Curcumin Protects Diabetic Mice against Isoproterenol-Induced Myocardial Infarction by Modulating CB2 Cannabinoid Receptors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Induction of Experimental Diabetes
2.4. Induction of Experimental Myocardial Infarction
2.5. Experimental Protocols
- Group I—Normal
- Group II—HFD + STZ + ISO
- Group III—HFD + STZ + ISO + Curcumin
- Group IV—HFD + STZ + ISO + Curcumin
- Group V—HFD + STZ + ISO + AM630 + Curcumin
- Group VI—HFD + STZ + ISO + AM630
2.6. Experimental Parameters
2.7. Biochemical Parameters
2.8. Estimation of Oxidative Stress
2.9. Estimation of Inflammatory Cytokines
2.10. Light Microscopy Examination
2.11. Molecular Docking Study
2.12. Statistical Analysis
3. Results
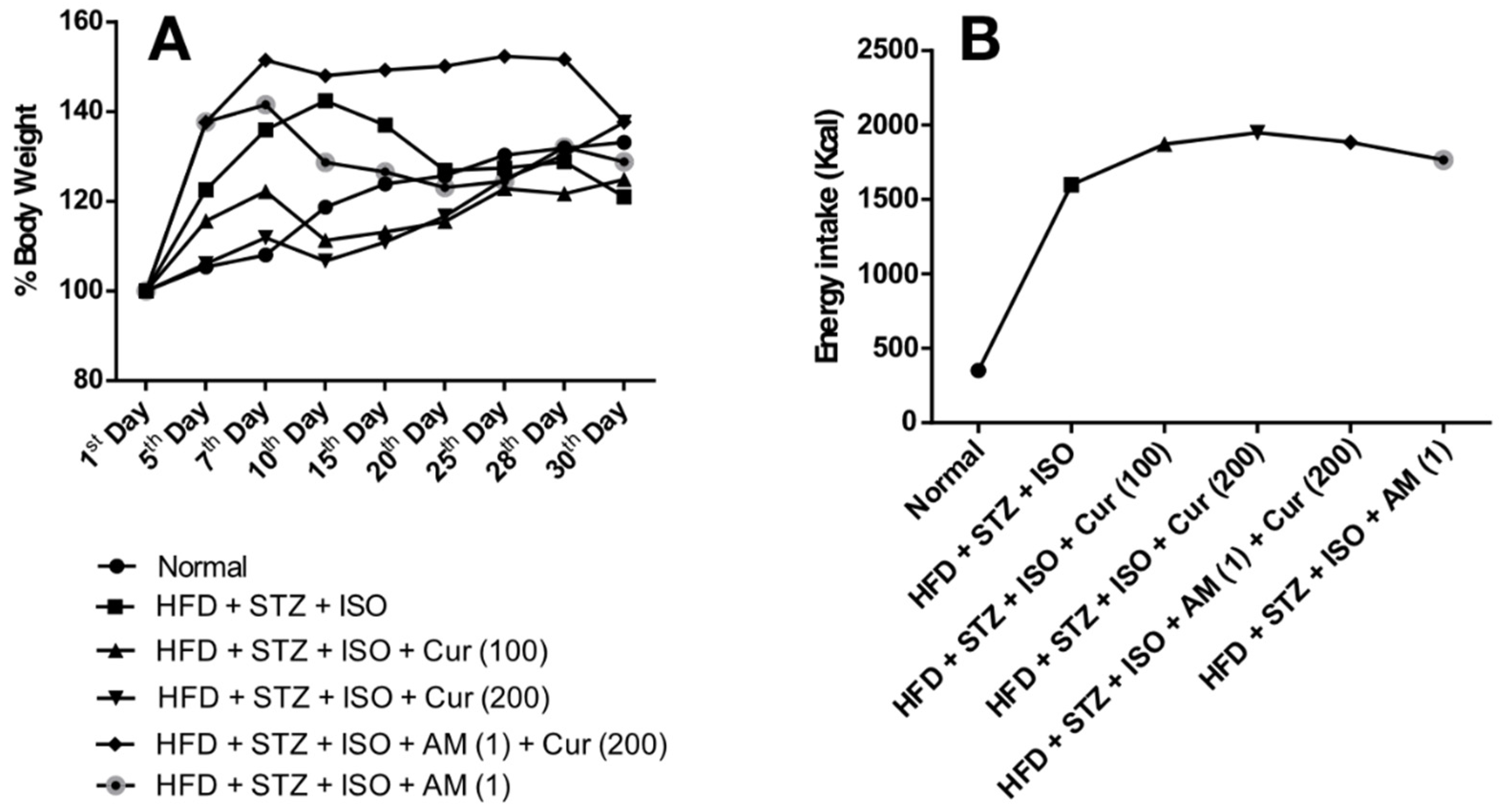
3.1. Effect of Curcumin on Body Weight and Food Intake in HFD Fed Myocardial Infarcted Diabetic Mice
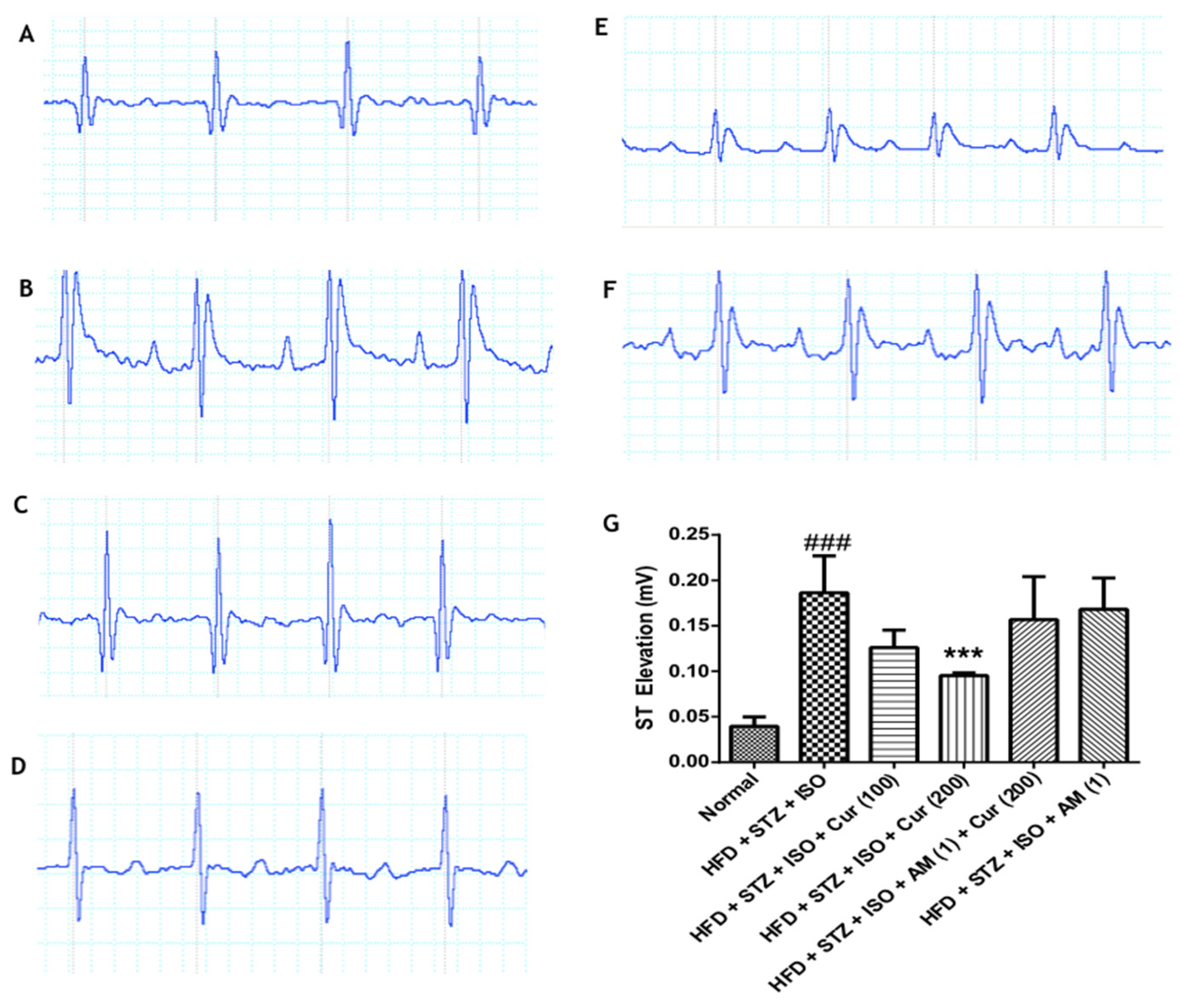
3.2. Effect of Curcumin on Electrocardiogram in HFD Fed Myocardial Infarcted Diabetic Mice
3.3. Effect of Curcumin on Blood Glucose Level and Lipid Profile in HFD Fed Myocardial Infarcted Diabetic Mice
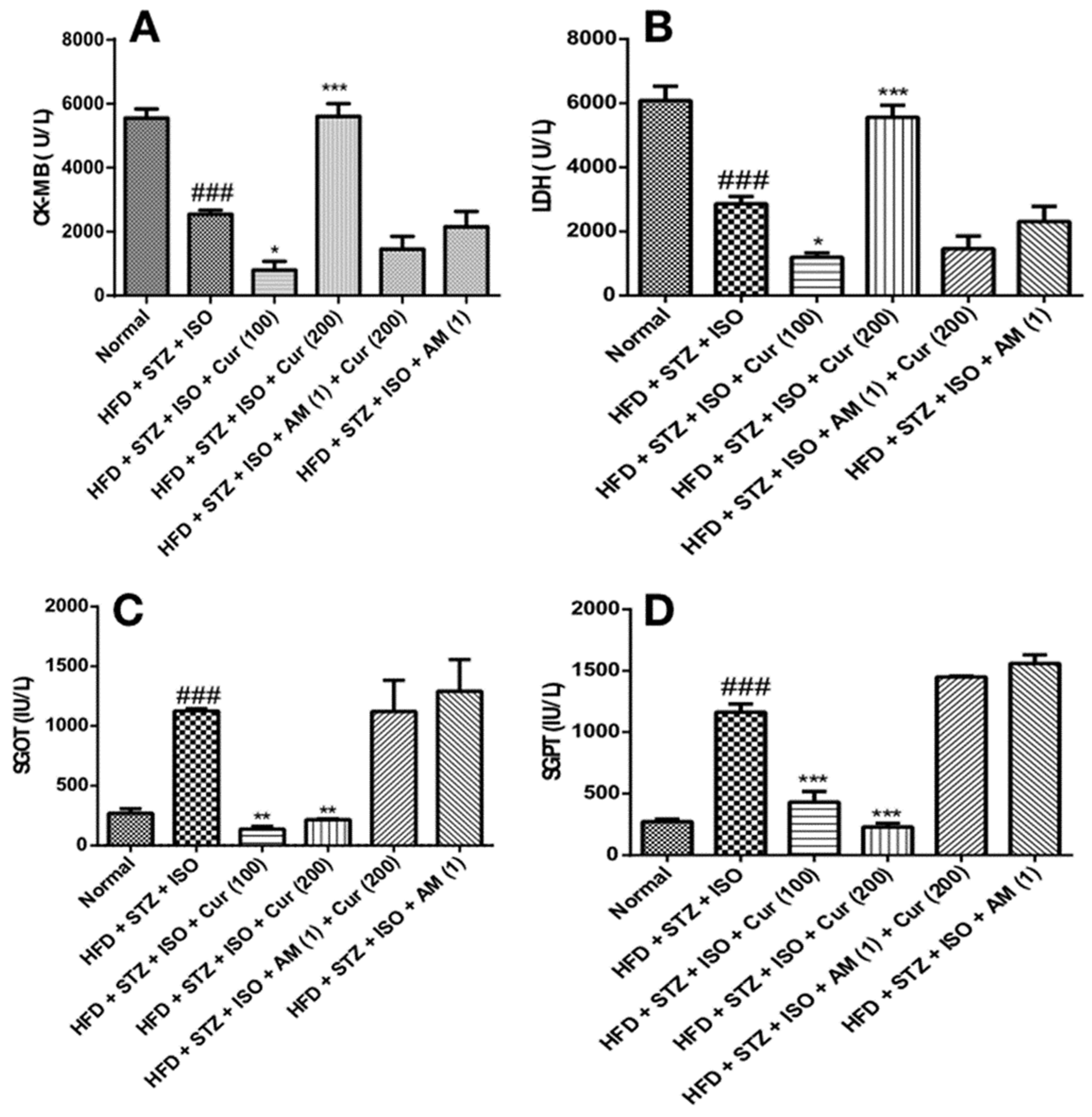
3.4. Effect of Curcumin on Cardiac and Liver Injury Markers in HFD Fed Myocardial Infarcted Diabetic Mice
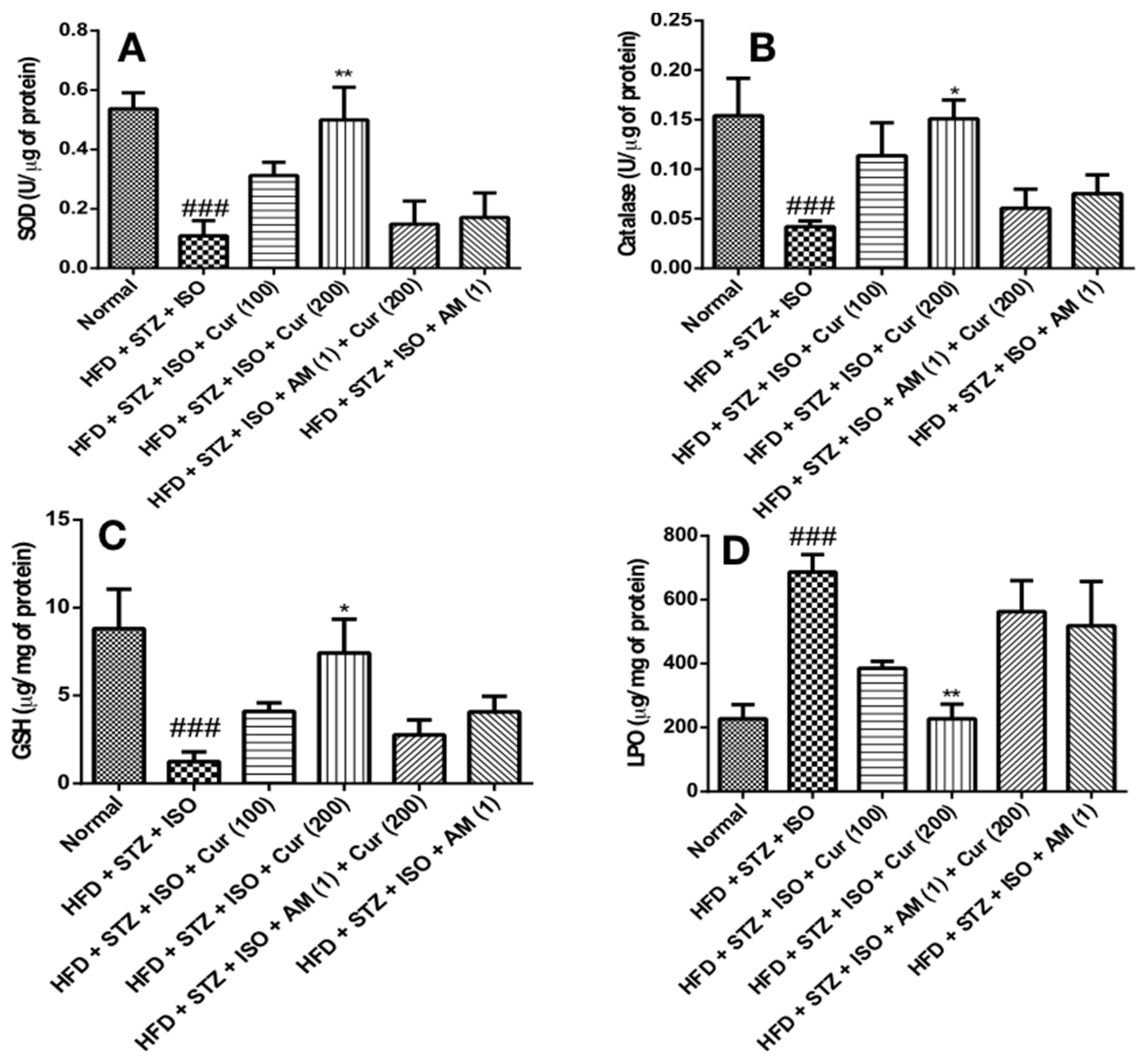
3.5. Effect of Curcumin on Oxidative Stress in HFD Fed Myocardial Infarcted Diabetic Mice
3.6. Effect of Curcumin on Inflammatory Cytokines in HFD Fed Myocardial Infarcted Diabetic Mice
3.7. Effect of Curcumin on Histopathology of Myocardium in HFD Fed Myocardial Infarcted Diabetic Mice
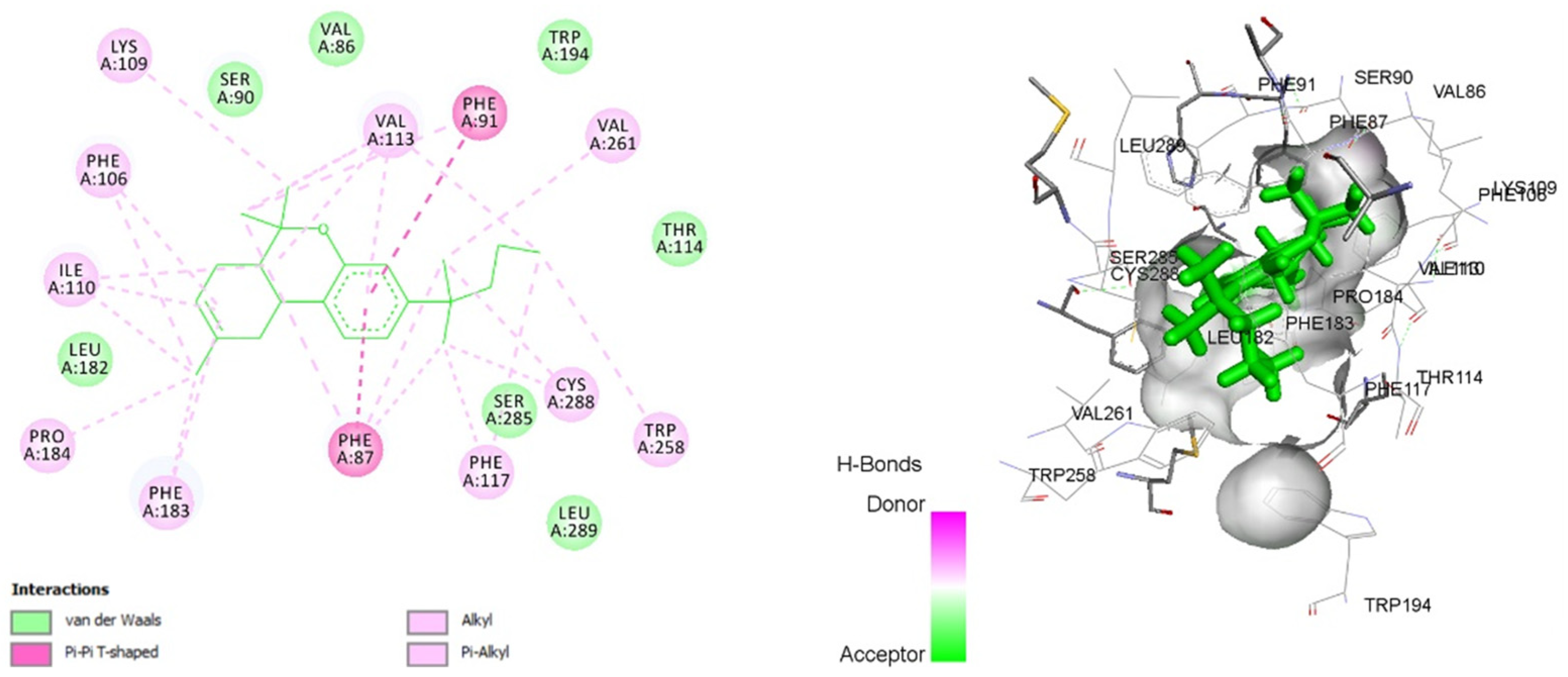
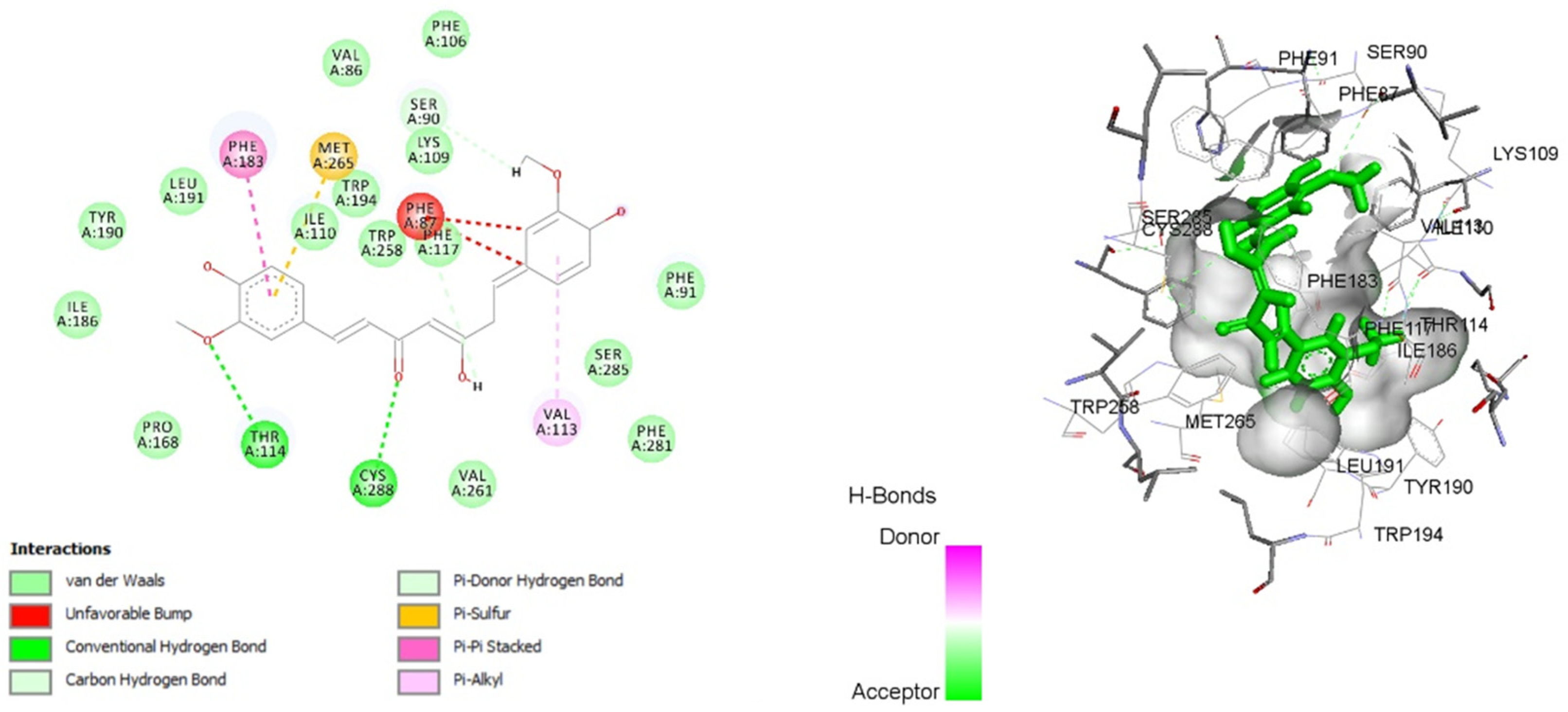
3.8. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hudspeth, B. The Burden of Cardiovascular Disease in Patients with Diabetes. Am. J. Manag. Care 2018, 24 (Suppl. S13), S268–S272. [Google Scholar] [PubMed]
- Strain, W.D.; Smith, C. Cardiovascular outcome studies in diabetes: How do we make sense of these new data? Diabetes Ther. 2016, 7, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Saleh, M.; Ambrose, J.A. Understanding myocardial infarction. F1000Research 2018, 7, 1378. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Pathophysiology of myocardial infarction. Compr. Physiol. 2011, 5, 1841–1875. [Google Scholar]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.-P. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wang, F.; Zhang, Y.-M.; Zhou, J.-J.; Zhang, Y. Activation of cannabinoid type 2 receptor by JWH133 protects heart against ischemia/reperfusion-induced apoptosis. Cell. Physiol. Biochem. 2013, 31, 693–702. [Google Scholar] [CrossRef]
- Dhopeshwarkar, A.; Mackie, K. CB2 cannabinoid receptors as a therapeutic target—What does the future hold? Mol. Pharmacol. 2014, 86, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Tao, Y.; Hu, J. Cannabinoid receptor 2 deletion deteriorates myocardial infarction through the down-regulation of AMPK-mTOR-p70S6K signaling-mediated autophagy. Biosci. Rep. 2019, 39, BSR20180650. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.-J.; Lu, Y.; Wang, H.-W.; Zhang, H.; Wang, S.-R.; Xu, W.-Y.; Fu, H.-L.; Yao, X.-Y.; Yang, F.; Yuan, H.-B. Activation of endocannabinoid receptor 2 as a mechanism of propofol pretreatment-induced cardioprotection against ischemia-reperfusion injury in rats. Oxid. Med. Cell. Longev. 2017, 2017, 2186383. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, P.; Hassanzadeh, A. The CB1 receptor-mediated endocannabinoid signaling and NGF: The novel targets of curcumin. Neurochem. Res. 2012, 37, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Sadek, B.; Goyal, S.N.; Sinha, S.; Kamal, M.A.; Ojha, S. Small molecules from nature targeting G-protein coupled cannabinoid receptors: Potential leads for drug discovery and development. Evid. Based Complement. Altern. Med. 2015, 2015, 238482. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Boarescu, P.-M.; Boarescu, I.; Bocșan, I.C.; Pop, R.M.; Gheban, D.; Bulboacă, A.E.; Nicula, C.; Râjnoveanu, R.-M.; Bolboacă, S.D. Curcumin nanoparticles protect against isoproterenol induced myocardial infarction by alleviating myocardial tissue oxidative stress, electrocardiogram, and biological changes. Molecules 2019, 24, 2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Agaty, S.M. Cardioprotective effect of vitamin D2 on isoproterenol-induced myocardial infarction in diabetic rats. Arch. Physiol. Biochem. 2019, 125, 210–219. [Google Scholar] [CrossRef]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes—A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem. Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef]
- Patil, A.S.; Singh, A.D.; Mahajan, U.B.; Patil, C.R.; Ojha, S.; Goyal, S.N. Protective effect of omeprazole and lansoprazole on β-receptor stimulated myocardial infarction in Wistar rats. Mol. Cell. Biochem. 2019, 456, 105–113. [Google Scholar] [CrossRef]
- Mahajan, U.B.; Chandrayan, G.; Patil, C.R.; Arya, D.S.; Suchal, K.; Agrawal, Y.O.; Ojha, S.; Goyal, S.N. The protective effect of apigenin on myocardial injury in diabetic rats mediating activation of the PPAR-γ pathway. Int. J. Mol. Sci. 2017, 18, 756. [Google Scholar] [CrossRef] [Green Version]
- Goyal, S.N.; Sharma, C.; Mahajan, U.B.; Patil, C.R.; Agrawal, Y.O.; Kumari, S.; Arya, D.S.; Ojha, S. Protective effects of cardamom in isoproterenol-induced myocardial infarction in rats. Int. J. Mol. Sci. 2015, 16, 27457–27469. [Google Scholar] [CrossRef] [Green Version]
- Panda, V.S.; Shah, T. A herbal premix containing Macrotyloma uniflorum, ginger, and whey curtails obesity in rats fed a high-fat diet by a novel mechanism. Appl. Physiol. Nutr. Metab. 2020, 45, 24–34. [Google Scholar] [CrossRef]
- Kamdi, S.P.; Badwaik, H.R.; Raval, A.; Ajazuddin; Nakhate, K.T. Ameliorative potential of phloridzin in type 2 diabetes-induced memory deficits in rats. Eur. J. Pharmacol. 2021, 913, 174645. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, K.T.; Bharne, A.P.; Verma, V.S.; Aru, D.N.; Kokare, D.M. Plumbagin ameliorates memory dysfunction in streptozotocin induced Alzheimer’s disease via activation of Nrf2/ARE pathway and inhibition of β-secretase. Biomed. Pharmacother. 2018, 101, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, C.; Rossi, F.; Rossi, S.; D’Amico, M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: Involvement of cytokine/chemokines and PMN. J. Leukoc. Biol. 2004, 75, 453–459. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Yedke, S.U.; Bharne, A.P.; Subhedar, N.K.; Kokare, D.M. Evidence for the involvement of neuropeptide y in the antidepressant effect of imipramine in type 2 diabetes. Brain Res. 2016, 1646, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Phloridzin ameliorates type 2 diabetes-induced depression in mice by mitigating oxidative stress and modulating brain-derived neurotrophic factor. J. Diabetes Metab. Disord. 2021, 20, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Effect of apple peel extract on diabetes-induced peripheral neuropathy and wound injury. J. Diabetes Metab. Disord. 2021, 20, 119–130. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, J.E.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Choi, Y.W.; Kim, K.M.; Song, H.; Hwang, D.Y. Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: Study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp. Ther. Med. 2019, 17, 2185–2193. [Google Scholar] [CrossRef] [Green Version]
- Wang-Fischer, Y.; Garyantes, T. Improving the reliability and utility of streptozotocin-induced rat diabetic model. J. Diabetes Res. 2018, 2018, 8054073. [Google Scholar] [CrossRef]
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018, 15, 151–166. [Google Scholar] [CrossRef]
- Kumawat, V.S.; Kaur, G. Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications. Eur. J. Pharmacol. 2019, 862, 172628. [Google Scholar] [CrossRef]
- Purohit, V.; Rapaka, R.; Shurtleff, D. Role of cannabinoids in the development of fatty liver (steatosis). AAPS J. 2010, 12, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallelli, C.A.; Calcagnini, S.; Romano, A.; Koczwara, J.B.; De Ceglia, M.; Dante, D.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Modulation of the oxidative stress and lipid peroxidation by endocannabinoids and their lipid analogues. Antioxidants 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argenziano, M.; Tortora, C.; Bellini, G.; Di Paola, A.; Punzo, F.; Rossi, F. The endocannabinoid system in pediatric inflammatory and immune diseases. Int. J. Mol. Sci. 2019, 20, 5875. [Google Scholar] [CrossRef] [Green Version]
- Parlar, A.; Arslan, S.O.; Doğan, M.F.; Çam, S.A.; Yalçin, A.; Elibol, E.; Özer, M.K.; Üçkardeş, F.; Kara, H. The exogenous administration of CB2 specific agonist, GW405833, inhibits inflammation by reducing cytokine production and oxidative stress. Exp. Ther. Med. 2018, 16, 4900–4908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swamy, A.V.; Gulliaya, S.; Thippeswamy, A.; Koti, B.C.; Manjula, D.V. Cardioprotective effect of curcumin against doxorubicin-induced myocardial toxicity in albino rats. Ind. J. Pharmacol. 2012, 44, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbaby, S.; Ewais, M.; Essawy, S.; Farag, N. Cardioprotective effects of curcumin and nebivolol against doxorubicin-induced cardiac toxicity in rats. Hum. Exp. Toxicol. 2014, 33, 800–813. [Google Scholar] [CrossRef]
- Hasan, S.T.; Zingg, J.M.; Kwan, P.; Noble, T.; Smith, D.; Meydani, M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis 2014, 232, 40–51. [Google Scholar] [CrossRef]
- Hatamipour, M.; Jamialahmadi, T.; Ramezani, M.; Tabassi, S.A.S.; Simental-Mendía, L.E.; Sarborji, M.R.; Banach, M.; Sahebkar, A. Protective effects of curcumin phytosomes against high-fat diet-induced atherosclerosis. Adv. Exp. Med. Biol. 2021, 1308, 37–44. [Google Scholar]

| Treatments | Blood Glucose (mg/dL) | TC (mg/dL) | TG (mg/dL) | LDL–C (mg/dL) | HDL–C (mg/dL) |
|---|---|---|---|---|---|
| Normal | 67.7 ± 5.80 | 113 ± 1.32 | 75.0 ± 2.23 | 29.9 ± 2.30 | 78.7 ± 2.51 |
| HFD + STZ + ISO | 288 ± 5.68 ### | 197 ± 2.83 ### | 188 ± 3.91 ### | 68.2 ± 1.95 ### | 50.4 ± 4.84 ### |
| HFD + STZ + ISO + Cur (100) | 164 ± 3.26 * | 161 ± 2.09 ** | 132 ± 14.9 *** | 30.1 ± 8.25 ** | 70.1 ± 2.24 *** |
| HFD + STZ + ISO + Cur (200) | 121 ± 7.49 ** | 121 ± 2.20 *** | 85.5 ± 4.27 *** | 31.4 ± 4.34 ** | 78.4 ± 2.58 *** |
| HFD + STZ + ISO + AM (1) + Cur (200) | 257 ± 4.85 | 228 ± 12.5 | 173 ± 4.63 | 67.6 ± 9.47 | 49.4 ± 1.84 |
| HFD + STZ + ISO + AM (1) | 215 ± 4.12 | 177 ± 6.67 | 139 ± 10.21 | 56.7 ± 10.03 | 44.3 ± 2.50 |
| Treatments | TNF-α (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) |
|---|---|---|---|
| Normal | 21.0 ± 1.20 | 182 ± 8.01 | 35.3 ± 3.99 |
| HFD + STZ + ISO | 34.4 ± 3.24 ### | 369 ± 3.50 ### | 66.4 ± 3.27 ### |
| HFD + STZ + ISO + Cur (100) | 21.8 ± 2.09 ** | 197 ± 1.18 ** | 39.7 ± 1.08 * |
| HFD + STZ + ISO + Cur (200) | 17.6 ± 1.81 *** | 177 ± 6.72 *** | 26.7 ± 1.65 *** |
| HFD + STZ + ISO + AM (1) + Cur (200) | 27.4 ± 1.35 | 282 ± 3.22 | 65.4 ± 9.20 |
| HFD + STZ + ISO + AM (1) | 29.1 ± 0.95 | 284 ± 4.69 | 50.8 ± 8.66 |
| Compound ID (s) | Binding Energy (kcal/mol) | Enzyme’s Binding Site Residue |
|---|---|---|
| JWH-133 | −14.8967 | VAL 86, PHE 87, SER 90, PHE 91, PHE 106, LYS 109, ILE 110, VAL 113, THR 114, PHE 117, LEU 182, PHE 183, PRO 184, TRP 194, TRP 258, VAL 261, SER 285, CYS 288, LEU 289 |
| Curcumin | −12.8272 | PHE 87, SER 90, PHE 91, LYS 109, ILE 110, VAL 113, THR 114, PHE 117, PHE 183, ILE 186, TYR 190, LEU 191, TRP 194, TRP 258, MET 265, SER 285, CYS 288 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawar, H.D.; Mahajan, U.B.; Nakhate, K.T.; Agrawal, Y.O.; Patil, C.R.; Meeran, M.F.N.; Sharma, C.; Ojha, S.; Goyal, S.N. Curcumin Protects Diabetic Mice against Isoproterenol-Induced Myocardial Infarction by Modulating CB2 Cannabinoid Receptors. Life 2022, 12, 624. https://doi.org/10.3390/life12050624
Pawar HD, Mahajan UB, Nakhate KT, Agrawal YO, Patil CR, Meeran MFN, Sharma C, Ojha S, Goyal SN. Curcumin Protects Diabetic Mice against Isoproterenol-Induced Myocardial Infarction by Modulating CB2 Cannabinoid Receptors. Life. 2022; 12(5):624. https://doi.org/10.3390/life12050624
Chicago/Turabian StylePawar, Harshal D., Umesh B. Mahajan, Kartik T. Nakhate, Yogeeta O. Agrawal, Chandragouda R. Patil, M. F. Nagoor Meeran, Charu Sharma, Shreesh Ojha, and Sameer N. Goyal. 2022. "Curcumin Protects Diabetic Mice against Isoproterenol-Induced Myocardial Infarction by Modulating CB2 Cannabinoid Receptors" Life 12, no. 5: 624. https://doi.org/10.3390/life12050624
APA StylePawar, H. D., Mahajan, U. B., Nakhate, K. T., Agrawal, Y. O., Patil, C. R., Meeran, M. F. N., Sharma, C., Ojha, S., & Goyal, S. N. (2022). Curcumin Protects Diabetic Mice against Isoproterenol-Induced Myocardial Infarction by Modulating CB2 Cannabinoid Receptors. Life, 12(5), 624. https://doi.org/10.3390/life12050624








