Osseointegration of Plasma Jet Treated Titanium Implant Surface in an Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
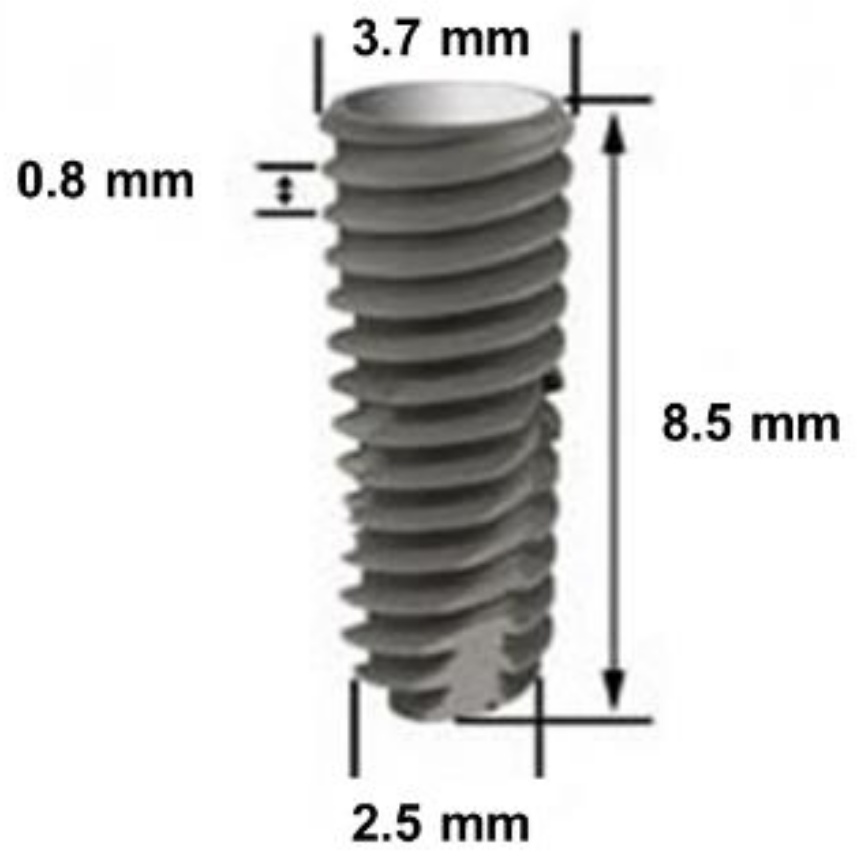
2.2. Titanium Implants
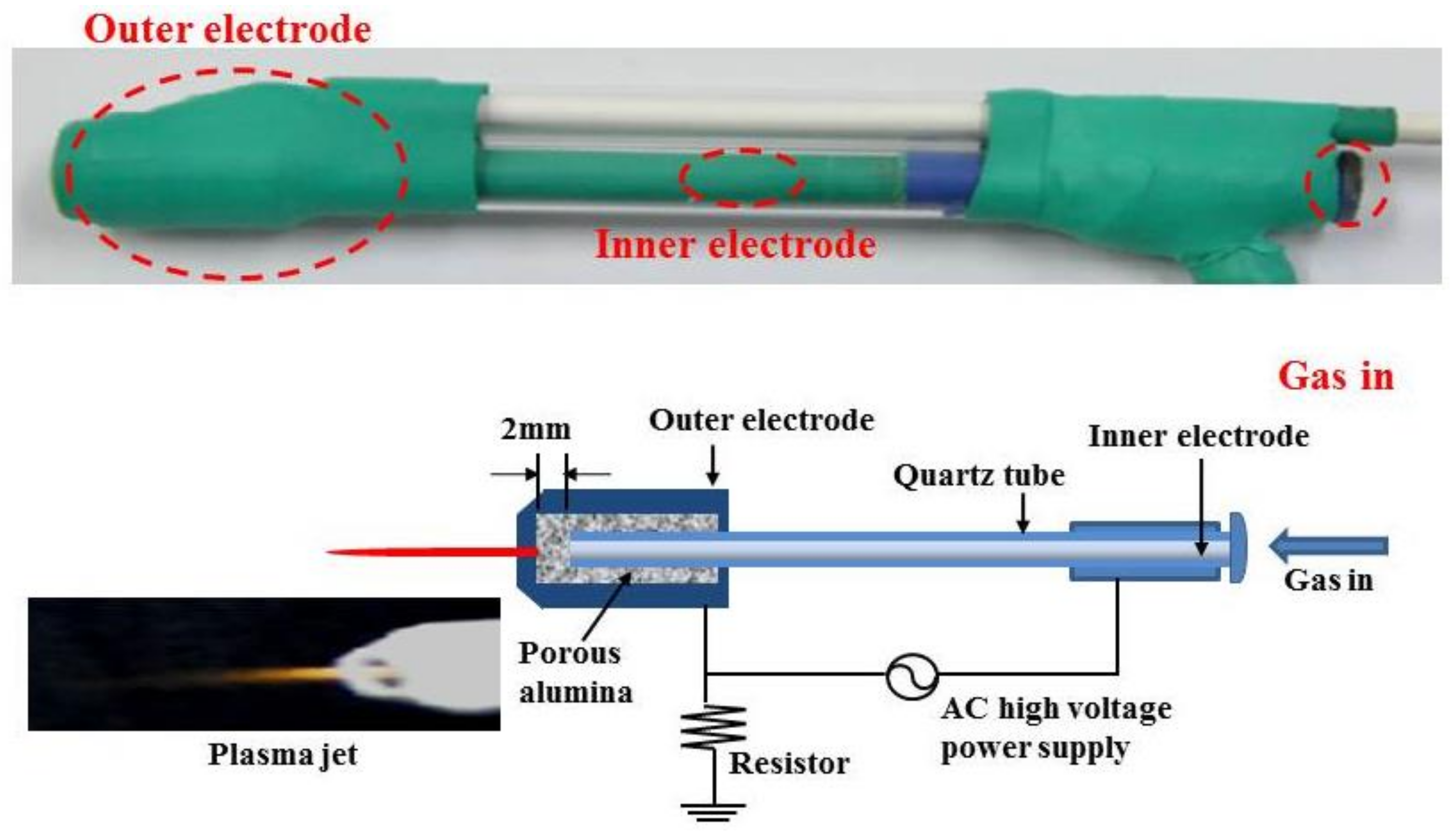
2.3. Treatment by Non-Thermal Atmospheric Pressure Plasma Jet
2.4. Surface Morphology and Chemical Characterization
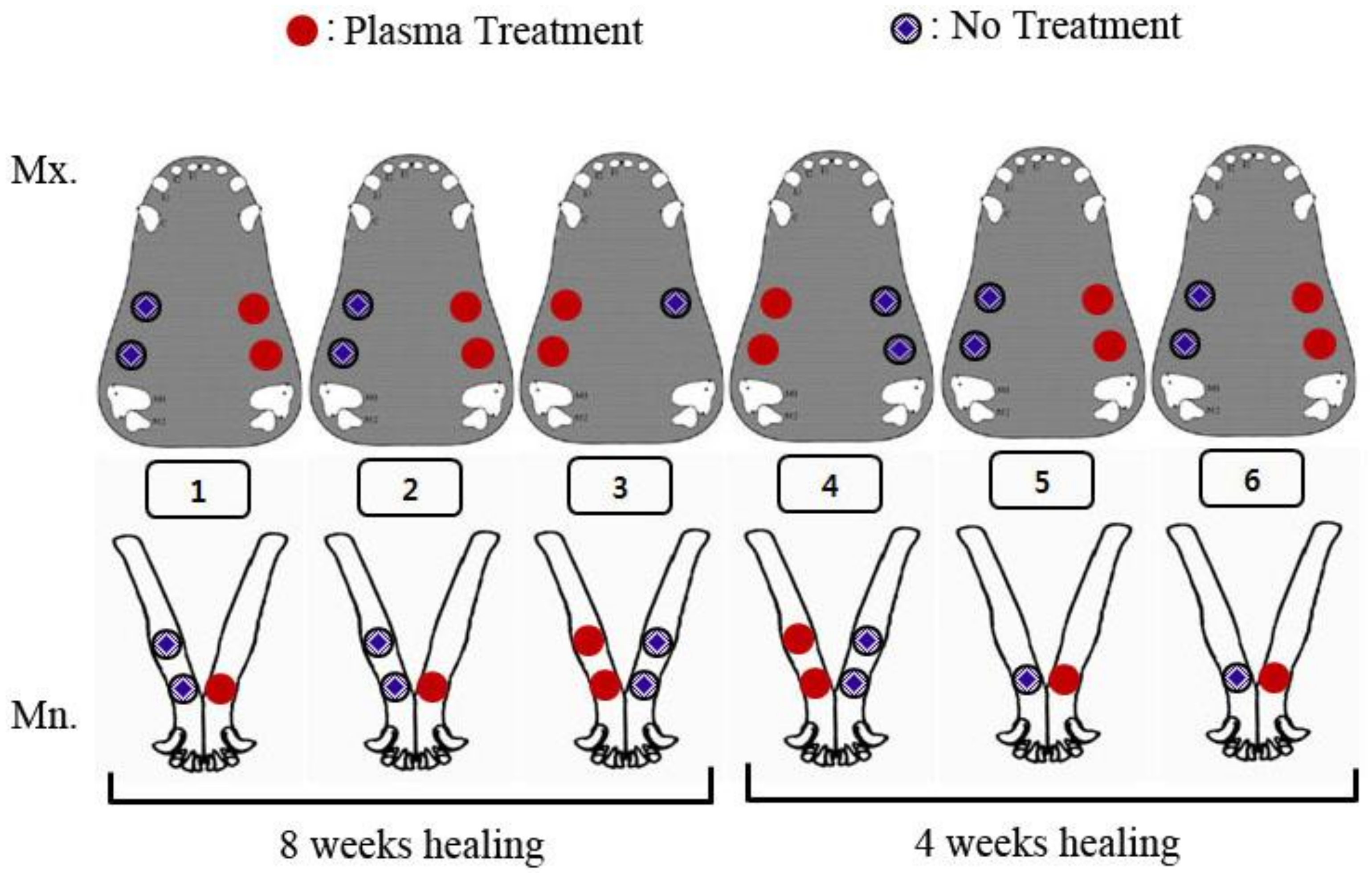
2.5. Surgical Protocol on Animal Model
2.6. Histomorphometric Analysis
2.7. Statistical Analysis
3. Results
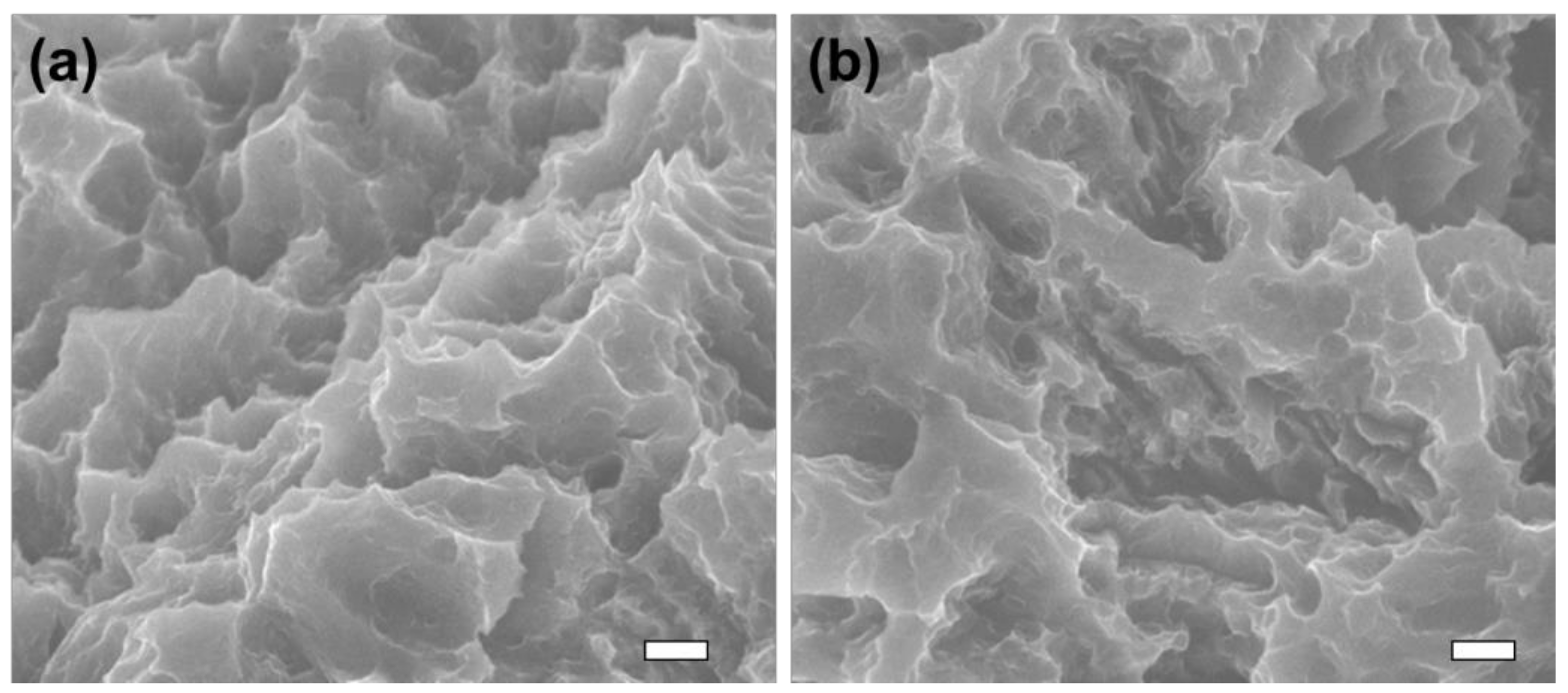
3.1. Surface Morphology
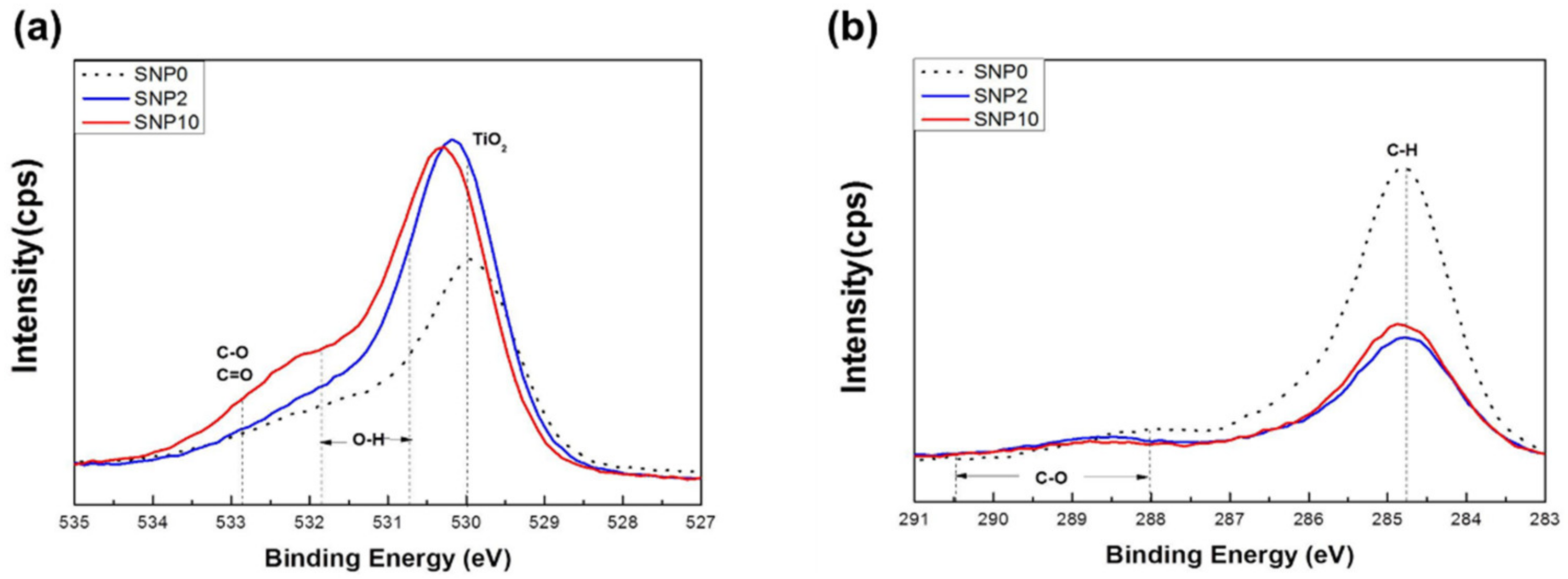
3.2. Surface Chemistry
3.3. Histomorphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Summary of Abbreviations
| NTAPPJ | Non-Thermal Atmospheric Pressure Plasma Jet |
| SA | Sandblasted Acid-etching |
| BIC | Bone-to-Implant Contact |
| BV | Bone Volume |
| TV | Bone Volume |
| ROI | Region Of Interest |
References
- Lemons, J.E.; Niemann, K.M.W.; Weiss, A.B. Biocompatibility studies on surgical-grade titanium-, cobalt-, and iron-base alloys. J. Biomed. Mater. Res. 1976, 10, 549–553. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudice, A.; Bennardo, F.; Antonelli, A.; Barone, S.; Wagner, F.; Fortunato, L.; Traxler, H. Influence of clinician’s skill on primary implant stability with conventional and piezoelectric preparation techniques: An ex-vivo study. J. Biol. Regul. Homeost. Agents 2020, 34, 739–745. [Google Scholar] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 5. [Google Scholar]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 2—Review focusing on clinical knowledge of different surfaces. Int. J. Prosthodont. 2004, 17, 5. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 1. [Google Scholar]
- Wennerberg, A.; Hallgren, C.; Johansson, C.; Danelli, S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin. Oral Implant. Res. 1998, 9, 11–19. [Google Scholar] [CrossRef]
- Ogawa, T.; Ozawa, S.; Shih, J.-H.; Ryu, K.; Sukotjo, C.; Yang, J.-M.; Nishimura, I. Biomechanical Evaluation of Osseous Implants Having Different Surface Topographies in Rats. J. Dent. Res. 2000, 79, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Weinlaender, M.; Kenney, E.B.; Lekovic, V.; Beumer, J., III; Moy, P.K.; Lewis, S. Histomorphometry of bone apposition around three types of endosseous dental implants. Int. J. Oral Maxillofac. Implant. 1992, 7, 198–211. [Google Scholar]
- Ogawa, T.; Nishimura, I. Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int. J. Oral Maxillofac. Implant. 2003, 18, 2. [Google Scholar]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium–cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nat. Cell Biol. 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kwon, J.-S.; Uhm, S.-H.; Song, D.-H.; Kim, Y.H.; Choi, E.H.; Kim, K.-N. The effects of non-thermal atmospheric pressure plasma jet on cellular activity at SLA-treated titanium surfaces. Curr. Appl. Phys. 2013, 13, S36–S41. [Google Scholar] [CrossRef]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Zhang, Y.; Andrukhov, O.; Berner, S.; Matejka, M.; Wieland, M.; Rausch-Fan, X.; Schedle, A. Osteogenic properties of hydrophilic and hydrophobic titanium surfaces evaluated with osteoblast-like cells (MG63) in coculture with human umbilical vein endothelial cells (HUVEC). Dent. Mater. 2010, 26, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Hamlet, S.; Salvi, G.; Huynh-Ba, G.; Bosshardt, D.; Lang, N.P.; Donos, N. Transcriptional profiling of osseointegration in humans. Clin. Oral Implant. Res. 2011, 22, 373–381. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; De Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. Part A 2006, 76, 323–334. [Google Scholar] [CrossRef]
- Teixeira, H.S.; Marin, C.; Witek, L.; Freitas, A., Jr.; Silva, N.R.; Lilin, T.; Tovar, N.; Janal, M.N.; Coelho, P.G. Assessment of a chair-side argon-based non-thermal plasma treatment on the surface characteristics and integration of dental implants with textured surfaces. J. Mech. Behav. Biomed. Mater. 2012, 9, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ao, X.; Xie, P.; Wu, J.; Dong, Y.; Yu, D.; Wang, J.; Zhu, Z.; Xu, H.H.K.; Chen, W. Effects of novel non-thermal atmospheric plasma treatment of titanium on physical and biological improvements and in vivo osseointegration in rats. Sci. Rep. 2020, 10, 10637. [Google Scholar] [CrossRef]
- Hung, Y.W.; Chen, H.L.; Lee, L.T.; Tung, K.C.; Bau, D.T.; Wong, Y.K. Effects of non-thermal plasma on sandblasted titanium dental implants in beagle dogs. J. Chin. Med. Assoc. 2018, 81, 920–925. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Tonino, A.J.; Thèrin, M.; Doyle, C. Hydroxyapatite-coated femoral stems. Histology and histomorphometry around five components retrieved at post mortem. J. Bone Joint. Surg. Br. 1999, 81, 148–154. [Google Scholar] [CrossRef]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent Degradation of the Protein Adsorption Capacity of Titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, J.E. A study on the mechanism of protein adsorption to TiO2. Biomaterials 1991, 12, 593–596. [Google Scholar] [CrossRef]
- Klinger, A.; Steinberg, D.; Kohavi, D.; Sela, M. Mechanism of adsorption of human albumin to titanium in vitro. J. Biomed. Mater. Res. Part A 1997, 36, 387–392. [Google Scholar] [CrossRef]
- Cho, S.; Jung, C. Surface modification of TiO₂ by atmospheric pressure plasma. J. Korean Vac. Soc. 2010, 19, 22–27. [Google Scholar] [CrossRef]
- Song, J.W.; Cha, J.Y.; Bechtold, T.E.; Park, Y.C. Influence of peri-implant artifacts on bone morphometric analysis with micro-computed tomography. Int. J. Oral Maxillofac. Implant. 2013, 28, 519–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, I.; Vagaska, B.; Park, B.J.; Lee, M.H.; Lee, S.J.; Park, J.-C. Selective fibronectin adsorption against albumin and enhanced stem cell attachment on helium atmospheric pressure glow discharge treated titanium. J. Korean Vac. Soc. 2011, 109, 124701. [Google Scholar] [CrossRef]
- Koban, I.; Duske, K.; Jablonowski, L.; Schröder, K.; Nebe, B.; Sietmann, R.; Weltmann, K.-D.; Hübner, N.-O.; Kramer, A.; Kocher, T. Atmospheric Plasma Enhances Wettability and Osteoblast Spreading on Dentin In Vitro: Proof-of-Principle. Plasma Process. Polym. 2011, 8, 975–982. [Google Scholar] [CrossRef]
- Kwon, J.-S.; Kim, Y.H.; Choi, E.H.; Kim, K.-N. The effects of non-thermal atmospheric pressure plasma jet on attachment of osteoblast. Curr. Appl. Phys. 2013, 13, S42–S47. [Google Scholar] [CrossRef]
| Duration of Implant and Groups | BV (Mean ± SD, %) | BIC (Mean ± SD, %) | |
|---|---|---|---|
| 4 weeks | Experimental group | 57.88 ± 6.52 * | 80.9 ± 9.85 * |
| Control group | 49.20 ± 6.83 * | 70.85 ± 17.65 * | |
| 8 weeks | Experimental group | 63.20 ± 11.28 | 81.9 ± 15.92 |
| Control group | 62.14 ± 10.20 | 77.95 ± 15.18 | |
| Author Name | Type and Mode of Non-Thermal Plasma Used | Animal Model Used | Parameter(s) Considered | Major Findings |
|---|---|---|---|---|
| Teixeira, et al. [21] | KinPenTM device1 for either 20 s or 60 s exposure | Radius diaphysis of beagle dogs | Removal torque of implants in 2 and 4 weeks | Significantly increased torque values following plasma exposure |
| Zheng, et al. [22] | CPActive device2 with argon gas flow | Maxillary first molar site of Sprague Dawley rats | BIC, BV, trabecular thickness, and trabecular separation at 2 to 6 weeks of implant | 25% to 40% increase in BIC |
| Hung, et al. [23] | Dielectric barrier discharge with argon flow of 1.8 L/min and oxygen flow of 0.01 L/min3 | Jawbone of beagle dogs | Implant stability quotient (ISQ) following 4, 8, and 12 weeks of implant | Increased the healing time slightly during the early recovery period |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, M.-H.; Park, Y.-B.; Kwon, J.-S.; Kim, Y.-J.; Lee, J.-H. Osseointegration of Plasma Jet Treated Titanium Implant Surface in an Animal Model. Materials 2021, 14, 1942. https://doi.org/10.3390/ma14081942
Jang M-H, Park Y-B, Kwon J-S, Kim Y-J, Lee J-H. Osseointegration of Plasma Jet Treated Titanium Implant Surface in an Animal Model. Materials. 2021; 14(8):1942. https://doi.org/10.3390/ma14081942
Chicago/Turabian StyleJang, Min-Ho, Young-Bum Park, Jae-Sung Kwon, Yeun-Ju Kim, and Jae-Hoon Lee. 2021. "Osseointegration of Plasma Jet Treated Titanium Implant Surface in an Animal Model" Materials 14, no. 8: 1942. https://doi.org/10.3390/ma14081942







