Encapsulation of EGCG by Zein-Gum Arabic Complex Nanoparticles and In Vitro Simulated Digestion of Complex Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zein-GA-EGCG (E-Z/G) Samples
2.3. The Particle Size, Polydispersity Index (PDI) and Zeta Potential of Complex Nanoparticles
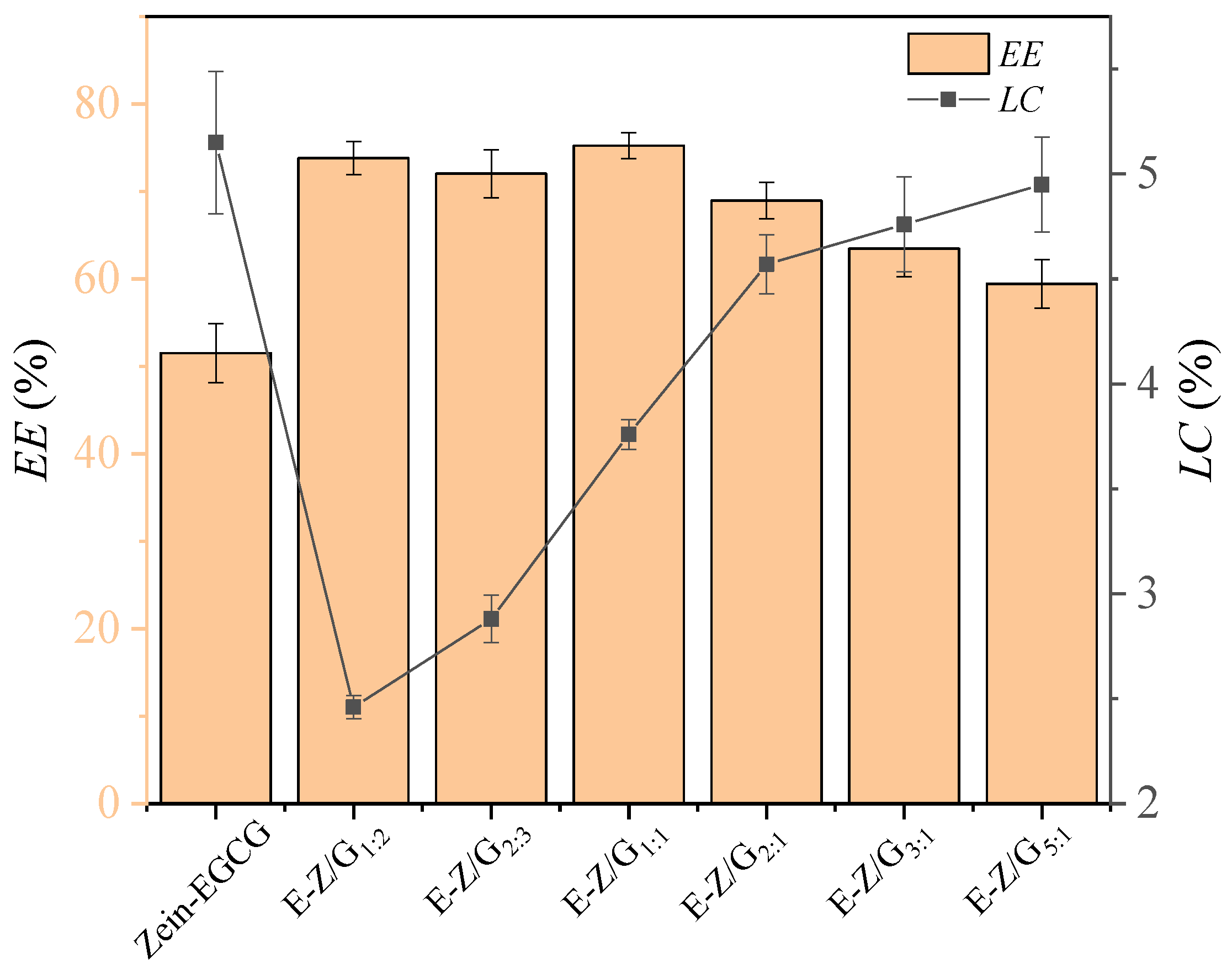
2.4. Encapsulation Efficiency (EE) and Loading Capacity (LC) Measurement
2.5. Morphology Characterization of Complex Nanoparticles
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. X-ray Diffraction (XRD) Test
2.8. Differential Scanning Calorimetry (DSC)
2.9. Antioxidant Activity Test
2.10. In Vitro Release Study
2.11. Statistical Analysis
3. Results and Discussion
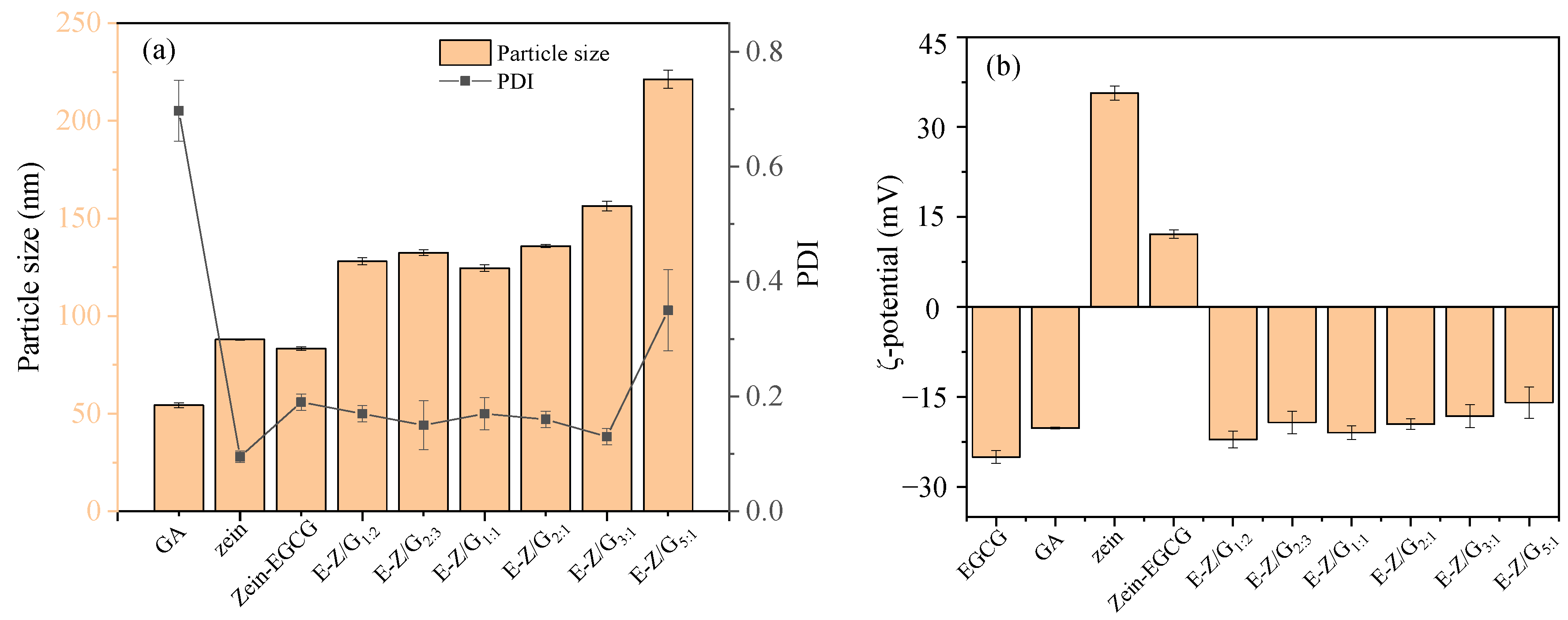
3.1. Analysis of Particle Size, Zeta Potential and Encapsulation Efficiency
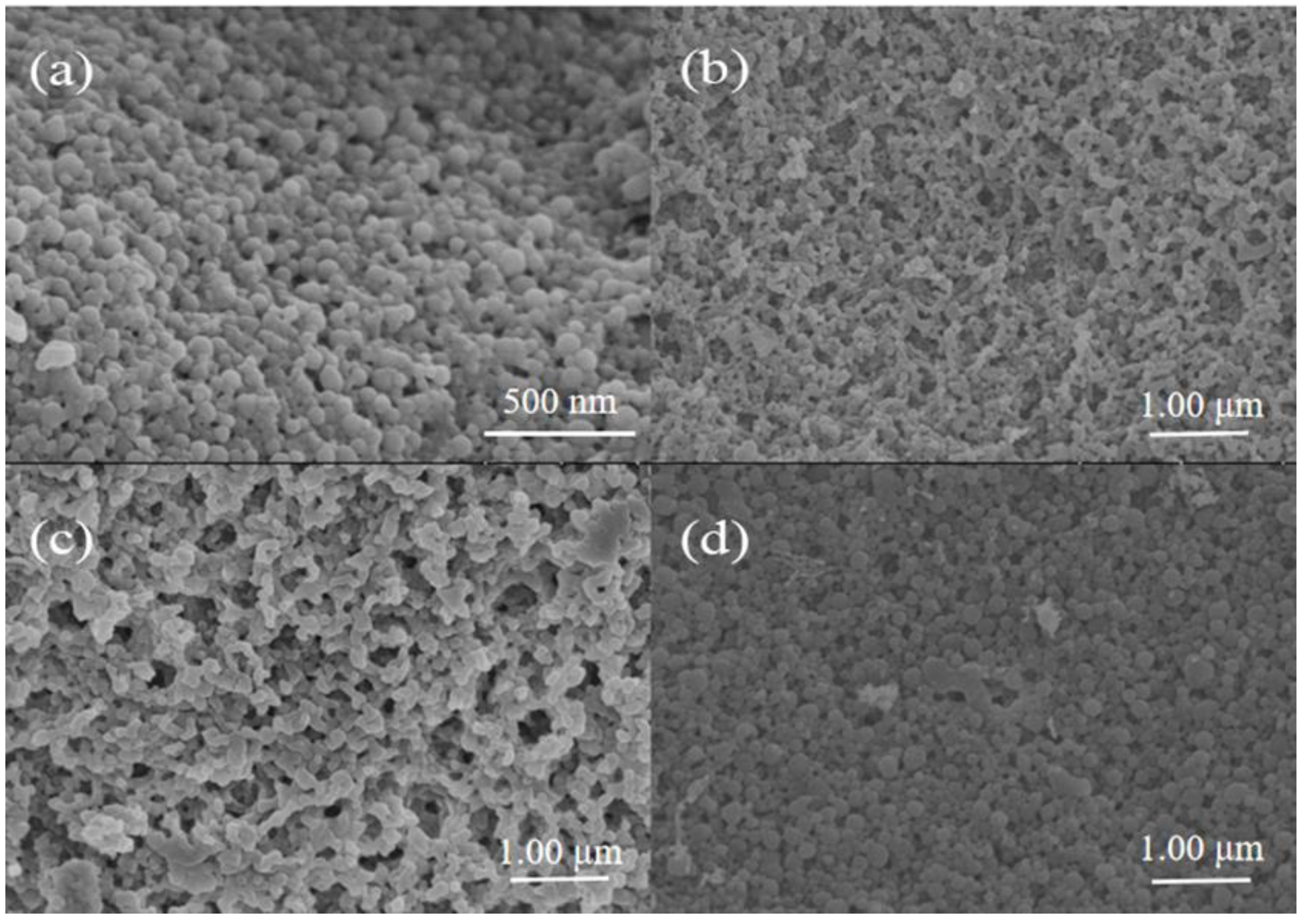
3.2. Microscopic Morphology Characterization
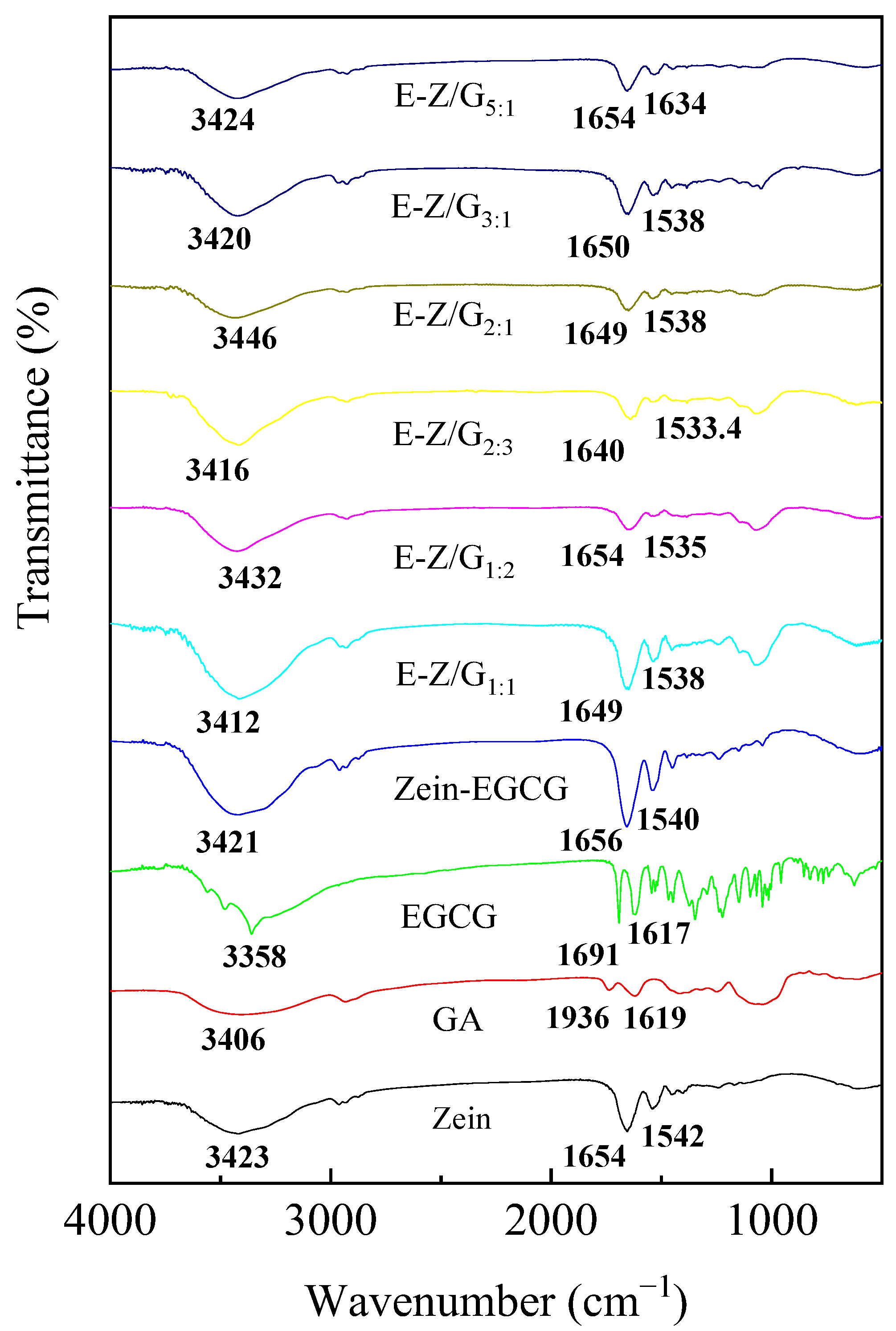
3.3. Analysis of FTIR
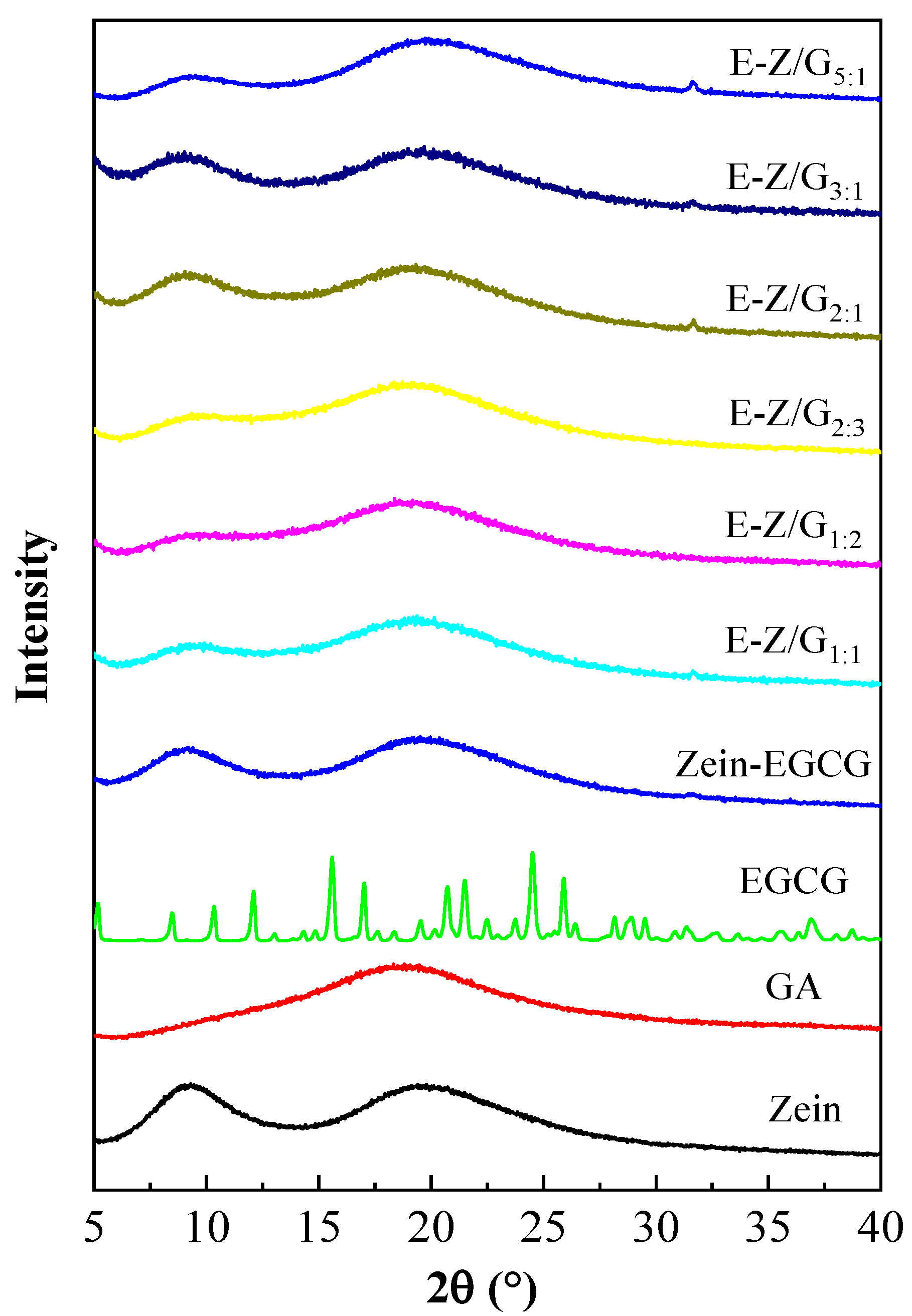
3.4. Analysis of XRD
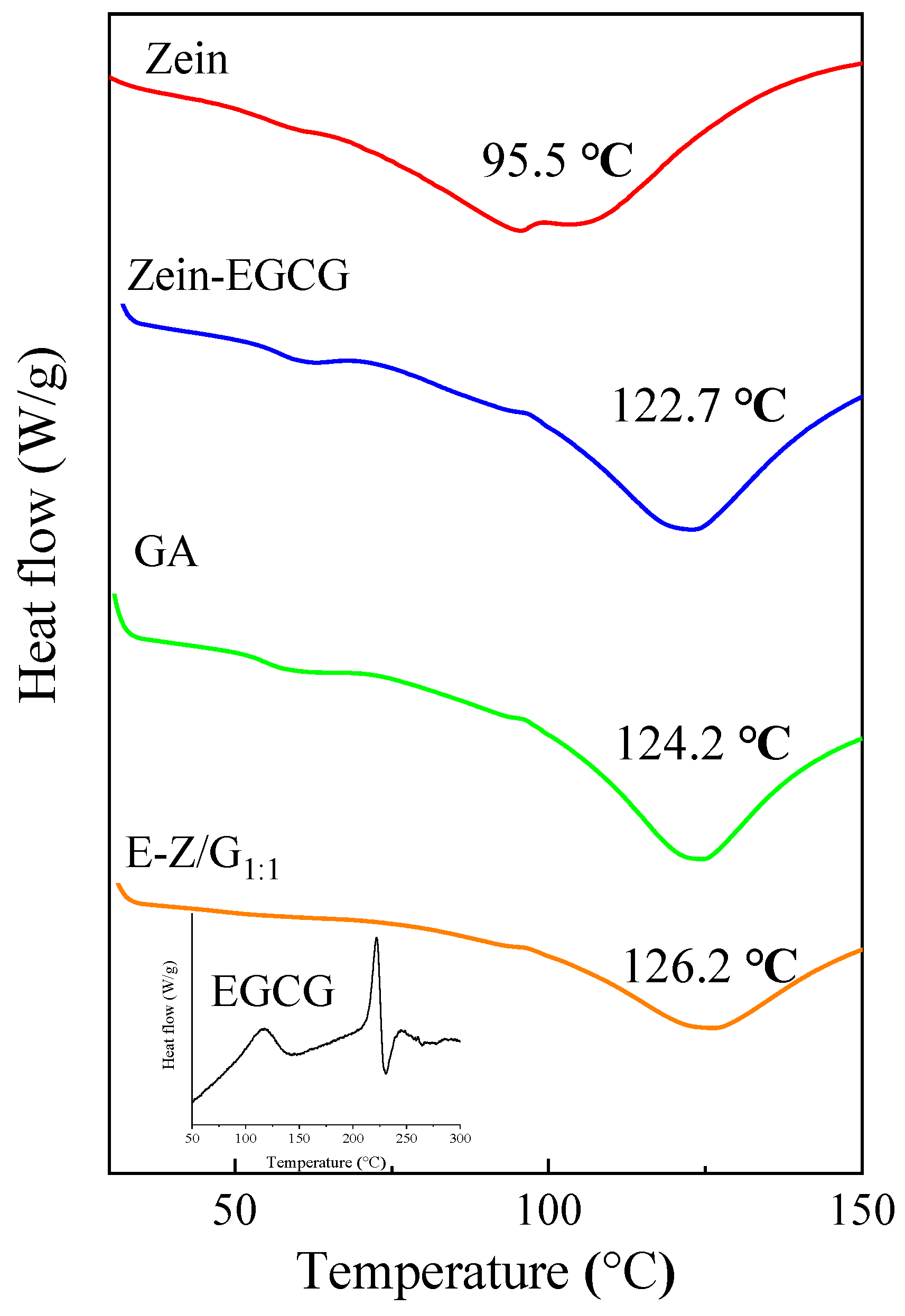
3.5. Analysis of DSC
3.6. Free Radical Scavenging Ability Analysis of DPPH and ABTS
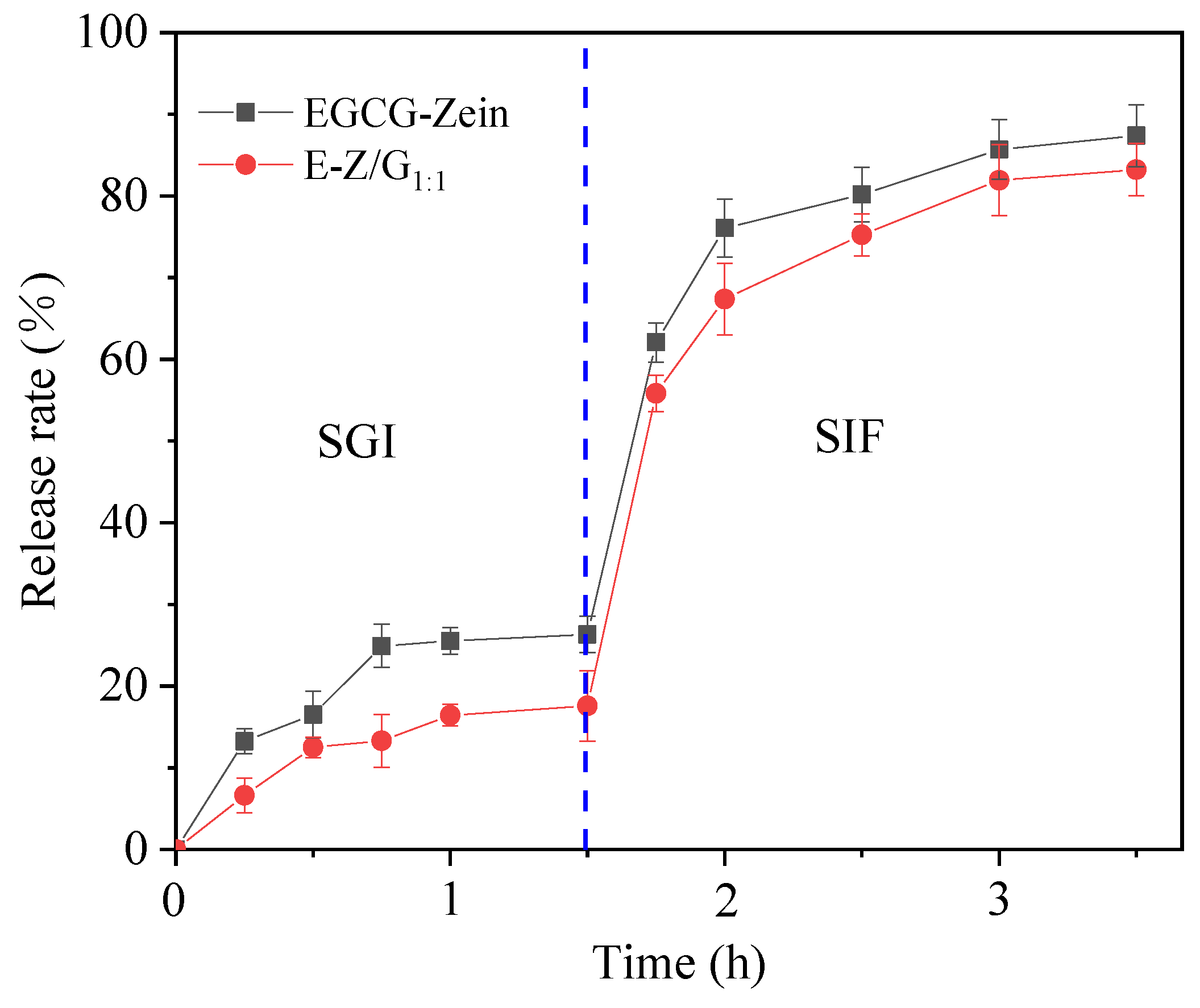
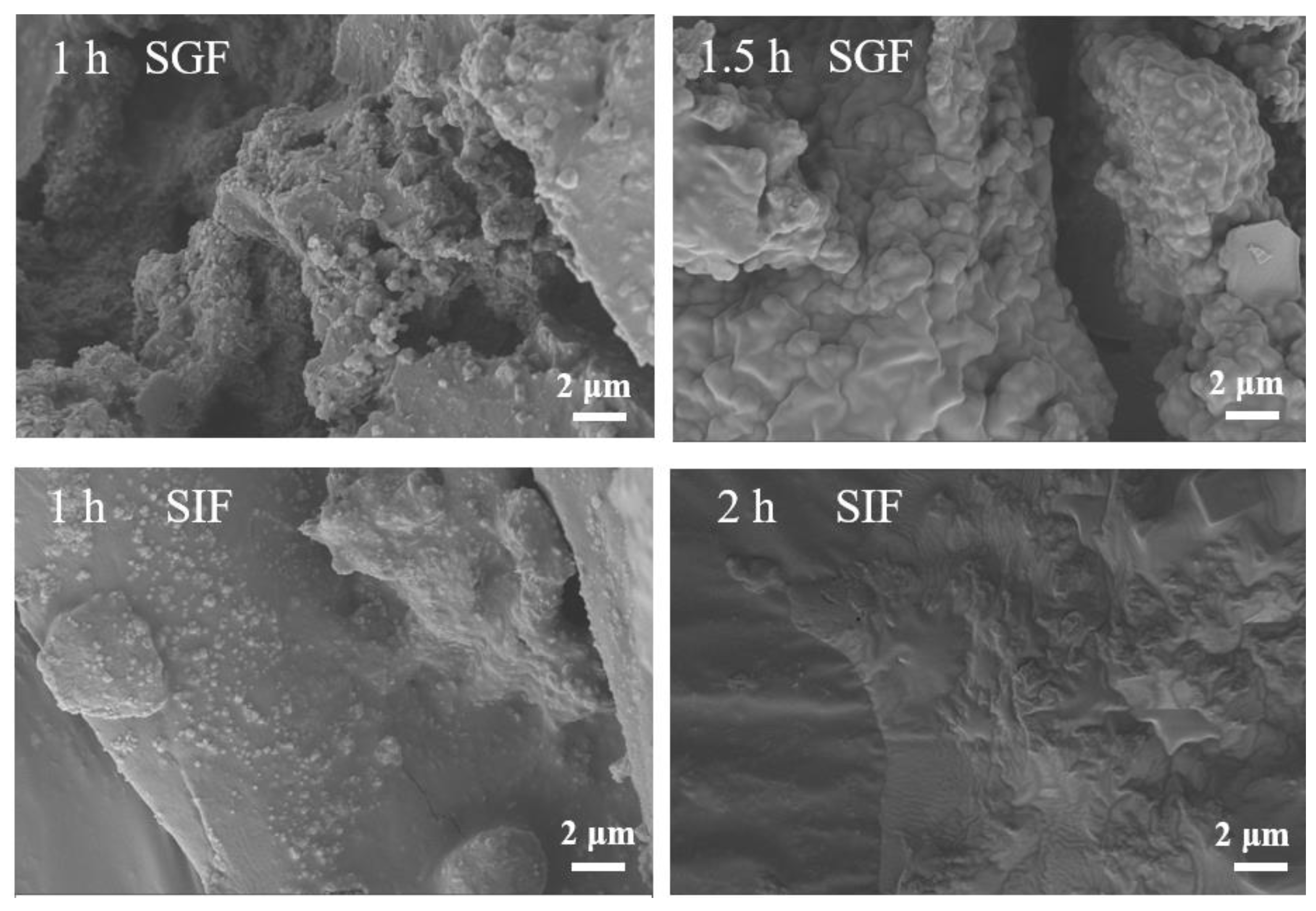
3.7. Release of EGCG during Simulated Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, G.J.; Wang, C.Z.; Qi, L.W.; Zhang, Z.Y.; Calway, T.; He, T.C.; Du, W.; Yuan, C.S. The synergistic apoptotic interaction of panaxadiol and epigallocatechin gallate in human colorectal cancer cells. Phytother. Res. 2013, 27, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.Z.; Lv, N.; Ren, G.R.; Wu, R.B.; Wang, B.J.; Cao, Z.X.; Xie, H.J. Explore the interaction mechanism between zein and EGCG using multi-spectroscopy and molecular dynamics simulation methods. Food Hydrocoll. 2021, 120, 106906. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Naghma, K.; Hasan, M. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar]
- Hirota, F.; Masami, S. Green tea: An effective synergist with anticancer drugs for tertiary cancer prevention. Cancer Lett. 2012, 324, 119–125. [Google Scholar]
- Zhang, S.; Zhu, Q.; Chen, J.Y.; OuYang, D.F.; Lu, J.H. The pharmacological activity of epigallocatechin-3-gallate (EGCG) on alzheimer’s disease animal model: A systematic review. Phytomedicine 2020, 79, 153316. [Google Scholar] [CrossRef]
- Zimeri, J.; Tong, C.H. Degradation kinetics of (−)-epigallocatechin gallate as a function of pH and dissolved oxygen in a liquid model system. J. Food Sci. 1999, 64, 753–758. [Google Scholar] [CrossRef]
- Zou, L.Q.; Peng, S.F.; Liu, W.; Gan, L.; Liu, W.L.; Liang, R.H.; Liu, C.M.; Niu, J.; Cao, Y.L.; Liu, Z. Improved in vitro digestion stability of (−)-epigallocatechin gallate through nanoliposome encapsulation. Food Res. Int. 2014, 64, 492–499. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, J.; Yang, H.N.; Zhang, B.L.; Zhang, P.; Sun, P.Y.; Zhang, N.; Wang, Y.; Sheng, J.; Wang, X.J. The oxidation of (−)-epigallocatechin-3-gallate inhibits T-cell acute lymphoblastic leukemia cell line HPB-ALL via the regulation of Notch1 expression. RSC Adv. 2020, 10, 1679–1684. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between β-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.; Chen, D.; Foldes, R.; Chan, T.H. Novel epigallocatechin gallate analogs as potential anticancer agents: A patent review (2009-present). Expert Opin. Ther. Pat. 2013, 23, 189–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülseren, I.; Guri, A.; Corredig, M. Encapsulation of tea polyphenols in nanoliposomes prepared with milk phospholipids and their effect on the viability of HT-29 human carcinoma cells. Food Dig. 2012, 3, 36–45. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Zhang, J.Y.; Dai, W.Z.; Gao, X.L.; Zhou, Y.B.; Wan, X.C.; Puligundla, P. Preparation and characterization of antioxidant edible chitosan films incorporated with epigallocatechin gallate nanocapsules. Carbohydr. Polym. 2017, 171, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.Q.; Deng, S.M.; Liu, W.; Yan, C.; Zhu, Y.Q.; Cheng, C.; Liu, C.M. Coencapsulation of (−)-epigallocatechin-3-gallate and quercetin in particle-stabilized W/O/W emulsion gels: Controlled release and bioaccessibility. J. Agric. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef]
- Feng, X.; Sun, Y.J.; Yang, Y.Y.; Zhou, X.; Cen, K.Y.; Yu, C.; Xu, T.; Tang, X.Z. Zein nanoparticle stabilized Pickering emulsion enriched with cinnamon oil and its effects on pound cakes. LWT 2020, 122, 109025. [Google Scholar] [CrossRef]
- Gali, L.; Bedjou, F.; Ferrari, G.; Donsì, F. Formulation and characterization of zein/gum Arabic nanoparticles for the encapsulation of a rutin-rich extract from Ruta chalepensis L. Food Chem. 2022, 367, 129982. [Google Scholar] [CrossRef]
- Liang, H.S.; Zhou, B.; He, L.; An, Y.P.; Lin, L.F.; Li, Y.; Liu, S.L.; Chen, Y.J.; Li, B. Fabrication of zein/quaternized chitosan nanoparticles for the encapsulation and protection of curcumin. RSC Adv. 2015, 5, 13891–13900. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, Y.Y.; Xu, Y.J.; Niu, F.G.; Li, Z.Y.; Ba, C.J.; Jin, B.; Chen, G.W.; Li, X.M. One-step assembly of zein/caseinate/alginate nanoparticles for encapsulation and improved bioaccessibility of propolis. Food Funct. 2019, 10, 635–645. [Google Scholar] [CrossRef]
- Kong, H.L.; Yang, J.X.; Zhang, Y.F.; Fang, Y.P.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liang, Q.F.; Liu, X.Q.; Raza, H.; Ma, H.L.; Ren, X.F. Treatment with ultrasound improves the encapsulation efficiency of resveratrol in zein-gum Arabic complex coacervates. LWT 2022, 153, 112331. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, G.Q.; Ren, F.Z.; Liu, N.; Tong, Y.; Li, Y.; Liu, A.N.; Wu, L.D.; Wang, P.J. Delivery of curcumin using Zein-Gum Arabic-Tannic Acid composite particles: Fabrication, characterization, and in vitro release properties. Front. Nutr. 2022, 9, 842850. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Liu, Y.X.; Gao, L.Y.; Yi, J.; Fan, Y.T.; Wu, X.L.; Zhang, Y.Z. Alpha-Lactalbumin and chitosan core-shell nanoparticles: Resveratrol loading, protection, and antioxidant activity. Food Funct. 2020, 11, 1525–1536. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Chen, J.J.; Qin, Y.; Jiang, B.; Zhang, T. Zein/fucoidan-based composite nanoparticles for the encapsulation of pterostilbene: Preparation, characterization, physicochemical stability, and formation mechanism. Int. J. Biol. Macromol. 2020, 158, 461–470. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.E.; Chen, Z.X.; Wang, T.; Wang, L.; Zhong, Q.X. Biological macromolecule delivery system fabricated using zein and gum arabic to control the release rate of encapsulated tocopherol during in vitro digestion. Food Res. Int. 2018, 114, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, C.X.; Wang, Y.Q.; Han, Y.H.; Dai, L.; Abliz, A.; Gao, Y.X. Quercetagetin-Loaded composite nanoparticles based on zein and hyaluronic acid: Formation, characterization, and physicochemical stability. J. Agric. Food Chem. 2018, 66, 7441–7450. [Google Scholar] [CrossRef]
- Zhang, S.L.; Jiang, W.P.; Zhang, Z.W.; Zhu, Y.L.; Wang, L.X.; Fu, J.J. A nanoparticle/oil double epigallocatechin gallate-loaded Pickering emulsion: Stable and delivery characteristics. LWT 2020, 130, 109369. [Google Scholar] [CrossRef]
- Donsi, F.; Voudouris, P.; Veen, S.J.; Velikov, K.P. Zein-based colloidal particles for encapsulation epigallocatechin gallate and delivery of epigallocatechin gallate. Food Hydrocoll. 2017, 63, 508–517. [Google Scholar] [CrossRef]
- Poureini, F.; Najafpour, G.D.; Nikzad, M.; Najafzadehvarzi, H.; Mohammadi, M. Loading of apigenin extracted from parsley leaves on colloidal core-shell nanocomposite for bioavailability enhancement. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126867. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.X.; Li, R.R.; Mao, L.K.; Liu, F.G.; Gao, Y.X. Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Huang, X.X.; Gao, Y.Q.; Huang, X.L.; Xiao, H.; McClements, D.J. Core-shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein-pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Su, J.Q.; Shu, X.; Yuan, F.; Mao, L.K.; Liu, J.F.; Gao, Y.X. Production and characterization of pea protein isolate-pectin complexes for delivery of curcumin: Effect of esterified degree of pectin. Food Hydrocoll. 2020, 105, 105777. [Google Scholar] [CrossRef]
- Wang, K.; Wu, K.; Xiao, M.; Kuang, Y.; Corke, H.; Ni, X.W.; Jiang, F.T. Structural characterization and properties of konjac glucomannan and zein blend films. Int. J. Biol. Macromol. 2017, 105, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.J.; Garcia-Rojas, E.E. Effects of salt and protein concentrations on the association and dissociation of ovalbumin-pectin complexes. Food Hydrocoll. 2015, 47, 124–129. [Google Scholar] [CrossRef]
- Istenič, K.; Cerc Korošec, R.; Poklar Ulrih, N. Encapsulation of (−)-epigallocatechin gallate into liposomes and into alginate or chitosan microparticles reinforced with liposomes. J. Sci. Food Agric. 2016, 96, 4623–4632. [Google Scholar] [CrossRef]
- Li, L.; Yao, P. High dispersity, stability and bioaccessibility of curcumin by assembling with deamidated zein peptide. Food Chem. 2020, 319, 126577. [Google Scholar] [CrossRef]
- Waku, T.; Terasawa, K.; Takimoto, K.; Ichikawa, M.; Handa, A.; Tanaka, N. Nanoparticle formation of ovalbumin with cationic oligopeptides. NLM 2017, 74, 285–292. [Google Scholar]
- Sekowski, S.; Terebka, M.; Veiko, A.; Lapshina, E.; Sulkowska, U.; Zavodnik, I.B.; Abdulladjanova, N.; Mavlyanov, S.; Roszkowska, A.; Zamaraeva, M. Epigallocatechin gallate (EGCG) activity against UV light-induced photo damages in erythrocytes and serum albumin-theoretical and experimental studies. JPPA 2018, 356, 379–388. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Yu, P.G.; Zhou, W.B. Combined effect of pH and temperature on the stability and antioxidant capacity of epigallocatechin gallate (EGCG) in aqueous system. J. Food Eng. 2019, 250, 46–54. [Google Scholar] [CrossRef]
- Chen, W.J.; Zou, M.M.; Ma, X.B.; Lv, R.L.; Ding, T.; Liu, D.H. Co-Encapsulation of EGCG and quercetin in liposomes for optimum antioxidant activity. J. Food Sci. 2019, 84, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.M.; Park, J.; Cho, J.Y. Skin protective effect of epigallocatechin gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.Q.; Guo, Q.; Chen, Y.L.; Mao, L.K.; Gao, Y.X.; Yuan, F. Utilization of β-lactoglobulin Epigallocatechin gallate (EGCG) composite colloidal nanoparticles as stabilizers for lutein pickering emulsion. Food Hydrocoll. 2020, 98, 105293. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Li, D.; Tang, H.L.; Yu, D.Y.; Wang, L.Q.; Jiang, L.Z. Effect of anionic polysaccharides on conformational changes and antioxidant properties of protein-polyphenol binary covalently-linked complexes. Process. Biochem. 2020, 89, 89–97. [Google Scholar] [CrossRef]
- Gao, J.; Mao, Y.Z.; Xiang, C.Y.; Cao, M.N.; Ren, G.R.; Wang, K.W.; Ma, X.J.; Wu, D.; Xie, H.J. Preparation of β-lactoglobulin/gum arabic complex nanoparticles for encapsulation and controlled release of EGCG in simulated gastrointestinal digestion model. Food Chem. 2021, 354, 129516. [Google Scholar] [CrossRef]
- Gao, J.; Liu, C.Z.; Shi, J.Y.; Ni, F.F.; Shen, Q.; Xie, H.J.; Wang, K.W.; Lei, Q.F.; Fang, W.J.; Ren, G.R. The regulation of sodium alginate on the stability of ovalbumin-pectin complexes for VD3 encapsulation and in vitro simulated gastrointestinal digestion study. Food Res. Int. 2021, 140, 110011. [Google Scholar] [CrossRef]
- Xiang, C.Y.; Gao, J.; Ye, H.X.; Ren, G.R.; Ma, X.J.; Xie, H.J.; Fang, S.; Lei, Q.F.; Fang, W.J. Development of ovalbumin-pectin nanocomplexes for vitamin D3 encapsulation: Enhanced storage stability and sustained release in simulated gastrointestinal digestion. Food Hydrocoll. 2020, 106, 105926. [Google Scholar] [CrossRef]
- Chang, Y.; Jiao, Y.; Li, D.J.; Liu, X.L.; Han, H. Glycosylated zein as a novel nanodelivery vehicle for lutein. Food Chem. 2021, 376, 131927. [Google Scholar] [CrossRef]
- Xie, H.J.; Liu, C.Z.; Gao, J.; Shi, J.Y.; Ni, F.F.; Luo, X.; He, Y.; Ren, G.R.; Luo, Z.S. Fabrication of Zein-Lecithin-EGCG complex nanoparticles: Characterization, controlled release in simulated gastrointestinal digestion. Food Chem. 2021, 365, 130542. [Google Scholar] [CrossRef]
- Li, Z.P.; Lin, Q.Q.; McClements, D.J.; Fu, Y.Y.; Xie, H.J.; Li, T.; Chen, G.W. Curcumin-loaded core-shell biopolymer nanoparticles produced by the pH-driven method: Physicochemical and release properties. Food Chem. 2021, 355, 129686. [Google Scholar] [CrossRef]
- Huang, W.N.; Wang, L.H.; Wei, Y.Q.; Cao, M.N.; Xie, H.J.; Wu, D. Fabrication of lysozyme/κ-carrageenan complex nanoparticles as a novel carrier to enhance the stability and in vitro release of curcumin. Int. J. Biol. Macrmol. 2020, 146, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.R.; He, Y.; Liu, C.Z.; Ni, F.F.; Luo, X.; Shi, J.Y.; Song, Y.L.; Li, T.; Huang, M.; Shen, Q.; et al. Encapsulation of curcumin in ZEIN-HTCC complexes: Physicochemical characterization, in vitro sustained release behavior and encapsulation mechanism. LWT 2022, 155, 112909. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Liu, C.; Tong, H.; Sun, Y.; Huang, M.; Ren, G.; Xie, H. Encapsulation of EGCG by Zein-Gum Arabic Complex Nanoparticles and In Vitro Simulated Digestion of Complex Nanoparticles. Foods 2022, 11, 2131. https://doi.org/10.3390/foods11142131
Jin J, Liu C, Tong H, Sun Y, Huang M, Ren G, Xie H. Encapsulation of EGCG by Zein-Gum Arabic Complex Nanoparticles and In Vitro Simulated Digestion of Complex Nanoparticles. Foods. 2022; 11(14):2131. https://doi.org/10.3390/foods11142131
Chicago/Turabian StyleJin, Jianchang, Chengzhi Liu, Huafei Tong, Yulu Sun, Min Huang, Gerui Ren, and Hujun Xie. 2022. "Encapsulation of EGCG by Zein-Gum Arabic Complex Nanoparticles and In Vitro Simulated Digestion of Complex Nanoparticles" Foods 11, no. 14: 2131. https://doi.org/10.3390/foods11142131
APA StyleJin, J., Liu, C., Tong, H., Sun, Y., Huang, M., Ren, G., & Xie, H. (2022). Encapsulation of EGCG by Zein-Gum Arabic Complex Nanoparticles and In Vitro Simulated Digestion of Complex Nanoparticles. Foods, 11(14), 2131. https://doi.org/10.3390/foods11142131




