Chemical Authentication and Speciation of Salvia Botanicals: An Investigation Utilizing GC/Q-ToF and Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Sample Preparation
2.4. GC/Q-ToF Analysis
2.5. Data Processing and Statistical Analysis
2.6. Establishment of a Personal Compound Database and Library (PCDL)
3. Results and Discussion
3.1. Extraction
3.2. GC/Q-ToF Analysis
3.3. Chemometric Analysis
3.4. Construction of a Personal Compound Database and Library (PCDL) for High-Throughput Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, S.E. Sage: The Genus Salvia; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Chun-Yan, S.; Qian-Liang, M.; Rahman, K.; Ting, H.; Lu-Ping, Q. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complementary Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Topçu, G. Bioactive triterpenoids from Salvia species. J. Nat. Prod. 2006, 69, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Bae, J.-Y.; Chittiboyina, A.G.; Wang, Y.-H.; Wang, M.; Srivedavyasasri, R.; Ali, Z.; Li, J.; Wu, C.; Khan, I.A. Comparative analysis of five Salvia species using LC-DAD-QToF. J. Pharm. Biomed. Anal. 2022, 209, 114520. [Google Scholar] [CrossRef]
- Krol, A.; Kokotkiewicz, A.; Luczkiewicz, M. White Sage (Salvia apiana)—A Ritual and Medicinal Plant of the Chaparral: Plant Characteristics in Comparison with Other Salvia Species. Planta Med. 2021, 88, 604–627. [Google Scholar] [CrossRef]
- Ali, Z.; Radhakrishnan, S.; Avula, B.; Chittiboyina, A.G.; Li, J.; Wu, C.; Khan, I.A. Eupatorin 3′-O-glucopyranoside, a trimethoxyflavonoid glucoside from the aerial parts of Salvia mellifera. Nat. Prod. Res. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.-H.; Jiang, C.; Lü, J. Tanshinones: Sources, pharmacokinetics and anti-cancer activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef] [Green Version]
- Benoni, H. Salvinorin A—A hallucinogen from Aztec sage. Nat. Rundsch 2001, 54, 575–578. [Google Scholar]
- Jermain, J.D.; Evans, H.K. Analyzing Salvia divinorum and its active ingredient salvinorin A utilizing thin layer chromatography and gas chromatography/mass spectrometry. J. Forensic Sci. 2009, 54, 612–616. [Google Scholar] [CrossRef]
- Cao, J.-L.; Wei, J.-C.; Hu, Y.-J.; He, C.-W.; Chen, M.-W.; Wan, J.-B.; Li, P. Qualitative and quantitative characterization of phenolic and diterpenoid constituents in Danshen (Salvia miltiorrhiza) by comprehensive two-dimensional liquid chromatography coupled with hybrid linear ion trap Orbitrap mass. J. Chromatogr. A 2016, 1427, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Dal Piaz, F.; Imparato, S.; Lepore, L.; Bader, A.; de Tommasi, N. A fast and efficient LC–MS/MS method for detection, identification and quantitative analysis of bioactive sesterterpenes in Salvia dominica crude extracts. J. Pharm. Biomed. Anal. 2010, 51, 70–77. [Google Scholar] [CrossRef]
- Gericke, S.; Lübken, T.; Wolf, D.; Kaiser, M.; Hannig, C.; Speer, K. Identification of new compounds from sage flowers (Salvia officinalis L.) as markers for quality control and the influence of the manufacturing technology on the chemical composition and antibacterial activity of sage flower extracts. J. Agric. Food Chem. 2018, 66, 1843–1853. [Google Scholar] [CrossRef]
- Haq, F.U.; Ali, A.; Akhtar, N.; Aziz, N.; Khan, M.N.; Ahmad, M.; Musharraf, S.G. A high-throughput method for dereplication and assessment of metabolite distribution in Salvia species using LC-MS/MS. J. Adv. Res. 2020, 24, 79–90. [Google Scholar] [CrossRef]
- Hu, P.; Liang, Q.-L.; Luo, G.-A.; Zhao, Z.-Z.; Jiang, Z.-H. Multi-component HPLC fingerprinting of Radix Salviae Miltiorrhizae and its LC-MS-MS identification. Chem. Pharm. Bull. 2005, 53, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhang, C.; Peng, L.; Liang, Z.; Yan, X.; Zhu, Y.; Liu, Y. Comparison of essential oil composition and phenolic acid content of selected Salvia species measured by GC–MS and HPLC methods. Ind. Crops Prod. 2015, 69, 329–334. [Google Scholar] [CrossRef]
- Qiao, X.; Zhang, Y.-T.; Ye, M.; Wang, B.-R.; Han, J.; Guo, D.-a. Analysis of chemical constituents and taxonomic similarity of Salvia species in China using LC/MS. Planta Med. 2009, 75, 1613–1617. [Google Scholar] [CrossRef]
- Shi, Z.; He, J.; Yao, T.; Chang, W.; Zhao, M. Simultaneous determination of cryptotanshinone, tanshinone I and tanshinone IIA in traditional Chinese medicinal preparations containing Radix salvia miltiorrhiza by HPLC. J. Pharm. Biomed. Anal. 2005, 37, 481–486. [Google Scholar] [CrossRef]
- Kintzios, S.E. The biological/pharmacological activity of the Salvia genus. In Sage: The Genus Salvia; CRC Press: Boca Raton, FL, USA, 2003; pp. 161–198. [Google Scholar]
- Lou, J.; Mao, Z.; Shan, T.; Wang, Q.; Zhou, L. Chemical composition, antibacterial and antioxidant properties of the essential oils from the roots and cultures of Salvia miltiorrhiza. J. Essent. Oil Bear. Plants 2014, 17, 380–384. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, H.-x.; Wang, L.; Wang, T.; Yang, R.-w.; Wang, X.-l.; Zhou, Y.-h.; Ding, C.-b.; Zhang, L. Potential use of DNA barcoding for the identification of Salvia based on cpDNA and nrDNA sequences. Gene 2013, 528, 206–215. [Google Scholar] [CrossRef]
- Ciesla, Ł.; Hajnos, M.; Staszek, D.; Wojtal, Ł.; Kowalska, T.; Waksmundzka-Hajnos, M. Validated binary high-performance thin-layer chromatographic fingerprints of polyphenolics for distinguishing different Salvia species. J. Chromatogr. Sci. 2010, 48, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzepa, J.; Wojtal, Ł.; Staszek, D.; Grygierczyk, G.; Labe, K.; Hajnos, M.; Kowalska, T.; Waksmundzka-Hajnos, M. Fingerprint of selected Salvia species by HS-GC-MS analysis of their volatile fraction. J. Chromatogr. Sci. 2009, 47, 575–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willard, M.A.B.; McGuffin, V.L.; Smith, R.W. Forensic analysis of Salvia divinorum using multivariate statistical procedures. Part I: Discrimination from related Salvia species. Anal. Bioanal. Chem. 2012, 402, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Trevizan, L.N.F.; do Nascimento, K.F.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; do Carmo Vieira, M.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016, 192, 510–515. [Google Scholar]
- Ali, A.; Tabanca, N.; Demirci, B.; Blythe, E.K.; Ali, Z.; Baser, K.H.C.; Khan, I.A. Chemical composition and biological activity of four salvia essential oils and individual compounds against two species of mosquitoes. J. Agric. Food Chem. 2015, 63, 447–456. [Google Scholar] [CrossRef]
- Martino, L.D.; Roscigno, G.; Mancini, E.; Falco, E.D.; Feo, V.D. Chemical composition and antigerminative activity of the essential oils from five Salvia species. Molecules 2010, 15, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Agilent. Agilent Mass Profiler Professional Operation for Chemometric Analysis, SW-MPP-3101c; Agilent Technologies: Lexington, MA, USA, 2019. [Google Scholar]
- Wylie, P.L.; Westland, J.; Wang, M.; Radwan, M.M.; Majumdar, C.G.; ElSohly, M.A. Screening for More than 1,000 Pesticides and Environmental Contaminants in Cannabis by GC/Q-TOF. Med. Cannabis Cannabinoids 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
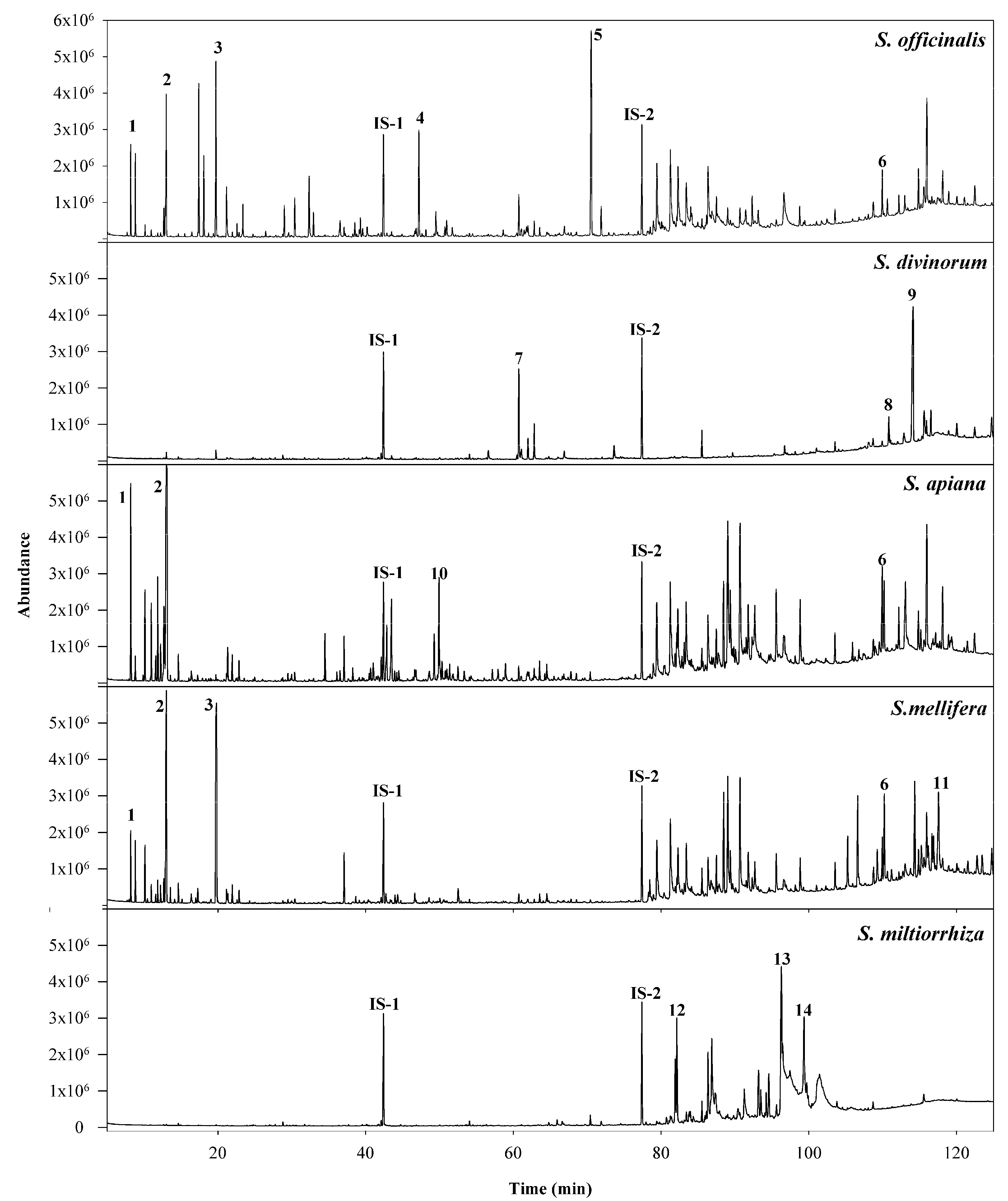
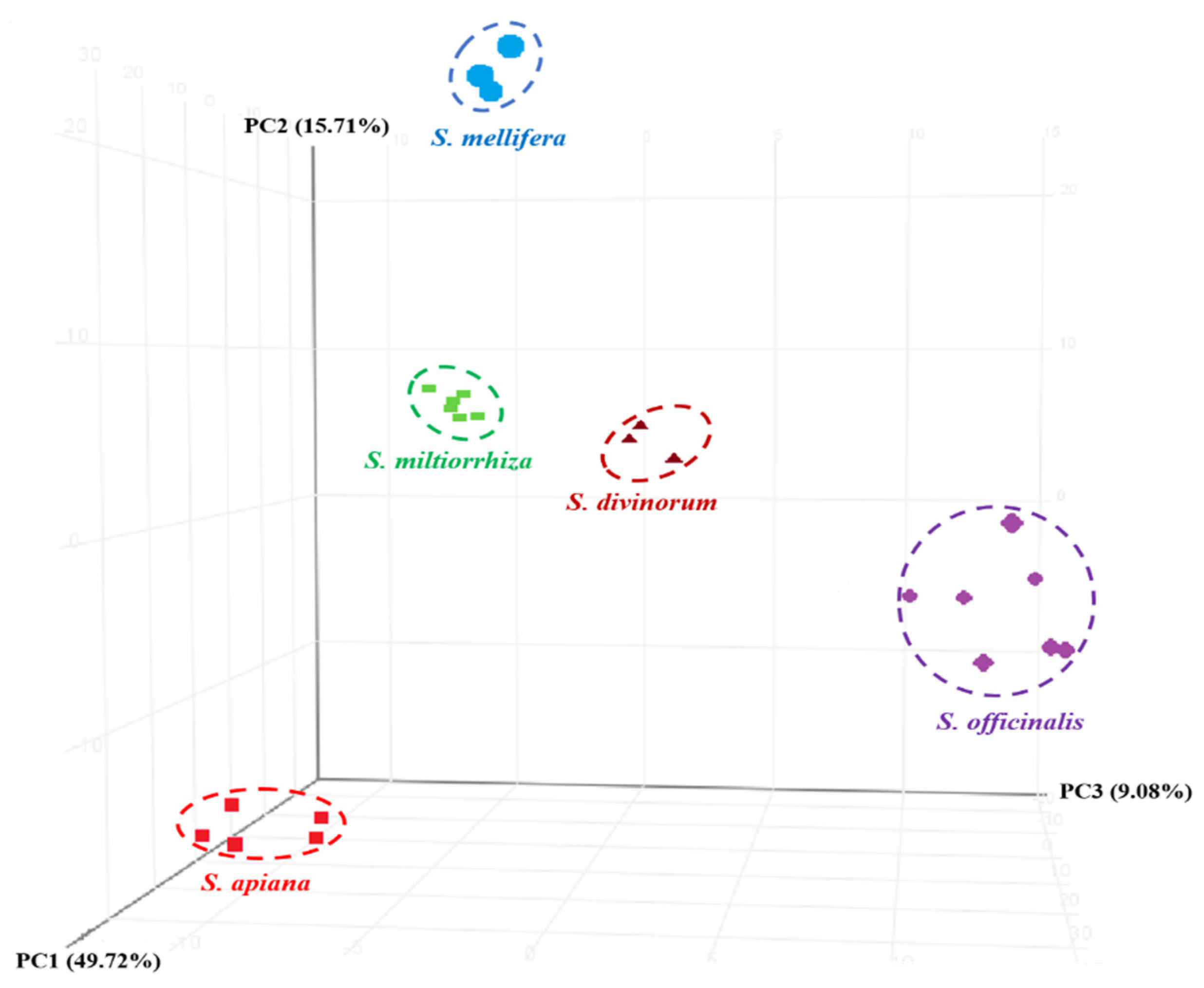
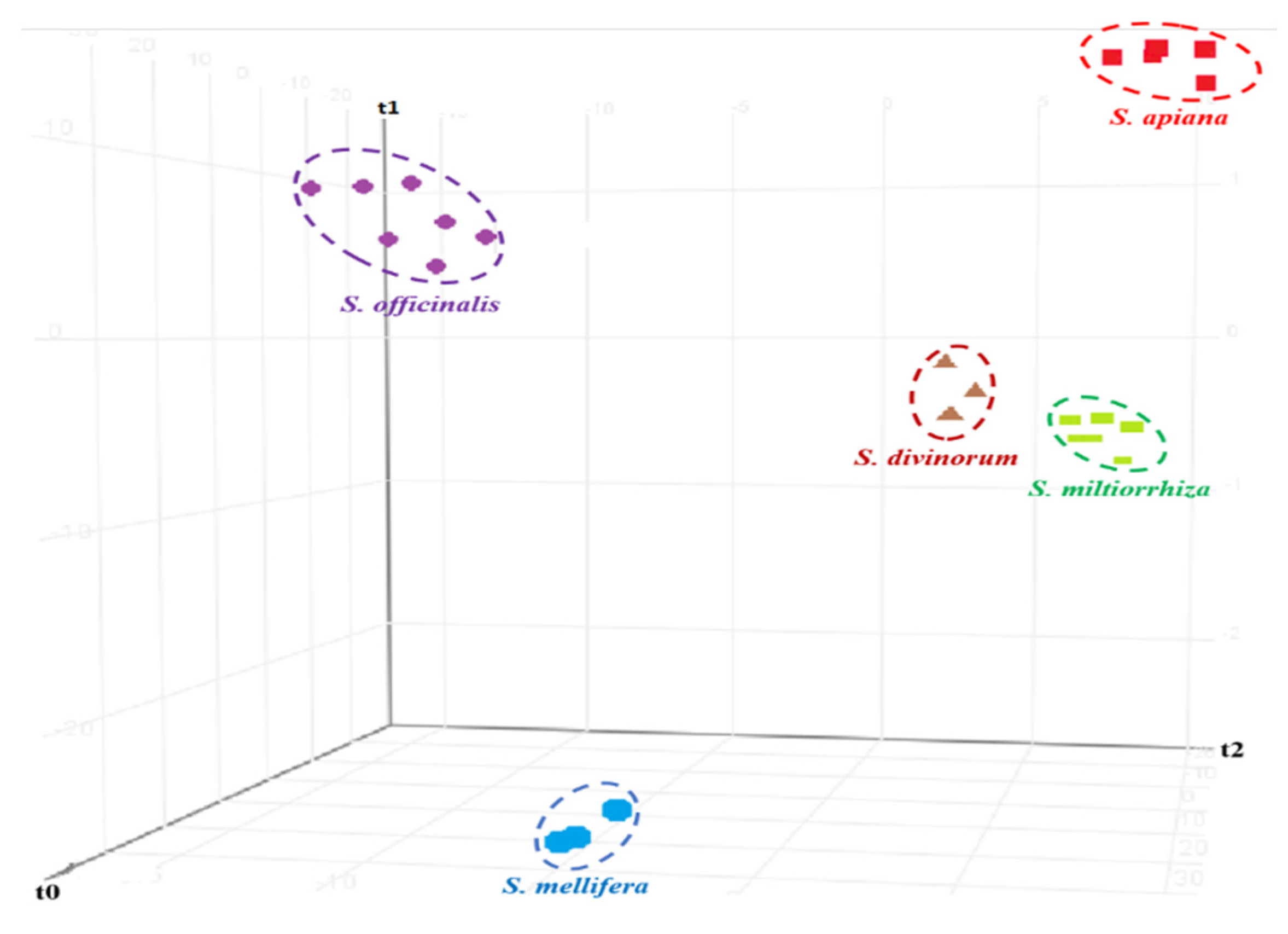
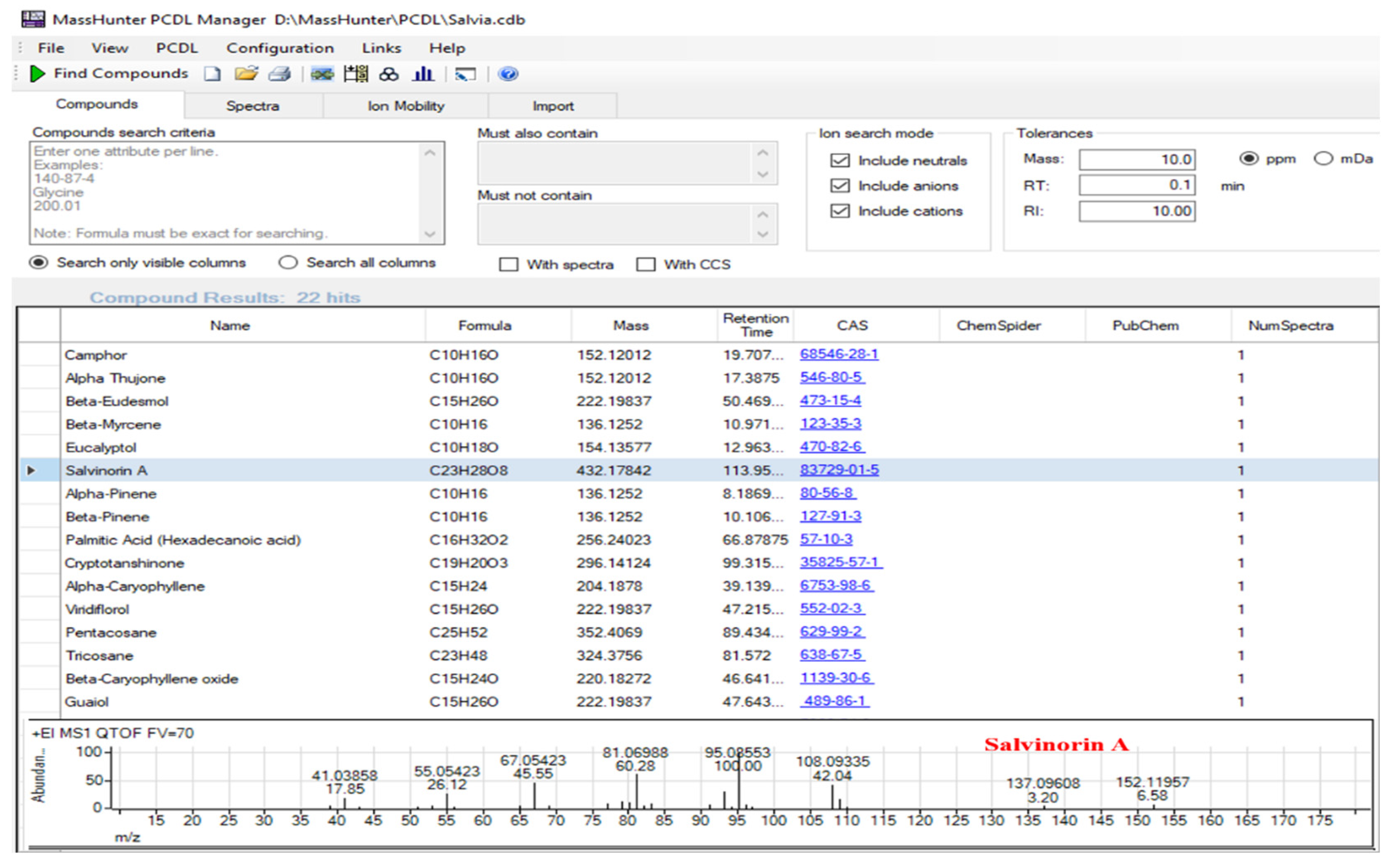
| No. | NCNPR Code | Part | Botanical Name |
|---|---|---|---|
| 1 | 1523 | Leaf | Salvia officinalis |
| 2 | 2852 | Leaf | |
| 3 | 7686 | Mixed Parts | |
| 4 | 7917 | - | |
| 5 | 13095 | Leaf | |
| 6 | 16732 | Leaf | |
| 7 | 20712 | - | |
| 8 | 13096 | Leaf | Salvia apiana |
| 9 | 22497 | Aerial | |
| 10 | 22498 | Aerial | |
| 11 | 22502 | Aerial | |
| 12 | 22773 | Leaf | |
| 13 | 578 | Leaf | Salvia divinorum |
| 14 | 18434 | Aerial | |
| 15 | 22491 | Leaf | |
| 16 | 22506 | Aerial | Salvia mellifera |
| 17 | 22771 | Leaf | |
| 18 | 22772 | Leaf | |
| 19 | 767 | Root | Salvia miltiorrhiza |
| 20 | 5399 | Root | |
| 21 | 8676 | Root | |
| 22 | 9729 | Root | |
| 23 | 11750 | Root | |
| 24 | 12535 | Root |
| A | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | tR (min) | S. officinalis | S. apiana | ||||||||||
| 1523 | 2852 | 7686 | 7917 | 13095 | 16732 | 20712 | 13096 | 22497 | 22498 | 22502 | 22773 | ||
| α-Pinene a | 8.194 | 0.11 | 0.20 | 1.57 | tr | 1.91 | 0.54 | tr | 3.52 | 1.43 | 1.25 | 2.25 | 0.09 |
| Camphene b | 8.817 | 0.29 | 0.20 | 1.99 | 0.15 | 1.66 | 0.48 | tr | 0.37 | 1.17 | 0.92 | 0.57 | 0.10 |
| β-Pinene a,b | 10.113 | tr | tr | 1.99 | tr | 0.25 | 0.28 | tr | 1.47 | 1.32 | 1.38 | 1.90 | 0.10 |
| 3-Carene a | 11.852 | nd | nd | nd | nd | nd | nd | nd | 1.88 | 0.57 | 0.38 | 1.23 | tr |
| Eucalyptol a,b | 12.983 | 3.14 | 2.11 | 4.34 | 1.36 | 4.41 | 1.16 | 1.47 | 10.90 | 6.20 | 5.89 | 9.59 | 3.04 |
| α-Thujone | 17.393 | 1.15 | 0.62 | 3.46 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| β-Thujone b | 18.086 | 1.53 | 0.73 | 1.51 | 0.30 | 2.19 | 1.33 | tr | nd | nd | nd | nd | nd |
| Camphor a | 19.697 | 3.73 | 5.32 | 6.33 | 3.61 | 6.18 | 3.40 | 4.32 | tr | 6.74 | 6.81 | 3.86 | 2.44 |
| endo-Borneol a | 21.160 | 1.50 | 0.74 | 1.78 | 1.84 | 1.66 | 1.23 | 0.47 | 0.12 | 0.30 | 0.21 | 0.2 | 0.21 |
| β-Caryophyllene a | 37.092 | 0.13 | 0.09 | 0.06 | 0.15 | 0.25 | 0.41 | 0.43 | 0.93 | 1.64 | 2.81 | 3.22 | 0.13 |
| Isoledene b | 43.496 | tr | tr | tr | tr | 0.11 | tr | 0.23 | 2.35 | 1.84 | 2.59 | 1.05 | 0.33 |
| Viridiflorol a | 47.199 | 2.73 | 3.14 | 5.38 | 3.93 | 3.54 | 4.14 | 1.94 | nd | nd | nd | nd | nd |
| Humulenol b | 49.514 | tr | 0.15 | 1.94 | 0.98 | 0.77 | 0.93 | 1.71 | nd | nd | nd | nd | nd |
| γ-Gurjunene b | 49.927 | nd | nd | nd | nd | nd | nd | nd | 2.73 | 1.46 | 2.06 | 1.01 | 0.23 |
| α-Bisabolol a | 52.517 | 0.23 | 0.36 | tr | nd | tr | nd | nd | 0.28 | 0.41 | 0.66 | 2.59 | 0.33 |
| 8-Hexadecyne b | 60.731 | 0.11 | 1.32 | 0.59 | 2.86 | 1.22 | 1.63 | 4.15 | 0.28 | 0.31 | 0.41 | 0.43 | 0.75 |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol b | 62.814 | tr | 0.47 | 0.24 | 1.16 | 0.42 | 0.53 | 1.26 | 0.21 | 0.21 | 0.35 | 0.36 | 0.32 |
| Verticiol b | 70.524 | 8.19 | 12.17 | 7.81 | 10.59 | 9.75 | 10.17 | 3.84 | nd | nd | nd | nd | nd |
| Aromandendrene b | 71.894 | 0.38 | 0.92 | 1.00 | 0.88 | 0.96 | 1.31 | 0.37 | nd | nd | nd | nd | nd |
| Ferruginol b | 82.097 | nd | nd | nd | nd | nd | nd | nd | 0.36 | 0.07 | 0.09 | 1.15 | 0.48 |
| Hexanedioic acid, mono(2-ethylhexyl)ester b | 85.533 | 0.65 | 2.88 | 2.83 | 0.96 | 0.25 | 0.67 | 1.24 | 0.42 | 0.44 | 0.50 | 0.45 | 0.49 |
| Unknown | 86.366 | 5.92 | 4.53 | 4.57 | 4.45 | 2.65 | 5.26 | 0.83 | 1.70 | 0.41 | 1.04 | 1.37 | 1.15 |
| Salvicanol b | 88.476 | nd | nd | nd | nd | nd | nd | nd | nd | 1.41 | 1.19 | 2.05 | 2.45 |
| Tanshinone II a | 96.184 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Cryptotanshinone a | 99.365 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Pectolinaringenin b | 105.420 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Heptacosane b | 109.945 | 1.47 | 1.96 | 2.02 | 2.26 | 1.57 | 2.25 | 4.35 | 2.12 | 1.51 | 1.79 | 1.25 | 3.69 |
| Salvigenin b | 110.291 | 0.60 | 0.06 | 0.05 | 0.06 | 0.64 | 0.64 | 0.29 | 1.72 | 4.26 | 3.45 | 2.01 | 5.90 |
| Salvinorin B b | 110.841 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Salvinorin A a | 114.026 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| β-Amyrone b | 116.668 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Lupeol b | 117.441 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| B | |||||||||||||
| Compound | tR (min) | S. divinorum | S. mellifera | S. miltiorrhiza | |||||||||
| 578 | 18434 | 22490 | 22506 | 22771 | 22772 | 767 | 5399 | 8676 | 9729 | 11750 | 12535 | ||
| α-Pinene a | 8.194 | nd | nd | nd | 1.28 | 0.24 | 0.67 | nd | nd | nd | nd | nd | nd |
| Camphene b | 8.817 | nd | nd | nd | 1.14 | 0.37 | 1.02 | nd | nd | nd | nd | nd | nd |
| β-Pinene a,b | 10.113 | nd | nd | nd | 1.09 | 0.26 | 0.69 | nd | nd | nd | nd | nd | nd |
| 3-Carene a | 11.852 | nd | nd | nd | 0.38 | tr | tr | nd | nd | nd | nd | nd | nd |
| Eucalyptol a,b | 12.983 | 1.12 | 0.31 | 1.13 | 6.44 | 3.55 | 4.20 | 0.67 | 0.81 | tr | 2.12 | nd | 1.67 |
| α-Thujone | 17.393 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| β-Thujone b | 18.086 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Camphor | 19.697 | 1.63 | tr | 3.71 | 10.21 | 8.75 | 9.73 | 0.40 | 2.13 | 1.95 | 0.90 | 0.05 | 0.84 |
| endo-Borneol a | 21.160 | nd | nd | nd | 0.33 | 0.52 | 0.52 | nd | nd | nd | nd | nd | nd |
| β-Caryophyllene a | 37.092 | nd | nd | nd | 1.28 | 0.73 | 0.82 | nd | nd | nd | nd | nd | nd |
| Isoledene b | 43.496 | nd | nd | nd | tr | 0.13 | 0.11 | nd | nd | nd | nd | nd | nd |
| Viridiflorol a | 47.199 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Humulenol b | 49.514 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| γ-Gurjunene b | 49.927 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| α-Bisabolol a | 52.517 | nd | nd | nd | 0.50 | 1.70 | 0.69 | nd | nd | nd | nd | nd | nd |
| 8-Hexadecyne b | 60.731 | 12.09 | 10.45 | 9.32 | 0.22 | 0.50 | 0.45 | nd | nd | nd | nd | nd | nd |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol b | 62.814 | 4.70 | 3.11 | 3.20 | 0.10 | 0.11 | 0.15 | nd | nd | nd | nd | nd | nd |
| Verticiol b | 70.524 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Aromandendrene b | 71.894 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Ferruginol b | 82.097 | nd | nd | nd | 0.20 | 0.31 | 0.39 | 9.14 | 2.61 | 1.05 | 8.11 | 9.18 | 1.61 |
| Hexanedioic acid, mono(2-ethylhexyl) ester b | 85.533 | 4.68 | 4.72 | 2.65 | 0.73 | 0.48 | 1.23 | 4.347 | 24.95 | 35.25 | 19.46 | 1.55 | 14.59 |
| Unknown | 86.366 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Salvicanol b | 88.476 | nd | nd | nd | 3.23 | 2.55 | 2.81 | nd | nd | nd | nd | nd | nd |
| Tanshinone II a | 96.184 | nd | nd | nd | nd | nd | nd | 15.25 | 13.19 | 12.70 | 16.91 | 12.57 | 12.14 |
| Cryptotanshinone a | 99.365 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 12.16 | nd |
| Pectolinaringenin b | 105.420 | nd | nd | nd | 1.63 | 4.22 | 3.98 | nd | nd | nd | nd | nd | nd |
| Heptacosane b | 109.945 | nd | nd | nd | 2.55 | 1.93 | 2.21 | nd | nd | nd | nd | nd | nd |
| Salvigenin b | 110.291 | nd | nd | nd | 2.55 | 1.93 | 2.21 | nd | nd | nd | nd | nd | nd |
| Salvinorin B b | 110.841 | 3.60 | 0.75 | 1.12 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Salvinorin A a | 114.026 | 33.48 | 20.24 | 22.70 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| β-Amyrone b | 116.668 | nd | nd | nd | 1.14 | 0.96 | 0.79 | nd | nd | nd | nd | nd | nd |
| Lupeol b | 117.441 | nd | nd | nd | 4.16 | 3.34 | 4.09 | nd | nd | nd | nd | nd | nd |
| S. apiana | S. divinorum | S. mellifera | S. miltiorrhiza | S. officinalis | Accuracy (%) | |
|---|---|---|---|---|---|---|
| Model Training | ||||||
| S. apiana | 5 | 0 | 0 | 0 | 0 | 100 |
| S. divinorum | 0 | 3 | 0 | 0 | 0 | 100 |
| S. mellifera | 0 | 0 | 3 | 0 | 0 | 100 |
| S. miltiorrhiza | 0 | 0 | 0 | 6 | 0 | 100 |
| S. officinalis | 0 | 0 | 0 | 0 | 7 | 100 |
| Recognition ability (%) | - | - | - | - | - | 100 |
| Model validation | ||||||
| S. apiana | 5 | 0 | 0 | 0 | 0 | 100 |
| S. divinorum | 0 | 3 | 0 | 0 | 0 | 100 |
| S. mellifera | 0 | 0 | 3 | 0 | 0 | 100 |
| S. miltiorrhiza | 0 | 0 | 0 | 6 | 0 | 100 |
| S. officinalis | 0 | 0 | 0 | 0 | 7 | 100 |
| Prediction ability (%) | - | - | - | - | - | 100 |
| No. | Compound ID | tR (min) | Formula | Base Peak | M+ | Diff (ppm) | CAS Number |
|---|---|---|---|---|---|---|---|
| S. officinalis | |||||||
| 1 | β-Thujone | 18.086 | C10H16O | 67.0542 | 152.1196 | 0.22 | 471-15-8 |
| 2 | Viridiflorol | 47.199 | C15H26O | 105.0697 | 222.1975 | −1.43 | 552-02-3 |
| 3 | Verticiol | 70.524 | C20H34O | 95.0853 | 290.2598 | −2.13 | 70000-19-0 |
| S. divinorum | |||||||
| 4 | 8-Hexadecyne * | 60.731 | C16H30 | 67.0542 | 222.2345 | 1.34 | 19781-86-3 |
| 5 | Salvinorin B | 110.800 | C21H26O7 | 94.0413 | 390.1679 | 1.53 | 92545-30-7 |
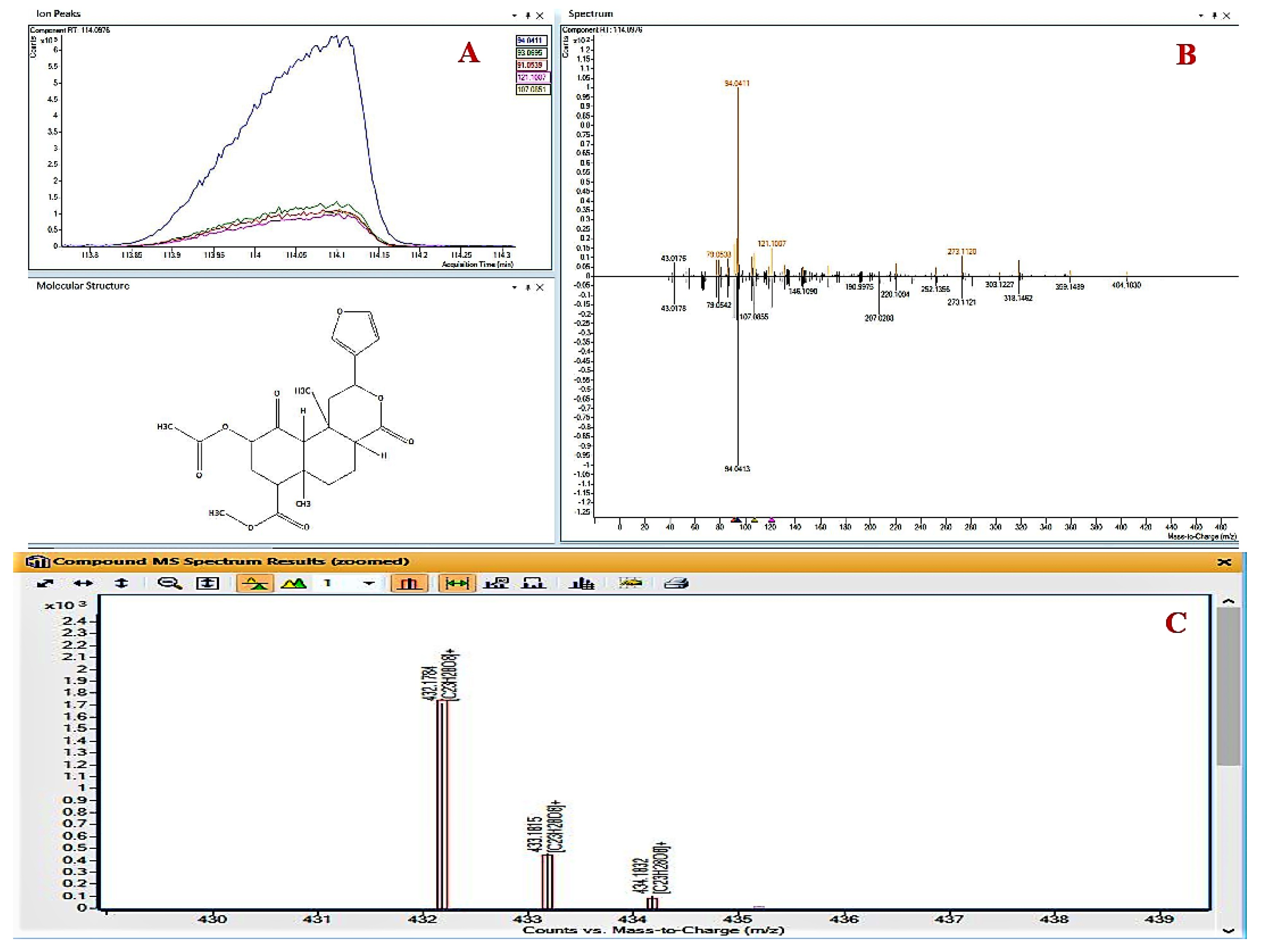
| 6 | Salvinorin A | 114.026 | C23H28O8 | 94.0412 | 432.1784 | 1.23 | 83729-01-5 |
| S. apiana | |||||||
| 7 | Isoledene * | 43.496 | C15H24 | 119.0854 | 204.1877 | 1.21 | 95910-36-4 |
| 8 | γ-Gurjunene | 49.927 | C15H24 | 105.0702 | 204.1875 | 1.21 | 22567-17-5 |
| S. mellifera | |||||||
| 10 | Camphor * | 19.697 | C10H16O | 95.0856 | 152.1195 | −0.44 | 464-49-3 |
| 11 | β-Amyrone | 116.670 | C30H48O | 218.2034 | 424.3700 | 0.08 | 638-97-1 |
| 12 | Lupeol | 117.441 | C30H50O | 189.1639 | 426.3855 | −0.28 | 545-47-1 |
| 13 | Pectolinaringenin | 105.42 | C17H14O6 | 271.0607 | 314.0785 | 2.58 | 520-12-7 |
| S. miltiorrhiza | |||||||
| 14 | Ferruginol * | 82.097 | C20H30O | 189.1275 | 286.2297 | 2.04 | 514-62-5 |
| 15 | Tanshinone II | 96.184 | C19H18O3 | 261.0912 | 294.1252 | 0.52 | 568-72-9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Wang, M.; Zhao, J.; Avula, B.; Chittiboyina, A.G.; Li, J.; Wu, C.; Khan, I.A. Chemical Authentication and Speciation of Salvia Botanicals: An Investigation Utilizing GC/Q-ToF and Chemometrics. Foods 2022, 11, 2132. https://doi.org/10.3390/foods11142132
Lee J, Wang M, Zhao J, Avula B, Chittiboyina AG, Li J, Wu C, Khan IA. Chemical Authentication and Speciation of Salvia Botanicals: An Investigation Utilizing GC/Q-ToF and Chemometrics. Foods. 2022; 11(14):2132. https://doi.org/10.3390/foods11142132
Chicago/Turabian StyleLee, Joseph, Mei Wang, Jianping Zhao, Bharathi Avula, Amar G. Chittiboyina, Jing Li, Charles Wu, and Ikhlas A. Khan. 2022. "Chemical Authentication and Speciation of Salvia Botanicals: An Investigation Utilizing GC/Q-ToF and Chemometrics" Foods 11, no. 14: 2132. https://doi.org/10.3390/foods11142132
APA StyleLee, J., Wang, M., Zhao, J., Avula, B., Chittiboyina, A. G., Li, J., Wu, C., & Khan, I. A. (2022). Chemical Authentication and Speciation of Salvia Botanicals: An Investigation Utilizing GC/Q-ToF and Chemometrics. Foods, 11(14), 2132. https://doi.org/10.3390/foods11142132







