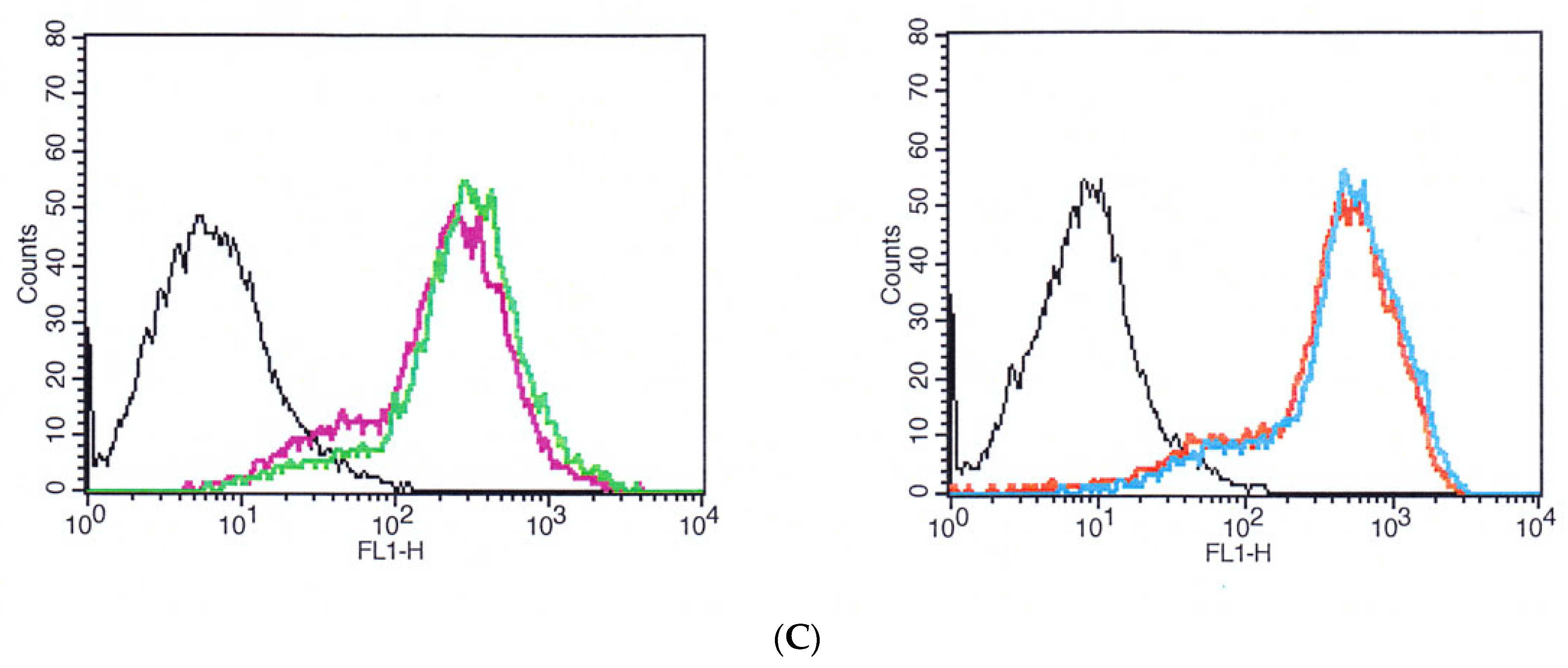
3.5. Expression of Actin and Vinculin in HGF-1 Cells upon Incubation on MD and ND by Flow Cytometry
3.6. Scanning Electron Microscope Images of HGF-1 Cells on Discs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Linder, E.; Wennerberg, A.; Lindhe, J. The Mucosal Attachment to Titanium Implants with Different Surface Characteristics: An Experimental Study in Dogs. J. Clin. Periodontol. 2002, 29, 448–455. [Google Scholar] [CrossRef]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Race to Invade: Understanding Soft Tissue Integration at the Transmucosal Region of Titanium Dental Implants. Dent. Mater. 2021, 37, 816–831. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-Implant Health and Disease. A Systematic Review of Current Epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-Implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef] [Green Version]
- Hussain, R.A.; Miloro, M.; Cohen, J.B. An Update on the Treatment of Periimplantitis. Dent. Clin. North Am. 2021, 65, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Astasov-Frauenhoffer, M.; Mukaddam, K.; Hauser-Gerspach, I.; Köser, J.; Glatzel, T.; Kisiel, M.; Marot, L.; Kühl, S. Antibacterial Effects of Bio-Inspired Nanostructured Materials. J. Oral Microbiol. 2017, 9, 1325241. [Google Scholar] [CrossRef]
- Molecular and Topographical Organization: Influence on Cicada Wing Wettability and Bactericidal Properties—Román-Kustas. Advanced Materials Interfaces. 2020. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/admi.202000112 (accessed on 28 October 2021).
- Bright, R.; Hayles, A.; Fernandes, D.; Visalakshan, R.M.; Ninan, N.; Palms, D.; Burzava, A.; Barker, D.; Brown, T.; Vasilev, K. In Vitro Bactericidal Efficacy of Nanostructured Ti6Al4V Surfaces Is Bacterial Load Dependent. ACS Appl. Mater. Interfaces 2021, 13, 38007–38017. [Google Scholar] [CrossRef] [PubMed]
- Jennes, M.-E.; Naumann, M.; Peroz, S.; Beuer, F.; Schmidt, F. Antibacterial Effects of Modified Implant Abutment Surfaces for the Prevention of Peri-Implantitis—A Systematic Review. Antibiotics 2021, 10, 1350. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural Bactericidal Surfaces: Mechanical Rupture of Pseudomonas Aeruginosa Cells by Cicada Wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal Activity of Black Silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Serrano, C.; García-Fernández, L.; Fernández-Blázquez, J.P.; Barbeck, M.; Ghanaati, S.; Unger, R.; Kirkpatrick, J.; Arzt, E.; Funk, L.; Turón, P.; et al. Nanostructured Medical Sutures with Antibacterial Properties. Biomaterials 2015, 52, 291–300. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial Effects of Nanopillar Surfaces Are Mediated by Cell Impedance, Penetration and Induction of Oxidative Stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Xu, Z.; He, Y.; Zeng, X.; Zeng, X.; Huang, J.; Lin, X.; Chen, J. Enhanced Human Gingival Fibroblast Response and Reduced Porphyromonas Gingivalis Adhesion with Titania Nanotubes. Biomed. Res. Int. 2020, 2020, 5651780. [Google Scholar] [CrossRef]
- Sengstock, C.; Lopian, M.; Motemani, Y.; Borgmann, A.; Khare, C.; Buenconsejo, P.J.S.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Structure-Related Antibacterial Activity of a Titanium Nanostructured Surface Fabricated by Glancing Angle Sputter Deposition. Nanotechnology 2014, 25, 195101. [Google Scholar] [CrossRef]
- Tsimbouri, P.M.; Fisher, L.; Holloway, N.; Sjostrom, T.; Nobbs, A.H.; Meek, R.M.D.; Su, B.; Dalby, M.J. Osteogenic and Bactericidal Surfaces from Hydrothermal Titania Nanowires on Titanium Substrates. Sci. Rep. 2016, 6, 36857. [Google Scholar] [CrossRef] [PubMed]
- Diu, T.; Faruqui, N.; Sjöström, T.; Lamarre, B.; Jenkinson, H.F.; Su, B.; Ryadnov, M.G. Cicada-Inspired Cell-Instructive Nanopatterned Arrays. Sci. Rep. 2014, 4, 7122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harawaza, K.; Cousins, B.; Roach, P.; Fernandez, A. Modification of the Surface Nanotopography of Implant Devices: A Translational Perspective. Mater. Today Biol. 2021, 12, 100152. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Okada, M.; Xie, S.C.; Jiao, Y.Y.; Hara, E.S.; Yanagimoto, H.; Fukumoto, T.; Matsumoto, T. Immediate Soft-Tissue Adhesion and the Mechanical Properties of the Ti–6Al–4V Alloy after Long-Term Acid Treatment. J. Mater. Chem. B 2021, 9, 8348–8354. [Google Scholar] [CrossRef] [PubMed]
- Konopatsky, A.S.; Teplyakova, T.O.; Popova, D.V.; Vlasova, K.Y.; Prokoshkin, S.D.; Shtansky, D.V. Surface Modification and Antibacterial Properties of Superelastic Ti-Zr-Based Alloys for Medical Application. Coll. Surfaces B Biointerfaces 2022, 209, 112183. [Google Scholar] [CrossRef]
- Variola, F.; Lauria, A.; Nanci, A.; Rosei, F. Influence of Treatment Conditions on the Chemical Oxidative Activity of H2SO4/H2O2 Mixtures for Modulating the Topography of Titanium. Adv. Eng. Mater. 2009, 11, B227–B234. [Google Scholar] [CrossRef]
- Vetrone, F.; Variola, F.; Tambasco de Oliveira, P.; Zalzal, S.F.; Yi, J.-H.; Sam, J.; Bombonato-Prado, K.F.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Nanoscale Oxidative Patterning of Metallic Surfaces to Modulate Cell Activity and Fate. Nano Lett. 2009, 9, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Lumetti, S.; Manfredi, E.; Ferraris, S.; Spriano, S.; Passeri, G.; Ghiacci, G.; Macaluso, G.; Galli, C. The Response of Osteoblastic MC3T3-E1 Cells to Micro- and Nano-Textured, Hydrophilic and Bioactive Titanium Surfaces. J. Mater. Sci. Mater. Med. 2016, 27, 68. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.-J.; Li, T.; Sudheesh Kumar, P.T.; Ivanovski, S. Titania Nanopores with Dual Micro-/Nano-Topography for Selective Cellular Bioactivity. Mater. Sci. Eng. C 2018, 91, 624–630. [Google Scholar] [CrossRef]
- Yi, J.-H.; Bernard, C.; Variola, F.; Zalzal, S.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Characterization of a Bioactive Nanotextured Surface Created by Controlled Chemical Oxidation of Titanium. Surface Sci. 2006, 600, 4613–4621. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, Y.; Zha, G.; Chen, Y.; Deng, X.; Zhang, K.; Zhu, W.; Zhao, S.; Li, X. Topography-Dependent Antibacterial, Osteogenic and Anti-Aging Properties of Pure Titanium. J. Mater. Chem. B 2015, 3, 784–795. [Google Scholar] [CrossRef]
- Hölscher, H. AFM, Tapping Mode. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; p. 99. ISBN 978-90-481-9751-4. [Google Scholar]
- Ohnesorge, F.; Binnig, G. True Atomic Resolution by Atomic Force Microscopy Through Repulsive and Attractive Forces. Science 1993, 260, 1451. [Google Scholar] [CrossRef]
- Huang, Y.; Zha, G.; Luo, Q.; Zhang, J.; Zhang, F.; Li, X.; Zhao, S.; Zhu, W.; Li, X. The Construction of Hierarchical Structure on Ti Substrate with Superior Osteogenic Activity and Intrinsic Antibacterial Capability. Sci. Rep. 2014, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and Bioinspired Nanostructured Bactericidal Surfaces. Adv. Coll. Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Kallas, P.; Kang, H.; Valen, H.; Haugen, H.J.; Andersson, M.; Hulander, M. Effect of Silica Nano-Spheres on Adhesion of Oral Bacteria and Human Fibroblasts. Biomater. Investig Dent. 2020, 7, 134–145. [Google Scholar] [CrossRef]
- Tengvall, P.; Hörnsten, E.G.; Elwing, H.; Lundström, I. Bactericidal Properties of a Titanium-Peroxy Gel Obtained from Metallic Titanium and Hydrogen Peroxide. J. Biomed. Mater. Res. 1990, 24, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Unosson, E.; Tsekoura, E.K.; Engqvist, H.; Welch, K. Synergetic Inactivation of Staphylococcus Epidermidis and Streptococcus Mutansin a TiO2/H2O2/UV System. Biomatter 2013, 3, e26727. [Google Scholar] [CrossRef] [Green Version]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A Gecko Skin Micro/Nano Structure—A Low Adhesion, Superhydrophobic, Anti-Wetting, Self-Cleaning, Biocompatible, Antibacterial Surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Hayes, M.J.; Levine, T.P.; Wilson, R.H. Identification of Nanopillars on the Cuticle of the Aquatic Larvae of the Drone Fly (Diptera: Syrphidae). J. Insect Sci. 2016, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Almaguer-Flores, A.; Olivares-Navarrete, R.; Wieland, M.; Ximénez-Fyvie, L.A.; Schwartz, Z.; Boyan, B.D. Influence of Topography and Hydrophilicity on Initial Oral Biofilm Formation on Microstructured Titanium Surfaces in Vitro. Clin. Oral. Implants Res. 2012, 23, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Bays, J.L.; DeMali, K.A. Vinculin in Cell–Cell and Cell–Matrix Adhesions. Cell. Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [Green Version]
- Babakhanova, G.; Krieger, J.; Li, B.-X.; Turiv, T.; Kim, M.-H.; Lavrentovich, O.D. Cell Alignment by Smectic Liquid Crystal Elastomer Coatings with Nanogrooves. J. Biomed. Mater. Res. A 2020, 108, 1223–1230. [Google Scholar] [CrossRef]
- Golji, J.; Mofrad, M.R.K. The Interaction of Vinculin with Actin. PLoS Comput. Biol. 2013, 9, e1002995. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Bohner, L.; Hanisch, M.; Kleinheinz, J.; Sielker, S. Influence of Implant Material and Surface on Mode and Strength of Cell/Matrix Attachment of Human Adipose Derived Stromal Cell. Int. J. Mol. Sci. 2020, 21, 4110. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Pierfelice, T.V.; D’Amico, E.; Di Pietro, N.; Pandolfi, A.; D’Arcangelo, C.; De Angelis, F.; Mandatori, D.; Schiavone, V.; Piattelli, A.; et al. Influence of Nano, Micro, and Macro Topography of Dental Implant Surfaces on Human Gingival Fibroblasts. Int. J. Mol. Sci. 2021, 22, 9871. [Google Scholar] [CrossRef] [PubMed]
- Ingenta Connect. Effects of Titanium Nano-Foveolae Surfaces on Human Gingival Fibroblasts. Available online: https://www.ingentaconnect.com/content/asp/jnn/2020/00000020/00000002/art00003;jsessionid=1qe05rixasu2f.x-ic-live-01 (accessed on 30 November 2021).
- Ferraris, S.; Warchomicka, F.; Barberi, J.; Cochis, A.; Scalia, A.C.; Spriano, S. Contact Guidance Effect and Prevention of Microfouling on a Beta Titanium Alloy Surface Structured by Electron-Beam Technology. Nanomaterials 2021, 11, 1474. [Google Scholar] [CrossRef]
- Meyle, J.; Gültig, K.; Brich, M.; Hämmerle, H.; Nisch, W. Contact Guidance of Fibroblasts on Biomaterial Surfaces. J. Mater. Sci. Mater. Med. 1994, 5, 463–466. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.-J.; Kumar, P.T.S.; Han, P.; Ivanovski, S. Anodized Anisotropic Titanium Surfaces for Enhanced Guidance of Gingival Fibroblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110860. [Google Scholar] [CrossRef] [PubMed]
- Guida, L.; Oliva, A.; Basile, M.A.; Giordano, M.; Nastri, L.; Annunziata, M. Human Gingival Fibroblast Functions Are Stimulated by Oxidized Nano-Structured Titanium Surfaces. J. Dent. 2013, 41, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Tambasco de Oliveira, P.; Nanci, A. Nanotexturing of Titanium-Based Surfaces Upregulates Expression of Bone Sialoprotein and Osteopontin by Cultured Osteogenic Cells. Biomaterials 2004, 25, 403–413. [Google Scholar] [CrossRef]
- Miao, X.; Wang, D.; Xu, L.; Wang, J.; Zeng, D.; Lin, S.; Huang, C.; Liu, X.; Jiang, X. The Response of Human Osteoblasts, Epithelial Cells, Fibroblasts, Macrophages and Oral Bacteria to Nanostructured Titanium Surfaces: A Systematic Study. IJN 2017, 12, 1415–1430. [Google Scholar] [CrossRef] [Green Version]
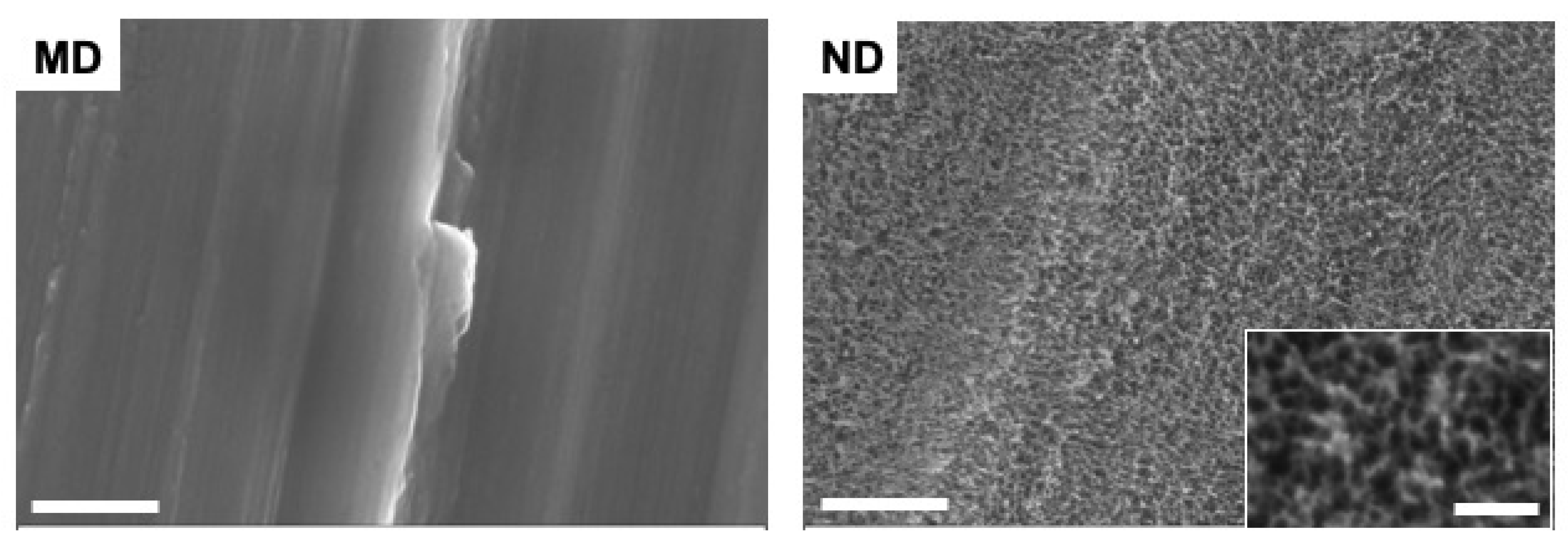
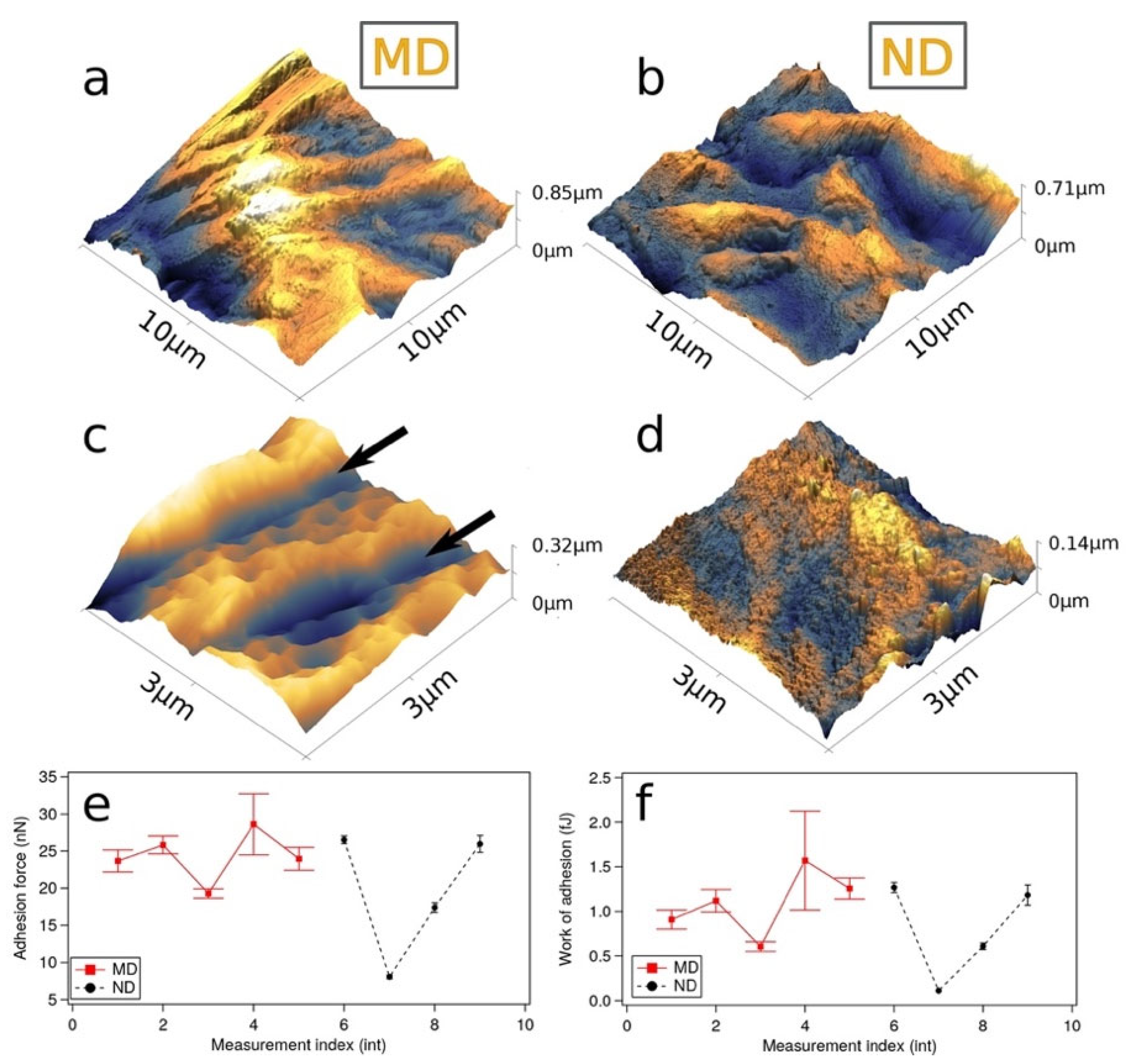

| MD Sample | ND Sample | |||
|---|---|---|---|---|
| Projected area | 100 nm2 | 9 nm2 | 100 nm2 | 9 nm2 |
| Surface area | 108.3 nm2 | 9.58 nm2 | 107.2 nm2 | 9.67 nm2 |
| rms roughness Sq | 101.7 nm | 17.27 nm | 157.4 nm | 49.77 nm |
| Mean roughness Sa | 82.6 nm | 13.30 nm | 130 nm | 39.68 nm |
| Skew Ssk | 0.084 | 0.1208 | −0.2236 | 0.08191 |
| Max. peak height Sp | 405.5 nm | 68.2 nm | 371.5 nm | 219.1 nm |
| Max. pit depth Sp | 304.9 nm | 70.8 nm | 478.1 nm | 134.8 nm |
| Max height Sz | 710.5 nm | 139 nm | 849.6 nm | 353.9 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukaddam, K.; Astasov-Frauenhoffer, M.; Fasler-Kan, E.; Marot, L.; Kisiel, M.; Meyer, E.; Köser, J.; Waser, M.; Bornstein, M.M.; Kühl, S. Effect of a Nanostructured Titanium Surface on Gingival Cell Adhesion, Viability and Properties against P. gingivalis. Materials 2021, 14, 7686. https://doi.org/10.3390/ma14247686
Mukaddam K, Astasov-Frauenhoffer M, Fasler-Kan E, Marot L, Kisiel M, Meyer E, Köser J, Waser M, Bornstein MM, Kühl S. Effect of a Nanostructured Titanium Surface on Gingival Cell Adhesion, Viability and Properties against P. gingivalis. Materials. 2021; 14(24):7686. https://doi.org/10.3390/ma14247686
Chicago/Turabian StyleMukaddam, Khaled, Monika Astasov-Frauenhoffer, Elizaveta Fasler-Kan, Laurent Marot, Marcin Kisiel, Ernst Meyer, Joachim Köser, Marcus Waser, Michael M. Bornstein, and Sebastian Kühl. 2021. "Effect of a Nanostructured Titanium Surface on Gingival Cell Adhesion, Viability and Properties against P. gingivalis" Materials 14, no. 24: 7686. https://doi.org/10.3390/ma14247686
APA StyleMukaddam, K., Astasov-Frauenhoffer, M., Fasler-Kan, E., Marot, L., Kisiel, M., Meyer, E., Köser, J., Waser, M., Bornstein, M. M., & Kühl, S. (2021). Effect of a Nanostructured Titanium Surface on Gingival Cell Adhesion, Viability and Properties against P. gingivalis. Materials, 14(24), 7686. https://doi.org/10.3390/ma14247686






