The Protein Composition and In Vitro Digestive Characteristics of Animal- versus Plant-Based Infant Nutritional Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Infant Formulae Sample Collection and Preparation
2.2. Nitrogen Fraction and Protein Analysis of Infant Formulae
2.3. Static In Vitro Digestion
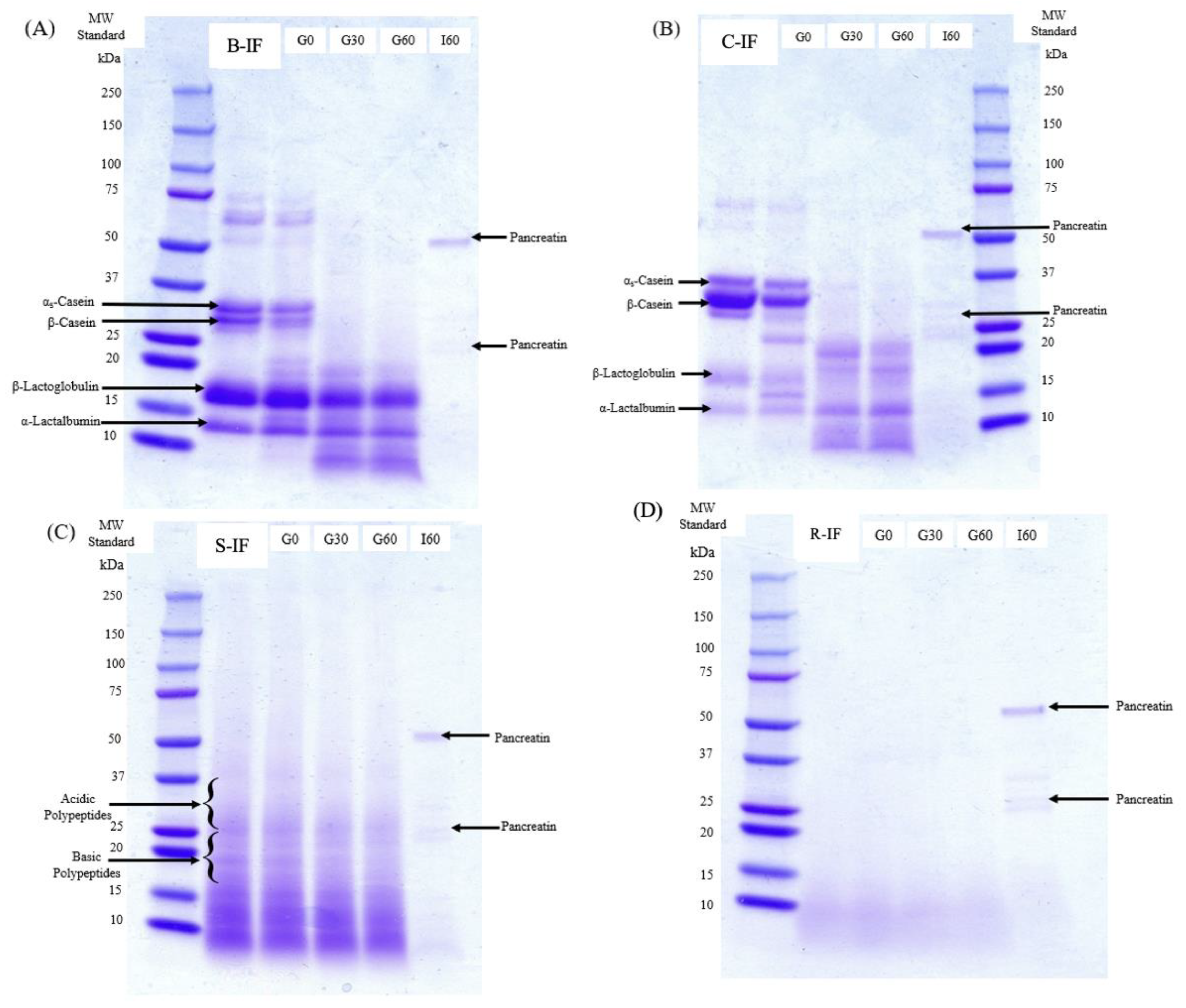
2.4. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis
2.5. Degree of Hydrolysis
2.6. Amino Acid Analysis
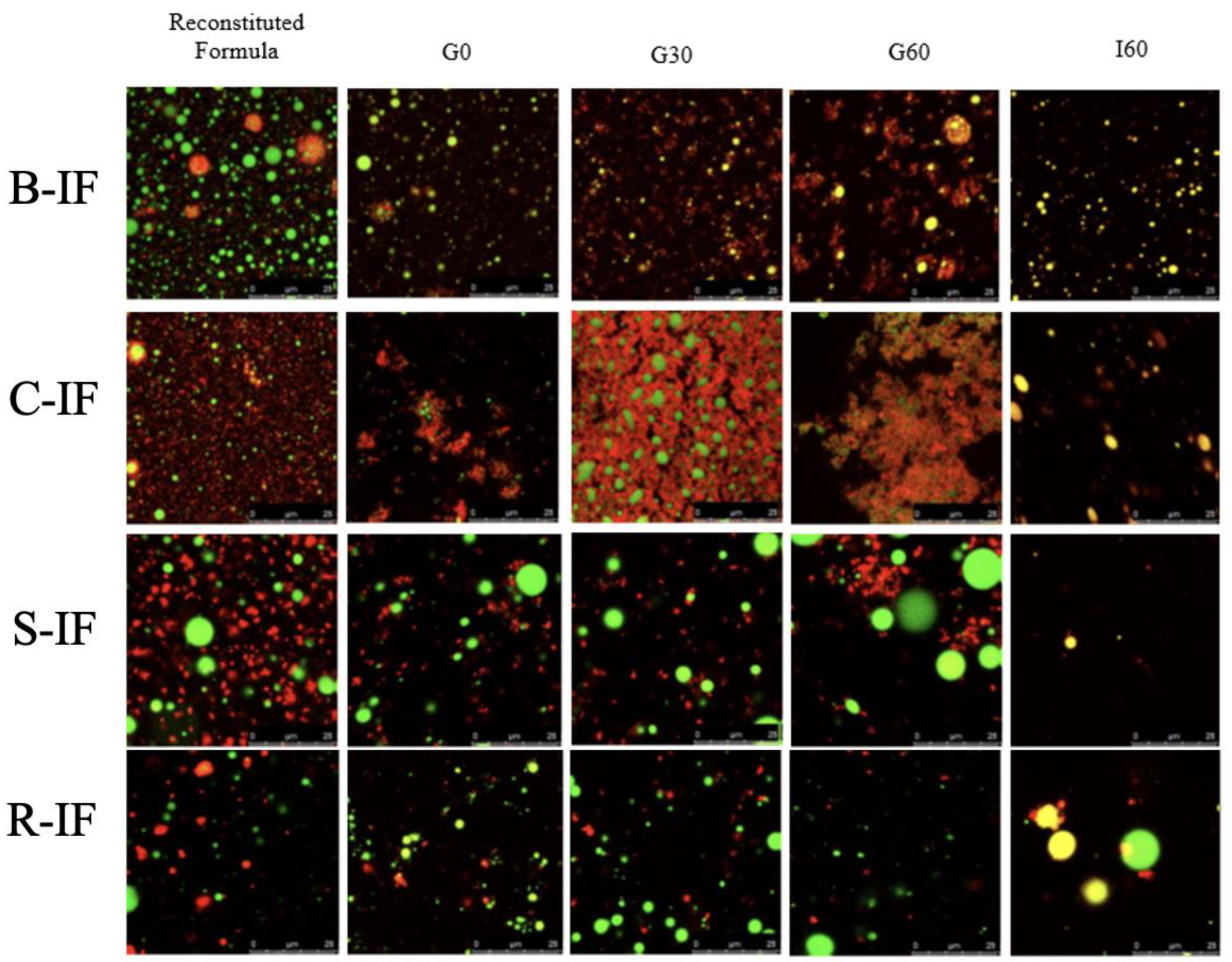
2.7. Microstructural Analysis
2.8. Simulated acid Coagulation Properties of Infant Formulae
2.9. Statistical Data Analysis
3. Results and Discussion
3.1. Protein and Amino Acid Composition of Formulae
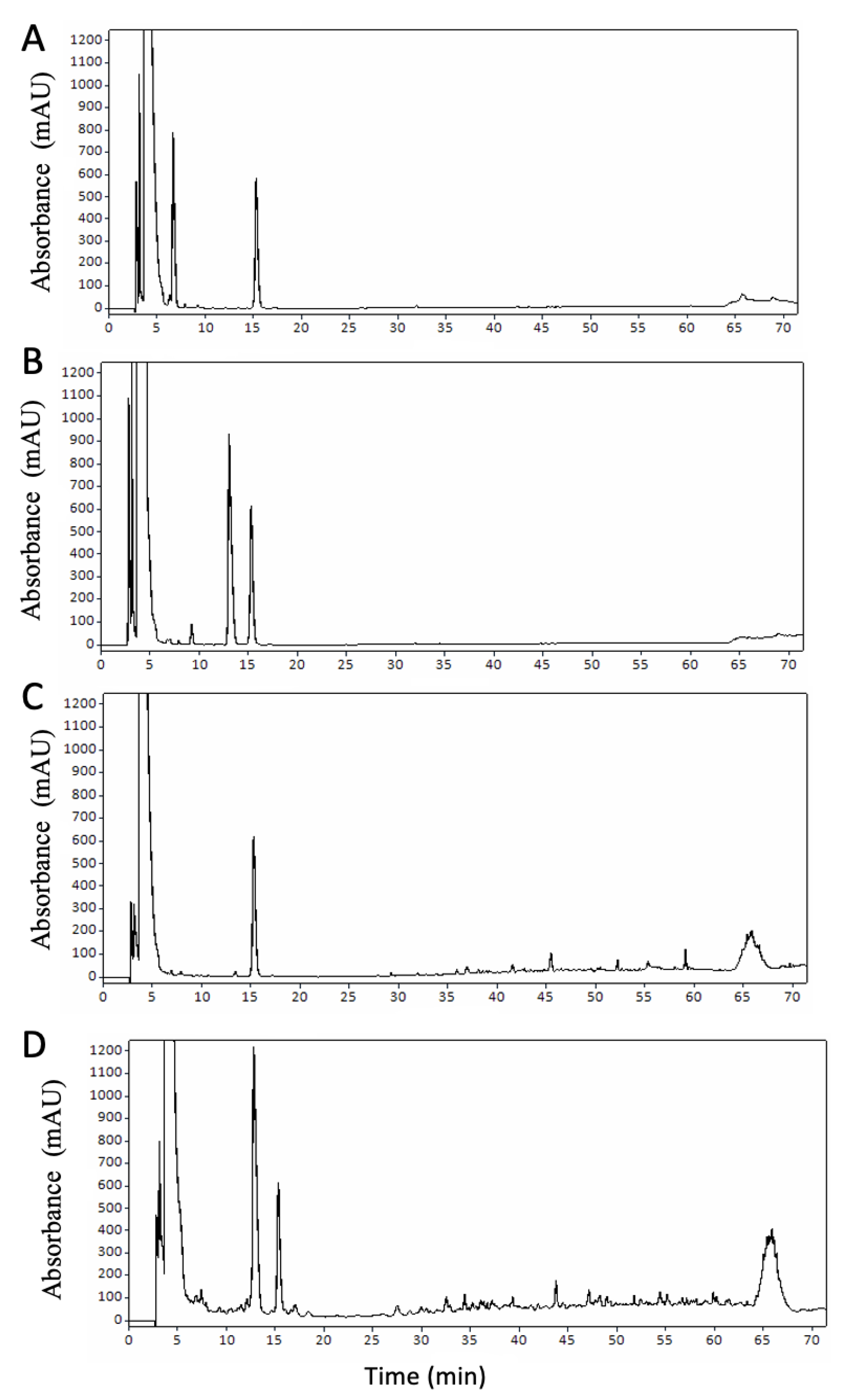
3.2. Protein Profile during Digestion
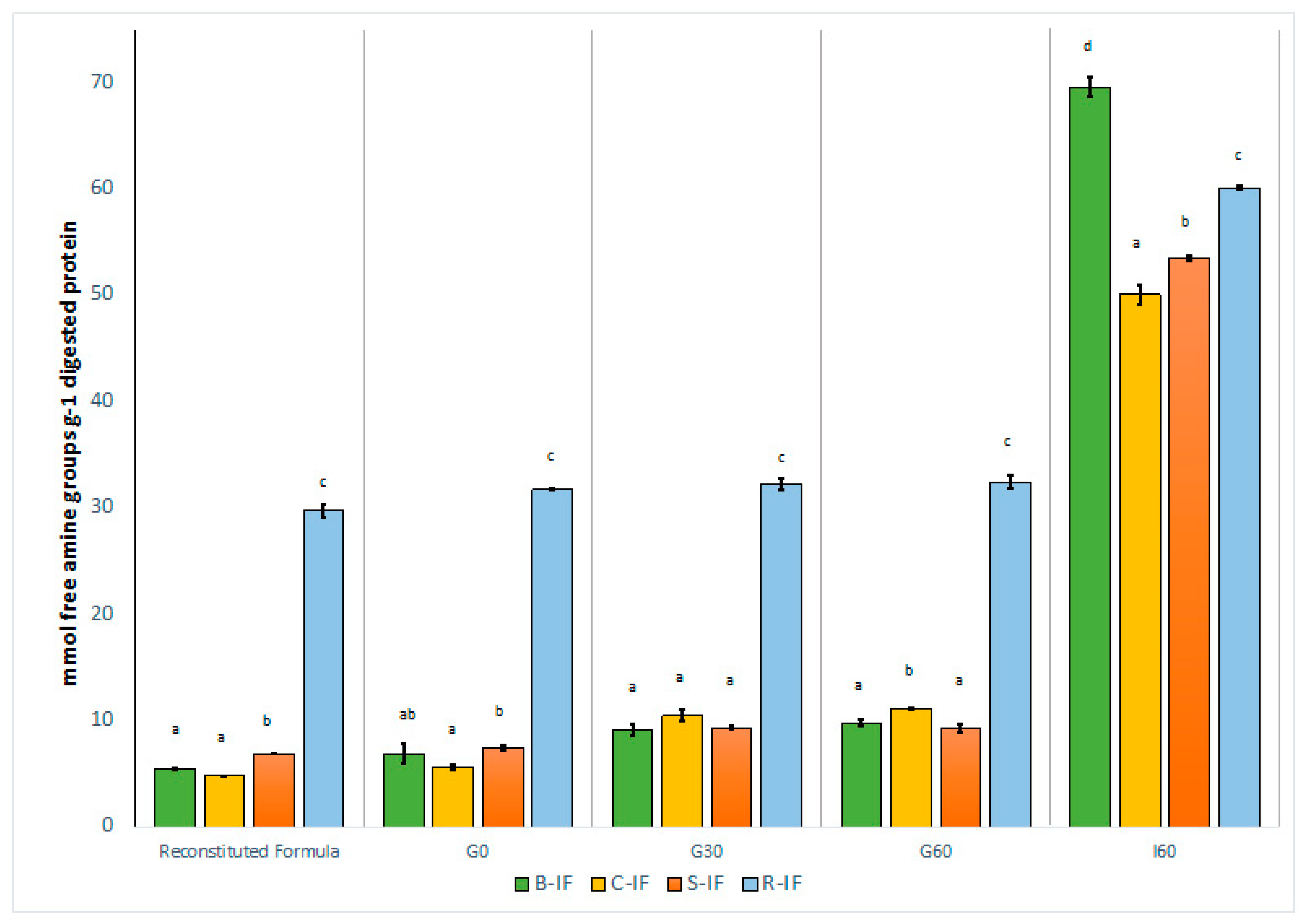
3.3. Degree of Protein Hydrolysis
3.4. Free Amino Acid Composition of Simulated Gastrointestinal Digests
3.5. Microstructural Changes during Digestion
3.6. Acid Coagulation Properties of Infant Formulae
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaelsen, K.F.; Greer, F.R. Protein needs early in life and long-term health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.V.; Kelly, A.L.; Lucey, J.A.; O’Mahony, J.A. Potential applications of non-bovine mammalian milk in infant nutrition. In Handbook of Milk of Non-Bovine Mammals, 2nd ed.; Park, Y.W., Haenlein, G.F.W., Wendorff, W.L., Eds.; John Wiley & Sons, Incorporated: Hoboken, UK, 2017; pp. 625–654. [Google Scholar]
- McSweeney, S.; O’Regan, J.; O’Callaghan, D. Nutritional formulae for infants and young children. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 458–476. [Google Scholar]
- Agostoni, C.; Axelsson, I.; Goulet, O.; Koletzko, B.; Michaelsen, K.F.; Puntis, J.; Rieu, D.; Rigo, J.; Shamir, R.; Szajewska, H.; et al. Soy protein infant formulae and follow-on formulae: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Busch, C.; Rodiger, M.; Hamm, U. Motives of consumers following a vegan diet and their attitudes towards animal agriculture. Appetite 2016, 105, 643–651. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; Barone, G.; Waldron, D.; Bez, J.; Joehnke, M.S.; Petersen, I.L.; Zannini, E.; Arendt, E.K.; O’Mahony, J.A. Formulation, pilot-scale preparation, physicochemical characterization and digestibility of a lentil protein-based model infant formula powder. J. Sci. Food Agric. 2021, 102, 5044–5054. [Google Scholar] [CrossRef]
- Le Roux, L.; Chacon, R.; Dupont, D.; Jeantet, R.; Deglaire, A.; Nau, F. In vitro static digestion reveals how plant proteins modulate model infant formula digestibility. Int. Food Res. J. 2020, 130, 108917. [Google Scholar] [CrossRef]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef]
- Gan, J.; Bornhorst, G.M.; Henrick, B.M.; German, J.B. Protein digestion of baby foods: Study approaches and implications for infant health. Mol. Nutr. Food Res. 2018, 62, 1700231. [Google Scholar] [CrossRef]
- Tari, N.R.; Fan, M.Z.; Archbold, T.; Kristo, E.; Guri, A.; Arranz, E.; Corredig, M. Effect of milk protein composition of a model infant formula on the physicochemical properties of in vivo gastric digestates. J. Dairy Sci. 2018, 101, 2851–2861. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Rioux, L.E. Food matrix impact on macronutrients nutritional properties. Food Hydrocoll. 2011, 25, 1915–1924. [Google Scholar] [CrossRef]
- Corrigan, B.; Brodkorb, A. The effect of pre-treatment of protein ingredients for infant formula on their in vitro gastro-intestinal behaviour. Int. Dairy J. 2020, 110, 104810. [Google Scholar] [CrossRef]
- Dupont, D.; Boutrou, R.; Menard, O.; Jardin, J.; Tanguy, G.; Schuck, P.; Haab, B.B.; Leonil, J. Heat treatment of milk during powder manufacture increases casein resistance to simulated infant digestion. Food Dig. 2010, 1, 28–39. [Google Scholar] [CrossRef]
- He, T.; Rombouts, W.; Einerhand, A.W.C.; Hotrum, N.; van de Velde, F. Gastric protein digestion of goat and cow milk infant formula and human milk under simulated infant conditions. Int. J. Food Sci. Nutr. 2021, 73, 28–38. [Google Scholar] [CrossRef]
- Maathuis, A.; Havenaar, R.; He, T.; Bellmann, S. Protein digestion and quality of goat and cow milk infant formula and human milk under simulated infant conditions. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Phosanam, A.; Chandrapala, J.; Huppertz, T.; Adhikari, B.; Zisu, B. In vitro digestion of infant formula model systems: Influence of casein to whey protein ratio. Int. J. Dairy 2021, 117, 105008. [Google Scholar] [CrossRef]
- Ye, A.Q.; Cui, J.; Carpenter, E.; Prosser, C.; Singh, H. Dynamic in vitro gastric digestion of infant formulae made with goat milk and cow milk: Influence of protein composition. Int. Dairy J. 2019, 97, 76–85. [Google Scholar] [CrossRef]
- Nguyen, T.T.P.; Bhandari, B.; Cichero, J.; Prakash, S. Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study. Food Res. Int. 2015, 76, 348–358. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Zhang, Y.; Zhang, S.; Liu, T.; Wang, D. Review on plant-derived bioactive peptides: Biological activities, mechanism of action and utilizations in food development. J. Future Foods 2022, 2, 143–159. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Ferraretto, A.; Arranz, E.; Stuknytė, M.; Bottani, M.; O’Connor, P.M.; Kelly, P.M.; De Noni, I.; Buckin, V.; Giblin, L. Bovine whey peptides transit the intestinal barrier to reduce oxidative stress in muscle cells. Food Chem. 2019, 288, 306–314. [Google Scholar] [CrossRef]
- Bourlieu, C.; Menard, O.; Bouzerzour, K.; Mandalari, G.; Macierzanka, A.; Mackie, A.R.; Dupont, D. Specificity of infant digestive conditions: Some clues for developing relevant in vitro models. Crit. Rev. Food Sci. Nutr. 2014, 54, 1427–1457. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.C.; Dellarosa, N.; Laghi, L.; Carriere, F.; Capozzi, F.; Dupont, D.; Deglaire, A. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef] [PubMed]
- ISO. Milk Determination of nitrogen content. In Part 1: Kjeldahl Method ISO 8968-1: 2001 (IDF 20-1:2001); International Standardisation Organisation: Geneva, Switzerland, 2001. [Google Scholar]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Bot, F.; O’Mahony, J.A.; Bez, J.; Arendt, E.K.; Zannini, E. Enzymatic hydrolysis of lentil protein concentrate for modification of physicochemical and techno-functional properties. Eur. Food Res. Technol. 2023, 249, 573–586. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- McDermott, A.; Visentin, G.; De Marchi, M.; Berry, D.; Fenelon, M.; O’connor, P.; Kenny, O.; McParland, S. Prediction of individual milk proteins including free amino acids in bovine milk using mid-infrared spectroscopy and their correlations with milk processing characteristics. J. Dairy Sci. 2016, 99, 3171–3182. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Mannion, D.; Apopei, D.; McCarthy, N.A.; Hogan, S.A.; Kilcawley, K.N.; Egan, M. Influence of Supplemental Feed Choice for Pasture-Based Cows on the Fatty Acid and Volatile Profile of Milk. Foods 2019, 8, 137. [Google Scholar] [CrossRef]
- Bhatia, J.; Greer, F.; Committee on Nutrition. Use of soy protein-based formulas in infant feeding. Pediatrics 2008, 121, 1062–1068. [Google Scholar] [CrossRef]
- Dupont, C.; Bocquet, A.; Tomé, D.; Bernard, M.; Campeotto, F.; Dumond, P.; Essex, A.; Frelut, M.-L.; Guénard-Bilbault, L.; Lack, G.; et al. Hydrolyzed rice protein-based formulas, a vegetal alternative in cow’s milk allergy. Nutrients 2020, 12, 2654. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Dairy Chemistry and Biochemistry, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Donovan, S.M.; Lonnerdal, B. Non-protein nitrogen and True protein in infant formulas. Acta Paediatr. Scand. 1989, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Fleddermann, M.; Fechner, A.; Rößler, A.; Bähr, M.; Pastor, A.; Liebert, F.; Jahreis, G. Nutritional evaluation of rapeseed protein compared to soy protein for quality, plasma amino acids, and nitrogen balance–a randomized cross-over intervention study in humans. Clin. Nutr. 2013, 32, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, K.; Govindaraju, K.; Singh, V.; Subramanian, R. Production of okara and soy protein concentrates using membrane technology. J. Food Sci. 2011, 76, E158–E164. [Google Scholar] [CrossRef]
- Prosser, C.G.; McLaren, R.D.; Frost, D.; Agnew, M.; Lowry, D.J. Composition of the non-protein nitrogen fraction of goat whole milk powder and goat milk-based infant and follow-on formulae. Int. J. Food Sci. Nutr. 2008, 59, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, A.; Dupont, C.; Chouraqui, J.-P.; Darmaun, D.; Feillet, F.; Frelut, M.-L.; Girardet, J.-P.; Hankard, R.; Lapillonne, A.; Rozé, J.-C. Efficacy and safety of hydrolyzed rice-protein formulas for the treatment of cow’s milk protein allergy. Arch. Pediatr. 2019, 26, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; Weiss, J. Alternative protein sources as technofunctional food ingredients. Annu. Rev. Food Sci. Technol. 2021, 12, 93–117. [Google Scholar] [CrossRef]
- Venkateswara Rao, M.; CK, S.; Rawson, A.; DV, C. Modifying the plant proteins techno-functionalities by novel physical processing technologies: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–22, ahead of print. [Google Scholar] [CrossRef]
- Jimenez-Munoz, L.; Brodkorb, A.; Gomez-Mascaraque, L.G.; Corredig, M. Effect of heat treatment on the digestion behavior of pea and rice protein dispersions and their blends, studied using the semi-dynamic INFOGEST digestion method. Food Funct. 2021, 12, 8747–8759. [Google Scholar] [CrossRef]
- Lee, K.H.; Ryu, H.S.; Rhee, K.C. Protein solubility characteristics of commercial soy protein products. J. Am. Oil Chem. Soc. 2003, 80, 85–90. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Prosser, C.G. Compositional and functional characteristics of goat milk and relevance as a base for infant formula. J. Food Sci. 2021, 86, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Juárez, M.; Ramos, M.; Haenlein, G. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Dupont, D.; Mandalari, G.; Molle, D.; Jardin, J.; Léonil, J.; Faulks, R.M.; Wickham, M.S.; Mills, E.N.; Mackie, A.R. Comparative resistance of food proteins to adult and infant in vitro digestion models. Mol. Nutr. Food Res. 2010, 54, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Tomé, D. Milk proteins: Digestion and absorption in the gastrointestinal tract. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2020; pp. 701–714. [Google Scholar]
- Medic, J.; Atkinson, C.; Hurburgh, C.R. Current knowledge in soybean composition. J. Am. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar] [CrossRef]
- Nguyen, T.T.P.; Bhandari, B.; Cichero, J.; Prakash, S. A comprehensive review on in vitro digestion of infant formula. Food Res. Int. 2015, 76, 373–386. [Google Scholar] [CrossRef]
- Zhou, S.J.; Sullivan, T.; Gibson, R.A.; Lönnerdal, B.; Prosser, C.G.; Lowry, D.J.; Makrides, M. Nutritional adequacy of goat milk infant formulas for term infants: A double-blind randomised controlled trial. Br. J. Nutr. 2014, 111, 1641–1651. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Darragh, A.J.; Hendriks, W.H.; Prosser, C.G.; Lowry, D. True ileal amino acid digestibility of goat and cow milk infant formulas. J. Dairy Sci. 2006, 89, 2408–2413. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Shamsia, S.; Helal, A.; Conte, A. Biological activities and peptidomic profile of in vitro-digested cow, camel, goat and sheep milk. Int. Dairy J. 2018, 81, 19–27. [Google Scholar] [CrossRef]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Vagadia, B.H.; Vanga, S.K.; Raghavan, V. Inactivation methods of soybean trypsin inhibitor—A review. Trends Food Sci. Technol. 2017, 64, 115–125. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Li, H.; Liang, M.C.; Yang, L. Glutelin and prolamin, different components of rice protein, exert differently in vitro antioxidant activities. J. Cereal Sci. 2016, 72, 108–116. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, structure, and digestive dynamics of milk from different species—A review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef] [PubMed]
- Mulet-Cabero, A.-I.; Mackie, A.R.; Wilde, P.J.; Fenelon, M.A.; Brodkorb, A. Structural mechanism and kinetics of in vitro gastric digestion are affected by process-induced changes in bovine milk. Food Hydrocoll. 2019, 86, 172–183. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Torcello-Gómez, A.; Saha, S.; Mackie, A.R.; Wilde, P.J.; Brodkorb, A. Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption. Food Chem. 2020, 319, 126514. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Rigby, N.M.; Brodkorb, A.; Mackie, A.R. Dairy food structures influence the rates of nutrient digestion through different in vitro gastric behaviour. Food Hydrocoll. 2017, 67, 63–73. [Google Scholar] [CrossRef]
- Wang, Y.R.; Eastwood, B.; Yang, Z.; de Campo, L.; Knott, R.; Prosser, C.; Carpenter, E.; Hemar, Y. Rheological and structural characterization of acidified skim milks and infant formulae made from cow and goat milk. Food Hydrocoll. 2019, 96, 161–170. [Google Scholar] [CrossRef]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef]
- Makinen, O.E.; Uniacke-Lowe, T.; O’Mahony, J.A.; Arendt, E.K. Physicochemical and acid gelation properties of commercial UHT-treated plant-based milk substitutes and lactose free bovine milk. Food Chem. 2015, 168, 630–638. [Google Scholar] [CrossRef]
| IF | Total N (%) | Crude Protein (%) | NPN (%) | NPN as % of Total N (%) | True Protein (%) | p-Value * |
|---|---|---|---|---|---|---|
| B-IF | 0.20 ± <0.01 c | 1.25 ± 0.01 c | 0.016 ± <0.01 d | 8.10 ± 0.20 d | 1.15 ± 0.01 b | <0.001 |
| C-IF | 0.20 ± <0.01 c | 1.25 ± 0.02 c | 0.023 ± <0.01 c | 11.5 ± 0.65 c | 1.11 ± 0.01 c | <0.001 |
| S-IF | 0.27 ± <0.01 a | 1.67 ± 0.01 a | 0.060 ± <0.01 b | 22.7 ± 0.69 b | 1.29 ± 0.02 a | <0.001 |
| R-IF | 0.24 ± < 0.01 b | 1.44 ± 0.01 b | 0.210 ± <0.01 a | 86.7 ± 0.69 a | 0.19 ± 0.01 d | <0.001 |
| Amino Acid | B-IF | C-IF | S-IF | R-IF | p-Value * | |
|---|---|---|---|---|---|---|
| Essential Amino Acids | Histidine | 141 ± 9.7 | 138 ± 5.3 | 131 ± 32.5 | 140 ± 8.4 | 0.881 |
| Isoleucine | 59.7 ± 5.8 a | 35.7 ± 1.7 b | 59.2 ± 16.3 ab | 102 ± 5.8 c | <0.001 | |
| Leucine | 250 ± 29.6 a | 122 ± 7.5 b | 200 ± 73.0 ab | 246 ± 14.7 a | 0.015 | |
| Lysine | 354 ± 23.7 a | 125 ± 9.4 b | 231 ± 62.1 b | 275 ± 13.0 ab | 0.009 | |
| Methionine | 46.5 ± 2.3 a | 12.3 ± 2.3 c | 33.4 ± 8.3 b | 13.3 ± 2.1 c | <0.001 | |
| Phenylalanine | 253 ± 24.1 a | 105 ± 23.5 b | 209 ± 57.3 a | 196 ± 12.8 a | 0.004 | |
| Threonine | 72.0 ± 22.9 b | 29.7 ± 2.8 c | 53.4 ± 15.7 bc | 123 ± 7.3 a | <0.001 | |
| Tryptophan | 127 ± 34.1 ab | 163 ± 18.2 a | 89.4 ± 31.9 b | 169 ± 7.9 a | 0.009 | |
| Valine | 105 ± 8.1 a | 55.8 ± 6.87 b | 87.8 ± 31.8 ab | 102 ± 4.9 a | 0.026 | |
| Conditionally Essential Amino Acids | Cysteine | 50.3 ± 7.5 | 62.2 ± 4.8 | 49.0 ± 16.5 | 62.8 ± 6.8 | 0.249 |
| Non-Essential Amino Acids 1 | Cysteic Acid | 51.4 ± 3.1 b | 71.4 ± 11.4 c | 15.1 ± 4.3 a | 12.97 ± 3.4 a | <0.001 |
| Tyrosine | 159 ± 16.8 | 125 ± 2.6 | 133 ± 52.2 | 184 ± 14.0 | 0.121 | |
| Alanine | 47.4 ± 5.2 a | 33.4 ± 2.2 a | 49.6 ± 13.5 a | 83.0 ± 1.8 b | <0.001 | |
| Arginine | 185 ± 11.5 b | 156 ± 4.5 b | 338 ± 94.2 a | 345 ± 12.7 a | 0.002 | |
| Aspartic Acid | 34.3 ± 5.6 | 25.7 ± 1.3 | 31.2 ± 7.6 | 36.6 ± 4.2 | 0.135 | |
| Glutamic Acid | 96.1 ± 6.8 | 82.7 ± 3.1 | 88.1 ± 24.1 | 99.3 ± 6.4 | 0.437 | |
| Glycine | 32.6 ± 1.4 | 28.8 ± 1.2 | 29.1 ± 7.7 | 35.9 ± 0.6 | 0.169 | |
| Serine | 40.3 ± 14.2 ab | 27.1 ± 1.1 b | 56.5 ± 15.4 a | 66.1 ± 3.3 a | 0.009 |
| Infant Formula | Gel Point (s) | Gel pH | G’ at pH 4.6 (Pa) | G* at pH 4.6 (Pa) |
|---|---|---|---|---|
| B-IF | 760 ± 35 a | 5.18 ± 0.05 a | 3.56 ± 0.11 a | 3.71 ± 0.12 a |
| C-IF | 1270 ± 35 b | 5.10 ± 0.02 a | 6.57 ± 0.22 b | 6.89 ± 0.23 b |
| S-IF | N/A | N/A | 0.368 ± 0.06 c | 0.393 ± 0.07 c |
| R-IF | N/A | N/A | 0.118 ± 0.03 c | 0.406 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne, M.E.; Arranz, E.; Bot, F.; Gómez-Mascaraque, L.G.; Tobin, J.T.; O’Mahony, J.A.; O’Callaghan, T.F. The Protein Composition and In Vitro Digestive Characteristics of Animal- versus Plant-Based Infant Nutritional Products. Foods 2023, 12, 1469. https://doi.org/10.3390/foods12071469
Byrne ME, Arranz E, Bot F, Gómez-Mascaraque LG, Tobin JT, O’Mahony JA, O’Callaghan TF. The Protein Composition and In Vitro Digestive Characteristics of Animal- versus Plant-Based Infant Nutritional Products. Foods. 2023; 12(7):1469. https://doi.org/10.3390/foods12071469
Chicago/Turabian StyleByrne, Margaret E., Elena Arranz, Francesca Bot, Laura G. Gómez-Mascaraque, John T. Tobin, James A. O’Mahony, and Tom F. O’Callaghan. 2023. "The Protein Composition and In Vitro Digestive Characteristics of Animal- versus Plant-Based Infant Nutritional Products" Foods 12, no. 7: 1469. https://doi.org/10.3390/foods12071469
APA StyleByrne, M. E., Arranz, E., Bot, F., Gómez-Mascaraque, L. G., Tobin, J. T., O’Mahony, J. A., & O’Callaghan, T. F. (2023). The Protein Composition and In Vitro Digestive Characteristics of Animal- versus Plant-Based Infant Nutritional Products. Foods, 12(7), 1469. https://doi.org/10.3390/foods12071469








