Abstract
Despite the growing prevalence of central precocious puberty (CPP), most cases are still diagnosed as “idiopathic” due to the lack of identifiable findings of other diagnostic etiology. We are gaining greater insight into some key genes affecting neurotransmitters and receptors and how they stimulate or inhibit gonadotropin-releasing hormone (GnRH) secretion, as well as transcriptional and epigenetic influences. Although the genetic contributions to pubertal regulation are more established in the hypogonadotropic hypogonadism (HH) literature, cases of CPP have provided the opportunity to learn more about its own genetic influences. There have been clinically confirmed cases of CPP associated with gene mutations in kisspeptin and its receptor (KISS1, KISS1R), Delta-like noncanonical Notch ligand 1 (DLK1), and the now most commonly identified genetic cause of CPP, makorin ring finger protein (MKRN3). In addition to these proven genetic causes, a number of other candidates continue to be evaluated. After reviewing the basic clinical aspects of puberty, we summarize what is known about the various genetic and epigenetic causes of CPP as well as discuss some of the potential effects of endocrine disrupting chemicals (EDCs) on some of these processes.
1. Introduction
Central precocious puberty (CPP) refers to the premature activation of the hypothalamic–pituitary–gonadal (HPG) axis, resulting in the gonadotropin-dependent production of sex hormones causing the well-known physical changes associated with puberty prior to the accepted age of onset. Although the age cutoffs for onset have been debated since Tanner and Marshall’s original definitions of CPP, they are still considered to be thelarche before age 8 years for girls, or gonadarche before age 9 years for boys [1,2,3,4]. The prevalence and incidence of CPP varies greatly depending on the study. It is generally estimated to affect 1 in 5000–10,000 children; however, multiple countries throughout the world have described the increasing incidence and/or prevalence including the United States, Spain, France, Denmark, Korea, and China since the 1990s [5,6,7,8,9,10].
The diagnosis of CPP in boys more commonly has organic causes, specifically those affecting the central nervous system, such as hamartomas, gliomas, or astrocytomas. When no organic cause is found, such as in 95% cases of CPP in females (and hence referred to as “idiopathic” CPP), it is thought to be a complex interplay between environmental and genetic factors. We focus mainly on the genetic factors that may contribute to CPP, as the details of most environmental effects including nutrition and adoptive status are beyond the scope of this review.
Specific environmental agents, known as endocrine disrupting chemicals (EDCs) are known to affect the HPG axis from the prenatal period throughout life. There are many proposed mechanisms of their actions, such as interference with the cellular organization of the hypothalamus including the molecular and cellular function of the GnRH neurons, and long-lasting epigenetic changes [11]. At a population level and in various cohort studies, some EDCs such as bisphenol A (BPA) and phthalates have been speculated to have estrogen-like properties and, hence, can cause precocious puberty in females and delayed puberty in males [12,13]. However, in addition to their likely effects on estrogen receptors, they likely interact with other neuroendocrine paths related to puberty. Furthermore, some EDCs likely also have epigenetic effects which can cause multi- and transgenerational effects on the timing of puberty [14]. A detailed review of EDCs in CPP is beyond the scope of this paper, but recently was thoroughly reviewed by Lopez Rodriguez et al. [11]. Here, we briefly mention whether there are any known EDC effects on the specific gene products.
The role of genetics in pubertal onset was initially described in the context of congenital hypogonadotropic hypogonadism (HH), including but not limited to normosmic and anosmic Kallman Syndrome. Over forty genes have been identified and some of these have been incorporated in next generation sequencing (NGS) panels, which resulted in confirming a genetic cause of HH in over 20% of cases [15]. In addition to playing a role in the diagnostic process, this knowledge has not only given us better insight into HH pathology, but also normal pubertal physiology. More recently, there has been increased interest in monogenetic causes of central precocious puberty, and there are a number of recent reviews on this topic [16,17,18]. Here, we briefly review the physiology of puberty and focus on how some mutations and epigenetic changes have been found to be associated with confirmed CPP in non-syndromic phenotypes, as well as other gene candidates proposed in the literature that likely contribute to normal pubertal timing and may also be implicated in pathology. We also mention a handful of syndromic forms of CPP.
2. Physiology of Normal Puberty
The decapeptide known as gonadotropin-releasing hormone (GnRH) is produced from a neuronal network primarily in the arcuate nucleus (ARC) area of the hypothalamus, and is known to be under both genetic and environmental influences [19]. What was initially referred to in the 1950s as the “gonadostat” is now accepted to be a complex interplay of numerous factors on the GnRH network, commonly referred to as the “GnRH pulse generator” [1]. The neuroendocrine control of this network as well as its genetic and epigenetic influences result in the suppression of GnRH during childhood and subsequent activation of its release at the onset of puberty.
During early embryonic development GnRH neurons migrate from the olfactory placode to the hypothalamus. GnRH is detected in the fetal hypothalamus at around 15 weeks of gestation. The GnRH neuronal network subsequently activates in a pulsatile fashion, resulting in the release of the pituitary gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH), while the placenta concurrently makes human chorionic gonadotropin (HCG), which acts similarly to LH. Although this important neuroendocrine network is first active mid-gestation, it is then “silenced”, likely by placental hormones. Its subsequent reactivation for an initial period in postnatal life is often referred to as “minipuberty”. This perinatal period is thought to be critical for neurobehavioral development and also phallic growth [20]. During this time GnRH secretion is present and LH and FSH are detectable, with differences in patterns between the sexes [21]. Subsequently, there is an inhibition of the HPG axis during childhood prior to the classic pubertal period.
The beginning of central puberty is characterized by a decrease in hypothalamic inhibitory signals coupled with an increase in excitatory impulses that result in GnRH release, which is initially diurnal and pulsatile [19]. The primary excitatory signals are provided by glutamatergic neurons as well as a family of proteins called kisspeptins [22]. The inhibitory signals are from various neurons, but transduction is mainly via gamma-aminobutyric acid (GABA), and other inhibitory signals including opiatergic, arginine-phenylalanine-amidated-related peptide neurotransmitters. Indirect inhibition of GnRH release is also achieved via the MKRN3 protein (to be discussed in more detail below). The pulsatile release of GnRH is also facilitated by glial cells including astrocytes and tanycytes, which release small molecules as well as communicate via direct cell–cell interactions [22].
A subset of neurons in the ARC are sometimes referred to as “KNDy” due to their secretion of kisspeptin, neurokinin B (NKB), and dynorphin (Dy). These neuropeptides along with their receptors are thought to be the basis of the “pulse generator” activity since they form oscillations in hormone levels resulting from positive feedback from kisspeptin and NKB, coupled with the phase-shifted inhibitory action of dynorphin [22,23]. It has been shown that nitric oxide (NO) stimulates the exocytosis of GnRH through various pathways [24]. Toro et al. (2018) described the numerous epigenetic influences (i.e., DNA methylation, miRNA processing, histone post-translational modifications, and noncoding RNA) that affect this delicate balance, including the effects on a number of inhibitory genes (GAD1, PDYN), activating genes (GNRH1, GLS, KISS1, TTF1), or other genes with dual effects (TAC3, IRF2BPL) [22].
GnRH signaling ultimately translates to nocturnal pulsatile LH release, which is the first biochemically detectable endocrine manifestation of puberty [22]. The secretion of LH and FSH results in gonadal production of sex steroids (i.e., estrogen, progesterone, and testosterone). Hence, it is the onset of the pulsatile activation of the HPG axis that ultimately causes not only gonadal development and pubertal changes, but also eventual reproductive functions including gametogenesis.
3. Diagnosis and Treatment of Central Precocious Puberty
Clinical examination is the cornerstone of diagnosing precocious puberty. The classic signs of precocious puberty are thelarche prior to age 8 years in girls and testicular enlargement > 4 mL before 9 years in boys [3,4]. However, data from the last several decades suggest earlier physiological onset of puberty. Results from a cross-sectional study from the Pediatric Research in Office Settings (PROS) network showed that white girls experience thelarche at a mean age of 9.96 +/− 1.82 years and black girls at a mean age of 8.87 +/− 2.93 years [25]. A multisite cohort study published in 2010 of 1239 girls showed that at age 7 years, 10.4% of white, 23.4% of black non-Hispanic, and 14.9% of Hispanic girls had attained breast Tanner stage ≥2, which is greater than previously reported from studies of girls born 10–30 years earlier [26]. Consequently, there is evidence linking the trend of earlier puberty with increasing BMI and obesity, particularly in girls [27]. Although some have proposed changing the cutoff age based on epidemiological factors, it has been demonstrated that lowering the age limit may miss the diagnosis of treatable underlying pathology, and hence, the classically accepted cutoffs continue to be used [28].
In order to confirm clinical findings, as well as to differentiate central from peripheral causes of precocious puberty, biochemical evaluation is usually obtained. Although a random basal LH level of >0.3 IU/L is generally considered diagnostic for CPP [29], Mucaria et al. summarized reports of other suggested LH cut off levels for the diagnosis of CPP [30].
In children with lower levels of LH but with physical manifestations concerning for progressive pubertal development, GnRH stimulation testing with leuprolide allows evaluation of LH and sex steroid response and may aid in distinguishing between central and peripheral precocious puberty [30,31]. Leuprolide stimulation is considered the gold standard in evaluating CPP; however, there are several cut-offs for leuprolide-stimulated LH levels that have been proposed, ranging from 5–8 IU/L, to indicate centrally mediated pubertal development [30,32].
Imaging modalities can also aid in the diagnosis of CPP. A bone age radiograph is indicated and is typically found to be advanced compared to chronological age. Bone age advancement that is disproportionate to height predicts compromised height potential or that is more than 20% greater than height age suggests an excess of sex hormones, and is an indication for a more extensive investigation to determine the cause of the precocity [1]. Additionally, pelvic ultrasound in girls may show enlarged ovarian volumes and uterine changes consistent with pubertal morphology to support the diagnosis. Although felt to be more complementary than necessary, Talarico et al. published a review summarizing various studies and uses of pelvic ultrasound parameters in CPP, including one indicating that uterine length was the best parameter for distinguishing between CPP and isolated premature thelarche [33,34]. Badouraki et al. suggested a uterine length cutoff of 3.83 cm in girls >6–8 years, giving a sensitivity of 82.4% and specificity of 90.9% for the 0–6 and >6–8 years age intervals, respectively [34].
Brain magnetic resonance imaging (MRI) is often performed in children with confirmed CPP to rule out CNS pathology. MRI is recommended in all boys with central pubertal changes before age 9 years. Since CNS lesions are uncommon in older girls, MRI is only necessary in girls less than 6 years, or in any child with neurologic manifestations such as headaches, vision changes, or seizures [35]. In 2009, members of the GnRH Analogs Consensus Conference Group suggested that “all boys with CPP and girls with CPP at <6 years of age should have a head MRI” and acknowledged that while it was controversial in girls aged 6–8 years, it should be considered if there is rapid pubertal progression or neurological signs or symptoms [36]. These suggestions are supported by a 2018 systematic review and meta-analysis of 15 studies including 1857 girls with CPP which confirmed the decreasing prevalence of intracranial lesions by age and supported the idea that few girls who are older than 6 years will be found to have an intracranial lesion [37].
The treatment of CPP aims to temporarily slow down progression of pubertal development, improve final adult height, and minimize psychosocial dissociation in an affected child who is not developmentally ready for pubertal changes. GnRH agonists remain the standard of care for the treatment of CPP. Continuous exposure to a GnRH agonist results in paradoxical suppression of the HPG axis by rendering the receptors insensitive to the usual pulsatile GnRH activity during physiological puberty. Several preparations are available, with extended-release depot leuprolide injections gaining more favor [38]. Monthly and quarterly intramuscular leuprolide injections have been FDA approved and available in the US for more than 2 decades. Another GnRH agonist, triptorelin, is available for the treatment of CPP with formulations available for either quarterly, half-yearly, or annual intramuscular injections. An alternative to injectable preparations is the subcutaneous histrelin implant, affording hormone suppression for at least 12 months [39]. Results of a prospective study conducted by Lewis et al. demonstrated continued puberty hormone suppression by histrelin for 2 years, potentially decreasing cost and frequency of surgical procedures for placement and removal of the device [40]. Occasionally the use of histrelin is limited in the pediatric population due to a lesser capacity for temporal management (i.e., if hormone suppression cannot be achieved).
Current data support improvement of final height in girls with CPP treated before 6 years and boys before 9 years of age, but with less impressive results for those started on treatment at a later age [41,42,43]. There is very limited and conflicting data on the psychosocial effects of CPP, and whether or not pharmacologic intervention can afford relief of stress associated with the condition, particularly in older girls [44,45]. Although current recommendations from the North American and European endocrine societies support treatment in girls less than 6 years with rapid and progressive development, they suggest individualizing treatment decisions for those with onset of pubertal development between 6–8 years of age. In boys, treatment should be considered for those with onset of progressive puberty before age nine, particularly if there is evidence of compromised height potential [36,37]. A 2021 systematic review and meta-analysis by Luo et al. evaluated 98 articles for the long-term efficacy and safety of GnRH agonists in 5475 children (98.5% female) with idiopathic CPP. The authors found that treatment increases final adult height and decreases BMI in girls with no evident increase in polycystic ovarian syndrome (PCOS), and that there was a paucity of high-quality evidence in other key long-term outcomes such as infertility and malignant or metabolic diseases, indicating further research is required before any conclusions can be drawn [46].
4. Confirmed Genetic and Epigenetic Causes of CPP
Whereas the genes encoding kisspeptin (KISS1) and its receptor (KISSR1) were the first implicated in the pathology of CPP, there has also been demonstrated causality in the mutation of DLK1 (gene encoding for NKB) and MECP2 (gene encoding for methyl-CpG-binding protein). Most notably, MKRN3 (gene encoding makorin ring finger protein) mutations have been demonstrated to be the most prevalent confirmed cause of CPP worldwide.
4.1. Kisspeptin (KISS1) and Kisspeptin Receptor (KISS1R or GPR54)
Kisspeptins are a family of neuropeptides which originate from a single precursor product from the KISS1 gene, which is located on chromosome 1 and contains four exons. There are many isoforms of kisspeptin which can be differentiated by mass spectrometry, but all share a common precursor from exons 3 and 4 resulting in a common decapeptide at the C-terminus believed to be essential for kisspeptin activity [47,48]. Kisspeptins were initially identified as metastasis suppressors (the isoform kisspeptin-54 was previously known as metastin) since they had a capacity to inhibit tumor metastasis [48]. However, there is now also established data on how kisspeptin and the kisspeptin receptor (KISS1R, also known as G-protein-coupled receptor 54 or GPR54) are key regulators of the onset of puberty through their direct role in the release of GnRH [49].
Kisspeptin neurons are found in hypothalamic and extra-hypothalamic areas. Kisspeptin provides input to the hypothalamic–pituitary–gonadal axis via direct or indirect effects on leptin, prolactin, and sex steroids [50,51,52]. As previously discussed and illustrated in Figure 1, kisspeptin is one of the key players in the KNDy network control of GnRH pulsatility [23,53]. Kisspeptin has a direct stimulatory effect via its receptor on GnRH neurons and, subsequently, causes a dose-dependent increase in gonadotropin levels. There is reported discordance between the role of kisspeptin during minipuberty and adolescent puberty [54]. In mice, over 90% of GnRH neurons depolarize in response to kisspeptin [48]. Furthermore, electrophysiological studies in mice indicate that when kisspeptin binds to its receptor it may activate phospholipase C and/or inositol phosphate, resulting in a calcium cascade which effects both potassium and non-selective ion channels to initiate depolarization in GnRH neurons [55]. GABA inhibits GnRH release at the level of kisspeptin.
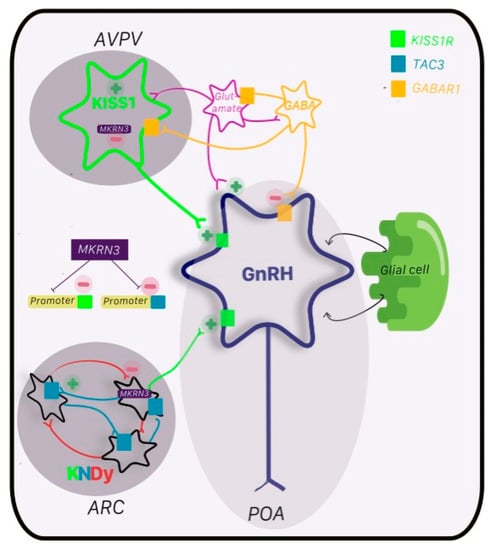
Figure 1.
Regulation of GnRH network. GnRH neurons (navy) reside primarily in the preoptic area (POA) and form the GnRH pulsatility that is the basis of subsequent pubertal changes downstream in the HPG axis. The KNDy network in the arcuate nucleus (ARC) is known as the pulse generator for GnRH, which is inhibited by dynorphin (Dy; red) and stimulated by neurokinin B (NKB; blue) via its receptor TAC3 (blue box) and kisspeptin (green) and its receptor KISSR1 (green box). Kisspeptin is also found in the anteroventral periventricular nucleus (AVPN) with stimulatory input from glutamate (pink) and inhibitory input from GABA (orange) and its receptor GABAAR1 (orange box). The major antagonist to kisspeptin activity, and hence GnRH, is via makorin ring finger protein (MKRN3; purple). MKRN3 inhibits promoters of KISS1 and TAC3, and hence, suppresses transcription of kisspeptin and NKB. Glial cells also play a critical role, as do other neuroendocrine regulators including feedback from the pituitary and gonads (not shown).
The clinical importance of kisspeptin signaling was initially brought to light by studies showing a higher incidence of loss-of-function mutations of KISS1R in individuals with HH, with about 5% of cases of normosmic HH being caused by mutations in KISS1R [56,57,58]. Although most of the KISS1/KISS1R mutations have been found to be associated with HH, we now know that some can result in CPP [47].
Although it seems intuitive that activating mutations of KISS1R would cause CPP, this seems to be rare. In 2008, Teles et al. published the first confirmed case of a heterozygous activating mutation of KISS1R in a girl with CPP [59]. Subsequently, in 2010 Silveira et al. presented a novel missense mutation of the KISS gene in a one-year-old boy from a cohort of 77 children with CPP, and demonstrated that this mutation afforded the kisspeptin neuropeptide a higher resistance to degradation with permanence and hyperstimulation of its own receptor [60]. Interestingly, in a 2019 review of the contribution of these genes to both HH and CPP, Ke et al. extensively reviewed previously reported cases and demonstrated the biochemical relevance of the location of the mutations on the functionality of the protein, and noted that some mutations of KISS1/KISS1R can be associated in both HH and CPP [47]. Since then, there have been a handful of studies from various countries that show polymorphisms of KISS1/KISSR1 in their cohorts [60,61,62,63,64].
Dysfunction in kisspeptin and its receptor proteins may also be affected by various EDCs. Although BPA has been associated with CPP in girls based on associations of increased urinary BPA, the relationship to serum kisspeptin was evaluated in a cohort of 28 girls with CPP or premature thelarche and no association was found compared to controls [65]. Interestingly, in 2019 Ruiz-Pino et al. demonstrated that mice that are perinatally exposed to BPA have premature physical changes, but also noted that there was persistent, but divergent, impairment of different populations of kisspeptin neuronal maturation, with more kisspeptin neurons in the rostral (RP3V) hypothalamus but fewer in the ARC [66]. Another EDC, perfluorohexane sulfonate, was found to decrease KISS1 expression in the hypothalamus, resulting in reproductive deficits [67]. Chronic exposure to anabolic–androgenic steroids also had suppressive effects on the presynaptic, kisspeptin-mediated excitatory drive of GnRH [68].
4.2. Makorin Ring Finger Protein 3 (MKRN3)
The MKRN3 gene is a maternally imprinted gene located adjacent to the Prader–Willi syndrome (PWS) region of chromosome 15q11.2. As such, all affected individuals inherit the mutation from their fathers. The protein is composed of five zinc finger domains: three C3H1 fingers or motifs responsible for RNA binding; one C3HC RING motif with ubiquitin ligase activity; and one MKRN-specific Cys-His domain. Whereas kisspeptin may be one of the major stimulating factors for the onset of puberty, this more recently described protein serves as a “brake” on pubertal onset and may help to regulate the “epigenetic switch” of puberty [69]. The MKRN3 protein inhibits hypothalamic activity upstream of GnRH neurons. Abreu et al. demonstrated that in mice, MKRN3 acts selectively within kisspeptin neurons to inhibit the expression of the stimulatory neuropeptides kisspeptin and neurokinin B, contributing to pubertal suppression as shown in Figure 1. Notably, MKRN3 did not alter the promoter activity of the inhibitory dynorphin [70].
Although classically loss-of-function mutations of MKRN3 are associated with CPP, other mutation variants including missense and complete deletions of MKRN3 have also been described [71,72]. When mutations affect the RING finger domain, the inherent ubiquinase activity of MKRN3 is altered, thus affecting the repression of KISS1 and TAC3 promoter activity and transcription. Additionally, novel genetic alterations in the promoter and 5′-UTR regulatory regions of the MKRN3 gene have also been associated with CPP [72,73].
In 2013, Aubreu et al. first reported four novel mutations in MKRN3 by whole exome sequencing (WES) in 40 members of 15 different families with CPP (including 32 individuals with CPP (27 females/5 males) and 8 with normal pubertal timing (5 females/3 males)). The mutations included three frameshift mutations predicted to encode truncated proteins, and one missense mutation predicted to disrupt protein function; they were found in five of the families, affecting both sexes [74].
At present, MKRN3 mutations are the most commonly identified monogenetic cause of CPP, with local prevalence rates ranging between 0–46% depending on the study, and with apparent differences in geographic distributions [75]. A 2019 systematic review and meta-analysis of over 800 individuals with CPP from 14 studies demonstrated an overall MKRN3 mutation prevalence of 9%. The subgroup analysis showed that prevalence estimates were higher in males (but girls were affected earlier), in familial cases, and in non-occidental countries [75].
In a more recently published multiethnic cohort study looking at genotype–phenotype correlations of MKRN3 mutations in CPP, 71 out of 716 individuals with CPP were found to have 18 different loss-of function mutations in MKRN3 [76]. These individuals came from nine different ethnic backgrounds including Brazilian, American, Spanish, Argentinean, Belgian, Israeli, Australian, Norwegian, and Turkish. There were similar clinical features in the individuals who were positive for MKRN3 mutations and those with idiopathic CPP, with a similar age of onset of puberty noted (6.2 ± 1.2 years in girls and 7.1 ± 1.5 years in boys). However, it appears that patients with severe MKRN3 mutations presented with more significant bone age advancement and higher basal luteinizing hormone levels [76]. In another study of 56 children (54 girls and 2 boys) with CPP from Cyprus, MKRN3 gene mutations were the most prevalent mutation found by WES and NGS analysis. The novel variants include mutations upstream to the transcription site (i.e., in the proximal promoter of the MKRN3 gene) and a novel missense loss-of-function variant, which are proposed as a possible explanation for the founder effect (i.e., high prevalence of CPP) in this population [72].
4.3. Delta-Like Noncanonical Notch Ligand 1 (DLK1)
Another recently discovered gene mutation causing familial CPP is a loss-of-function mutation in the maternally imprinted (and hence, paternally inherited) Delta-like noncanonical Notch ligand 1 DLK1 (also known as preadipocyte factor 1 (Pref-1) or fetal antigen 1 (FA1)), found on chromosome 14q32 [12]. This gene encodes an epidermal growth factor (EGF)-like membrane protein which is involved in the well-conserved Notch signaling pathway. This pathway is known to be inhibited by its two noncanonical ligands (one of which is DLK1). Amongst other functions, the Notch pathway is involved in cell differentiation during embryonic development, and is also a modulator of adipogenesis [17]. Specifically, the formation of kisspeptin neurons and their later development in adulthood are dependent on the Notch pathway [77]. Although the exact mechanism of how DLK1 affects pubertal timing is still unknown, it is postulated that the negative regulatory role of DLK1 in Notch signaling may have an effect on the development of kisspeptin neurons in the hypothalamus [78,79].
The DLK1 gene was one of the many identified in a large population study evaluating relationships between genome studies and age of menarche in European women [80]. The initial report of the direct causality of epigenetic changes in DLK1 with CPP was reported in 2017 by Dauber et al. in a family with five females with CPP. Linkage analysis and whole genome sequencing demonstrated deletions that included the exon of DLK1 and which lead to undetectable levels of the DLK1 protein [81]. In a population of children from Cyprus, nine girls with CPP that underwent genetic testing had rare variants in the DLK1 gene [72]. The DLK1 gene is also implicated in Temple syndrome [81,82], which is discussed in detail later.
4.4. Methyl-CpG-Binding Protein 2 (MECP2)
Methylation of DNA is one of the key mechanisms of epigenetic regulation. Methylated DNA is recognized and bound by various proteins, of which the methyl-CpG-binding protein 2 (MeCP2) is one of the best known. There is a region within the encoding gene MECP2, referred to as the methyl-CpG-binding domain (MBD), which binds methylated DNA including methylated CpG dinucleotides (mCG) [83]. Mouse models show that either the gain or loss of MECP2 results in gene expression changes in multiple brain regions including the hypothalamus and cerebellum and suggest that this can contribute to some of the neurodevelopmental phenotypes affecting either mutations or duplications in this gene [84,85,86]. MECP2 is found on chromosome Xq28. MECP2 duplication syndrome (MDS) has been linked to CPP, especially in males. In 2017, Tsuji-Hosokawa et al. reported on a 6-year-old male with MDS presenting with severe intellectual disability and who also presented with central precocious puberty [87].
Multiple studies have shown the association of MDS with precocious puberty, with modified gene expression in the hypothalamus and/or resulting hyperandrogenism. In addition to the known effects of MDS on CNS gene expression in the hypothalamus and cerebellum [84], in 2021 Wang et al. demonstrated (using a mouse model of MDS) that MECP2 is highly expressed in the Leydig cells of the testis [86]. The authors suggested that duplication of MECP2 increases androgen synthesis and decreases androgen to estrogen conversion through the upregulation of the luteinizing hormone receptor (LHCGR) in the testis. They proposed that MeCP2 binds the LHGCR promoter and may also have effects on the transcription activator CREB1 and aromatase activity in the testis. Thus, MeCP2 plays not only an important role in androgen synthesis but also supports a novel non-CNS function of MeCP2 in the process of sex hormone synthesis [86].
Interestingly, in addition to the gain-of-function effects of MECP2 on puberty seen in MDS, the loss of function of MECP2 may also impact puberty, as seen in Rett syndrome, which is discussed in more detail later.
5. Other Potential Genetic Contributors to Normal and/or Precocious Puberty
5.1. Gamma-Aminobutyric Acid Receptor Subunit Alpha-1 (GABRA1)
Gamma-aminobutyric acid (GABA) is a dominant neurotransmitter whose mediation of GnRH neurons is via the GABA receptor. Although there are at least 18 different subunits, the receptor with the alpha1 subunit known as GABAAR (encoded by GABRA1) is proposed to be the major receptor for GnRH inhibition. The interaction between the stimulatory glutamate neurotransmitter GABA and its receptor is complex and may be one of the key players in the coordination of pubertal initiation [88]. Support for the important role of GABA in pubertal inhibition has been demonstrated in a number of animal studies, including those using bicuculline (a GABAAR antagonist) which results in increases in both kisspeptin and GnRH secretion [89]. However, in one study of 31 girls with CPP, no functional mutations in GABRA1 were found, and none of the polymorphisms identified were statistically different between unrelated girls with CPP and the controls [90].
Multiple animal studies have shown that exposure to BPA either in the early developmental stages or during the pubertal developmental window can alter GABA effects on kisspeptin and GnRH neurons in a variety of dose-dependent ways and are further influenced by the time of day and estradiol [91,92,93]. Additionally, another EDC, manganese, may result in CPP via stimulation of GABAAR in the hypothalamus, resulting in an increase in nitric oxide production and GnRH secretion [94].
5.2. Neurokinin B (NKB, or Tachykinin 3 TAC3) and Tachykinin 3 Receptor (TACR3)
Neurokinin B (NKB, also known as tachykinin 3 or TAC3) is a member of the tachykinin family, which is encoded by the TAC3/tac2 gene and is expressed throughout the central and peripheral nervous systems. Specifically, TAC3 is expressed in the same KNDy neurons as kisspeptin, as shown in Figure 1 [64]. It is a non-adrenergic/non-cholinergic neurotransmitter and acts through a G-protein-coupled receptor (encoded by TACR3 in humans). Genetic network modeling (using the Genemania platform and MKRN3 as an anchor) shows that MKRN3 directly interacts with TAC3, and that this gene is also only one node away from KISS/KISSR1 [72]. Although the mechanism remains to be elucidated, NKB is proposed to be involved in GnRH release.
Mutations in the genes encoding this ligand–receptor pair have been identified in normosmic HH patients [95,96], and hence, it remains plausible that different (i.e., activating) mutations could cause CPP. In one study of 108 children with CPP in which TAC3 and TACR3 were studied, a new variant of the TAC3 gene was found (c.187G>C) in a Brazilian girl with CPP, and segregation analysis revealed that her mother was also heterozygous for this genetic variant [97]. Furthermore, a study of girls with CPP from China demonstrated a number of polymorphisms in TAC3 and TACR3, with one polymorphism (A63P) of TACR3 meeting statistical significance compared to their control population, leading the authors to conclude that it has “at least minor contributions to the pathology of idiopathic central precocious puberty in Chinese girls” [98]. A Brazilian study of these genes in both CPP and HH showed the same variant (A63P) in TAC3 in a girl with CPP, whereas TACR3 loss-of-function mutations were associated only with HH cases [99]. On the other hand, a study from Macedonia that sequenced both TAC3 and TACR3 genes in individuals with CPP did not find mutations in this cohort [100].
In the same 2019 animal study by Ruiz-Pino et al. that demonstrated that perinatal exposure to BPA resulted in persistently lower KISS1 expression during (pre)pubertal maturation, there were also lower Tac2 levels identified (i.e., lower levels of the gene than encodes NKB in mice) [66].
5.3. Leptin (ob)
The concomitant rise in the incidence of overweight status and obesity in children and the trends of earlier puberty has sparked debate and research on the link between adiposity and puberty. There are many studies that establish the association between increased adiposity and early puberty changes in girls [27,101], but such correlation remains unclear in boys [102].
Leptin is best known as an adipokine hormone produced by fat cells that is sensed by pro-opiomelanocortin (POMC) and neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons in the ARC to regulate food intake [103]. It helps to regulate energy balance by inhibiting hunger, and is now also known to have multiple functions involved in pubertal timing. A product of the obesity (ob) gene, leptin controls the amount of body energy stores by reducing food intake and increasing thermogenesis [104].
Pioneering studies by Frisch et al. established that pubertal onset and progression is associated with a critical body weight and composition [105,106]. Leptin’s primary function is to regulate fat stores and guarantee normal reproductive function. Leptin receptors are present in the hypothalamus as well in gonadotrope cells of the anterior pituitary. Leptin exerts a direct stimulatory effect on the HPG axis by promoting GnRH secretion [24,107]. Furthermore, leptin acts on the anterior pituitary to directly stimulate the release of LH via nitric oxide synthase activation in the gonadotropes and also gonads (as implied by the expression of functional leptin receptors on the surface of ovarian follicular cells, including granulosa, theca, and interstitial cells, as well as Leydig cells) [107].
Leptin can be affected by gestational exposure to EDCs [95]. Although not studied specifically in the context of puberty, EDCs are known to affect the functioning of hypothalamic neurocircuitry in the context of feeding. Mackay et al. demonstrated that BPA can disrupt hypothalamic feeding circuitry both structurally and functionally, resulting in leptin resistance [108].
5.4. Neuropeptide Y Receptor (NPY1R)
The NPY1R gene encodes for the G-protein-coupled receptor for neuropeptide Y (NPY). Neuropeptide Y has a stimulating effect on GnRH via its antagonistic effects on GABA. Although its role in puberty in animal models has been demonstrated, in humans it is best known for its inhibitory role during the quiescent period of childhood [109]. In one cohort including 33 people from Brazil with CPP, a girl had an inherited heterozygous polymorphism of NPY1R; however, it was felt to be clinically insignificant since in vitro assays failed to detect altered activity [110]. Other polymorphisms were also described in another small cohort of children with CPP, but pathogenicity could not be confirmed due to the small sample size [111].
Similar to leptin (since it is also associated with hypothalamic feeding neurocircuitry) EDC effects on NPY have been studied mainly in the context of feeding, where perinatal exposure of mice to BPA reduced NPY expression in the ARC and paraventricular nucleus, resulting in obesogenic effects [108,112].
5.5. Lin-28 Homolog B (Gene LIN28B)
The human LIN28B gene is a homolog of the nematode gene lin-28 and encodes a highly conserved RNA-binding protein that blocks microRNAs of the LET7 family. In other species, deleterious effects on this gene lead to rapid physical development (i.e., deletions in the nematode Caenorhabditis elegans result in an abnormal rapid tempo of development through larval stages to adult cuticle formation), whereas gains of function lead to delays in the developmental process [113]. In humans, in addition to their better known roles in oncology, LIN28B and LET7 also play roles in embryonic stem cell preservation as well as other cell developmental processes [77].
A genome-wide association study (GWAS) evaluating the presence of SNPs that correlated with the age of menarche and other variables in over 4000 women from three prior studies using the same Affymetrix GeneChip 500K found that the rs314276 in intron 2 of LIN28B was the only one to reach genome-wide statistical significance with age of menarche [113]. Similarly, a case–control study of over 500 girls from China with CPP reported that the LIN28B SNPs rs314280 and rs314276 were significantly associated with CPP [114]. Although this seems promising, a smaller study of 30 girls with CPP from Denmark failed to identify any significant mutations in LIN28B (although one girl with CPP did have a missense change His199Arg in LIN28B, this variant was also detected in one of the 132 controls) [115]. These inconsistencies could potentially be explained by the small sample size or the ethnic differences in populations, and hence, warrant further evaluation [77].
5.6. Melanoma Antigen L2 (MAGEL2)
Melanoma antigen L2 (MAGEL2) is one of the genes in the same PWS imprinted area of chromosome 15 as MKRN3. It encodes a protein in the MAGE/necdin family of ubiquitin ligase receptors [71,116,117]. It is highly expressed in hypothalamic tissue, although the neuroendocrine effects are not fully understood. It seems to impact oxytocin production [117]. Since this gene is also postulated to be affected in PWS, loss of function of the MAGEL2 gene has been associated with the known reproductive deficits seen in PWS [116]. In one study of 16 patients with CPP who had normal Sanger sequencing of MKRN3, a large deletion involving MAGEL2, MKRN3, and NDN was found in one patient without classic features of PWS but with pubertal onset at 5.5 years. Furthermore, in the study of 44 children with CPP from Cyprus, 9 of the girls had one of two rare variants in the MAGEL2 gene [72].
6. Genetic and Epigenetic Causes of Syndromic CPP
Some known genetic syndromes have CPP as an associated major or minor feature. This review describes a few of these conditions along with the affected gene/protein and/or epigenetic changes.
6.1. Prader–Willi Syndrome (PWS)
Prader–Willi syndrome is a rare genetic imprinting disorder resulting from the lack of expression of the paternally inherited chromosome 15q11–q13. Classic phenotypic features include short stature with hyperphagic obesity and metabolic complications. Hypothalamic dysfunction in PWS results in hyperphagia as well as GH, TSH, and ACTH deficiencies, and hypogonadism with variable clinical presentations. Most children with PWS have normal minipuberty changes, although cryptorchidism is reported in 66–100% of males and hypoplasia of the labia majora and clitoris at birth is noted in 76% of females [118]. The onset of puberty is typically within the normal range with subsequent gonadal failure, which is typically due to a combination of HH and primary gonadal failure [118].
Interestingly, despite the classic association with hypogonadism, CPP has also been described to occur in 4–10% of PWS cases. However, one recent review noted five reported cases in the literature [119,120]. A report from 2009 of the genotype–phenotype correlations of three patients described a paternal deletion of MKRN3, MAGEL2, and NDN in a child who had only a few features of PWS (i.e., obesity, developmental delay, and a high pain threshold), but also had a diagnosis of CPP which was supported by her response to treatment with triptorelin [121]. It is possible that changes to the imprinted MKRN3 and/or MAGEL2 genes may be involved in pubertal development in PWS, but the exact roles remain to be elucidated [116,120]. Another hypothesized mechanism of the more commonly noted hypogonadism is the inhibitory effect of ghrelin on the HPG axis [120].
6.2. Russell–Silver Syndrome (RSS)
Russell–Silver syndrome (RSS) is another genetic imprinting condition. An underlying molecular cause is identified in around 60% of patients clinically diagnosed with RSS, with the most common underlying mechanisms being loss of methylation on chromosome 11p15 (11p15 LOM; seen in 30–60% of patients) and maternal uniparental disomy for chromosome 7 (upd(7)mat; seen in 5–10% of patients). Infants with RSS have intrauterine growth restriction (IUGR), resulting in birth parameters consistent with small-for-gestational-age (SGA) infants with a head circumference that is “normal” but relatively larger compared to their height and weight percentiles (often referred to as “relative macrocephaly”). In addition to postnatal growth issues including feeding difficulty, these children are also at risk for variations in growth and puberty [122].
In addition to an increased risk of premature adrenarche, children with RSS can experience CPP in up to 25% of cases [119]. Although studies are limited, some note that in patients with RSS that experience premature adrenarche, the onset of central puberty might be earlier and with a relatively faster progression than typically expected. Children with upd(7)mat are likely to progress to central puberty at an even younger age than patients with RSS and 11p15 LOM (mean starting age 8.5 years in girls and 9.5 years in boys) [122]. There are no proposed mechanisms for the differences in pubertal progression, but it is possible that epigenetics may play a role due to the pathophysiology of the condition.
6.3. Temple Syndrome (TS14)
The chromosomal region 14q32.2 is another key area of imprinting with both paternally and maternally expressed genes and also includes at least two unique differentially methylated regions (DMRs): (i) the intergenic (IG)-DMR MEG3/DLK1 in the placenta, and (ii) the transcription start site (TSS)-DMR MEG3 in the body. Maternal uniparental disomy of this region (UPD(14)mat), as well as other associated epimutations and deletions, have recently been coined as Temple syndrome. Clinically and phenotypically, Temple syndrome (also referred to as TS14) overlaps with both PWS (growth failure, hypotonia, and brachydactyly) and RSS (growth failure and feeding difficulties, especially in infancy). Like RSS, it also can be present in otherwise non-syndromic infants that were born SGA [82]. Hence, it is suggested that testing for this condition be considered in individuals suspected to have either PWS or RSS but the respective testing comes back negative. It should also be considered in the differential of CPP since this is associated in 90% of cases [119], providing additional support for the DLK1 role in puberty.
6.4. Neurofibromatosis Type 1 (NF1)
Neurofibromatosis 1 (NF1) is an autosomal dominant neurocutaneous disorder characterized by increased risk of benign and malignant tumor formation. The condition is caused by the mutation of the NF1 gene, located in chromosome 17q11.2. This gene regulates the production of the protein neurofibromin. Loss of function of NF1 results in the hyperactivation of the proto-oncogene RAS, leading to an increased risk of tumor formation, predominantly affecting the skin, bone, and nervous system. NF1 has been associated with an increased incidence of CPP, diencephalic syndrome, GH deficiency, and GH hypersecretion. Endocrinopathies in NF1 (most common of which is CPP) are thought to arise as complications of optic gliomas that involve the hypothalamic and sellar regions [123,124]. The prevalence of CPP in NF1 is about 3% [124,125]. It is postulated that lesions close to the hypothalamus interfere with tonic central nervous system inhibition of the hypothalamic–pituitary–gonadal axis, resulting in precocious puberty [126]. Although there are reported cases of CPP in NF1 in the absence of optic gliomas [127,128], it is proposed that these occurrences are incidental, as the incidence is about the same as in the general population [129].
6.5. Rett Syndrome
Rett syndrome (RTT) is a neurodevelopmental disorder that is attributed to mutations in the MECP2 gene in >95% of classic cases. The main diagnostic criteria for classic RTT include loss of hand skills, loss of acquired spoken language, gait abnormalities, and stereotypic hand movements. Additional developmental issues include growth retardation and orthopedic complications [130].
Many studies of gonadal and adrenal steroids in RTT suggest normal sex hormone levels [131]. However, in 2014 Killian et al. (as part of the Rett Syndrome Natural History Study) found that more than 25% of girls with RTT initiated puberty early, yet entered menarche later (median age 13.0 years). The authors also demonstrated that BMI correlated with earlier thelarche and adrenarche, but not with the age of menarche [130].
Other patterns of puberty and hypotheses of the pubertal variations seen in RTT have been proposed. For instance, in a large cohort of 494 ethnically homogeneous girls with RTT, precocious pubertal onset was noted, but with normal age of pubertal completion [132]. This study suggested that the presence of FYXD1 (also known as phospholemman, a gene that encodes a protein involved in the homeostatic regulation of cell function, including being a modulator of Na+, K+-ATPase activity) is increased in the frontal cortex of patients with RTT. This might contribute to the homeostatic system that modulates the excitatory trans-synaptic control of neuroendocrine neurons, including the GnRH neuronal network [132]. Garcia-Rudaz et al. demonstrated that the FYXD1 and FXYD7 genes are “expressed in the developing female rat and mouse hypothalamus, and that at least 50% of GnRH neurons contain FXYD1 mRNA, and that the absence of FYXD1 results in decreased GnRH neuronal excitability” [132].
The association between mutation and pubertal milestones varies among studies [85]. In a smaller study, MECP2 mutations did not affect age of pubertal onset [133]. However, in a larger study, R168X was associated with later adrenarche, thelarche, and menarche, R255X was associated with later menarche, and C-terminal mutations and early truncating mutations were associated with earlier thelarche and menarche [134]. More recently, a novel mutation in MECP2 has also described precocious puberty in two girls with atypical Rett syndrome [135].
6.6. Williams–Beuren Syndrome
Williams–Beuren syndrome (WBS) is a multi-systemic disorder that results from deletions from contiguous genes located on the long arm of chromosome 7 (7q11.23). Approximately 10–18% of individuals with this syndrome develop CPP [106], with menarche occurring two years earlier than in the general population [136]. The pathogenesis of CPP in WBS is unknown.
7. Future Directions
Advancing genetic technologies including GWAS, NGS panels, WES, and epigenetics have added to the growing knowledge of the genetic contributors to puberty and will facilitate future research and more accurate diagnosis. Other techniques such as genetic modeling and proteomics should be further studied in CPP to provide additional information regarding etiological mechanisms [72,121]. For instance, in one CPP study describing proteomic profiles, the authors conclude that the “altered members of the metabolic signaling are valuable and potential novel therapeutic targets of central precocious puberty” [137]. As we learn more about the neuroendocrine signaling, it may be possible to measure the protein molecules in the blood (e.g., kisspeptin or MKRN3) to make the diagnosis of CPP without the need for more expensive and complicated analysis [138,139].
Similar to the ever-expanding field of genetics, studies of the implications of EDCs on the HPG axis and pubertal onset have been growing over the past decades. Further studies of both known and new EDCs (e.g., plastics and microplastics) and of disrupting stimuli of other origins (e.g., excessive visual stimulation, emotional or physical stress, etc.) is required. Learning how EDCs impact neuroendocrine cells and transmitters can potentially give greater insight into their functioning. This could also serve as a platform for advocacy of reducing exposure to these toxic environmental agents.
In addition to the consideration of alternate GnRH analogs and neurotransmitters for treatment of CPP, there have been some steps towards using targeted treatments such as kisspeptin and NKB antagonists. Despite these advances, their use has been relatively limited by the lack of supporting studies and FDA approval, high cost, modes of administration, and side effects [140,141]. As more targets are identified, these could be further optimized and/or other treatments could be developed.
8. Conclusions
There has been much progress over the past decades towards a better understanding of the physiology of pubertal timing in animals and humans, as well as potential genetic and environmental contributors to CPP. Although most cases of CPP are still labeled as idiopathic, we are increasingly identifying underlying genetic and epigenetic underpinnings. Mutations in MKRN3 are now the most commonly identifiable genetic cause for CPP. Additionally, there have also been described clinical cases with causal associations attributed to mutations in KISS1/KISS1R, DLK1, and MECP2. A number of other genetic candidates (i.e., GABRA1, NKB, TAC3R, ob, NPY, LIN28B, and MAGEL2) warrant farther evaluation to elucidate their potential roles. The influences of EDCs on the increasing prevalence of CPP should also be clarified.
Author Contributions
Conceptualization, A.M. and E.C.; writing—original draft preparation, A.M. and E.C.; writing—review and editing, A.M. and E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosenfield, R.L.; Cooke, D.W.; Radovick, S. Puberty in the Female and Its Disorders. In Sperling Pediatric Endocrinology, 5th ed.; Sperling, M., Majzoub, J.A., Menon, R.K., Stratakis, C.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 528–626. [Google Scholar] [CrossRef]
- Palmert, M.R.; Chan, Y.-M.; Dunkel, L. Puberty and Its Disorders in the Male, 5th ed; Sperling, M., Majzoub, J.A., Menon, R.K., Stratakis, C.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Obstet. Gynecol. Surv. 1969, 25, 694–696. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bräuner, E.V.; Busch, A.S.; Eckert-Lind, C.; Koch, T.; Hickey, M.; Juul, A. Trends in the Incidence of Central Precocious Puberty and Normal Variant Puberty Among Children in Denmark, 1998 to 2017. JAMA Netw. Open 2020, 3, e2015665. [Google Scholar] [CrossRef]
- Teilmann, G.; Pedersen, C.B.; Jensen, T.K.; Skakkebaek, N.E.; Juul, A. Prevalence and incidence of precocious pubertal development in Denmark: An epidemiologic study based on national registries. Pediatrics 2005, 116, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Guillén, L.; Corripio, R.; Labarta, J.I.; Cañete, R.; Castro-Feijóo, L.; Espino, R.; Argente, J. Central precocious puberty in children living in Spain: Incidence, prevalence, and influence of adoption and immigration. J. Clin. Endocrinol. Metab. 2010, 95, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, J.; Rigou, A.; Le Tertre, A.; De Crouy-Channel, P.; Léger, J.; Carel, J.C. Marked geographic patterns in the incidence of idiopathic central precocious puberty: A nationwide study in France. Eur. J. Endocrinol. 2018, 178, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Huh, K.; Won, S.; Lee, K.W.; Park, M.J. A Significant Increase in the Incidence of Central Precocious Puberty among Korean Girls from 2004 to 2010. PLoS ONE 2015, 10, e0141844. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, A.; Jung, M.K.; Kim, K.E.; Suh, J.; Chae, H.W.; Kim, D.H.; Ha, S.; Seo, G.H.; Kim, H.S. Incidence and Prevalence of Central Precocious Puberty in Korea: An Epidemiologic Study Based on a National Database. J. Pediatr. 2019, 208, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, D.; Franssen, D.; Heger, S.; Parent, A.S. Endocrine-disrupting chemicals and their effects on puberty. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 1–12. [Google Scholar] [CrossRef]
- Spaziani, M.; Tarantino, C.; Tahani, N.; Gianfrilli, D.; Sbardella, E.; Lenzi, A.; Radicioni, A.F. Hypothalamo-Pituitary axis and puberty. Mol. Cell. Endocrinol. 2021, 520, 111094. [Google Scholar] [CrossRef] [PubMed]
- Buluş, A.D.; Aşci, A.; Erkekoglu, P.; Balci, A.; Andiran, N.; Koçer-Gümüşel, B. The evaluation of possible role of endocrine disruptors in central and peripheral precocious puberty. Toxicol. Mech. Methods 2016, 26, 493–500. [Google Scholar] [CrossRef]
- Rattan, S.; Flaws, J.A. The epigenetic impacts of endocrine disruptors on female reproduction across generations. Biol. Reprod. 2019, 101, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Butz, H.; Nyírő, G.; Kurucz, P.A.; Likó, I.; Patócs, A. Molecular genetic diagnostics of hypogonadotropic hypogonadism: From panel design towards result interpretation in clinical practice. Hum. Genet. 2021, 140, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kusa, T.O.; Chan, Y.M. Genetics of pubertal timing. Curr. Opin. Pediatr. 2018, 30, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Kaiser, U.B. Genetics in endocrinology genetic etiologies of central precocious puberty and the role of imprinted genes. Eur. J. Endocrinol. 2020, 183, R107–R117. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, R.S.; Eugster, E.A. Central precocious puberty: From genetics to treatment. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 343–354. [Google Scholar] [CrossRef]
- Manotas, M.C.; González, D.M.; Céspedes, C.; Forero, C.; Rojas Moreno, A.P. Genetic and Epigenetic Control of Puberty. Sex. Dev. 2021, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kuiri-Hänninen, T.; Sankilampi, U.; Dunkel, L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: Minipuberty. Horm. Res. Paediatr. 2014, 82, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Schwarz, H.P. Serum concentrations of LH and FSH in the healthy newborn. Eur. J. Endocrinol. 2000, 143, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Toro, C.A.; Aylwin, C.F.; Lomniczi, A. Hypothalamic epigenetics driving female puberty. J. Neuroendocrinol. 2018, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Uenoyama, Y.; Nagae, M.; Tsuchida, H.; Inoue, N.; Tsukamura, H. Role of KNDy Neurons Expressing Kisspeptin, Neurokinin B, and Dynorphin A as a GnRH Pulse Generator Controlling Mammalian Reproduction. Front. Endocrinol. 2021, 12, 724632. [Google Scholar] [CrossRef] [PubMed]
- Lebrethon, M.C.; Vandersmissen, E.; Gérard, A.; Parent, A.S.; Junien, J.L.; Bourguignon, J.P. In vitro stimulation of the prepubertal rat gonadotropin-releasing hormone pulse generator by leptin and neuropeptide Y through distinct mechanisms. Endocrinology 2000, 141, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Herman-Giddens, M.E.; Slora, E.J.; Wasserman, R.C.; Bourdony, C.J.; Bhapkar, M.V.; Koch, G.G.; Hasemeier, C.M. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the pediatric research in office settings network. Pediatrics 1997, 99, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Galvez, M.P.; Greenspan, L.C.; Succop, P.A.; Vangeepuram, N.; Pinney, S.M.; Teitelbaum, S.; Windham, G.C.; Kushi, L.H.; Wolff, M.S. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 2010, 126, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Reinehr, T.; Roth, C.L. Connections between obesity and puberty. Curr. Opin. Endocr. Metab. Res. 2020, 14, 160–168. [Google Scholar] [CrossRef]
- Sørensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Mogensen, S.S.; Juul, A. Recent secular trends in pubertal timing: Implications for evaluation and diagnosis of precocious puberty. Horm. Res. Paediatr. 2012, 77, 137–145. [Google Scholar] [CrossRef]
- Houk, C.P.; Kunselman, A.R.; Lee, P.A. Adequacy of a single unstimulated luteinizing hormone level to diagnose central precocious puberty in girls. Pediatrics 2009, 123, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Mucaria, C.; Tyutyusheva, N.; Baroncelli, G.; Peroni, D.; Bertelloni, S. Central Precocious Puberty in Boys and Girls: Similarities and Differences. Sexes 2021, 2, 119–131. [Google Scholar] [CrossRef]
- Ibanez, L.; Potau, N.; Zampolli, M.; Virdis, R.; Gussinyé, M.; Carrascosa, A.; Saenger, P.; Vicens-Calvet, E. Use of leuprolide acetate response patterns in the early diagnosis of pubertal disorders: Comparison with the gonadotropin-releasing hormone test. J. Clin. Endocrinol. Metab. 1994, 78, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Latronico, A.C.; Brito, V.N.; Carel, J.C. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016, 4, 265–274. [Google Scholar] [CrossRef]
- Talarico, V.; Rodio, M.B.; Viscomi, A.; Galea, E.; Galati, M.C.; Raiola, G. The role of pelvic ultrasound for the diagnosis and management of central precocious puberty: An update. Acta Biomed. 2021, 92, e2021480. [Google Scholar] [CrossRef] [PubMed]
- Badouraki, M.; Christoforidis, A.; Economou, I.; Dimitriadis, A.S.; Katzos, G. Evaluation of pelvic ultrasonography in the diagnosis and differentiation of various forms of sexual precocity in girls. Ultrasound Obstet. Gynecol. 2008, 32, 819–827. [Google Scholar] [CrossRef]
- Kaplowitz, P.B.; Slora, E.J.; Wasserman, R.C.; Pedlow, S.E.; Herman-Giddens, M.E. Earlier onset of puberty in girls: Relation to increased body mass index and race. Pediatrics 2001, 108, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Carel, J.C.; Eugster, E.A.; Rogol, A.; Ghizzoni, L.; Palmert, M.R. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009, 123, e752–e762. [Google Scholar] [CrossRef] [PubMed]
- Cantas-Orsdemir, S.; Garb, J.L.; Allen, H.F. Prevalence of cranial MRI findings in girls with central precocious puberty: A systematic review and meta-analysis. J. Pediatr. Endocrinol. Metab. 2018, 31, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Freire, A.; Gryngarten, M.G.; Kletter, G.B.; Benson, M.; Miller, B.S.; Dajani, T.S.; A Eugster, E.; Mauras, N. Phase 3 trial of a small-volume subcutaneous 6-month duration leuprolide acetate treatment for central precocious puberty. J. Clin. Endocrinol. Metab. 2020, 105, 3660–3671. [Google Scholar] [CrossRef]
- Hirsch, H.J.; Gillis, D.; Strich, D.; Chertin, B.; Farkas, A.; Lindenberg, T.; Gelber, H.; Spitz, I.M. The histrelin implant: A novel treatment for central precocious puberty. Pediatrics 2005, 116, e798–e802. [Google Scholar] [CrossRef]
- Lewis, K.A.; Goldyn, A.K.; West, K.W.; Eugster, E.A. A single histrelin implant is effective for 2 years for treatment of central precocious puberty. J. Pediatr. 2013, 163, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Lazar, L.; Padoa, A.; Phillip, M. Growth pattern and final height after cessation of gonadotropin-suppressive therapy in girls with central sexual precocity. J. Clin. Endocrinol. Metab. 2007, 92, 3483–3489. [Google Scholar] [CrossRef]
- Pasquino, A.M.; Pucarelli, I.; Accardo, F.; Demiraj, V.; Segni, M.; Di Nardo, R. Long-term observation of 87 girls with idiopathic central precocious puberty treated with gonadotropin-releasing hormone analogs: Impact on adult height, body mass index, bone mineral content, and reproductive function. J. Clin. Endocrinol. Metab. 2008, 93, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Conte, F.A.; Grumbach, M.M.; Kaplan, S.L. Long Term Effect of Gonadotropin-Releasing on Final and Near-Final Age of Less Than 5 Years. J. Clin. Endocrinol. Metab. 1995, 80, 546–551. [Google Scholar] [PubMed]
- Xhrouet-Heinrichs, D.; Lagrou, K.; Heinrichs, C.; Craen, M.; Dooms, L.; Malvaux, P.; Kanen, F.; Bourguignon, J.-P. Longitudinal study of behavioral and affective patterns in girls with central precocious puberty during long-acting triptorelin therapy. Acta Paediatr. Int. J. Paediatr. 1997, 86, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Schoelwer, M.J.; Donahue, K.L.; Didrick, P.; Eugster, E.A. One-Year Follow-Up of Girls with Precocious Puberty and Their Mothers: Do Psychological Assessments Change over Time or with Treatment? Horm. Res. Paediatr. 2017, 88, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liang, Y.; Hou, L.; Wu, W.; Ying, Y.; Ye, F. Long-term efficacy and safety of gonadotropin-releasing hormone analog treatment in children with idiopathic central precocious puberty: A systematic review and meta-analysis. Clin. Endocrinol. 2021, 94, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.; Ma, X.; Lee, L.T. Understanding the functions of kisspeptin and kisspeptin receptor (Kiss1R) from clinical case studies. Peptides 2019, 120, 170019. [Google Scholar] [CrossRef]
- Pinilla, L.; Aguilar, E.; Dieguez, C.; Millar, R.P.; Tena-Sempere, M. Kisspeptins and reproduction: Physiological roles and regulatory mechanisms. Physiol. Rev. 2012, 92, 1235–1316. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Babwah, A.V. Kisspeptin: Beyond the brain. Endocrinology 2015, 156, 1218–1227. [Google Scholar] [CrossRef]
- Martin, C.; Navarro, V.M.; Simavli, S.; Vong, L.; Carroll, R.S.; Lowell, B.B.; Kaiser, U.B. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J. Neurosci. 2014, 34, 6047–6056. [Google Scholar] [CrossRef] [PubMed]
- Cravo, R.M.; Frazao, R.; Perello, M.; Osborne-Lawrence, S.; Williams, K.; Zigman, J.M.; Vianna, C.; Elias, C.F. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS ONE 2013, 8, e58698. [Google Scholar] [CrossRef]
- Dungan, H.M.; Clifton, D.K.; Steiner, R.A. Minireview: Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 2006, 147, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Leka-Emiri, S.; Chrousos, G.P.; Kanaka-Gantenbein, C. The mystery of puberty initiation: Genetics and epigenetics of idiopathic central precocious puberty (ICPP). J. Endocrinol. Investig. 2017, 40, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.; Lippincott, M.; Chan, Y.-M.; Davies, A.; Merino, P.M.; Plummer, L.; Mericq, V.; Seminara, S. Discordance in the Dependence on Kisspeptin Signaling in Minipuberty vs Adolescent Puberty: Human Genetic Evidence. J. Clin. Endocrinol. Metab. 2018, 103, 1273–1276. [Google Scholar] [CrossRef]
- Liu, X.; Lee, K.; Herbison, A.E. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 2008, 149, 4605–4614. [Google Scholar] [CrossRef] [PubMed]
- De Roux, N.; Genin, E.; Carel, J.C.; Matsuda, F.; Chaussain, J.L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef] [PubMed]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef]
- Bianco, S.D.C.; Kaiser, U.B. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat. Rev. Endocrinol. 2009, 5, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.G.; Bianco, S.D.; Brito, V.N.; Trarbach, E.B.; Kuohung, W.; Xu, S.; Seminara, S.B.; Mendonca, B.B.; Kaiser, U.B.; Latronico, A.C. A GPR54 -Activating Mutation in a Patient with Central Precocious Puberty. N. Engl. J. Med. 2008, 358, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.G.; Noel, S.D.; Silveira-Neto, A.P.; Abreu, A.P.; Brito, V.N.; Santos, M.; Bianco, S.; Kuohung, W.; Xu, S.; Gryngarten, M.; et al. Mutations of the KISS1 gene in disorders of puberty. J. Clin. Endocrinol. Metab. 2010, 95, 2276–2280. [Google Scholar] [CrossRef] [PubMed]
- Pagani, S.; Calcaterra, V.; Acquafredda, G.; Montalbano, C.; Bozzola, E.; Ferrara, P.; Gasparri, M.; Villani, A.; Bozzola, M. MKRN3 and KISS1R mutations in precocious and early puberty. Ital. J. Pediatr. 2020, 46, 1–6. [Google Scholar] [CrossRef]
- Rhie, Y.J.; Lee, K.H.; Ko, J.M.; Lee, W.J.; Kim, J.H.; Kim, H.S. KISS1 gene polymorphisms in Korean girls with central precocious puberty. J. Korean Med. Sci. 2014, 29, 1120–1125. [Google Scholar] [CrossRef]
- Ghaemi, N.; Ghahraman, M.; Asl, S.N.; Vakili, R.; Golyan, F.F.; Moghbeli, M.; Abbaszadegan, M.R. Novel DNA variation of GPR54 gene in familial central precocious puberty. Ital. J. Pediatr. 2019, 45, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, Y.; Cheng, J.; Liu, L.; Li, X.; Chen, D.; Huang, S.; Wen, Y.; Ke, Y.; Yao, Y.; et al. Association of polymorphisms in the kisspeptin/GPR54 pathway genes with risk of early puberty in Chinese girls. J. Clin. Endocrinol. Metab. 2020, 105, E1458–E1467. [Google Scholar] [CrossRef] [PubMed]
- Özgen, I.T.; Torun, E.; Bayraktar-Tanyeri, B.; Durmaz, E.; Klllç, E.; Cesur, Y. The relation of urinary bisphenol A with kisspeptin in girls diagnosed with central precocious puberty and premature thelarche. J. Pediatr. Endocrinol. Metab. 2016, 29, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pino, F.; Miceli, D.; Franssen, D.; Vazquez, M.J.; Farinetti, A.; Castellano, J.M.; Panzica, G.; Tena-Sempere, M. Environmentally relevant perinatal exposures to bisphenol a disrupt postnatal Kiss1/NKB neuronal maturation and puberty onset in female mice. Env. Health Perspect. 2019, 127, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Di, T.; Cao, X.; Liu, Z.; Xie, J.; Zhang, S. Chronic exposure to perfluorohexane sulfonate leads to a reproduction deficit by suppressing hypothalamic kisspeptin expression in mice. J. Ovarian Res. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Penatti, C.A.A.; Oberlander, J.G.; Davis, M.C.; Porter, D.M.; Henderson, L.P. Chronic exposure to anabolic androgenic steroids alters activity and synaptic function in neuroendocrine control regions of the female mouse. Neuropharmacology 2011, 61, 653–664. [Google Scholar] [CrossRef]
- Li, C.; Lu, W.; Yang, L.; Li, Z.; Zhou, X.; Guo, R.; Wang, J.; Wu, Z.; Dong, Z.; Ning, G.; et al. MKRN3 regulates the epigenetic switch of mammalian puberty via ubiquitination of MBD3. Natl. Sci. Rev. 2020, 7, 671–685. [Google Scholar] [CrossRef]
- Abreu, A.P.; Toro, C.A.; Song, Y.B.; Navarro, V.M.; Bosch, M.A.; Eren, A.; Liang, J.N.; Carroll, R.S.; Latronico, A.C.; Ronnekleiv, O.K.; et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J. Clin. Investig. 2020, 140, 4486–4500. [Google Scholar] [CrossRef]
- Meader, B.N.; Albano, A.; Sekizkardes, H.; Delaney, A. Heterozygous Deletions in MKRN3 Cause Central Precocious Puberty without Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 2732–2739. [Google Scholar] [CrossRef]
- Neocleous, V.; Fanis, P.; Toumba, M.; Gorka, B.; Kousiappa, I.; Tanteles, G.A.; Iasonides, M.; Nicolaides, N.C.; Christou, Y.P.; Michailidou, K.; et al. Pathogenic and Low-Frequency Variants in Children With Central Precocious Puberty. Front. Endocrinol. 2021, 12, 1187. [Google Scholar] [CrossRef]
- Fanis, P.; Skordis, N.; Toumba, M.; Papaioannou, N.; Makris, A.; Kyriakou, A.; Neocleous, V.; Phylactou, L.A. Central Precocious Puberty Caused by Novel Mutations in the Promoter and 5′-UTR Region of the Imprinted MKRN3 Gene. Front. Endocrinol. 2019, 10, 677. [Google Scholar] [CrossRef]
- Abreu, A.P.; Dauber, A.; Macedo, D.B.; Noel, S.D.; Brito, V.N.; Gill, J.C.; Cukier, P.; Thompson, I.R.; Navarro, V.M.; Gagliardi, P.C.; et al. Central Precocious Puberty Caused by Mutations in the Imprinted Gene MKRN3. N. Engl. J. Med. 2013, 368, 2467–2475. [Google Scholar] [CrossRef]
- Valadares, L.P.; Meireles, C.; De Toledo, I.P.; De Oliveira, R.S.; de Castro, L.C.G.; Abreu, A.P.; Carroll, R.S.; Latronico, A.C.; Kaiser, U.B.; Guerra, E.N.S.; et al. MKRN3 mutations in central precocious puberty: A systematic review and meta-analysis. J. Endocr. Soc. 2019, 3, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Seraphim, C.E.; Canton, A.P.M.; Montenegro, L.; Piovesan, M.R.; Macedo, D.B.; Cunha, M.; Guimaraes, A.; Ramos, C.O.; Benedetti, A.F.F.; Leal, A.D.C.; et al. Genotype-Phenotype Correlations in Central Precocious Puberty Caused by MKRN3 Mutations. J. Clin. Endocrinol. Metab. 2021, 106, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.S.; Sang, L.H.; Hwang, J.S. Genetic Factors in Precocious Puberty. Clin. Exp. Pediatr. 2021, 65, 172–181. [Google Scholar] [CrossRef]
- Andersson, E.R.; Sandberg, R.; Lendahl, U. Notch signaling: Simplicity in design, versatility in function. Development 2011, 138, 3593–3612. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.B.; Kaiser, U.B. DLK1, Notch Signaling and the Timing of Puberty. Semin. Reprod. Med. 2019, 37, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.R.B.; Australian Ovarian Cancer Study; Day, F.; Elks, C.E.; Sulem, P.; Thompson, D.J.; Ferreira, T.; He, C.; Chasman, D.I.; Esko, T.; et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 2014, 514, 92–97. [Google Scholar] [CrossRef]
- Dauber, A.; Cunha-Silva, M.; Macedo, D.B.; Brito, V.N.; Abreu, A.P.; Roberts, S.A.; Montenegro, L.R.; Andrew, M.; Kirby, A.; Weirauch, M.T.; et al. Paternally Inherited DLK1 deletion associated with familial central precocious puberty. J. Clin. Endocrinol. Metab. 2017, 102, 1557–1567. [Google Scholar] [CrossRef]
- Kagami, M.; Nagasaki, K.; Kosaki, R.; Horikawa, R.; Naiki, Y.; Saitoh, S.; Tajima, T.; Yorifuji, T.; Numakura, C.; Mizuno, S.; et al. Temple syndrome: Comprehensive molecular and clinical findings in 32 Japanese patients. Genet. Med. 2017, 19, 1356–1366. [Google Scholar] [CrossRef]
- D’Mello, S.R. MECP2 and the biology of MECP2 duplication syndrome. J. Neurochem. 2021, 159, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, S.; Chahrour, M.; Thaller, C.; Shaw, C.A.; Zoghbi, H.Y. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum. Mol. Genet. 2009, 18, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Westberry, J.M.; Trout, A.L.; Wilson, M.E. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology 2010, 151, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Zheng, Y.F.; Wang, H.Y. MeCP2 duplication causes hyperandrogenism by upregulating LHCGR and downregulating ROR. Cell Death Dis. 2021, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Tsuji-Hosokawa, A.; Matsuda, N.; Kurosawa, K.; Kashimada, K.; Morio, T. A Case of MECP2 Duplication Syndrome with Gonadotropin-Dependent Precocious Puberty. Horm. Res. Paediatr. 2017, 87, 271–276. [Google Scholar] [CrossRef]
- Parent, A.S.; Matagne, V.; Bourguignon, J.P. Control of puberty by excitatory amino acid neurotransmitters and its clinical implications. Endocrine 2005, 28, 281–285. [Google Scholar] [CrossRef]
- Terasawa, E.; Garcia, J.P. Neuroendocrine mechanisms of puberty in non–human primates. Curr. Opin. Endocr. Metab. Res. 2020, 14, 145–151. [Google Scholar] [CrossRef]
- Brito, V.N.; Mendonca, B.B.; Guilhoto, L.M.F.F.; Monteiro Freitas, K.C.; Prado Arnhold, I.J.; Latronico, A.C. Allelic variants of the γ-aminobutyric acid-A receptor α1-subunit gene (GABRA1) are not associated with idiopathic gonadotropin-dependent precocious puberty in girls with and without electroencephalographic abnormalities. J. Clin. Endocrinol. Metab. 2006, 91, 2432–2436. [Google Scholar] [CrossRef][Green Version]
- Defazio, R.A.; Elias, C.F.; Moenter, S.M. GABAergic transmission to kisspeptin neurons is differentially regulated by time of day and estradiol in female mice. J. Neurosci. 2014, 34, 16296–16308. [Google Scholar] [CrossRef]
- Franssen, D.; Gérard, A.; Hennuy, B.; Donneau, A.F.; Bourguignon, J.P.; Parent, A.S. Delayed neuroendocrine sexual maturation in female rats after a very low dose of bisphenol a through altered gabaergic neurotransmission and opposing effects of a high dose. Endocrinology 2016, 157, 1740–1750. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, D.; Franssen, D.; Bakker, J.; Lomniczi, A.; Parent, A.S. Cellular and molecular features of EDC exposure: Consequences for the GnRH network. Nat. Rev. Endocrinol. 2021, 17, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tan, J.; Xu, X.; Yang, H.; Wu, F.; Xu, B.; Liu, W.; Shi, P.; Xu, Z.; Deng, Y. Prepubertal overexposure to manganese induce precocious puberty through GABAA receptor/nitric oxide pathway in immature female rats. Ecotoxicol. Environ. Saf. 2020, 188, 109898. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, A.K.; Reimann, F.; Guclu, M.; Yalin, A.S.; Kotan, L.D.; Porter, K.M.; Serin, A.; O Mungan, N.; Cook, J.R.; Ozbek, M.N.; et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat. Genet. 2009, 41, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Gianetti, E.; Tusset, C.; Noel, S.D.; Au, M.G.; Dwyer, A.; Hughes, V.A.; Abreu, A.P.; Carroll, J.; Trarbach, E.; Silveira, L.; et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin- releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J. Clin. Endocrinol. Metab. 2010, 95, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.G.; Silveira, L.F.G.; Tusset, C.; Latronico, A.C. New genetic factors implicated in human GnRH-dependent precocious puberty: The role of kisspeptin system. Mol. Cell. Endocrinol. 2011, 346, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhang, J.; Chang, Y.; Wu, Y. Association Study of TAC3 and TACR3 gene polymorphisms with idiopathic precocious puberty in Chinese girls. J. Pediatr. Endocrinol. Metab. 2015, 28, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Tusset, C.; Noel, S.D.; Trarbach, E.B.; Silveira, L.F.; Jorge, A.A.; Brito, V.N.; Cukier, P.; Seminara, S.B.; de Mendonça, B.B.; Kaiser, U.B.; et al. Mutational Analysis of TAC3 and TACR3 Genes in Patients with Idiopathic Central Pubertal Disorders Cintia. Arq. Bras. Endocrinol. Metab. 2012, 56, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Groenendyk, J.; Dabrowska, M.; Michalak, M. Mutational Analysis of TAC and TACR3 in Central Precocious Puberty. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 35, 129–132. [Google Scholar] [CrossRef][Green Version]
- Eckert-Lind, C.; Busch, A.S.; Petersen, J.H.; Biro, F.M.; Butler, G.; Bräuner, E.; Juul, A. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development among Girls: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 174, e195881. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Roth, C.L. The link between obesity and puberty: What is new? Curr. Opin. Pediatr. 2021, 33, 449–457. [Google Scholar] [CrossRef]
- Walley, S.N.; Roepke, T.A. Perinatal exposure to endocrine disrupting compounds and the control of feeding behavior—An overview. Horm. Behav. 2018, 101, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Frisch, R.E. Pubertal adipose tissue: Is it necessary for normal sexual maturation? Evidence from the rat and human female. Fed. Proc. 1980, 39, 2395–2400. [Google Scholar] [PubMed]
- Frisch, R.E.; Revelle, R.; Cook, S. Components of weight at menarche and the initiation of the adolescent growth spurt in girls: Estimated total water, lean body weight and fat. Hum. Biol. 1973, 45, 469–483. [Google Scholar]
- Shalitin, S.; Kiess, W. Putative effects of obesity on linear growth and puberty. Horm. Res. Paediatr. 2017, 88, 101–110. [Google Scholar] [CrossRef]
- MacKay, H.; Patterson, Z.R.; Abizaid, A. Perinatal exposure to low-dose bisphenol-a disrupts the structural and functional development of the hypothalamic feeding circuitry. Endocrinology 2017, 158, 768–777. [Google Scholar] [CrossRef]
- Plant, T.M.; Barker-Gibb, M.L. Neurobiological mechani87sms of puberty in higher primates. Hum. Reprod. Update 2004, 10, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Freitas, K.C.M.; Ryan, G.; Brito, V.N.; Tao, Y.-X.; Costa, E.M.F.; Mendonca, B.B.; Segaloff, D.; Latronico, A.C. Molecular analysis of the neuropeptide Y1 receptor gene in human idiopathic gonadotropin-dependent precocious puberty and isolated hypogonadotropic hypogonadism. Fertil. Steril. 2007, 87, 627–634. [Google Scholar] [CrossRef]
- Barker-Gibb, M.; Plant, T.M.; White, C.; Lee, P.A.; Feldman Witchel, S. Genotype analysis of the neuropeptide Y (NPY) Y1 and NPY Y5 receptor genes in gonadotropin-releasing hormone-dependent precocious gonadarche. Fertil. Steril. 2004, 82, 491–494. [Google Scholar] [CrossRef]
- MacKay, H.; Patterson, Z.R.; Khazall, R.; Patel, S.; Tsirlin, D.; Abizaid, A. Organizational effects of perinatal exposure to bisphenol-a and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice. Endocrinology 2013, 154, 1465–1475. [Google Scholar] [CrossRef]
- Ong, K.K.; Elks, C.E.; Li, S.; Zhao, J.H.; Luan, J.; Andersen, L.B.; Bingham, A.S.; Brage, S.; Smith, D.G.; Ekelund, U.; et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat. Genet. 2009, 41, 729–733. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, R.; Cai, C. Association of genetic polymorphisms around the LIN28B gene and idiopathic central precocious puberty risks among Chinese girls. Pediatr. Res. 2016, 80, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Tommiska, J.; Sørensen, K.; Aksglaede, L.; Koivu, R.; Puhakka, L.; Juul, A.; Raivio, T. LIN28B, LIN28A, KISS1, and KISS1R in idiopathic central precocious uberty. BMC Res. Notes 2011, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.E.; Wevrick, R. Loss of Magel2, a candidate gene for features of Prader- Willi syndrome, impairs reproductive function in mice. PLoS ONE 2009, 4, e4291. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Potts, P.R. Cellular and disease functions of the Prader-Willi Syndrome gene MAGEL2. Biochem. J. 2018, 474, 2177–2190. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cho, S.Y.; Jin, D.-K. Prader-Willi syndrome: An update on obesity and endocrine problems. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Canton, A.P.M.; Seraphim, C.E.; Brito, V.N.; Latronico, A.C. Pioneering studies on monogenic central precocious puberty. Arch. Endocrinol. Metab. 2019, 63, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Correa-da-Silva, F.; Fliers, E.; Swaab, D.F.; Yi, C.X. Hypothalamic neuropeptides and neurocircuitries in Prader Willi syndrome. J. Neuroendoc. 2021, 33, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kanber, D.; Giltay, J.; Wieczorek, D.; Zogel, C.; Hochstenbach, R.; Caliebe, A.; Kuechler, A.; Horsthemke, B.; Buiting, K. A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader-Willi syndrome. Eur. J. Hum. Genet. 2009, 17, 582–590. [Google Scholar] [CrossRef]
- Wakeling, E.L.; Brioude, F.; Lokulo-Sodipe, O.; O’Connell, S.M.; Salem, J.; Bliek, J.; Canton, A.P.M.; Chrzanowska, K.H.; Davies, J.H.; Dias, R.P.; et al. Diagnosis and management of Silver-Russell syndrome: First international consensus statement. Nat. Rev. Endocrinol. 2017, 13, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.M. Endocrine manifestations of neurofibromatosis in children. Am. J. Dis. Child. 1970, 120, 265–271. [Google Scholar] [CrossRef]
- Cnossen, M.H.; Stam, E.N.; Cooiman, L.C.M.G.; Simonsz, H.J.; Stroink, H.; Oranje, A.P.; Halley, D.J.J.; de Goede-Bolder, A.; Niermeijer, M.F.; Keizer-Schrama, S.M.P.F.D.M. Endocrinologic disorders and optic pathway gliomas in children with neurofibromatosis type 1. Pediatrics 1997, 100, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Habiby, R.; Silverman, B.; Listernick, R.; Charrow, J. Precocious puberty in children with neurofibromatosis type 1. J. Pediatr. 1995, 126, 364–367. [Google Scholar] [CrossRef]
- Grumbach, M.; Kaplan, S. The neuroendocrinology of human puberty: An ontogenetic perspective. In Control of the Onset of Puberty; Sizonenko, P., Aubert, M., Eds.; William & Wilkins: Philadelphia, PA, USA, 1990; pp. 1–68. [Google Scholar]
- Laue, L.; Comite, F.; Hench, K.; Loriaux, D.L.; Cutler, G.B.; Pescovitz, O.H. Precocious puberty associated with neurofibromatosis and optic gliomas. Treatment with luteinizing hormone releasing hormone analogue. Am. J. Dis. Child. 1985, 139, 1097–1100. [Google Scholar] [CrossRef]
- Listernick, R.; Darling, C.; Greenwald, M.; Strauss, L.; Charrow, J. Optic pathway tumors in children: The effect of neurofibromatosis type 1 on clinical manifestations and natural history. J. Pediatr. 1995, 127, 718–722. [Google Scholar] [CrossRef]
- Bizzarri, C.; Bottaro, G. Endocrine implications of neurofibromatosis 1 in childhood. Horm. Res. Paediatr. 2015, 83, 232–241. [Google Scholar] [CrossRef]
- Killian, J.T.; Lane, J.B.; Cutter, G.R.; Skinner, S.A.; Kaufmann, W.E.; Tarquinio, D.C.; Glaze, D.G.; Motil, K.J.; Neul, J.M.; Percy, A.K. Pubertal Development in Rett Syndrome Deviates from Typical Females. Pediatr. Neurol. 2014, 51, 769–775. [Google Scholar] [CrossRef]
- Huppke, P.; Roth, C.; Christen, H.J.; Brockmann, K.; Hanefeld, F. Endocrinological study on growth retardation in Rett syndrome. Acta Paediatr. 2001, 90, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rudaz, C.; Deng, V.; Matagne, V.; Ronnekleiv, O.K.; Bosch, M.; Han, V.; Ojeda, S.R. FXYD1, a modulator of Na,K-ATPase activity, facilitates female sexual development by maintaining gonadotrophin-releasing hormone neuronal excitability. J. Neuroendocrinol. 2009, 21, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Tarquinio, D.C.; Motil, K.J.; Hou, W.; Lee, H.-S.; Glaze, D.G.; Skinner, S.A.; Neul, J.L.; Annese, F.; McNair, L.; Barrish, J.O.; et al. Growth failure and outcome in Rett syndrome: Specific growth references. Neurology 2012, 79, 1653–1661. [Google Scholar] [CrossRef]
- Knight, O.; Bebbington, A.; Siafarikas, A.; Woodhead, H.; Girdler, S.; Leonard, H. Pubertal trajectory in females with Rett syndrome: A population-based study. Brain Dev. 2013, 35, 912–920. [Google Scholar] [CrossRef]
- Bernstein, U.; Demuth, S.; Puk, O.; Eichhorn, B.; Schulz, S. Novel MECP2 Mutation c.1162_1172del; p.Pro388* in Two Patients with Symptoms of Atypical Rett Syndrome. Mol. Syndromol. 2019, 10, 223–228. [Google Scholar] [CrossRef]
- Pober, B.R. Williams–Beuren Syndrome. New Eng. 2010, 362, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Q.; Yuan, K.; He, M.; Zhu, J.; Fang, Y.; Hu, J.; Yan, Q. The first central precocious puberty proteomic profiles revealed multiple metabolic networks and novel key disease-associated proteins. Aging 2021, 13, 24236–24250. [Google Scholar] [CrossRef] [PubMed]
- Cintra, R.G.; Wajnsztejn, R.; Trevisan, C.M.; Zaia, V.; Laganà, A.S.; Bianco, B.; Montagna, E. Kisspeptin Levels in Girls with Precocious Puberty: A Systematic Review and Meta-Analysis. Horm. Res. Paediatr. 2020, 93, 589–598. [Google Scholar] [CrossRef]
- Ge, W.; Wang, H.L.; Shao, H.J.; Liu, H.W.; Xu, R.Y. Evaluation of serum makorin ring finger protein 3 (MKRN3) levels in girls with idiopathic central precocious puberty and premature thelarche. Physiol. Res. 2020, 69, 127–133. [Google Scholar] [CrossRef]
- Blumenfeld, Z. Investigational and experimental GnRH analogs and associated neurotransmitters. Expert Opin Investig. Drugs 2017, 26, 661–667. [Google Scholar] [CrossRef]
- Newton, C.L.; Anderson, R.C.; Millar, R.P. Therapeutic neuroendocrine agonist and antagonist analogs of hypothalamic neuropeptides as modulators of the hypothalamic-pituitary-gonadal axis. Endocr. Dev. 2016, 30, 106–129. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
