Effects of Packaging Materials on Structural and Simulated Digestive Characteristics of Walnut Protein during Accelerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Walnut Protein Isolate (WPI)
2.3. Analysis of Amino Acid Composition
2.4. Measurement of Protein Carbonyl Groups
2.5. Dityrosine Content Measurement
2.6. Determination of Free Sulfhydryl Groups
2.7. Determination of Protein Solubility
2.8. Characterization of the Secondary Structure—FT-IR
2.9. Measurement of Intrinsic Fluorescence
2.10. Surface Hydrophobicity (H0) Measurement
2.11. Particle Size Determination
2.12. SDS-PAGE of WPI
2.13. Determination of Protein Molecular Weight Distribution
2.14. In Vitro Digestion of WPI
2.15. Degree of Hydrolysis (DH) Measurement
2.16. Molecular Weight Distribution of Peptide Determination
2.17. Data Analysis
3. Results
3.1. Amino Acid Composition Analysis in Different Packaging Strategies
3.2. Oxidation of WPI in Different Packaging Strategies
3.3. Characterization of Protein Structure in Different Packaging Strategies
3.3.1. Secondary Structure (FT-IR) Analysis
3.3.2. Intrinsic Fluorescence Analysis
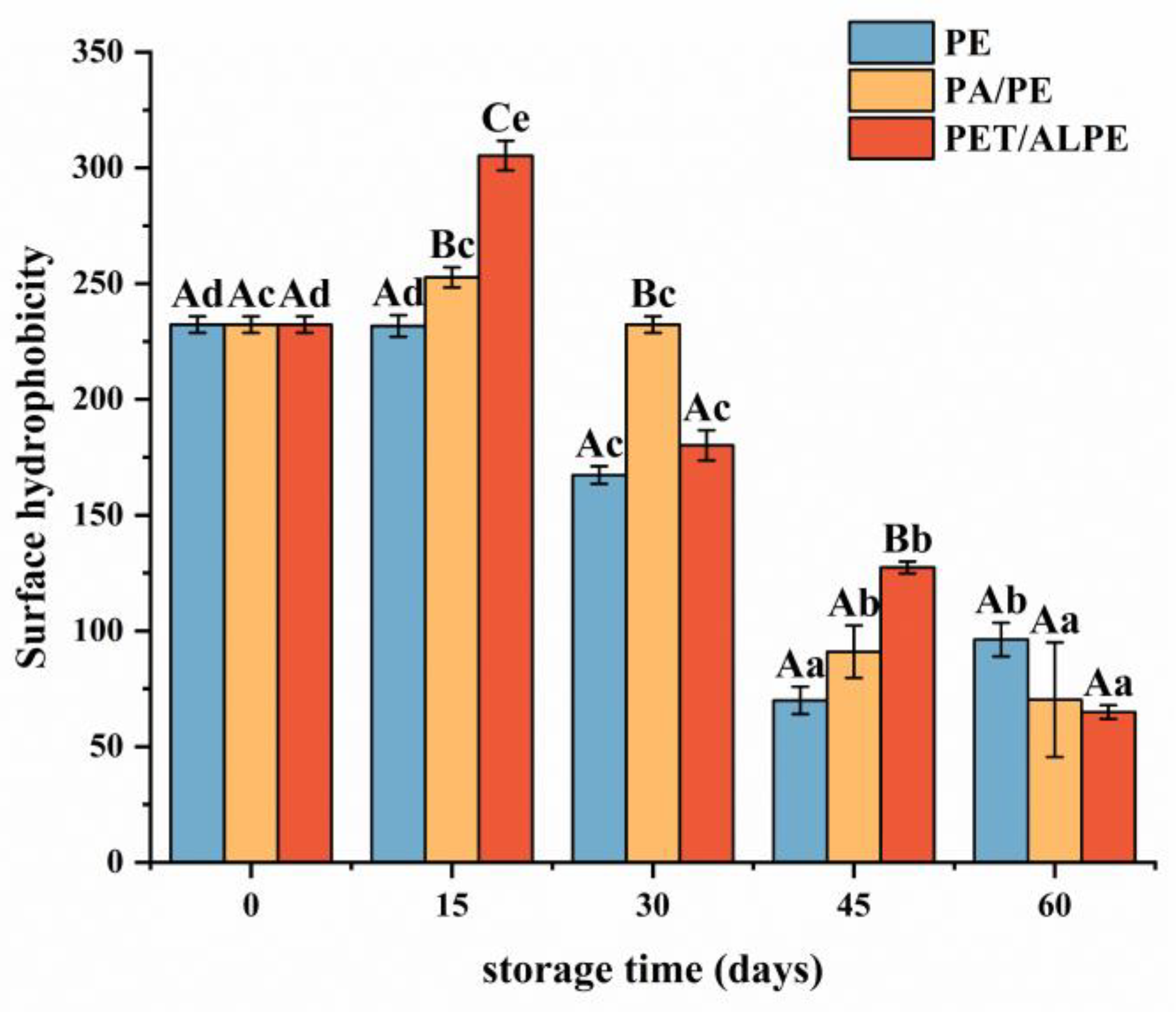
3.3.3. Surface Hydrophobicity (H0) Measurement
3.4. Aggregation of Walnut Protein Isolates during Storage
3.4.1. Solubility and Particle Size Analysis
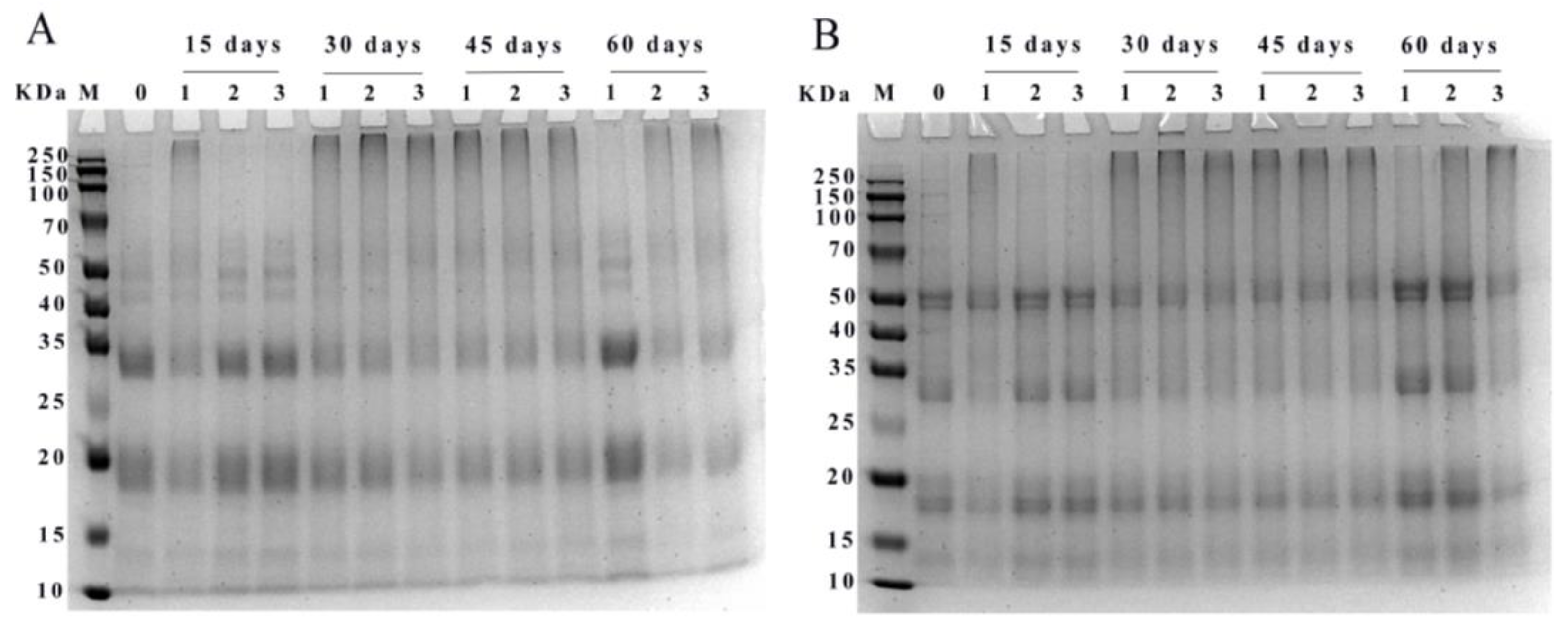
3.4.2. SDS-PAGE
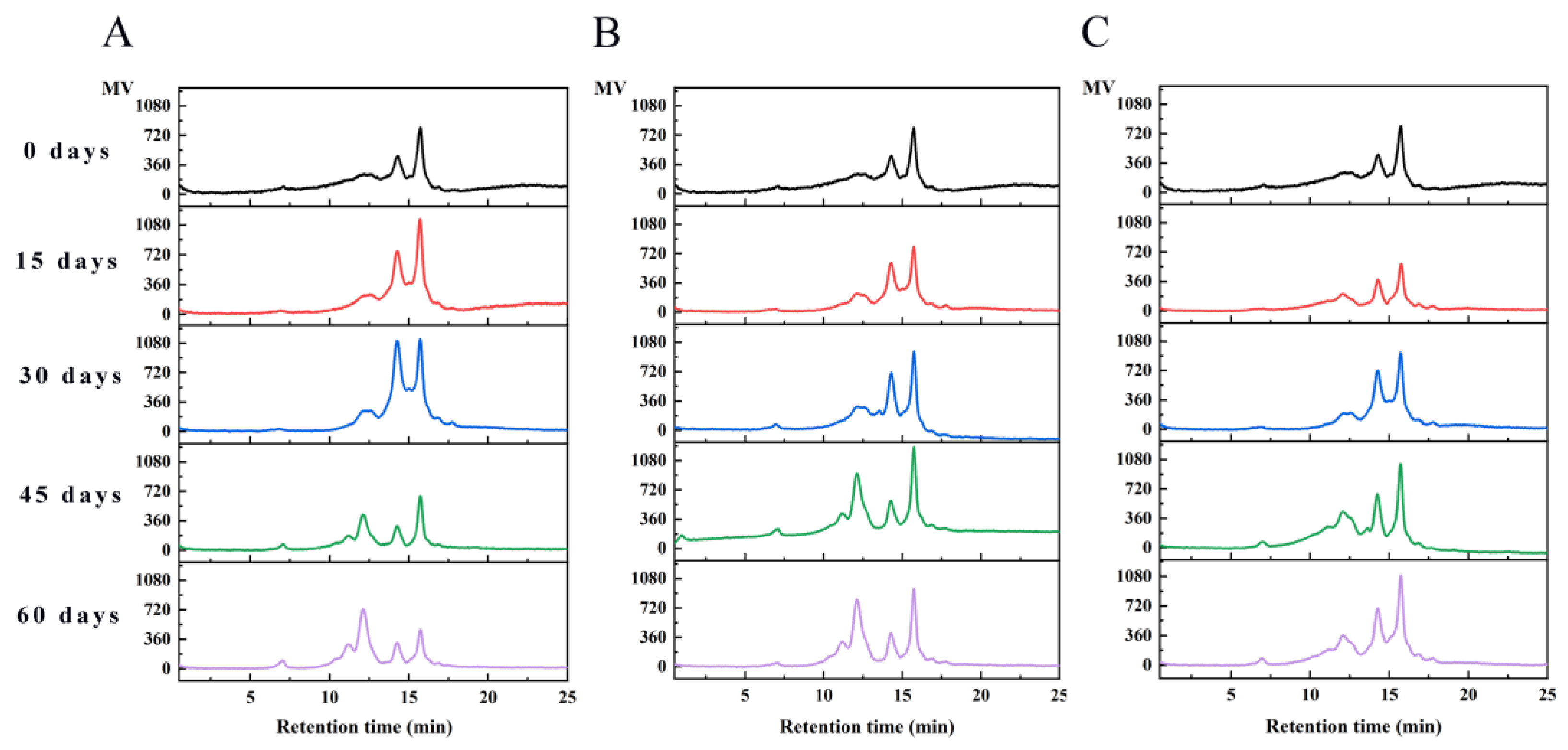
3.4.3. Molecular Weight Distribution of WPI Analysis
3.5. Properties of In Vitro Digestion in Different Packaging Strategies
3.5.1. Degree of Hydrolysis Analysis
3.5.2. Characterization of Peptides Molecular Weight Distribution
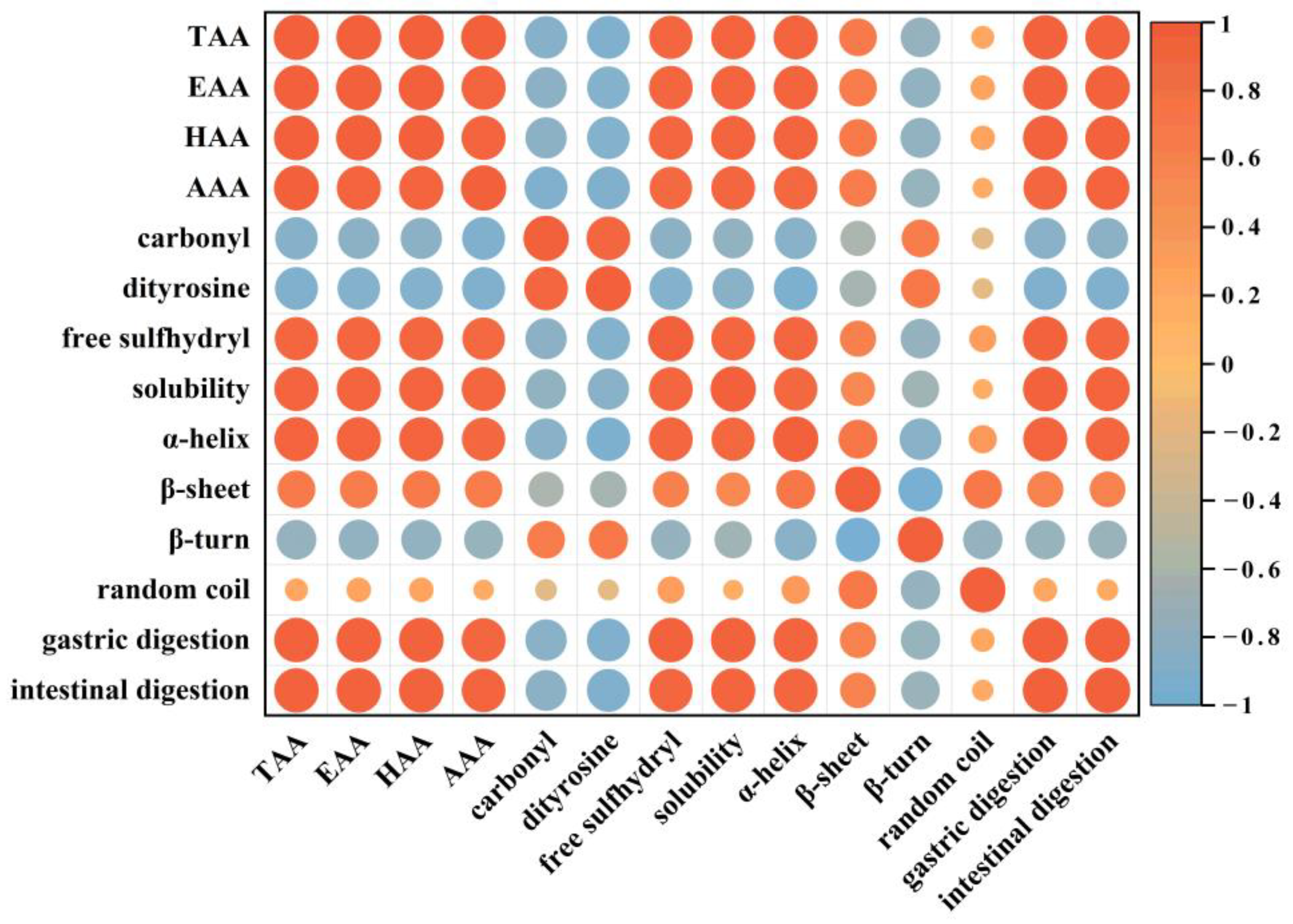
3.6. Pearson Correlation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rebufa, C.; Artaud, J.; Le Dreau, Y. Walnut (Juglans regia L.) oil chemical composition depending on variety, locality, extraction process and storage conditions: A comprehensive review. J. Food Compos. Anal. 2022, 110, 104534. [Google Scholar] [CrossRef]
- Wang, W.; Wen, H.; Jin, Q.; Yu, W.; Li, G.; Wu, M.; Bai, H.; Shen, L.; Wu, C. Comparative transcriptome analysis on candidate genes involved in lipid biosynthesis of developing kernels for three walnut cultivars in Xinjiang. Food Sci. Hum. Wellness 2022, 11, 1201–1214. [Google Scholar] [CrossRef]
- Gu, X.Z.; Zhang, L.; Li, L.T.; Ma, N.; Tu, K.; Song, L.J.; Pan, L.Q. Multisource fingerprinting for region identification of walnuts in Xinjiang combined with chemometrics. J. Food Process Eng. 2018, 41, e12687. [Google Scholar] [CrossRef]
- Wang, P.; Zhong, L.L.; Yang, H.B.; Zhu, F.; Hou, X.J.; Wu, C.Y.; Zhang, R.; Cheng, Y.J. Comparative analysis of antioxidant activities between dried and fresh walnut kernels by metabolomic approaches. LWT-Food Sci. Technol. 2022, 155, 112875. [Google Scholar] [CrossRef]
- Ozturk, I.; Sagdic, O.; Yalcin, H.; Capar, T.D.; Asyali, M.H. The effects of packaging type on the quality characteristics of fresh raw pistachios (Pistacia vera L.) during the storage. LWT-Food Sci. Technol. 2016, 65, 457–463. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci. Hortic. 2011, 131, 49–57. [Google Scholar] [CrossRef]
- Jensen, P.N.; Sorensen, G.; Engelsen, S.B.; Bertelsen, G. Evaluation of quality changes in walnut kernels (Juglans regia L.) by Vis/NIR spectroscopy. J. Agric. Food Chem. 2001, 49, 5790–5796. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Oil composition in stored walnut cultivars-quality and nutritional value. Eur. J. Lipid Sci. Technol. 2015, 117, 338–348. [Google Scholar] [CrossRef]
- Labuckas, D.; Maestri, D.; Lamarque, A. Lipid and protein stability of partially defatted walnut flour (Juglans regia L.) during storage. Int. J. Food Sci. Technol. 2011, 46, 1388–1397. [Google Scholar] [CrossRef]
- Ampofo, J.; Grilo, F.S.; Langstaff, S.; Wang, S.C. Oxidative Stability of Walnut Kernel and Oil: Chemical Compositions and Sensory Aroma Compounds. Foods 2022, 11, 3151. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Dalsgaard, T.K. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Wu, X.J.; Li, F.; Wu, W. Effects of rice bran rancidity on the oxidation and structural characteristics of rice bran protein. LWT-Food Sci. Technol. 2020, 120, 108943. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Li, X.; Wang, P.; Yu, X.; Fu, Q.; Gao, S. Effect of AAPH oxidation on digestion characteristics of seed watermelon (Citrullus lanatus var) kernels protein isolates. Food Sci. Hum. Wellness 2020, 9, 402–410. [Google Scholar] [CrossRef]
- Li, D.-Y.; Tan, Z.-F.; Liu, Z.-Q.; Wu, C.; Liu, H.-L.; Guo, C.; Zhou, D.-Y. Effect of hydroxyl radical induced oxidation on the physicochemical and gelling properties of shrimp myofibrillar protein and its mechanism. Food Chem. 2021, 351, 129344. [Google Scholar] [CrossRef]
- Zhang, W.G.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef]
- Zhu, Z.B.; Zhu, W.D.; Yi, J.H.; Liu, N.; Cao, Y.G.; Lu, J.L.; Decker, E.A.; McClements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chang, R.; Lu, H.; Qiu, L.; Tian, Y. Effect of amino acids composing rice protein on rice starch digestibility. LWT-Food Sci. Technol. 2021, 146, 111417. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.-J.; Wu, W. Effects of malondialdehyde-induced protein oxidation on the structural characteristics of rice protein. Int. J. Food Sci. Technol. 2020, 55, 760–768. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Li, Q.; Zhang, L.; Wang, Z.; Han, L.; Yu, Q. Oxidation of myofibrillar protein and crosslinking behavior during processing of traditional air-dried yak (Bos grunniens) meat in relation to digestibility. LWT-Food Sci. Technol. 2021, 142, 110984. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, T.; Liu, C.; Guo, F.; Zhao, J. L-Histidine improves solubility and emulsifying properties of soy proteins under various ionic strengths. LWT-Food Sci. Technol. 2021, 152. [Google Scholar] [CrossRef]
- Wu, W.; Li, F.; Wu, X. Effects of rice bran rancidity on oxidation, structural characteristics and interfacial properties of rice bran globulin. Food Hydrocoll. 2021, 110, 106123. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochimica et biophysica acta 1980, 624, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, P.; Deng, X.; Guo, X.; Mao, X.; Guo, X.; Zhang, J. Effects of hydroxyl radical oxidation on myofibrillar protein and its susceptibility to μ-calpain proteolysis. LWT 2021, 137, 110453. [Google Scholar] [CrossRef]
- Gong, X.; Hui, X.D.; Wu, G.; Morton, J.D.; Brennan, M.A.; Brennan, C.S. In vitro digestion characteristics of cereal protein concentrates as assessed using a pepsin-pancreatin digestion model. Food Res. Int. 2022, 152, 110715. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Yu, Y.H.; Hu, Q.H.; Liu, J.H.; Su, A.X.; Xu, H.; Li, X.T.; Huang, Q.R.; Zhou, J.L.; Mariga, A.M.; Yang, W.J. Isolation, purification and identification of immunologically active peptides from Hericium erinaceus. Food Chem. Toxicol. 2021, 151, 112111. [Google Scholar] [CrossRef]
- Cheng, Y.; Chi, Y.; Geng, X.H.; Chi, Y.J. Effect of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) induced oxidation on the physicochemical properties, in vitro digestibility, and nutritional value of egg white protein. LWT-Food Sci. Technol. 2021, 143, 111103. [Google Scholar] [CrossRef]
- Chen, N.N.; Zhao, M.M.; Sun, W.Z. Effect of protein oxidation on the in vitro digestibility of soy protein isolate. Food Chem. 2013, 141, 3224–3229. [Google Scholar] [CrossRef]
- Vogt, W. Oxidation of Methionyl Residues in Proteins—Tools, Targets, And Reversal. Free Radic. Biol. Med. 1995, 18, 93–105. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [Green Version]
- Weng, S.L.; Huang, K.Y.; Kaunang, F.J.; Huang, C.H.; Kao, H.J.; Chang, T.H.; Wang, H.Y.; Lu, J.J.; Lee, T.Y. Investigation and identification of protein carbonylation sites based on positionspecific amino acid composition and physicochemical features. BMC Bioinform. 2017, 18, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Kryndushkin, D.; Wu, W.W.; Venna, R.; Norcross, M.A.; Shen, R.F.; Rao, V.A. Complex Nature of Protein Carbonylation Specificity after Metal-Catalyzed Oxidation. Pharm. Res. 2017, 34, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, C.M.; Kong, X.Z.; Hua, Y.F. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Gurbuz, G.; Heinonen, M. LC-MS investigations on interactions between isolated beta-lactoglobulin peptides and lipid oxidation product malondialdehyde. Food Chem. 2015, 175, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.N.; Zhao, M.M.; Sun, W.Z.; Ren, J.Y.; Cui, C. Effect of oxidation on the emulsifying properties of soy protein isolate. Food Res. Int. 2013, 52, 26–32. [Google Scholar] [CrossRef]
- Wu, W.; Wu, X.J.; Hu, Y.F. Structural modification of soy protein by the lipid peroxidation product acrolein. Lwt-Food Sci. Technol. 2010, 43, 133–140. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhou, X.; Yu, J.; Wang, F.; Wang, J. Effects of glutaminase deamidation on the structure and solubility of rice glutelin. LWT-Food Sci. Technol. 2011, 44, 2205–2210. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Lin, J.H.; Wang, C.; Yousaf, L.; Xue, Y.; Shen, Q. Protein structural properties and proteomic analysis of rice during storage at different temperatures. Food Chem. 2021, 361, 130028. [Google Scholar] [CrossRef]
- Singh, T.P.; Sogi, D.S. Comparative study of structural and functional characterization of bran protein concentrates from superfine, fine and coarse rice cultivars. Int. J. Biol. Macromol. 2018, 111, 281–288. [Google Scholar] [CrossRef]
- Peng, Q.; Khan, N.A.; Wang, Z.; Yu, P. Relationship of feeds protein structural makeup in common Prairie feeds with protein solubility, in situ ruminal degradation and intestinal digestibility. Anim. Feed Sci. Technol. 2014, 194, 58–70. [Google Scholar] [CrossRef]
- Bao, Z.-j.; Wu, J.-p.; Cheng, Y.; Chi, Y.-j. Effects of lipid peroxide on the structure and gel properties of ovalbumin. Process Biochem. 2017, 57, 124–130. [Google Scholar] [CrossRef]
- Mitra, B.; Lametsch, R.; Akcan, T.; Ruiz-Carrascal, J. Pork proteins oxidative modifications under the influence of varied time-temperature thermal treatments: A chemical and redox proteomics assessment. Meat Sci. 2018, 140, 134–144. [Google Scholar] [CrossRef]
- Wu, X.J.; Li, F.; Wu, W. Effects of oxidative modification by 13-hydroperoxyoctadecadienoic acid on the structure and functional properties of rice protein. Food Res. Int. 2020, 132, 109096. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Shen, L. Role of Conformational Flexibility in the Emulsifying Properties of Bovine Serum Albumin. J. Agric. Food Chem. 2013, 61, 3097–3110. [Google Scholar] [CrossRef]
- Mune, M.A.M.; Sogi, D.S.; Minka, S.R. Response surface methodology for investigating structure-function relationship of grain legume proteins. J. Food Process. Preserv. 2018, 42, e13524. [Google Scholar] [CrossRef]
- Chen, J.W.; Mu, T.H.; Zhang, M.; Goffin, D. Effect of heat treatments on the structure and emulsifying properties of protein isolates from cumin seeds (Cuminum cyminum). Food Sci. Technol. Int. 2018, 24, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.T.; Jin, H.; Li, Y.Y.; Feng, H.Y.; Liu, C.H.; Xu, J. The Molecular Properties of Peanut Protein: Impact of Temperature, Relative Humidity and Vacuum Packaging during Storage. Molecules 2018, 23, 2618. [Google Scholar] [CrossRef]
- Grune, T.; Merker, K.; Sandig, G.; Davies, K.J.A. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem. Biophys. Res. Commun. 2003, 305, 709–718. [Google Scholar] [CrossRef]
- Ziegler, V.; Ferreira, C.D.; Hoffmann, J.F.; de Oliveira, M.; Elias, M.C. Effects of moisture and temperature during grain storage on the functional properties and isoflavone profile of soy protein concentrate. Food Chem. 2018, 242, 37–44. [Google Scholar] [CrossRef]
- Ye, L.; Liao, Y.; Zhao, M.M.; Sun, W.Z. Effect of Protein Oxidation on the Conformational Properties of Peanut Protein Isolate. J. Chem. 2013, 2013, 423254. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.N.; Zhao, Q.Z.; Sun, W.Z.; Zhao, M.M. Effects of Malondialdehyde Modification on the in Vitro Digestibility of Soy Protein Isolate. J. Agric. Food Chem. 2013, 61, 12139–12145. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M. Analysis of Protein Oxidation in Food and Feed Products. J. Agric. Food Chem. 2020, 68, 12870–12885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Li, M.; Guo, S.; Lv, Y. Effects of heat treatment on protein molecular structure and in vitro digestion in whole soybeans with different moisture content. Food Res. Int. 2022, 155, 111115. [Google Scholar] [CrossRef] [PubMed]
- Bisinotto, M.S.; da Silva, D.C.; Fino, L.D.; Simabuco, F.M.; Bezerra, R.M.N.; Antunes, A.E.C.; Pacheco, M.T.B. Bioaccessibility of cashew nut kernel flour compounds released after simulated in vitro human gastrointestinal digestion. Food Res. Int. 2021, 139, 109906. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, S.K.C. Comparative studies on ACE inhibition, degree of hydrolysis, antioxidant property and phenolic acid composition of hydrolysates derived from simulated in vitro gastrointestinal proteolysis of three thermally treated legumes. Food Chem. 2019, 281, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.C.; Li, C.Y.; Ullah, N.; Cao, J.Q.R.; Lan, Y.L.; Ge, W.P.; Hackman, R.M.; Li, Z.X.; Chen, L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef]
- Ren, L.K.; Fan, J.; Yang, Y.; Xu, Y.; Chen, F.L.; Bian, X.; Xing, T.L.; Liu, L.L.; Yu, D.H.; Zhang, N. Enzymatic Hydrolysis of Broken Rice Protein: Antioxidant Activities by Chemical and Cellular Antioxidant Methods. Front. Nutr. 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Wang, R.; Sui, X.; Qi, B.; Han, F.; Li, Y.; Jiang, L. Secondary Structure and Subunit Composition of Soy Protein In Vitro Digested by Pepsin and Its Relation with Digestibility. BioMed Res. Int. 2016, 2016, 5498639. [Google Scholar] [CrossRef]
- Zhao, J.; Su, G.W.; Zhao, M.M.; Sun, W.Z. Physicochemical Changes and in Vitro Gastric Digestion of Modified Soybean Protein Induced by Lipoxygenase Catalyzed Linoleic Acid Oxidation. J. Agric. Food Chem. 2019, 67, 13978–13985. [Google Scholar] [CrossRef]
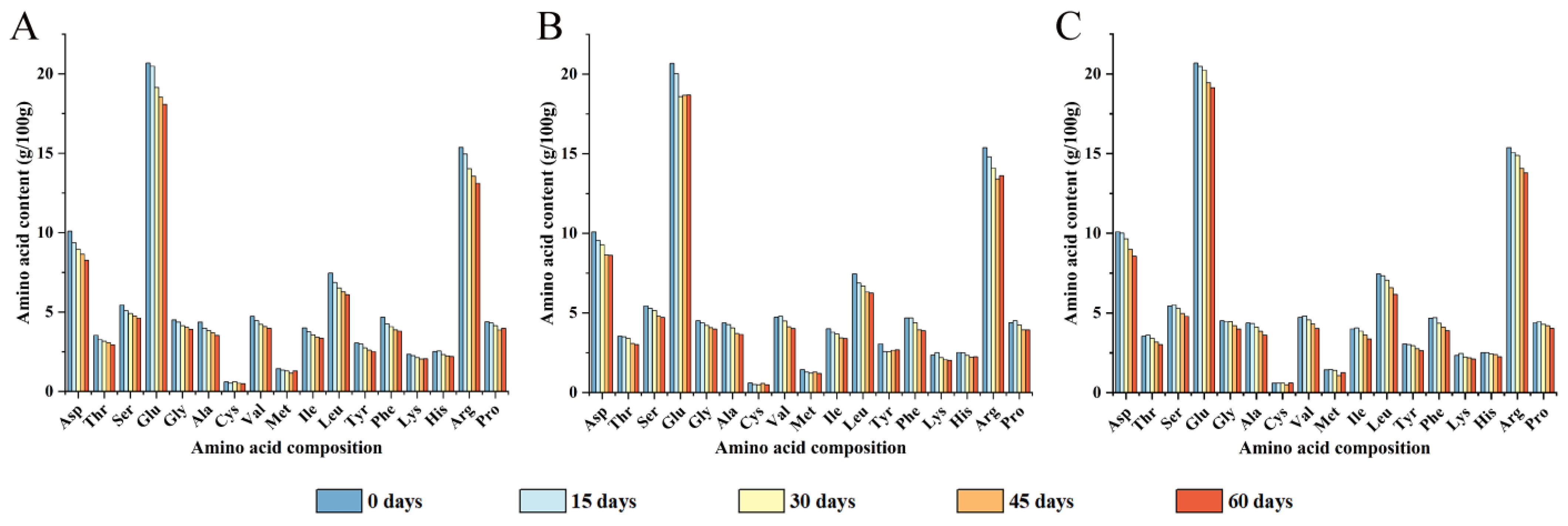





| Storage Time (Days) | Packaging Methods | Amino Acid Content (g/100 g) | |||
|---|---|---|---|---|---|
| TAA | EAA | HAA | AAA | ||
| 0 | — | 99.21 | 30.69 | 31.06 | 30.77 |
| PE | 94.78 | 28.72 | 28.94 | 29.85 | |
| 15 | PA/PE | 95.83 | 29.92 | 30.19 | 29.58 |
| PET/AL/PE | 98.90 | 30.97 | 31.18 | 30.49 | |
| PE | 89.81 | 27.31 | 27.65 | 28.10 | |
| 30 | PA/PE | 91.04 | 28.39 | 28.76 | 27.86 |
| PET/AL/PE | 95.79 | 29.35 | 29.66 | 29.88 | |
| PE | 86.37 | 26.18 | 26.40 | 27.17 | |
| 45 | PA/PE | 86.87 | 26.43 | 26.73 | 27.31 |
| PET/AL/PE | 90.45 | 27.45 | 27.74 | 28.44 | |
| PE | 84.21 | 25.71 | 26.03 | 26.35 | |
| 60 | PA/PE | 86.40 | 26.03 | 26.33 | 27.30 |
| PET/AL/PE | 87.28 | 26.09 | 26.37 | 27.70 | |
| Storage Time/Days | Packaging Methods | Percentage of Peak Area (%) and Its Corresponding Retention Time | ||||
|---|---|---|---|---|---|---|
| 7.00/min >1000 KDa | 11.16/min 105.93 KDa | 12.11/min 53.44 KDa | 14.25/min 11.49 KDa | 15.72/min 3.97 KDa | ||
| 0 | — | — | — | 39.27 | 60.73 | |
| PE | — | — | — | 45.50 | 54.50 | |
| 15 | PA/PE | — | — | — | 45.24 | 54.76 |
| PE/PET/AL | — | — | — | 32.53 | 67.47 | |
| PE | — | — | 2.46 | 55.72 | 41.82 | |
| 30 | PA/PE | 1.60 | — | — | 38.43 | 59.97 |
| PE/PET/AL | — | — | — | 47.01 | 52.99 | |
| PE | 2.14 | 3.71 | 36.15 | 16.53 | 43.61 | |
| 45 | PA/PE | 2.21 | 6.85 | 41.52 | 13.37 | 36.05 |
| PE/PET/AL | — | — | 17.85 | 29.62 | 52.53 | |
| PE | 3.67 | 12.04 | 50.40 | 12.58 | 21.32 | |
| 60 | PA/PE | — | 7.74 | 45.01 | 13.93 | 33.33 |
| PE/PET/AL | 2.74 | — | 5.61 | 37.14 | 54.51 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Zhao, J.; Wu, Q.; Mao, X.; Zhang, J. Effects of Packaging Materials on Structural and Simulated Digestive Characteristics of Walnut Protein during Accelerated Storage. Foods 2023, 12, 620. https://doi.org/10.3390/foods12030620
Han M, Zhao J, Wu Q, Mao X, Zhang J. Effects of Packaging Materials on Structural and Simulated Digestive Characteristics of Walnut Protein during Accelerated Storage. Foods. 2023; 12(3):620. https://doi.org/10.3390/foods12030620
Chicago/Turabian StyleHan, Miaomiao, Jinjin Zhao, Qingzhi Wu, Xiaoying Mao, and Jian Zhang. 2023. "Effects of Packaging Materials on Structural and Simulated Digestive Characteristics of Walnut Protein during Accelerated Storage" Foods 12, no. 3: 620. https://doi.org/10.3390/foods12030620






