Effects of Lactobacillus on the Differentiation of Intestinal Mucosa Immune Cells and the Composition of Gut Microbiota in Soybean-Sensitized Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lactobacillus Solution
2.3. Preparation of Protein Solution
2.4. Establishment of Soybean ALLERGY Model in Mice
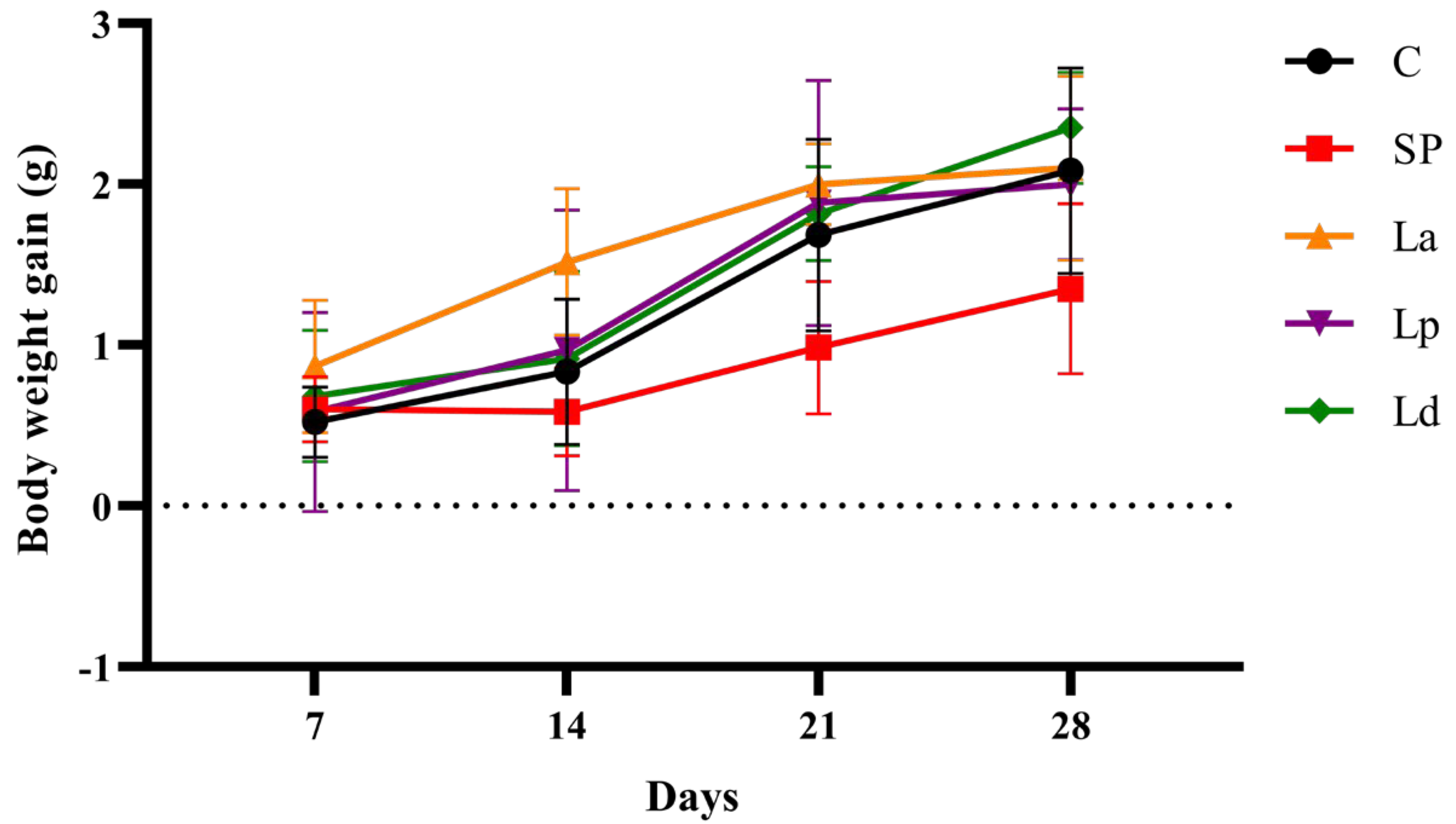
2.5. Body Weight and Allergic Symptom Scores of Mice
2.6. Analysis of Immune Cell Differentiation in Intestinal Mucosal Immunity
2.6.1. Preparation of Mouse MLN and PP Cell Suspension
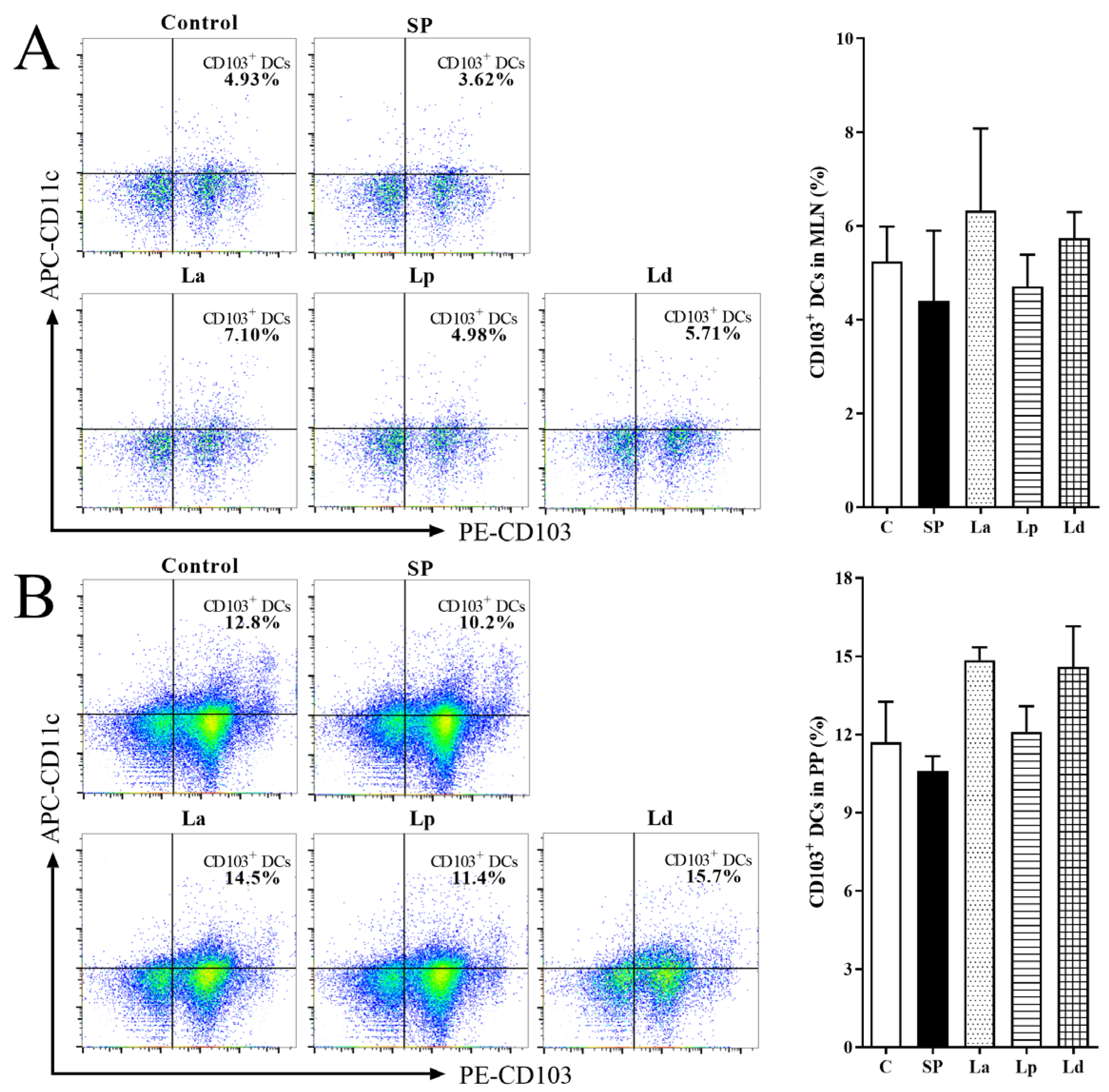
2.6.2. Identification of DCs and CD4+/CD8+ T Cells in MLNs and PPs
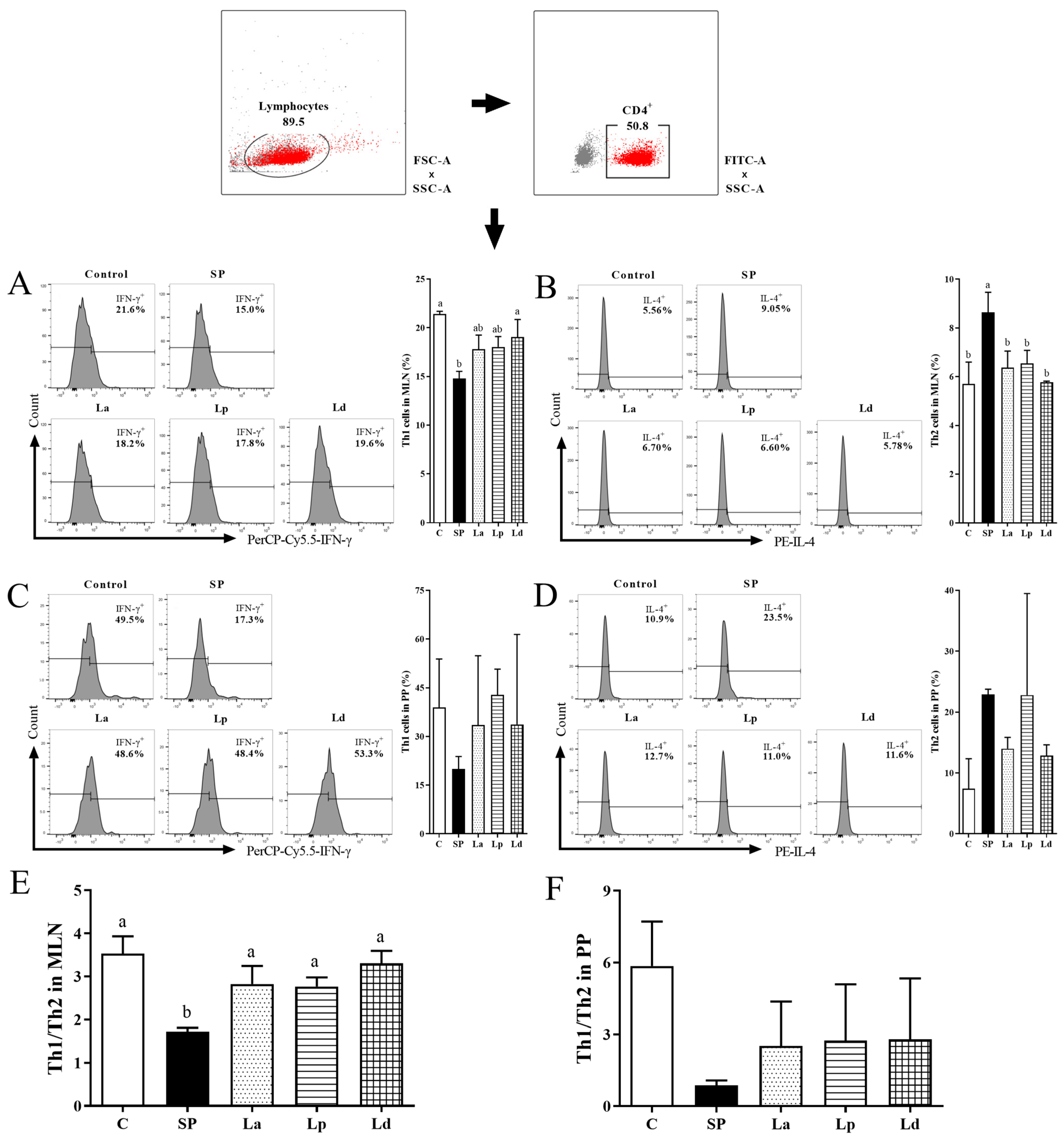
2.6.3. Detection of IFN-γ and IL-4 in MLNs and PPs
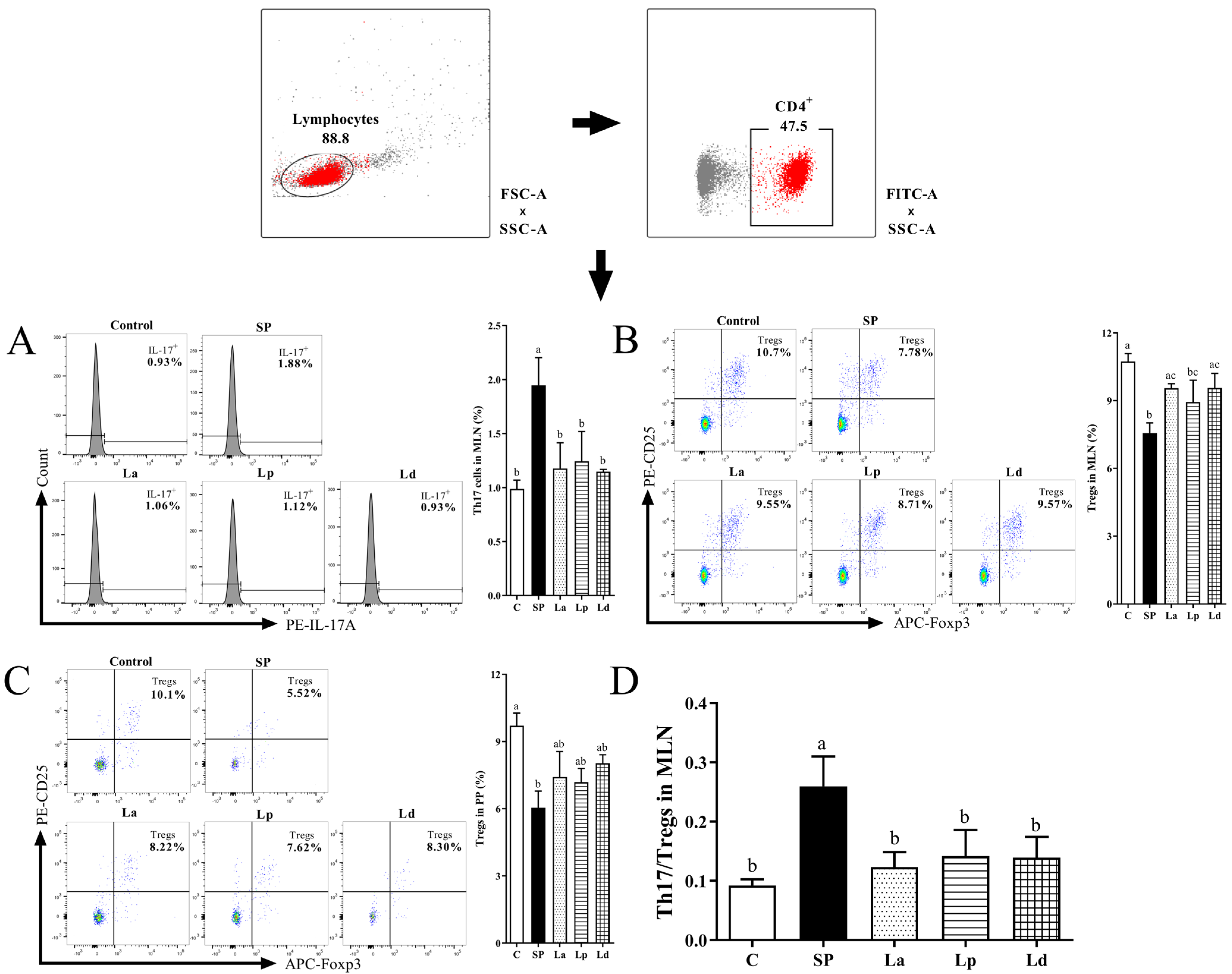
2.6.4. Identification of Th17 Cells and Tregs in MLNs and PPs
2.7. Microbial Composition Analysis of Cecal Contents in Mice
2.8. Statistical Analysis
3. Results
3.1. Allergic Response in Mice
3.2. Percentage of DCs in MLNs and PPs
3.3. Percentage of CD4+ and CD8+ Cells in MLNs and PPs
3.4. Differentiation Balance of Th1 and Th2 Cells in MLNs and PPs
3.5. Differentiation Balance of Th17 Cells and Tregs in MLNs and PPs
3.6. Changes of Gut Microbiota in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballmer-Weber, B.K.; Holzhauser, T.; Scibilia, J.; Mittag, D.; Zisa, G.; Ortolani, C.; Oesterballe, M.; Poulsen, L.K.; Vieths, S.; Bindslev-Jensen, C. Clinical characteristics of soybean allergy in Europe: A double-blind, placebo-controlled food challenge study. J. Allergy Clin. Immunol. 2007, 119, 1489–1496. [Google Scholar] [CrossRef]
- Katz, Y.; Gutierrez-Castrellon, P.; González, M.G.; Rivas, R.; Lee, B.W.; Alarcon, P. A comprehensive review of sensitization and allergy to soy-based products. Clin. Rev. Allergy Immunol. 2014, 46, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy 2018, 73, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hogenkamp, A.; Li, X.; Chen, H.; Garssen, J.; Knippels, L.M.J. Role of selenium in IgE mediated soybean allergy development. Crit. Rev. Food Sci. Nutr. 2022, 14, 1–9. [Google Scholar] [CrossRef]
- Lorenz, R.G.; Newberry, R.D. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann. N. Y. Acad. Sci. 2010, 1029, 44–57. [Google Scholar] [CrossRef]
- Nakajima-Adachi, H.; Kikuchi, A.; Fujimura, Y.; Shibahara, K.; Hachimura, S. Peyer’s Patches and Mesenteric Lymph Nodes Cooperatively Promote Enteropathy in a Mouse Model of Food Allergy. PLoS ONE 2014, 9, e107492. [Google Scholar] [CrossRef] [PubMed]
- Mair, F.; Liechti, T. Comprehensive phenotyping of human dendritic cells and monocytes. Cytom. A 2021, 99, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Granot, T.; Sen Da, T.; Carpenter, D.J.; Matsuoka, N.; Weiner, J.; Gordon, C.L.; Miron, M.; Kumar, B.V.; Griesemer, A.; Ho, S.H.; et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 2017, 46, 504–513. [Google Scholar] [CrossRef]
- Shiokawa, A.; Kotaki, R.; Takano, T.; Nakajima-Adachi, H.; Hachimura, S. Mesenteric lymph node CD11b CD103+ PD-L1High dendritic cells highly induce regulatory T cells. Immunology 2017, 152, 52–64. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.-Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Natália, P.; Lícia, T.; Mariana, D.; Andrade, D.; Andrade, D.; Macedo, C.M.; Juliana, D.; Uceli, M.T.; Caetano, F. Oral tolerance as antigen-specific immunotherapy. Immunother. Adv. 2021, 1, ltab017. [Google Scholar] [CrossRef]
- Spahn, T.W.; Weiner, H.L.; Rennert, P.D.; Lügering, N.; Fontana, A.; And, W.D.; Kucharzik, T. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer’s patches. Eur. J. Immunol. 2002, 32, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Hao, J.; He, Y.; Dong, X.; Fu, Y.-X.; Guo, X. The Interaction between Lymphoid Tissue Inducer-Like Cells and T Cells in the Mesenteric Lymph Node Restrains Intestinal Humoral Immunity. Cell Rep. 2020, 32, 107936. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Porsche, C.; Kozik, A.J.; Lynch, S.V. Microbiome–Immune Interactions in Allergy and Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 2244–2251. [Google Scholar] [CrossRef]
- Romano-Keeler, J.; Moore, D.J.; Wang, C.; Brucker, R.M.; Fonnesbeck, C.; Slaughter, J.C.; Li, H.; Curran, D.P.; Meng, S.; Correa, H.; et al. Early life establishment of site-specific microbial communities in the gut. Gut Microbes. 2014, 5, 192–201. [Google Scholar] [CrossRef]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Duffy, D. Understanding immune variation for improved translational medicine. Curr. Opin. Immunol. 2020, 65, 83–88. [Google Scholar] [CrossRef]
- Shu, S.A.; Yuen, A.W.T.; Woo, E.; Chu, K.H.; Kwan, H.S.; Yang, G.X.; Yang, Y.; Leung, P.S.C. Microbiota and Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 83–97. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Salmonella infection-prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 2018, 164, 1327–1344. [Google Scholar] [CrossRef]
- Geng, T.; He, F.; Su, S.; Sun, K.; Zhao, L.; Zhao, Y.; Bao, N.; Pan, L.; Sun, H. Probiotics Lactobacillus rhamnosus GG ATCC53103 and Lactobacillus plantarum JL01 induce cytokine alterations by the production of TCDA, DHA, and succinic and palmitic acids, and enhance immunity of weaned piglets. Res. Vet. Sci. 2021, 137, 56–67. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Haar, J. Lacticaseibacillus rhamnosus GR-1, a.k.a. Lactobacillus rhamnosus GR-1: Past and Future Perspectives. Trends Microbiol. 2021, 29, 747–761. [Google Scholar] [CrossRef]
- Bjrkstén, B. Effects of intestinal microflora and the environment on the development of asthma and allergy. Springer Semin. Immunopathol. 2004, 25, 257–270. [Google Scholar] [CrossRef]
- Lan, H.; Gui, Z.; Zeng, Z.; Li, D.; Qian, B.; Qin, L.-Y.; Dai, L.; Song, J.-L. Oral administration of Lactobacillus plantarum CQPC11 attenuated the airway inflammation in an ovalbumin (OVA)-induced Balb/c mouse model of asthma. J. Food Biochem. 2022, 46, e14036. [Google Scholar] [CrossRef]
- Fu, L.; Peng, J.; Zhao, S.; Zhang, Y.; Su, X.; Wang, Y. Lactic acid bacteria-specific induction of CD4+Foxp3+T cells ameliorates shrimp tropomyosin-induced allergic response in mice via suppression of mTOR signaling. Sci. Rep. 2017, 7, 1987. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yang, J.; Zhang, C.; Chi, H.; Zhang, C.; Li, T.; Zhang, J.; Du, P. Lactobacillus acidophilus KLDS 1.0738 inhibits TLR4/NF-κB inflammatory pathway in β-lactoglobulin-induced macrophages via modulating miR-146a. J. Food Biochem. 2021, 45, e13662. [Google Scholar] [CrossRef] [PubMed]
- Anatriello, E.; Cunha, M.; Nogueira, J.; Carvalho, J.L.C.; Fialho, A.K.C.d.S.; Miranda, M.; Castro-Faria-Neto, H.; Keller, A.C.; Aimbire, F. Oral feeding of Lactobacillus bulgaricus N45.10 inhibits the lung inflammation and airway remodeling in murine allergic asthma: Relevance to the Th1/Th2 cytokines and STAT6/T-bet. Cell. Immunol. 2019, 341, 103928. [Google Scholar] [CrossRef] [PubMed]
- Perdigon, G. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 2001, 2, 27–42. [Google Scholar] [PubMed]
- Bai, J.; Zhao, X.; Zhang, M.; Xia, X.; Yang, A.; Chen, H. Gut microbiota: A target for prebiotics and probiotics in the intervention and therapy of food allergy. Crit. Rev. Food Sci. Nutr. 2022, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Liao, Y.; Zhu, J.; Zhang, J.; Wu, Z.; Li, X.; Tong, P.; Chen, H.; Wang, S.; Liu, Z. Screening of anti-allergy Lactobacillus and its effect on allergic reactions in BALB/c mice sensitized by soybean protein. J. Funct. Foods 2021, 87, 104858. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, H.; Yang, A.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Effect of microbial transglutaminase cross-linking on the quality characteristics and potential allergenicity of tofu. Food Funct. 2019, 10, 5485–5497. [Google Scholar] [CrossRef]
- Anshu, Y.; Lingling, Z.; Youfei, C.; Zhihua, W.; Xin, L.; Ping, T.; Hongbing, C. Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms. Food Funct. 2018, 9, 1899–1909. [Google Scholar] [CrossRef]
- Gill, H.S.; Rutherfurd, K.J. Viability and dose–response studies on the effects of the immunoenhancing lactic acid bacterium Lactobacillus rhamnosus in mice. Br. J. Nutr. 2001, 86, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Hui, J.; Lu, Q.; Yang, A.; Yuan, J.; Gao, J.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Effect of transglutaminase cross-linking on the allergenicity of tofu based on a BALB/c mouse model. Food Funct. 2020, 11, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Gross, F.; Metzner, G.; Behn, U. Mathematical Modelling of Allergy and Specific Immunotherapy: Th1-Th2-Treg Interactions. J. Theor. Biol. 2011, 269, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Mckenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Li, Q.; Shi, Z.; Wu, H.; Zhang, H.; Tang, L.; Yi, R.; Su, H.; Sun, X. Oral administration of a mixture of probiotics protects against food allergy via induction of CD103+ dendritic cells and modulates the intestinal microbiota. J. Funct. Foods 2019, 55, 65–75. [Google Scholar] [CrossRef]
- Chu, H.; Khosravi, A.; Kusumawardhani, I.P.; Kwon, A.H.K.; Vasconcelos, A.C.; Cunha, L.D.; Mayer, A.E.; Shen, Y.; Wu, W.L.; Kambal, A.; et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 2016, 352, 1116–1120. [Google Scholar] [CrossRef]
- Wu, R.; Yuan, X.; Li, X.; Ma, N.; Jiang, H.; Tang, H.; Xu, G.; Liu, Z.; Zhang, Z. The bile acid-activated retinoic acid response in dendritic cells is involved in food allergen sensitization. Allergy 2022, 77, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Wambre, E.; Bajzik, V.; DeLong, J.H.; O’Brien, K.; Nguyen, Q.A.; Speake, C.; Gersuk, V.H.; DeBerg, H.A.; Whalen, E.; Ni, C.; et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci. Transl. Med. 2017, 9, eaam9171. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shi, Y.; Zhang, H.; Zhou, H. The Role of Th17 Cells and IL-17 in Th2 Immune Responses of Allergic Conjunctivitis. J. Ophthalmol. 2020, 2020, 6917185. [Google Scholar] [CrossRef]
- Shi, T.; Li, N.; He, Y.; Feng, J.; Mei, Z.; Du, Y.; Jie, Z. Th17/Treg cell imbalance played an important role in respiratory syncytial virus infection compromising asthma tolerance In mice. Microb. Pathog. 2021, 156, 104867. [Google Scholar] [CrossRef]
- Zhang, J.; Hui, S.; Li, Q.; Wu, H.; Liu, M.; Huang, J.; Zeng, M.; Zheng, Y.; Xin, S. Oral administration of Clostridium butyricum CGMCC0313-1 inhibits β-lactoglobulin-induced intestinal anaphylaxis in a mouse model of food allergy. Gut Pathog. 2017, 9, 667–673. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, W.; Wang, W. Bifidobacterium lactis Ameliorates the Risk of Food Allergy in Chinese Children by Affecting Relative Percentage of Treg and Th17 Cells. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 4561038. [Google Scholar] [CrossRef]
- Alcon-Giner, C.; Dalby, M.J.; Caim, S.; Ketskemety, J.; Shaw, A.; Sim, K.; Lawson, M.A.E.; Kiu, R.; Leclaire, C.; Chalklen, L.; et al. Microbiota Supplementation with Bifidobacterium and Lactobacillus Modifies the Preterm Infant Gut Microbiota and Metabolome: An Observational Study. Cell Rep. Med. 2020, 1, 100077. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Zhao, K.; Chen, H.; Wang, Y.; Wan, C. Effect of 3 lactobacilli on immunoregulation and intestinal microbiota in a β-lactoglobulin–induced allergic mouse model. J. Dairy Sci. 2019, 102, 1943–1958. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2015, 10, 742–750. [Google Scholar] [CrossRef]
- Kim, W.G.; Kang, G.D.; Kim, H.I.; Han, M.J.; Kim, D.H. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef. Microbes 2019, 10, 55–67. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Picchietti, S.; Fausto, A.M.; Randelli, E.; Carnevali, O.; Taddei, A.R.; Buonocore, F.; Scapigliati, G.; Abelli, L. Early treatment with Lactobacillus delbrueckii strain induces an increase in intestinal T-cells and granulocytes and modulates immune-related genes of larval Dicentrarchus labrax (L.). Fish Shellfish Immunol. 2009, 26, 368–376. [Google Scholar] [CrossRef] [PubMed]



| Score | Symptoms |
|---|---|
| 0 | No symptoms |
| 1 | Scratching nose and head |
| 2 | Swelling around the eyes and mouth; diarrhea; reduced or stationary activity; accelerating breathing |
| 3 | Blue rash around the mouth and tail; labored breathing |
| 4 | Loss of consciousness, tremors or seizures |
| 5 | Shocking and death |
| Groups (n = 6) | Score | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| C | 6 | 0 | 0 | 0 | 0 | 0 |
| SP | 0 | 1 | 5 | 0 | 0 | 0 |
| La | 4 | 2 | 0 | 0 | 0 | 0 |
| Lp | 2 | 4 | 0 | 0 | 0 | 0 |
| Ld | 3 | 3 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zhu, J.; Bai, J.; Zhang, J.; Wu, Z.; Li, X.; Tong, P.; Chen, H.; Yang, A. Effects of Lactobacillus on the Differentiation of Intestinal Mucosa Immune Cells and the Composition of Gut Microbiota in Soybean-Sensitized Mice. Foods 2023, 12, 627. https://doi.org/10.3390/foods12030627
Yang C, Zhu J, Bai J, Zhang J, Wu Z, Li X, Tong P, Chen H, Yang A. Effects of Lactobacillus on the Differentiation of Intestinal Mucosa Immune Cells and the Composition of Gut Microbiota in Soybean-Sensitized Mice. Foods. 2023; 12(3):627. https://doi.org/10.3390/foods12030627
Chicago/Turabian StyleYang, Chunhua, Jierui Zhu, Jing Bai, Jie Zhang, Zhihua Wu, Xin Li, Ping Tong, Hongbing Chen, and Anshu Yang. 2023. "Effects of Lactobacillus on the Differentiation of Intestinal Mucosa Immune Cells and the Composition of Gut Microbiota in Soybean-Sensitized Mice" Foods 12, no. 3: 627. https://doi.org/10.3390/foods12030627
APA StyleYang, C., Zhu, J., Bai, J., Zhang, J., Wu, Z., Li, X., Tong, P., Chen, H., & Yang, A. (2023). Effects of Lactobacillus on the Differentiation of Intestinal Mucosa Immune Cells and the Composition of Gut Microbiota in Soybean-Sensitized Mice. Foods, 12(3), 627. https://doi.org/10.3390/foods12030627







