Abstract
This review considers experimental findings on splenic repair, obtained in two types of small animal (mouse, rat, and rabbit) models: splenic resections and autologous transplantations of splenic tissue. Resection experiments indicate that the spleen is able to regenerate, though not necessarily to the initial volume. The recovery lasts one month and preserves the architecture, albeit with an increase in the relative volume of lymphoid follicles. The renovated tissues, however, exhibit skewed functional profiles; notably, the decreased production of antibodies and the low cytotoxic activity of T cells, consistent with the decline of T-dependent zones and prolonged reduction in T cell numbers. Species–specific differences are evident as well, with the post-repair organ mass deficiency most pronounced in rabbit models. Autotransplantations of splenic material are of particular clinical interest, as the procedure can possibly mitigate the development of post-splenectomy syndrome. Under these conditions, regeneration lasts 1–2 months, depending on the species. The transplants effectively destroy senescent erythrocytes, assist in microbial clearance, and produce antibodies, thus averting sepsis and bacterial pneumonia. Meanwhile, cellular sources of splenic recovery in such models remain obscure, as well as the time required for T and B cell number reconstitution.
1. Introduction
The spleen is the largest lymphoid unpaired parenchymal organ of the abdominal cavity found in all vertebrates. The spleen is functionally and morphologically divided into the red and white pulp with a marginal (in rodents) or perifollicular zone (in humans) between them (Figure 1) [1]. Blood circulation in the spleen is open: blood enters the tissues via the trabecular artery and becomes the central artery which gives rise to many branches which enter the red pulp and surround the white pulp [2]. The red pulp is responsible for blood filtration and removes opsonized, damaged, and dying cells from circulation. It also serves as the depot of the organism’s iron, as well as red blood cells, monocytes, and platelets. The spleen, as the largest secondary lymphoid organ, contains about a quarter of the body’s lymphocytes and initiates an immune response to blood antigens [3]. Although adaptive immune responses to such antigens are realized in the white pulp, the cells of innate immunity (neutrophils, monocytes, dendritic cells, and macrophages) could be easily found as residents of red pulp. The white pulp is divided into T- and B-zones, similar to the lymph nodes of the immune system. The T cell zone is also called the periarteriolar lymphoid sheath because it surrounds the central arterioles and is composed of lymphocytes, reticular cells, and reticular fibers [3]. B cell zones represent follicles where clonal expansion of activated B cells can take place [4]. The marginal zone serves to monitor the circulation for antigens and pathogens and plays an important role in antigen processing and for lymphocytes releasing from the circulation and entering the white pulp. The marginal zone mainly contains macrophages and a special subset of B cells (marginal zone B cells) [1].
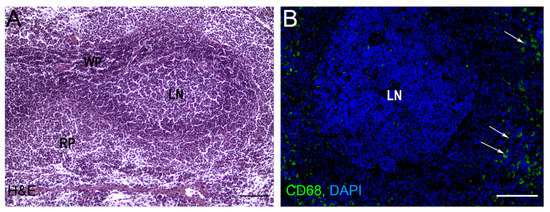
Figure 1.
Rat spleen histology. (A) Light microscopy, hematoxylin-eosin staining (H&E), bars, 200 μm. (B) Cryosection of spleen tissue after anti-CD68 (FITC) immunostaining. The nuclei are counterstained with DAPI. RP—red pulp, WP—white pulp, LN—lymphatic nodule (follicle), bars, 200 μm. Arrows indicate CD68 + macrophages in red pulp. Original image generated in the author’s laboratory.
Total resection of the spleen is an option in a number of clinical situations including traumatic injury, thrombocytopenia, and severe portal hypertension [5]. Despite the apparent simplicity of the procedure, 2–10 years in the aftermath, a number of patients develop complications collectively referred to as post-splenectomy syndrome, most typically manifested by recurrent infections of varying severity [5]. Clinical examples demonstrate a different patient survival rate after splenectomy [6]. In the early work of Bonnet-Gajdos and colleagues, it was shown that splenectomy performed in 21 patients with age from one to 23 years old had no pronounced negative clinical manifestations and did not affect the normal maturation of children [7]. In another study with a 19/21 survival rate, splenectomy caused by sporadic haemophagocytic lymphohistiocytosis (HLHs) showed a real diagnostic benefit in establishing the cause of HLHs and had a therapeutic effect [8]. Splenectomy leads to an increased risk of septic complications associated with high mortality, the most serious of which is the development of functional hyposplenism associated with overwhelming post-splenectomy infection (OPSI), which can progress from flu-like illness to fulminant sepsis in a short period of time and is accompanied by high mortality [9]. The first descriptions of OPSI date back to 1952, when King and Schumaker first described bacterial sepsis after splenectomy in infants and children [10]. In a large retrospective study of 6942 patients, Bisharat and colleagues showed a low risk of postsplenectomy sepsis; however, approximately 40–50% of all people who develop postsplenectomy sepsis died [11]. Now it is known that the predominant causative agents of postsplenectomy sepsis are resistant to phagocytosis encapsulated bacteria, Streptococcus pneumoniae [12]; therefore, the long-term antibiotic therapy is designed to prevent the development of OPSI after splenectomy [13]. Some studies also claim increased risks of tumorigenesis after splenectomy [6,14,15,16]. Such complications indicate significant problems in both humoral and cellular wings of immunity. Considering the prevalence of such complications, regeneration of the spleen is extremely relevant. Apart from the issues of splenic repair per se, this review considers effects of the spleen on hepatic recovery after various kinds of damage. These effects are substantial due to the close anatomical and functional relationship between the two organs.
2. Splenic Regeneration after Resections
The most common indications for splenectomy are a wide variety of diseases and conditions: raptured or enlarged spleen, blood disorders like idiopathic thrombocytopenic purpura and thalassemia, cancer (chronic lymphocytic leukemia, Hodgkin’s lymphoma, and non-Hodgkin’s lymphoma), infection, etc. The study of spleen regeneration after splenectomy is often limited to patients and small laboratory animals, while for larger species, splenectomy has only been studied in the context of survival. Thus, it was shown that post-splenectomy, post-operative survival rate was 52% for dogs [17], 87.5% for Theileria haneyi-infected, splenectomized horses [18], and almost complete post-operative recovery was observed for cattle in more earlier works [19,20].
The first studies on mammalian spleen regeneration date back to the 19th century [21,22]. Dedicated research on the dynamics, cellular sources, and spleen microanatomy after resections peaked in the 20th century [21,22]. Over recent years, the focus has shifted from post-splenectomy regrowth to post-transplantation recovery. Data on spleen regeneration after resection are summarized in Table 1.
The earliest known experimental studies on regeneration of the spleen after resections were carried out in the 19th century by Jean-Marie Philipeaux [21]. In early experiments, resections of the spleen ended with restoration of its shape and mass; occasionally, the remnant overgrew the initial size of the organ. Later on, these results were questioned and, by the turn of the century, the general opinion was that the spleen does not regenerate after resections and the site heals by scarring [21].
A bunch of experimental studies on regeneration of the spleen after resections was implemented by academic staff of the Laboratory of Growth and Development at the Scientific Research Institute of Human Morphology, Moscow, Russia. One series involved mice undergoing 50% splenectomy. The extent of regenerative outcomes considerably varied: after 1 month, most of the remnants grew significantly in length and volume, while preserving the architecture and showing no outgrowth at the wound surface. It should be noted that, even in cases of intensive regrowth, the organs never reached their initial size. Moreover, in a number of cases, the remnants progressively shrunk [22,23,24].
Resections of 90% of the splenic volume in mice produced functional spleen equivalents of loosely cubic shape in 38 days [21,22]. Regeneration was accompanied by an increase in the density of lymphoid follicles and their relative area, as assessed by light microscopy [25,26]. The earliest observable reaction of splenic tissues to the resection involved the emergence of macrophages burdened with particles of destroyed nuclei. Six hours post resection (p/r), reticular tissue cells started to proliferate and were followed by erythrocyte and lymphocyte lineages enriched in cells with blast morphology [21,22]. Erythrocyte progenitors reached their maximal mitotic activity by day 3 p/r. Interestingly, repeated resections promoted a sharp increase in the content of erythrocyte lineages within the remnant and a reciprocal decrease in the content of lymphocyte lineages [21,22]. Transplantations of cells isolated from intact and regenerating spleens to lethally irradiated mice yielded equal numbers of colonies, indicating the rapid recovery of stem cell populations [27]. The rates of recovery for T and B cell populations in regenerating spleens differed: the slower proliferation of T cells resulted in their significantly reduced numbers as late as 3 months after the resection [21,22].
In addition to the morphological and cell lineage studies, the focus was expanded to the functional profiles of splenic lymphocytes, including the production of antibodies and participation in cellular immunity reactions. Following the resection and regrowth, splenic lymphocytes produced lower amounts of antibodies and showed weaker graft-versus-host reactions compared with the cells from intact spleens [28]. The observed functional decline was explained by the enrichment with immature, functionally compromised lymphocytes in the course of regeneration [21,22].
Species–specific differences in splenic regeneration are pronounced. Rabbits, by contrast with mice and rats, are incapable of splenic regrowth [25]. The difference is interesting in terms of adaptive immunology: rabbits respond to splenectomy by compensatory increase in the volume of lymph nodes, which acquire structural features of the spleen. Splenic regeneration in rabbits can be induced chemically [29]. In other model animals, regeneration rates significantly depend on age (higher in younger animals) and season (higher in summer). In addition, the regrowth can be inhibited by thymectomy [28] and stimulated by injections of certain antigens [21,22].
Together, these studies indicate that classical experiments on spleen resection unequivocally indicate its ability to regenerate, which is accompanied by the preservation of tissue architectonics and an increase in the size of lymphoid follicles. In the next section, we will turn to the analysis of heterotopic (not in original location) transplantations of the spleen as a frequent model for studying regeneration.

Table 1.
Spleen regeneration after resection.
Table 1.
Spleen regeneration after resection.
| Authors | Volume of Resection | Regeneration Period | Antibody Production | T Cell Activity | Other | |
|---|---|---|---|---|---|---|
| 1. | L. D. Liozner and Kharlova [24] | 50% | 30 days | Decreased production of antibodies | Decreased T cell activity | The observed functional decline was explained by the enrichment with immature, functionally compromised lymphocytes in the course of regeneration |
| 2. | Cameron and Rhee [23] | 50% | 30 days | |||
| 3. | Macka and Scott Polland [25] | 50% | 30 days | An increase in the density of lymphoid follicles and their relative area | ||
| 4. | Kharlova [22] | 90% | 38 days | Slower recovery of T cells compared to B lymphocytes | ||
| 5. | Pouché et al. [26] | 50% | 90 days | The results of histologic study demonstrate a readjustment of the vascular net and the lymphoid tissue of the white pulp |
3. Autotransplantations of the Spleen
The orthotopic regeneration of the spleen is clinically unfeasible. At the same time, unattended splenectomies are fraught with delayed immunological complications [5]. The problem can be solved by transplantation of splenic material to heterotopic locations.
The idea was born in connection with clinical cases of spontaneous splenic engraftment. In the aftermath of splenectomy, functionally active fragments of splenic lymphoid tissue may occasionally settle in the abdominal cavity, resembling accessory spleens. This phenomenon is known as ‘splenosis’ [30,31].
Studies on the heterotopic recovery of autologous splenic grafts in rodent models began in the early 20th century. Two major sites of heterotopic engraftment were abdominal (mostly intraomental) and subcutaneous. The course of regeneration for these two sites was similar and took about 1 month [32,33]. Data on heterotopic spleen transplantation are summarized in Table 2.
An early series of such experiments was, oddly enough, performed on rabbits [34,35], incapable of orthotopic splenic regrowth under standard conditions [25]. Nevertheless, the splenic architecture was restored within 80 days after subcutaneous autotransplantations. In younger rabbits or rabbits after total splenectomy, the regeneration proceeded faster [34,35].
These pioneering models were meticulously reproduced in other species of laboratory animals [36]. In rats and mice, the abdominal engraftments proceeded similarly and lasted about 1 month [33]. Other studies indicated longer regeneration in rats, about 2 months [23,37]. The early stage (days 0–3 post-transplantation, p/t) was marked with degradation of the tissue architecture and massive death of lymphocytes. At 12–18 h p/t, two zones could be clearly distinguished within the graft: the central zone of necrosis and the peripheral zone of viable cells, with reticular cells projecting towards the central zone. First capillaries emerged at 48 h p/t. On day 7–9, clusters of cells with round dark nuclei and lymphocytic morphologies appeared in the peripheral zone and around capillaries. Starting from day 13–15, formation of lymphoid follicles with enlarged lymphocyte-like cells was evident. By day 29 p/t, the graft acquired characteristic tissue architecture of the spleen [23,33,38,39] and comprised differentiated B cells and red pulp macrophages [40].
Consistent with the previous findings on rabbits, in rat models, the engraftment was more robust in younger animals, with accelerated vascularization and re-innervation of the grafts, and higher numbers of Ki67+ cells and their distributions typical of the intact spleens in young rats [39,41,42]. In contrast to rabbit models, in rats, the engraftment was more robust after incomplete splenectomies [23,33,43].
Over recent decades, these techniques were refined. The optimal site for peritoneal engraftments is the greater omentum, although autologous transplantations to the mesentery or the inside of abdominal wall work as well [44,45,46]. Moreover, the whole ectomized spleen can be attached surgically to the liver, with successful outcomes [47].
Subcutaneous splenic grafts provide a major alternative, although the scenario is basically similar to the intraperitoneal splenic regeneration. In rats, the subcutaneous engraftments are finalized within 1–1.5 months p/t. Identical to the peritoneal grafts, the early phase is marked with necrosis. Subsequent morphological landmarks are blood vessel ingrowth (day 3) and accumulation of larger lymphocytes with dark nuclei in peripheral parts of the transplant (day 7). By day 14, the lymphocytes increase in number. By day 28, the transplant recovers characteristic splenic architecture with red and white pulp compartments [32]. These observations are corroborated by more recent studies of neovasculogenesis in murine transplants, with precise measurements enabled by injection of tracers (fluorescent polystyrene microspheres) and electron microscopy. Although the new vessels at the periphery of the grafts started to form on day 3 p/t, microcirculation within the marginal zone of white pulp remained rudimentary until week 10 p/t [48].
Most of the experiments indicate the universal capacity of autologous splenic grafts to regenerate at a variety of anatomical sites. At the same time, several studies argue that subcutaneous environments are less favorable due to the lower rates of angiogenesis and specify the greater omentum as the optimal site for the engraftment [49]. Such conclusions are based on functional metrics: only intraomental transplants, as opposed to intramuscular, etc., ensured the ‘intact’ rates of pneumococcal clearance after regeneration [50].
Comprehensive functional assessment of the outcomes is important in connection with the clinical relevance of splenic autotransplantations. In this regard, hematopoietic status and resistance to particular infections and tumors in the aftermath of the intervention are chief indicators of its success or otherwise.
Within 2 weeks p/t, the autografts begin to effectively sort erythrocytes, with the concomitant clearance of senescent erythrocytes from the blood [51,52,53]. The first week p/t is marked with a sharp increase in platelet counts and fibrinogen levels. These indicators return to normal within 3–6 weeks as the splenic tissue regenerates heterotopically [51,52].
The main clinical goal of splenic autografts is to prevent the development of severe infectious complications [54,55,56]. According to experimental findings, the prospects are realistic. For instance, splenic autotransplantations to the great omentum in rat models ensured the anti-pneumococcal defense [57,58,59], and similar effects were achieved with subcutaneous grafts [60]. Primary indicators are the ability of splenic autografts to produce antibodies and promote bacterial clearance [49]. After experimental autotransplantations in laboratory animals, total blood levels of IgM are restored within 8 months [61,62]. In functional tests with pneumococcal vaccination, autologous transplantations provide partial response in the form of specific IgM and IgG antibodies, albeit not to all pneumococcal serotypes [40]. Apart from the antibody production, the grafts effectively promote bacterial clearance; this was demonstrated for infections with E. coli, as well as pneumococci [38,50,63]. Participation of splenic autografts in the immune response to Staphylococcus aureus was explored in mouse model. Splenectomized animals with splenic autografts produced lesser colonies in seeded blood cultures along with higher titers of S. aureus-specific IgM and IgG1 in comparison with flat splenectomies [64]. Despite the encouraging results, the influence of autologous grafts on the circulating pools of T and B cells remains understudied. In several studies, splenic autografts did not rescue the lymphocyte blood counts, and after 8 months the circulating numbers of both CD3+ T and CD19+ B cells remained reduced [52,61,62]. In other studies, the ability of spleen autografts to confer immunity against fatal infections was either negligible, or the effect was short-term [49].
In translational perspective, it is important to determine the critical mass of splenic transplant, competent of protecting the body from fatal infections. Although such a value may be of pure theoretical interest [49], several studies loosely define it as 30–50% of the intact spleen mass [49,63].
Efficacy and safety of autologous splenic grafts were assessed in a number of clinical trials (≥18). Sixteen of those involved engraftments in the greater omentum and two trials involved engraftments at retro- and extraperitoneal locations. The general blood test indicators returned to their pre-operative (or similar) values in all participants, and regeneration of the transplant was confirmed scintigraphically in 95.3% of the cases. In 12 clinical trials, the levels of IgM returned to normal values; in 3 trials, IgM levels were higher in patients with splenic autografts than in patients after flat resections; and the other 3 trials found no difference in IgM levels between these groups. Complications of the engraftment per se were encountered in 3.7% of the cases; these included intestinal obstruction in four patients and subdiaphragmatic abscess in one patient. The incidence of delayed severe infections was evaluated in five trials, with a total of one case (pneumonia) recorded [65].
Apart from their pre-clinical significance, experimental models of splenic transplantation are of undoubted theoretical interest. The famously complex tissue architecture of the spleen is reconstituted both morphologically and functionally after the total collapse. The depth of the initial degradation can be explained by details of the technique: the surgeon creates no anastomosis between the graft vasculature and the local microcirculatory bed at the host site. Thus, the limiting stage (and also the critical stage, burdening and jeopardizing the whole process) is the ingrowth of new vessels into the autograft. Regeneration of splenic parenchyma is delayed until the vascular connection has been established. In terms of the repair process dynamics and angiogenesis, as well as hematopoietic and immune recovery at systemic level, regeneration of splenic autografts represents a unique model. Cellular sources of splenic regeneration are also of special interest. It is not clear which cells are responsible for the replacement of the destroyed splenic architecture: hematopoietic stem cells that arrive from circulation to populate the niche formed de novo by reticular cells or the resident stem cell lineages that survive within the graft despite the severity of the early necrosis. These questions have long been asked [49], but are only recently being answered [66].
The splenic stroma is known to consist of reticular tissue constituted by respective cells and fibers [66,67]. In seminal works on hematopoiesis, spleens devoid of hematopoietic cells by a lethal dose of ionizing radiation were capable of harboring proliferation and differentiation of newly transplanted hematopoietic cells [68]. These findings indicate the possibility of colonization of the autograft by circulating hematopoietic progenitors after elimination of its own hematopoietic lineages through necrosis. Such a scenario is all the more likely given that migration of hematopoietic stem cells from the bone marrow to the spleen in intact animals has been confirmed experimentally [69].
The spleen has a niche for hematopoietic stem cells, allegedly located in perivascular spaces [70]. The niche comprises endothelial cells, PDGFRb+ mesenchymal cells, and perivascular reticular cells of the red pulp which produce the stem cell factor (SCF) and CXCL12 necessary for hematopoiesis [71]. Some of the perivascular reticular cells of the red pulp also express Tcf21 and PDGFRb, thus being a unique Tcf21/PDGFRb/SCF/CXCL12 quadruple-positive cell type [72]. Stromal cells of the splenic hematopoietic niche are capable of myelopoiesis maintenance in vitro, as well as upon transplantation beneath the renal capsule in NOD/SCID mice [68,73].
Despite the existence of the authentic splenic niche that supports proliferation and differentiation of hematopoietic cells, determination of cellular sources for the splenic autograft regeneration is still an issue. One study showed that splenic capsule of 3-day-old mice transplanted allogeneically beneath the kidney capsule was able to induce genesis of a spleen with histological structure indistinguishable from the normal mouse spleen. Importantly, lymphocytes of the newly formed spleen were of the host origin [74]; the same was true in transplantations of whole embryonic spleens [75]. Stromal cells of the splenic capsule, which induced the heterotopic splenogenesis, exhibited the CD45+CD32 CD192CD11b2CD4+IL-7R+ hematopoietic lymphoid tissue inducer (LTi) phenotypes. Dynamic phenotyping of stromal cells isolated from splenic capsule in the course of postnatal development revealed a specific capacity of CD31+MAdCAM-1+LTbR+ cells with the assistance of lymphotoxin α1β2 (LT) signaling to induce the heterotopic splenogenesis [74,76]. These interesting findings are consistent with the higher regenerative capacity of splenic autografts from younger animals observed in earlier studies. The authors demonstrated that splenic capsules of >8-day-old animals failed to induce splenogenesis due to a sharp decline of CD31+MAdCAM-1+LTbR+ cells [74,76]. Nevertheless, as splenic autografts of mature animals regenerate well, the model may need a refinement.
Another potential cellular source of the splenic parenchyma in regenerating autologous splenografts consists of their own low-differentiated cells that survive during the necrotic phase. Some early findings suggested that the perivascular reticular cells may trans-differentiate into hematopoietic cells [23,33].
The evidence presented in this section suggests that the heterotopic transplantations of the spleen model is more complex for investigating splenic regeneration, but this approach may be a promising method for alleviating post-splenectomy syndrome. The spleen transplant is capable of properly clearing aging erythrocytes and bacteria and producing antibodies. Although in the contemporary perspective this is ambiguous, it is important to recognize the primacy of the hypothesis that autografts regenerate at the expense of their own cells. A more convincing concept has yet to be developed. The proper functional activity of the spleen certainly affects other organs of the abdominal cavity, and the issue of this mutual influence will be considered in the next section of our review.

Table 2.
Spleen regeneration after heterotopic transplantations.
Table 2.
Spleen regeneration after heterotopic transplantations.
| Authors | Animal, Autograft Localization | Regeneration Period | The Effect of Autotransplantation | |
|---|---|---|---|---|
| 1. | Manley and Marine [34,35] | Rabbit, subcutaneous | 80 days | |
| 2. | Perla [36] | Rat, abdomen wall | 12–21 days | |
| 3. | Calder and Scholar [33] | Rat, mouse, omentum | 30 days | |
| 4. | Cameron and Rhee [23] | Rat, mouse, omentum | 60 days | |
| 5. | Braga et al. [37] | Rat, mesenterium | 60 days | |
| 6. | Han et al. [47] | Rat, liver lobe | 35 days | |
| 7. | Han et al. [47] | Rat, mesenterium | 84 days | |
| 8. | Miko et al. [52] | Mouse, omentum | 42 days | Clearance of senescent erythrocytes from the blood, decreased platelet count and fibrinogen levels, recovery of IgM levels, a numbers of the circulating CD3+ T and CD19+ B cells remained reduced |
| 9. | Sipka et al. [53] | Mouse, omentum | Clearance of senescent erythrocytes from the blood, decreased platelet count and fibrinogen levels | |
| 10. | Patel et al. [57] | Rat, omentum | Anti-pneumococcal defense | |
| 11. | Leemans et al. [40] | Rat, omentum | Spleen autotransplants improve humoral response to pneumococcal capsular polysaccharides | |
| 12. | Marques et al. [38,63] | Rat, omentum | Efficient clearance of E. coli and pneumococci | |
| 13. | Teixeira [64] | Mouse, retroperitoneum | Production of high titers of S. aureus-specific IgM and IgG1 |
4. Splenic Influence on Repair Processes in the Liver
The immune system should be vigilant. Any minor failure in its functioning immediately affects the homeostasis, as exemplified by the post-splenectomy syndrome. Meanwhile, splenectomy is absolutely indicated in certain pathological conditions of the liver. The close relationship between the two organs is reflected by the concept of hepatosplenic axis (Figure 2) [77,78]. The apparent participation of the spleen in the regulation of hepatic repair can be further redefined as the role of immunity in regeneration. Pioneering research in this field was carried out by Anna G. Babaeva and her school [79,80,81].
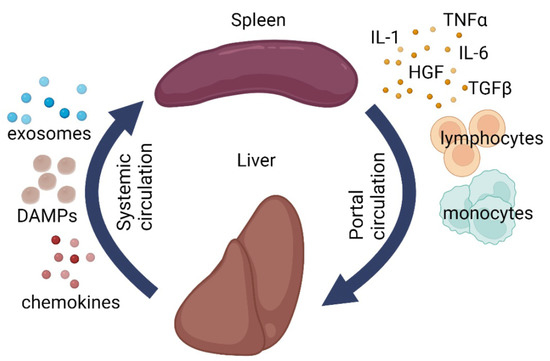
Figure 2.
Schematic illustration of liver-splenic axis. DAMPs—damage-associated molecular patterns, IL1—interleukin 1, IL6—interleukin 6, TGF-β—transforming growth factor beta, TNFα—tumor necrosis factor alpha, HGF—hepatocyte growth factor.
The spleen and the liver are anatomically connected via portal circulation and have shared responsibilities (immune, barrier, metabolic, and hematopoietic). Clinical experience shows that liver diseases often disrupt the normal splenic architecture [77,78]. At the same time, splenectomy has a positive effect on hepatic repair, though its mechanisms remain understudied [82,83].
Apart from experimental models, a significant portion of the evidence on the potential splenic involvement was obtained in patients with liver cirrhosis. The disease is accompanied by excessive production of extracellular matrix by the activated stellate cells (Ito cells) of the liver [84,85]. The activation is triggered by multiple soluble factors, most prominently TGFβ1 [85]. The elevated levels of pro-fibrotic TGFβ1, characteristic of hepatitis eventually resolved in cirrhosis, may significantly depend on the increased production of this factor by activated macrophages of the splenic red pulp. In a rat model of thioacetamide-induced cirrhosis, splenectomy leads to a decrease in blood levels of TGFβ1, considered beneficial for reparative processes in the damaged liver [86]. Consistent with these experimental findings, immunohistochemical assessment of splenic tissues in patients with cirrhosis reveals increased content of TGFβ1 and its colocalization with CD68+ cells (macrophages) [87].
The impact of splenectomy on macrophage and lymphocyte populations of the liver in cirrhosis was emphasized in a number of studies. In a mouse model of concanavalin-induced hepatitis and cirrhosis, splenectomy promotes polarization of liver macrophages towards anti-inflammatory M2 phenotypes, which support the recovery [88]. In mice with thioacetamide-induced cirrhosis, splenectomy reduces the degree of fibrotic lesions while enhancing hepatocyte proliferation and augmenting the numbers of Ly-6C(lo) monocytes and macrophages [89]. On the other hand, splenectomy in rats with induced cirrhosis alleviates the damage, in particular, through increased production of TNFα by liver macrophages; at that, the macrophage numbers remain unaltered [90]. It is noteworthy that in rats without liver damage, the liver reacts to splenectomy by enhanced proliferation of hepatocytes. Hepatic macrophages react as well: CD68+ cells increase in number, whereas the numbers of CD206+ cells decrease, with concomitant enhancement of Il6, Il10, Tnfa, Hgf, and Nos2 expression in the liver [91].
The heavy-duty hepatosplenic circulation provides a permanent opportunity for the transportation of splenic monocytes/macrophages to the liver. On systemic scale, the spleen is viewed as a stock of monocytes to be released on demand for transportation and deployment at inflammatory foci. This is true for a number of murine models, including ischemic myocardial damage [92], ischemic brain damage [93], concussion spinal cord injury [94], and muscular dystrophy in mice [95]. However, the effect may as well be disease-specific; for instance, in a mouse model of lung carcinoma, the majority of monocytes arrive to the tumor directly from the bone marrow, bypassing the spleen [96]. The arrival of monocytes/macrophages to the liver in the aftermath of hepatotoxic damage or resection has been demonstrated [97,98], albeit without clear specification of their source. In our setting, intrasplenic injections of mesenchymal stromal cells (MSCs) labeled with a vital dye PKH26 led to appearance of PKH26-positive CD68+ cells (macrophages) in the liver 24 h after the injections [99]. However, whether these are liver macrophages that have engulfed the labeled MSCs arriving from the spleen, rather than splenic macrophages that have engulfed MSCs on the spot before migrating to the liver, remains unknown.
Migration of monocytes from the spleen to the liver is disputable; for splenic lymphocytes, this route has been confirmed. In a murine model of cirrhosis, the spleen becomes progressively depleted of CD4+ (helper) T lymphocytes, with a simultaneous increase in the content of Th2 lymphocytes (thought to augment fibrosis) in the liver. Under these circumstances splenectomy restores the Th1/Th2 balance and alleviates the fibrosis [100]. Apart from the lymphocytes arriving from the spleen, the liver harbors several minor lymphocyte subpopulations including γδT cells [101], NK cells [102,103], and NKT cells [104], which exert modulatory effects on liver repair [105]. The impact of splenectomy on these subpopulations remains unknown.
Splenectomy (splenectomized status) is also beneficial for regeneration of the liver after massive resections. The majority of studies emphasize the elevated rates of hepatocyte proliferation in splenectomized animals, although its mechanistic causes have to be specified. The variants include (1) resolution of portal hypertension; (2) mitigation of the damage to sinusoidal endothelium; (3) alleviation of the inflammatory side effects by inhibiting the synthesis of pro-inflammatory cytokines, as well as the rates of macrophage and neutrophil infiltration; and (4) hepatocyte apoptosis inhibition [77,106].
Splenectomy complementing 90% resections of the liver volume is accompanied by decreased expression of multiple acute phase markers in the liver remnant and increased expression of heme oxygenase-1 gene, considered beneficial for the repair [107]. Physical removal of the spleen abrogates the inflow of pro-inflammatory cytokines that cause hepatocyte damage, as well as the proliferation blocker TGFβ1 [108,109,110]. These conditions enhance the synthesis of HMOX1 in the liver, which inhibits the activity of TNFα with a net cytoprotective effect on hepatocytes; moreover, the synthesis of TGFβ1 and its receptor TGFβ RII decreases, while the synthesis of HGF and its receptor c-met increases [108,109,111,112]. Other studies argue that the beneficial effect may be due to the withdrawal of IL10, which is a confirmed inhibitor of hepatocyte proliferation. IL10-deficient mice exhibit higher rates of hepatocyte proliferation in response to resections compared to ordinary animals. Partial hepatectomy promotes increased expression of IL10 not only in the liver, but also in the spleen; accordingly, splenectomy cuts off the inflow of IL10 via portal vein [113]. A similar positive correlation between splenectomy and liver repair is observed in liver transplantation models. The benefits include a decrease in portal hypertension and alleviation of endothelial damage, apoptosis, and pro-inflammatory cytokine synthesis [114].
On the other hand, some models question, and even disprove, the benefits of splenectomy for liver repair. For instance, Babaeva et al. observed the opposite, inhibitory effect of splenectomy on the compensatory growth of the liver after resections. The strength of the effect did not depend on the time lapse between the two interventions (splenectomy followed by liver resection); [115]. At the same time, splenectomy promoted a significant increase in the volume of intact liver through increased hepatocyte proliferation [115].
Contemporary studies on the role of immunity in regeneration involve model animals depleted of particular lymphocyte populations. The data obtained in such models are often controversial, which reflects the complexity of the regulatory mechanisms. In mice depleted of T cells, resections of 70% liver volume have lower rates of necrotic complications than in ordinary mice [116]. Similar results were obtained in a model of lipopolysaccharide-induced hepatitis; animals depleted of T or B cells revealed lower degree of hepatotoxic damage and better survival [117]. At the same time, the block of hepatocyte proliferation in rats depleted of T cells or NK cells is accompanied by the lack of proliferative response from hepatic oval cells, by contrast with the control animals, in which only the hepatocyte proliferation remained blocked [118].
Other examples of lymphoid cell participation in repair processes include experiments with adoptive transfer of lymphocytes from actively regenerating organs to the orthotopic locations in non-operated syngeneic animals [115,119]. Upon the transfer, lymphocytes retained their regeneration-supporting capacity to a degree depending on the organ, the phase of repair, and the type (population) of the lymphocytes. Transfers of helper T cells had the most pronounced effect [115,119]. The nature of regeneration-activating signals in this case is obscure; possible transmitters are microRNA molecules contained in microvesicles and exosomes secreted by lymphocytes and other cell types [120].
Thus, immune cells inside the liver, and some of those outside it, may influence repair processes within the organ or its remnant. The impact can be either activating or inhibiting; the latter is exerted by T killers, NK cells, and NKT cells. The stimulatory effect of splenectomy on the hepatic recovery after various types of damage, as well as the adoptive transfer of lymphocytes as the means for boosting liver repair, are subject to further investigation. Overall, these results indicate that the liver and spleen actively influence each other both at the level of cell migration and at the level of cytokine balance.
5. Conclusions
The spleen has been traditionally regarded an accessory immune organ. However, this view is challenged by severe infectious and tumorigenic consequences of splenectomy. Apart from its prominent role in immunogenesis, the spleen appears to control hepatic repair, as has been confirmed in a number of experimental models. The spleen is also a depot of monocytes, wherefrom they migrate to damaged organs. The problem of splenic regeneration is of high clinical relevance; in this regard, the most important frontier is splenic autografts. The possibility of heterotopic autologous transplantation of splenic fragments has been comprehensively assessed in experimental models and a number of clinical trials. The autografts successfully restore the normal splenic architecture of red pulp and white pulp with the newly formed periarteriolar lymphoid sheaths and lymphoid follicles. The issues still waiting to be explored include the extent of functional recovery and stability of T and B lymphopoiesis, as well as cellular sources of de novo splenogenesis in heterotopic autografts.
Author Contributions
Writing—original draft preparation, A.E.; writing—review and editing, A.E., P.V., T.F. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was performed within the framework of State Assignment “Development of a method for the treatment of ovarian cancer using macrophages with persistent pro-inflammatoryproperties” (No 121040600409-1). This paper has been supported by the RUDN University Strategic Academic Leadership Program. Part of the work concerning monocytes was supported by a grant for young Russian scientists MK-1573.2022.3. This work was supported by Russian Science Foundation [grant number 22-14-00152].
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
We acknowledge Natalia Usman for helpful discussions.
Conflicts of Interest
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef] [PubMed]
- Delves, J.P. (Ed.) Encyclopedia of Immunology, 2nd ed; Academic Press: Cambridge, MA, USA, 1998; ISBN 978-0-12-226765-9. [Google Scholar]
- Cesta, M.F. Normal Structure, Function, and Histology of the Spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Buzelé, R.; Barbier, L.; Sauvanet, A.; Fantin, B. Medical complications following splenectomy. J. Visc. Surg. 2016, 153, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Gridley, G.; Hoover, R.N.; Check, D.; Landgren, O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: A Cohort Study with up to 27 Years Follow-up. Haematologica 2014, 99, 392. [Google Scholar] [CrossRef]
- Fouquet, G.; Larroche, C.; Carpentier, B.; Terriou, L.; Urbanski, G.; Lacout, C.; Lazaro, E.; Salmon Gandonnière, C.; Perlat, A.; Lifermann, F.; et al. Splenectomy for haemophagocytic lymphohistiocytosis of unknown origin: Risks and Benefits in 21 Patients. Br. J. Haematol. 2021, 194, 638–642. [Google Scholar] [CrossRef]
- Bonnet-Gajdos, M.; Berger, J.P.; Gerota, I.; Vergoz, D.; Ferrer, M.; Gruner, M.L. Asplenia: A Study of 21 Subjects Splenectomized During Childhood for Spleen Injury (author’s transl). La Nouv. Press. Médicale 1981, 10, 313–316. [Google Scholar]
- Sinwar, P.D. Overwhelming post splenectomy infection syndrome—Review study. Int. J. Surg. 2014, 12, 1314–1316. [Google Scholar] [CrossRef]
- King, H.; Shumacker, H.B. Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann. Surg. 1952, 136, 239–242. [Google Scholar] [CrossRef]
- Bisharat, N.; Omari, H.; Lavi, I.; Raz, R. Risk of infection and death among post-splenectomy patients. J. Infect. 2001, 43, 182–186. [Google Scholar] [CrossRef]
- Morozov, D.A.; Klyuev, S.A. Hyposlenism After Splenectomy. Ann. Russ. Acad. Med. Sci. 2015, 70, 413–418. [Google Scholar] [CrossRef]
- Luu, S.; Spelman, D.; Woolley, I.J. Post-splenectomy sepsis: Preventative Strategies, Challenges, and Solutions. Infect. Drug Resist. 2019, 12, 2839–2851. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.G.; Cheong, J.H.; Hyung, W.J.; Kim, J.; Choi, S.H.; Noh, S.H. Adverse effect of splenectomy on recurrence in total gastrectomy cancer patients with perioperative transfusion. Am. J. Surg. 2006, 192, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Mellemkjøer, L.; Olsen, J.R.H.; Linef, M.S.; Gridley, G.; McLaughlin, J.K. Cancer Risk after Splenectomy. Cancer 1995, 75, 577–583. [Google Scholar] [CrossRef]
- Arlashkina, O.M.; Struchko, G.Y.; Merkulova, L.M.; Mikhailova, L.P. Changes in the white pulp of the spleen in the offspring of splenectomy rats of different age periods after the administration of 1,2-dimethylhydrazine. Clin. Exp. Morphol. 2019, 8, 48–59. [Google Scholar] [CrossRef]
- Spangler, W.L.; Kass, P.H. Pathologic Factors Affecting Postsplenectomy Survival in Dogs. J. Vet. Intern. Med. 1997, 11, 166–171. [Google Scholar] [CrossRef]
- Knowles, D.P.; Sears, K.P.; Knowles, D.P.; Fry, L.M. Clinical Progression of Theileria haneyi in Splenectomized Horses Reveals Decreased Virulence Compared to Theileria equi. Pathogens 2022, 11, 254. [Google Scholar] [CrossRef]
- Thompson, J.R.; Kersting, K.W.; Wass, W.M.; Davis, I.A. Splenectomy in cattle via transthoracic approach. Am. J. Vet. Res. 1992, 53, 143–144. [Google Scholar]
- Boyce, W.L.; Wellde, B.T.; Reardon, M.J.; Bhogal, M.S.; Chumo, D.A. Effects of splenectomy on Trypanosoma congolense infection in cattle. Ann. Trop. Med. Parasitol. 1989, 83, 195–200. [Google Scholar] [CrossRef]
- Liozner, L. Organ Regeneration: A Study of Developmental Biology in Mammals (Studies in Soviet Science), 1st ed.; Liozner, L.D., Ed.; Springer: New York, NY, USA, 1974; ISBN 978-1-4684-8458-8. [Google Scholar]
- Kharlova, G.V. Regeneration of Lymphoid Organs in Mammals; Meditsina: Moscow, Russia, 1975. [Google Scholar]
- Cameron, G.R.; Rhee, K.-S. Compensatory hypertrophy of the spleen: A Study of Splenic Growth. J. Pathol. Bacteriol. 1959, 78, 335–349. [Google Scholar] [CrossRef]
- Liozner, L.D.; Kharlova, G.V. Regeneration of the spleen in mice after removal of a large part of the organ. Biull. Eksp. Biol. Med. 1960, 49, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Macka, E.M.; Scott Polland, W. Comensatory Hypertrophy of the spleen. J. Exp. Med. 1931, 53, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Pouche, A.; Chiodera, P.; Bosio, P.; Allevi, G.; Tiberio, G. Experimental splenic regeneration. Surg. Gynecol. Obstet. 1986, 162, 25–28. [Google Scholar] [PubMed]
- Sukhova, G.K.; Podrabinek, T.R.; Kharlova, G.V. The influence of the regenerating hemopoietic organs on the number and type of splenic colonies. Byulleten Eksp. Biol. I Meditsiny 1978, 85, 219–221. [Google Scholar]
- Kharlova, G.V. Antibody formation processes in the regenerated spleen of intact and thymectomy mice. Byulleten Eksp. Biol. I Meditsiny 1971, 72, 52–54. [Google Scholar]
- Mezhlumian, A.A. On stimulation of spleen regeneration in rabbits. Biull. Eksp. Biol. Med. 1964, 57, 103–106. [Google Scholar]
- Fremont, R.D.; Rice, T.W. Splenosis: A Review. South. Med. J. 2007, 100, 589–593. [Google Scholar] [CrossRef]
- Smoot, T.; Revels, J.; Soliman, M.; Liu, P.; Menias, C.O.; Hussain, H.H.; Savas, H.; Gaballah, A.H. Abdominal and pelvic splenosis: Atypical Findings, Pitfalls, and Mimics. Abdom. Radiol. 2022, 47, 923–947. [Google Scholar] [CrossRef]
- Dijkstra, C.D.; Langevoort, H.L. Regeneration of splenic tissue after autologous subcutaneous implantation: Development of Non-lymphoid Cells in the White Pulp of the Rat Spleen. Cell Tissue Res. 1982, 222, 69–79. [Google Scholar] [CrossRef]
- Calder, R.M.; Scholar, G. Autoplastic splenic grafts: Their Use in the Study of the Growth of Splenic Tissue. J. Pathol. Bacteriol. 1939, 49, 351–362. [Google Scholar] [CrossRef]
- Manley, O.T.; Marine, D. The Transplantation of splenic tissue into the subcutaneous fascia of the abdomen in rabbits. J. Exp. Med. 1917, 25, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Marine, D.; Manley, O.T. Homeotransplantation and Autotransplantation of the Spleen in Rabbits: III. Further Data on Growth, Permanence, Effect of Age, and Partial or Complete Removal of the Spleen J. Exp. Med. 1920, 32, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Perla, D. The Regeneration of Autoplastic Splenic Transplants. Am. J. Pathol. 1936, 12, 665. [Google Scholar] [PubMed]
- Braga, A.A.; Malagó, R.; Anacleto, T.P.; da Silva, C.R.N.; Andreollo, N.A.; Fernandes, F.L.F. Histological aspects of autologous transplantation of different fragments of the spleen in rats. Acta Cir. Bras. 2012, 27, 880–884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marques, R.G.; Petroianu, A.; Coelho, J.M.C.O.; Portela, M.C. Regeneration of splenic autotransplants. Ann. Hematol. 2002, 81, 622–626. [Google Scholar] [CrossRef]
- Westermann, J.; Peschel, P.; Pabst, R. Immunoarchitecture of regenerated splenic transplants: Influence of Donor and Host Age on the Regeneration of Splenic Compartments. Cell Tissue Res. 1988, 254, 403–413. [Google Scholar] [CrossRef]
- Leemans, R.; Harms, G.; Rijkers, G.T.; Timens, W. Spleen autotransplantation provides restoration of functional splenic lymphoid compartments and improves the humoral immune response to pneumococcal polysaccharide vaccine. Clin. Exp. Immunol. 1999, 117, 596–604. [Google Scholar] [CrossRef]
- Westermann, J.; Michel, S.; Lopez-Kostka, S.; Bode, U.; Rothkötter, H.J.; Bette, M.; Weihe, E.; Straub, R.H.; Pabst, R. Regeneration of implanted splenic tissue in the rat: Reinnervation is Host Age-Dependent and Necessary for Tissue Development. J. Neuroimmunol. 1998, 88, 67–76. [Google Scholar] [CrossRef]
- Westermann, J.; Willführ, K.U.; Pabst, R. Influence of donor and host age on the regeneration and blood flow of splenic transplants. J. Pediatr. Surg. 1988, 23, 835–838. [Google Scholar] [CrossRef]
- Metcalf, D. Spleen graft growth in splenectomised mice. Aust. J. Exp. Biol. Med. Sci. 1963, 41, 51–60. [Google Scholar] [CrossRef]
- Malagó, R.; Reis, N.S.; Araújo, M.R.; Andreollo, N.A. Late histological aspects of spleen autologous transplantation in rats. Acta Cir. Bras. 2008, 23, 274–281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miko, I.; Brath, E.; Furka, I.; Kovacs, J.; Kelvin, D.; Zhong, R. Spleen autotransplantation in mice: A Novel Experimental Model for immunology study. Microsurgery 2001, 21, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, I.; Pulvirenti, E.; Toro, A. A new technique for spleen autotransplantation. Surg. Innov. 2012, 19, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Meng, B.; Cui, G.; Wu, Z.; Yu, L.; Zhu, H.; Ma, H.; Shi, J.; Lv, Y. Regeneration of Splenic Autotransplants Attached on Liver by a Tissue Adhesive. Transplant. Proc. 2010, 42, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.J.; Viana, G.; Magalha, N.M.; Arantes, R.M.E.; Coelho, P.M.Z.; Cunha-melo, R. Kinetics of neovascularisation of splenic autotransplants in mice. J. Anat. 1999, 195, 387–392. [Google Scholar] [CrossRef]
- Holdsworth, R.J. Regeneration of the spleen and splenic autotransplantation. Br. J. Surg. 1991, 78, 270–278. [Google Scholar] [CrossRef]
- Iinuma, H.; Okinaga, K.; Sato, S.; Tomioka, M.; Matsumoto, K. Optimal site and amount of splenic tissue for autotransplantation. J. Surg. Res. 1992, 53, 109–116. [Google Scholar] [CrossRef]
- Jacob, H.S.; Macdonald, R.A.; Jandl, J.H. Regulation OF Spleen Growth and Sequestering Function. J. Clin. Investig. 1963, 42, 1476. [Google Scholar] [CrossRef]
- Miko, I.; Brath, E.; Nemeth, N.; Toth, F.F.; Sipka, S.; Kovacs, J.; Sipka, S.; Fachet, J.; Furka, A.; Furka, I.; et al. Hematological, hemorheological, immunological, and morphological studies of spleen autotransplantation in mice: Preliminary Results. Microsurgery 2003, 23, 483–488. [Google Scholar] [CrossRef]
- Sipka, S.; Brath, E.; Toth, F.F.; Fabian, A.; Krizsan, C.; Barath, S.; Sipka, S.; Nemeth, N.; Balint, A.; Furka, I.; et al. Distribution of peripheral blood cells in mice after splenectomy or autotransplantation. Microsurgery 2006, 26, 43–49. [Google Scholar] [CrossRef]
- Pabst, R. Regeneration of autotransplanted splenic fragments: Basic Immunological and Clinical Relevance. Clin. Exp. Immunol. 1999, 117, 423. [Google Scholar] [CrossRef] [PubMed]
- Pabst, R.; Westermann, J.; RothköTter, H.J. Immunoarchitecture of regenerated splenic and lymph node transplants. Int. Rev. Cytol. 1991, 128, 215–260. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Buczacki, S.; Khan, Z.A.J. Splenic regeneration following splenectomy and impact on sepsis: A Clinical Review. J. R. Soc. Med. 2009, 102, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Williams, J.S.; Naim, J.O.; Hinshaw, J.R. Protection against pneumococcal sepsis in splenectomized rats by implantation of splenic tissue into an omental pouch. Curr. Surg. 1981, 38, 323–325. [Google Scholar]
- Patel, J.; Williams, J.S.; Naim, J.O.; Hinshaw, J.R. Protection against pneumococcal sepsis in splenectomized rats by implantation of splenic tissue into an omental pouch. Surgery 1982, 91, 638–641. [Google Scholar] [CrossRef]
- Steely, W.M.; Satava, R.M.; Harris, R.W.; Quispe, G. Comparison of omental splenic autotransplant to partial splenectomy. Protective effect against septic death. Am. Surg. 1987, 53, 702–705. [Google Scholar]
- Likhite, V.V. Protection against fulminant sepsis in splenectomized mice by implantation of autochthonous splenic tissue. Exp. Hematol. 1978, 6, 433–439. [Google Scholar]
- Sipka, S.; Bráth, E.; Tóth, F.F.; Aleksza, M.; Kulcsár, A.; Fábián, Á.; Baráth, S.; Balogh, P.; Sipka, S.; Furka, I.; et al. Cellular and serological changes in the peripheral blood of splenectomized and spleen autotransplanted mice. Transpl. Immunol. 2006, 16, 99–104. [Google Scholar] [CrossRef]
- Miko, I.; Brath, E.; Nemeth, N.; Furka, A.; Sipka, S.; Peto, K.; Serfozo, J.; Kovacs, J.; Imre, S.; Benko, I.; et al. Spleen autotransplantation. Morphological and functional follow-up after spleen autotransplantation in mice: A Research Summary. Microsurgery 2007, 27, 312–316. [Google Scholar] [CrossRef]
- Marques, R.G.; Caetano, C.E.R.; Diestel, C.F.; Lima, E.; Portela, M.C.; Oliveira, A.V.; Oliveira, M.B.N.; Bernardo-Filho, M. Critical mass of splenic autotransplant needed for the development of phagocytic activity in rats. Clin. Exp. Immunol. 2012, 170, 77–85. [Google Scholar] [CrossRef]
- Teixeira, F.M.; Fernandes, B.F.; Rezende, A.B.; Machado, R.R.P.; Alves, C.C.S.; Perobelli, S.M.; Nunes, S.I.; Farias, R.E.; Rodrigues, M.F.; Ferreira, A.P.; et al. Staphylococcus aureus infection after splenectomy and splenic autotransplantation in BALB/c mice. Clin. Exp. Immunol. 2008, 154, 255–263. [Google Scholar] [CrossRef]
- Surendran, A.; Smith, M.; Houli, N.; Usatoff, V.; Spelman, D.; Choi, J. Splenic autotransplantation: A Systematic Review. ANZ J. Surg. 2020, 90, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.H.; Watanabe, T. Determinants of postnatal spleen tissue regeneration and organogenesis. NPJ Regen. Med. 2018, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Brendolan, A.; Rosado, M.M.; Carsetti, R.; Selleri, L.; Dear, T.N. Development and function of the mammalian spleen. BioEssays 2007, 29, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Short, C.; Lim, H.K.; Tan, J.; O’Neill, H.C. Targeting the Spleen as an Alternative Site for Hematopoiesis. Bioessays 2019, 41, 1800234. [Google Scholar] [CrossRef]
- Cao, Y.A.; Wagers, A.J.; Beilhack, A.; Dusich, J.; Bachmann, M.H.; Negrin, R.S.; Weissman, I.L.; Contag, C.H. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 221. [Google Scholar] [CrossRef]
- Kiel, M.J.; Yilmaz, Ö.H.; Iwashita, T.; Yilmaz, O.H.; Terhorst, C.; Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Inra, C.N.; Zhou, B.O.; Acar, M.; Murphy, M.M.; Richardson, J.; Zhao, Z.; Morrison, S.J. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 2015, 527, 466–471. [Google Scholar] [CrossRef]
- O’Neill, H.C.; Lim, H.K.; Periasamy, P.; Kumarappan, L.; Tan, J.K.H.; O’Neill, T.J. Transplanted spleen stromal cells with osteogenic potential support ectopic myelopoiesis. PLoS ONE 2019, 14, e0223416. [Google Scholar] [CrossRef]
- Tan, J.K.H.; Watanabe, T. Murine Spleen Tissue Regeneration from Neonatal Spleen Capsule Requires Lymphotoxin Priming of Stromal Cells. J. Immunol. 2014, 193, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Glanville, S.H.; Bekiaris, V.; Jenkinson, E.J.; Lane, P.J.L.; Anderson, G.; Withers, D.R. Transplantation of embryonic spleen tissue reveals a role for adult non-lymphoid cells in initiating lymphoid tissue organization. Eur. J. Immunol. 2009, 39, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.H.; Watanabe, T. Stromal Cell Subsets Directing Neonatal Spleen Regeneration. Sci. Rep. 2017, 7, 40401. [Google Scholar] [CrossRef]
- Li, L.; Duan, M.; Chen, W.; Jiang, A.; Li, X.; Yang, J.; Li, Z. The spleen in liver cirrhosis: Revisiting an Old Enemy with Novel Targets. J. Transl. Med. 2017, 15, 111. [Google Scholar] [CrossRef]
- Tarantino, G.; Scalera, A.; Finelli, C. Liver-spleen axis: Intersection between Immunity, Infections and Metabolism. World J. Gastroenterol. 2013, 19, 3534. [Google Scholar] [CrossRef]
- Babaeva, A.G. Cellular and humoral immunity factors as regulators of regenerative morphogenesis. Ontogenez 1989, 20, 453–460. [Google Scholar] [PubMed]
- Babaeva, A.G. Lymphocytes as regulators of cell proliferation and differentiation of non-lymphoid organs. Vestn. Akad. Med. Nauk SSSR 1990, 43–45, 2356655. [Google Scholar]
- Babaeva, A.G.; Druzhkova, T.A.; Yudina, N.V.; Gimmelpharb, E.I.; Medvedev, A. Lymphoid cell-derived humoral factors as possible mediators in regeneration information transfer. Monogr. Dev. Biol. 1992, 23, 223–229. [Google Scholar]
- Yamada, S.; Morine, Y.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Iwahashi, S.; Saito, Y.; Yoshikawa, M.; Teraoku, H.; Shimada, M. Liver regeneration after splenectomy in patients with liver cirrhosis. Hepatol. Res. 2016, 46, 443–449. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Jin, Y.; Zhao, L.; Zhao, F.; Feng, J.; Li, A.; Wei, Y. Splenectomy Leads to Amelioration of Altered Gut Microbiota and Metabolome in Liver Cirrhosis Patients. Front. Microbiol. 2018, 9, 963. [Google Scholar] [CrossRef]
- Romanelli, R.G.; Stasi, C. Recent Advancements in Diagnosis and Therapy of Liver Cirrhosis. Curr. Drug Targets 2016, 17, 1804–1817. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, T.; Hashizume, M.; Tanoue, K.; Shimabukuro, R.; Gotoh, N.; Tomikawa, M.; Sugimachi, K. Role of the spleen in liver fibrosis in rats may be mediated by transforming growth factor beta-1. J. Gastroenterol. Hepatol. 2002, 17, 59–65. [Google Scholar] [CrossRef]
- Asanoma, M.; Ikemoto, T.; Mori, H.; Utsunomiya, T.; Imura, S.; Morine, Y.; Iwahashi, S.; Saito, Y.; Yamada, S.; Shimada, M. Cytokine expression in spleen affects progression of liver cirrhosis through liver-spleen cross-talk. Hepatol. Res. 2014, 44, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, X.; Jiao, G.; Luo, L.; Zhou, L.; Zhang, J.; Wang, B. Splenectomy Promotes Macrophage Polarization in a Mouse Model of Concanavalin A- (ConA-) Induced Liver Fibrosis. BioMed Res. Int. 2019, 2019, 5756189. [Google Scholar] [CrossRef] [PubMed]
- Yada, A.; Iimuro, Y.; Uyama, N.; Uda, Y.; Okada, T.; Fujimoto, J. Splenectomy attenuates murine liver fibrosis with hypersplenism stimulating hepatic accumulation of Ly-6Clo macrophages. J. Hepatol. 2015, 63, 905–916. [Google Scholar] [CrossRef]
- Murata, K.; Shiraki, K.; Sugimoto, K.; Takase, K.; Nakano, T.; Furusaka, A.; Tameda, Y. Splenectomy enhances liver regeneration through tumor necrosis factor (TNF)-alpha following dimethylnitrosamine-induced cirrhotic rat model. Hepatogastroenterology 2001, 48, 1022–1027. [Google Scholar]
- Elchaninov, A.V.; Fatkhudinov, T.K.; Vishnyakova, P.A.; Nikitina, M.P.; Lokhonina, A.V.; Makarov, A.V.; Arutyunyan, I.V.; Kananykhina, E.Y.; Poltavets, A.S.; Butov, K.R.; et al. Molecular mechanisms of splenectomy-induced hepatocyte proliferation. PLoS ONE 2020, 15, e0233767. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.-L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Kim, E.; Yang, J.; Beltran, C.D.; Cho, S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J. Cereb. Blood Flow Metab. 2014, 34, 1411–1419. [Google Scholar] [CrossRef]
- Blomster, L.; Brennan, F.; Lao, H.; Harle, D.; Harvey, A.; Ruitenberg, M. Mobilisation of the splenic monocyte reservoir and peripheral CX3CR1 deficiency adversely affects recovery from spinal cord injury. Exp. Neurol. 2013, 247, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Maggio, R.D.; Benedetti, A.; Morroni, J.; Bouche, M.; Lozanoska-Ochser, B. Splenic Ly6Chi monocytes are critical players in dystrophic muscle injury and repair. JCI Insight 2020, 5, e130807. [Google Scholar] [CrossRef] [PubMed]
- Shand, F.; Ueha, S.; Otsuji, M.; Koid, S.; Shichino, S.; Tsukui, T.; Kosugi-Kanaya, M.; Abe, J.; Tomura, M.; Ziogas, J.; et al. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc. Natl. Acad. Sci. USA. 2014, 111, 7771–7776. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, E.; Samia-Grinberg, S.; Pasmanik-Chor, M.; Brazowski, E.; Shibolet, O.; Halpern, Z.; Varol, C. Infiltrating Monocyte-Derived Macrophages and Resident Kupffer Cells Display Different Ontogeny and Functions in Acute Liver Injury. J. Immunol. 2014, 193, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Elchaninov, A.; Nikitina, M.; Vishnyakova, P.; Lokhonina, A.; Makarov, A.; Sukhikh, G.; Fatkhudinov, T. Macro- and microtranscriptomic evidence of the monocyte recruitment to regenerating liver after partial hepatectomy in mouse model. Biomed. Pharmacother. 2021, 138, 111516. [Google Scholar] [CrossRef]
- Arutyunyan, I.; Elchaninov, A.; Fatkhudinov, T.; Makarov, A.; Kananykhina, E.; Usman, N.; Bolshakova, G.; Glinkina, V.; Goldshtein, D.; Sukhikh, G. Elimination of allogeneic multipotent stromal cells by host macrophages in different models of regeneration. Int. J. Clin. Exp. Pathol. 2015, 8, 4469–4480. [Google Scholar]
- Tanabe, K.; Taura, K.; Koyama, Y.; Yamamoto, G.; Nishio, T.; Okuda, Y.; Nakamura, K.; Toriguchi, K.; Takemoto, K.; Yamanaka, K.; et al. Migration of splenic lymphocytes promotes liver fibrosis through modification of T helper cytokine balance in mice. J. Gastroenterol. 2015, 50, 1054–1068. [Google Scholar] [CrossRef]
- Marquez-Medina, D.; Salla-Fortuny, J.; Salud-Salvia, A. Role of gamma-delta T-cells in cancer: Another Opening Door to Immunotherapy. Clin. Transl. Oncol. 2012, 14, 891–895. [Google Scholar] [CrossRef]
- Bi, J.; Zheng, X.; Chen, Y.; Wei, H.; Sun, R.; Tian, Z. TIGIT safeguards liver regeneration through regulating natural killer cell-hepatocyte crosstalk. Hepatology 2014, 60, 1389–1398. [Google Scholar] [CrossRef]
- Huang, W.; Dong, Z.; Wei, H.; Ding, C.; Sun, R.; Tian, Z. Selective elimination of hepatic natural killer T cells with concanavalin A improves liver regeneration in mice. Liver Int. 2006, 26, 339–345. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Stankovic, S.; Baxter, A.G. Raising the NKT cell family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hua, J. Immune cells in liver regeneration. Oncotarget 2017, 8, 3628–3639. [Google Scholar] [CrossRef]
- Ito, K.; Ozasa, H.; Horikawa, S. Effects of prior splenectomy on remnant liver after partial hepatectomy with Pringle maneuver in rats. Liver Int. 2005, 25, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, Y.; Shimada, M.; Utsunomya, T.; Imura, S.; Morine, Y.; Ikemoto, T.; Takasu, C. Effects of splenectomy on hepatic gene expression profiles after massive hepatectomy in rats. J. Gastroenterol. Hepatol. 2013, 28, 1669–1677. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.-J.; Ko, I.-G.; Joo, S.H.; Ahn, H.J. Splenectomy affects the balance between hepatic growth factor and transforming growth factor-β and its effect on liver regeneration is dependent on the amount of liver resection in rats. J. Korean Surg. Soc. 2012, 82, 238. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Yamanoi, A.; Hishikawa, Y.; Dhar, D.K.; Tachibana, M.; Nagasue, N. Transforming growth factor-beta1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Lab. Investig. 2003, 83, 1595–1603. [Google Scholar] [CrossRef]
- Morinaga, A.; Ogata, T.; Kage, M.; Kinoshita, H.; Aoyagi, S. Comparison of liver regeneration after a splenectomy and splenic artery ligation in a dimethylnitrosamine-induced cirrhotic rat model. HPB 2010, 12, 22–30. [Google Scholar] [CrossRef]
- Lee, S.C.; Jeong, H.J.; Choi, B.-J.; Kim, S.-J. Role of the spleen in liver regeneration in relation to transforming growth factor-β1 and hepatocyte growth factor. J. Surg. Res. 2015, 196, 270–277. [Google Scholar] [CrossRef]
- Tang, W.-P.; Akahoshi, T.; Piao, J.-S.; Narahara, S.; Murata, M.; Kawano, T.; Hamano, N.; Ikeda, T.; Hashizume, M. Splenectomy enhances the therapeutic effect of adipose tissue-derived mesenchymal stem cell infusion on cirrhosis rats. Liver Int. 2016, 36, 1151–1159. [Google Scholar] [CrossRef]
- Yin, S.; Wang, H.; Park, O.; Wei, W.; Shen, J.; Gao, B. Enhanced liver regeneration in IL-10-deficient mice after partial hepatectomy via stimulating inflammatory response and activating hepatocyte STAT3. Am. J. Pathol. 2011, 178, 1614–1621. [Google Scholar] [CrossRef]
- Kuriyama, N.; Isaji, S.; Kishiwada, M.; Ohsawa, I.; Hamada, T.; Mizuno, S.; Usui, M.; Sakurai, H.; Tabata, M.; Yamada, T. Dual cytoprotective effects of splenectomy for small-for-size liver transplantation in rats. Liver Transplant 2012, 18, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Babaeva, A.G.; Zotikov, E.A. Immunology of Processes of Adaptive Growth, Proliferation and their Disorders; Medicine: Moscow, Russia, 1987. [Google Scholar]
- Rudich, N.; Zamir, G.; Pappo, O.; Shlomai, Z.; Faroja, M.; Weiss, I.D.; Wald, H.; Galun, E.; Peled, A.; Wald, O. Focal liver necrosis appears early after partial hepatectomy and is dependent on T cells and antigen delivery from the gut. Liver Int. 2009, 29, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Yokoi, Y.; Kurachi, K.; Uno, A.; Suzuki, S.; Konno, H.; Nakamura, S. Implication of B Lymphocytes in Endotoxin-Induced Hepatic Injury After Partial Hepatectomy in Rats. J. Surg. Res. 2007, 137, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Strick-Marchand, H.; Masse, G.X.; Weiss, M.C.; Di Santo, J.P. Lymphocytes support oval cell-dependent liver regeneration. J. Immunol. 2008, 181, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Babaeva, A.G. Immunological Mechanisms of Regulation of Recovery Processes; Medicine: Moscow, Russia, 1972. [Google Scholar]
- Piotto, C.; Julier, Z.; Martino, M.M. Immune Regulation of Tissue Repair and Regeneration via miRNAs—New Therapeutic Target. Front. Bioeng. Biotechnol. 2018, 6, 98. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).