Enhancing Photocatalytic Hydrogen Evolution with Oxygen Vacancy-Modified P/Ag/Ag2O/Ag3PO4/TiO2 by Using Optimized NaBH4 Reduction Strategy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic H2 Evolution Rate and Stability of R-PAgT Photocatalysts
2.1.1. First Cycle H2 Evolution Rate of R-PAgT Photocatalysts
2.1.2. Cyclic Experiment of R-PAgT Photocatalysts
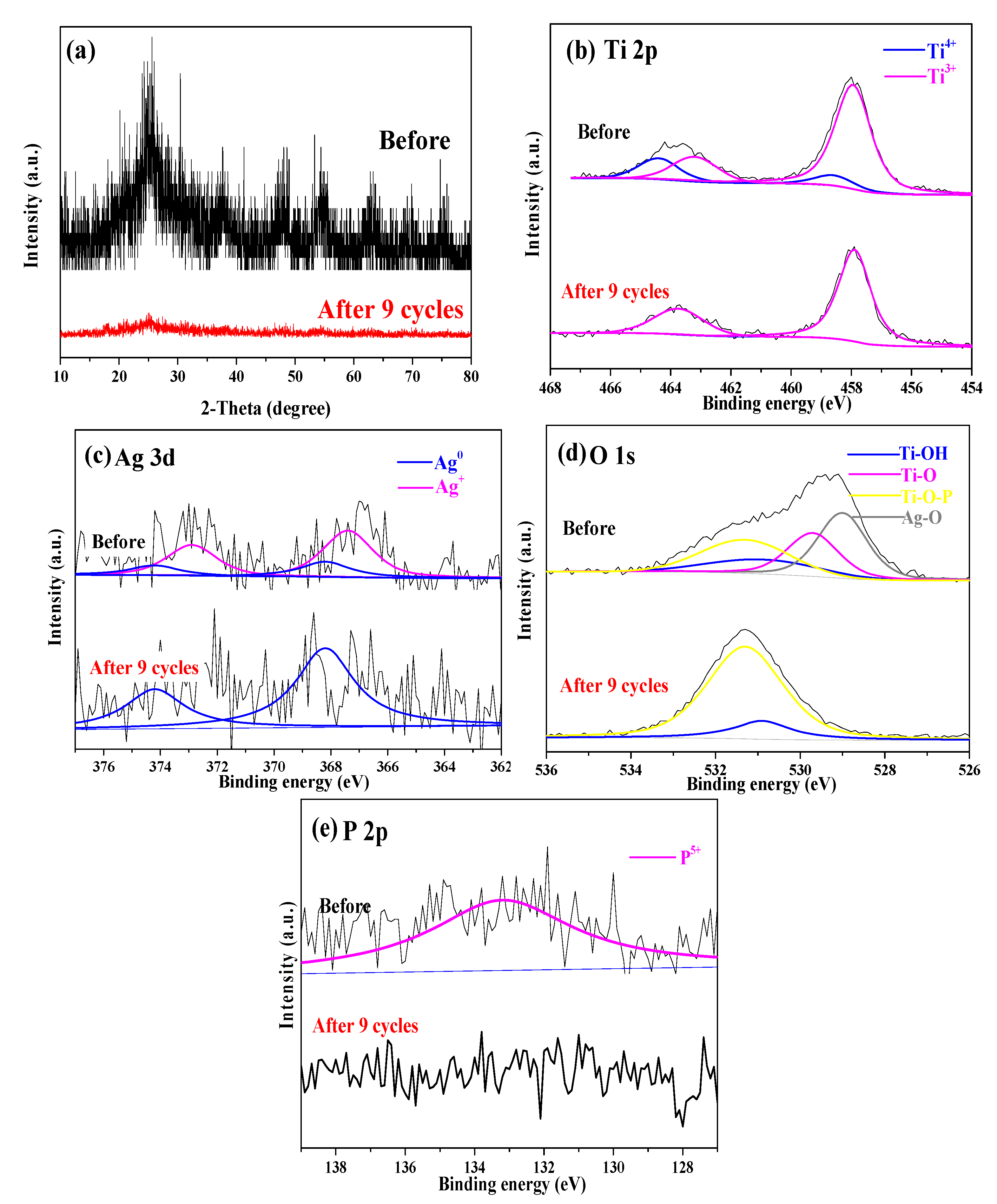
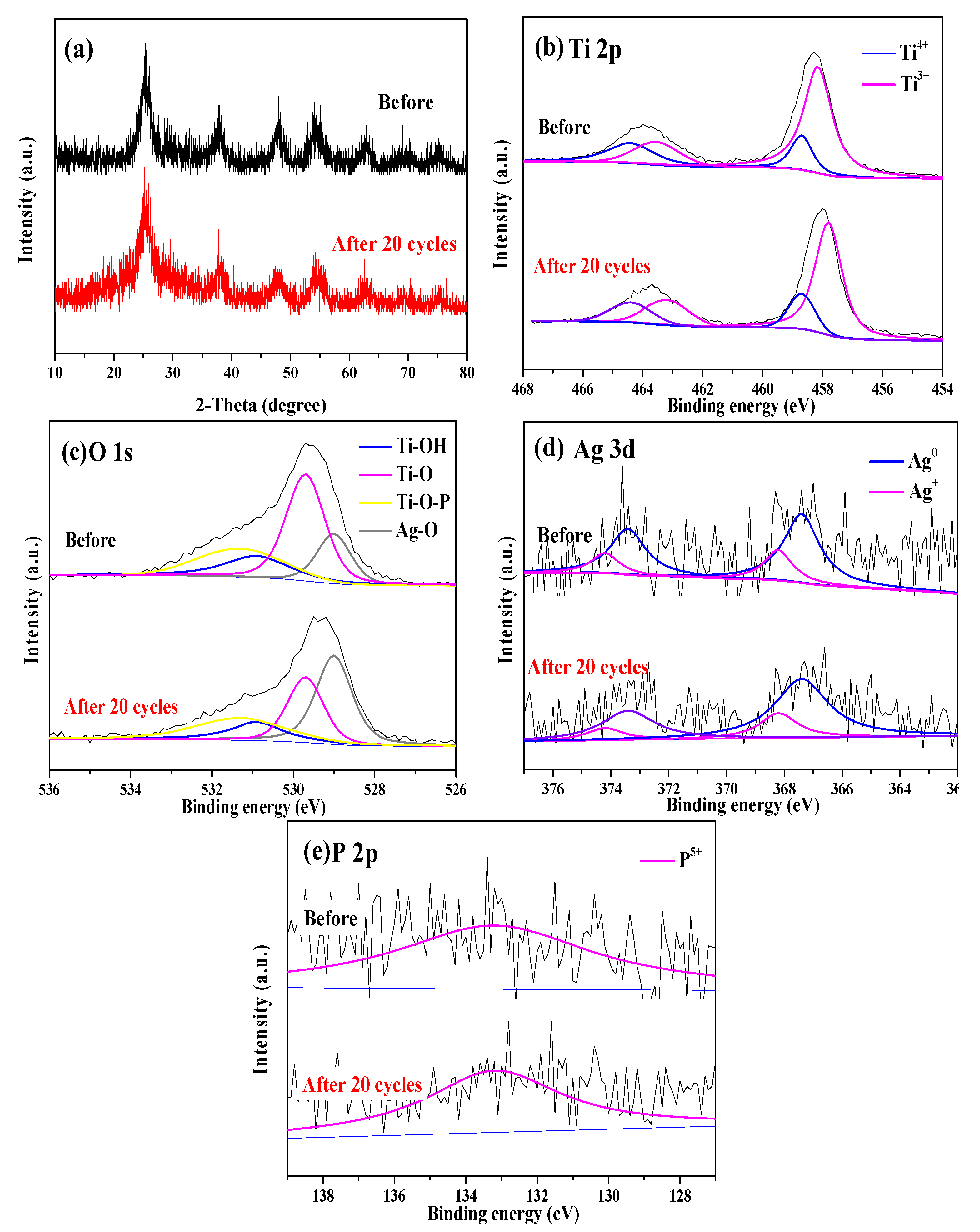
2.2. Characterization of R-PAgT Photocatalysts
2.2.1. Crystal Structures of R-PAgT Photocatalysts
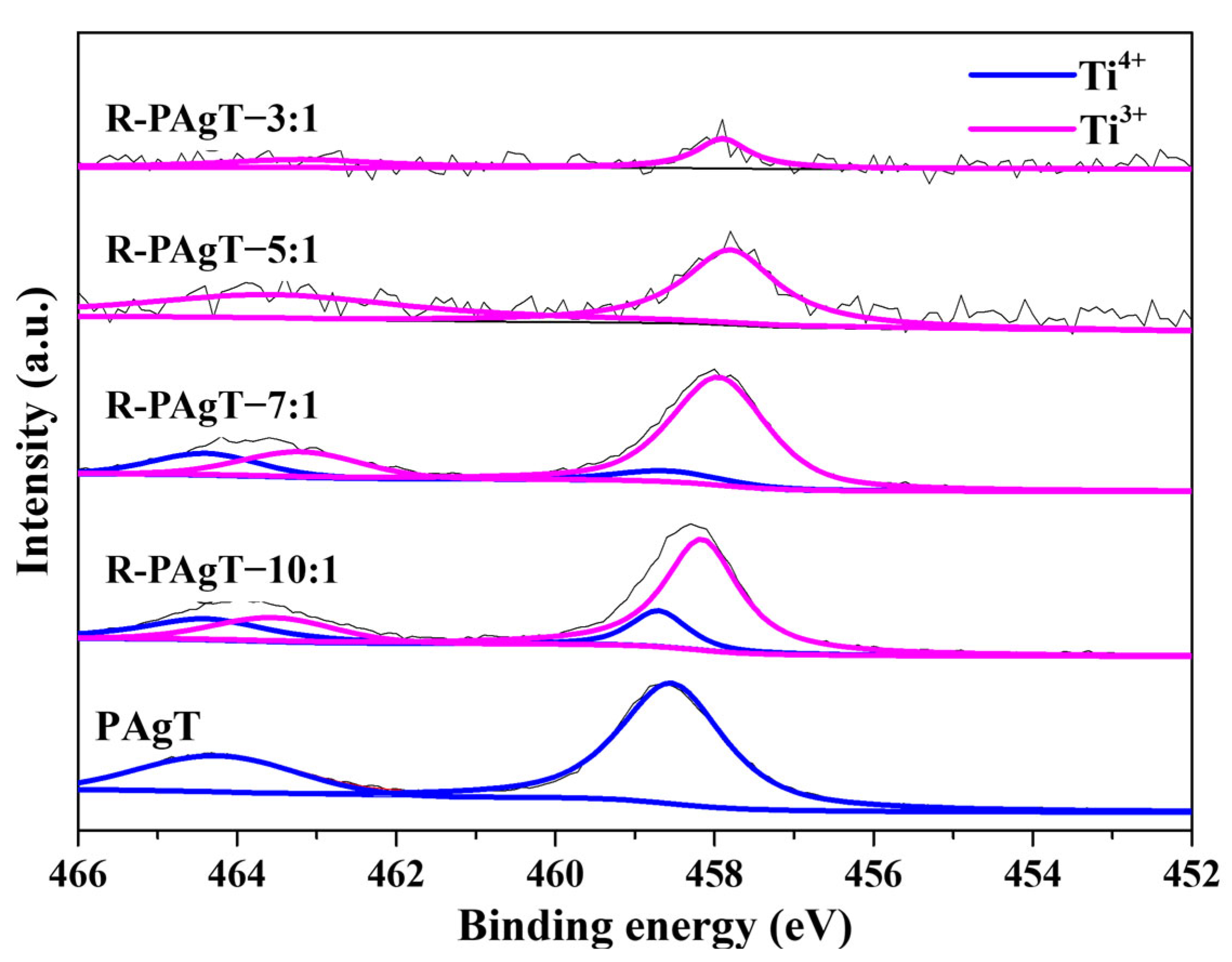
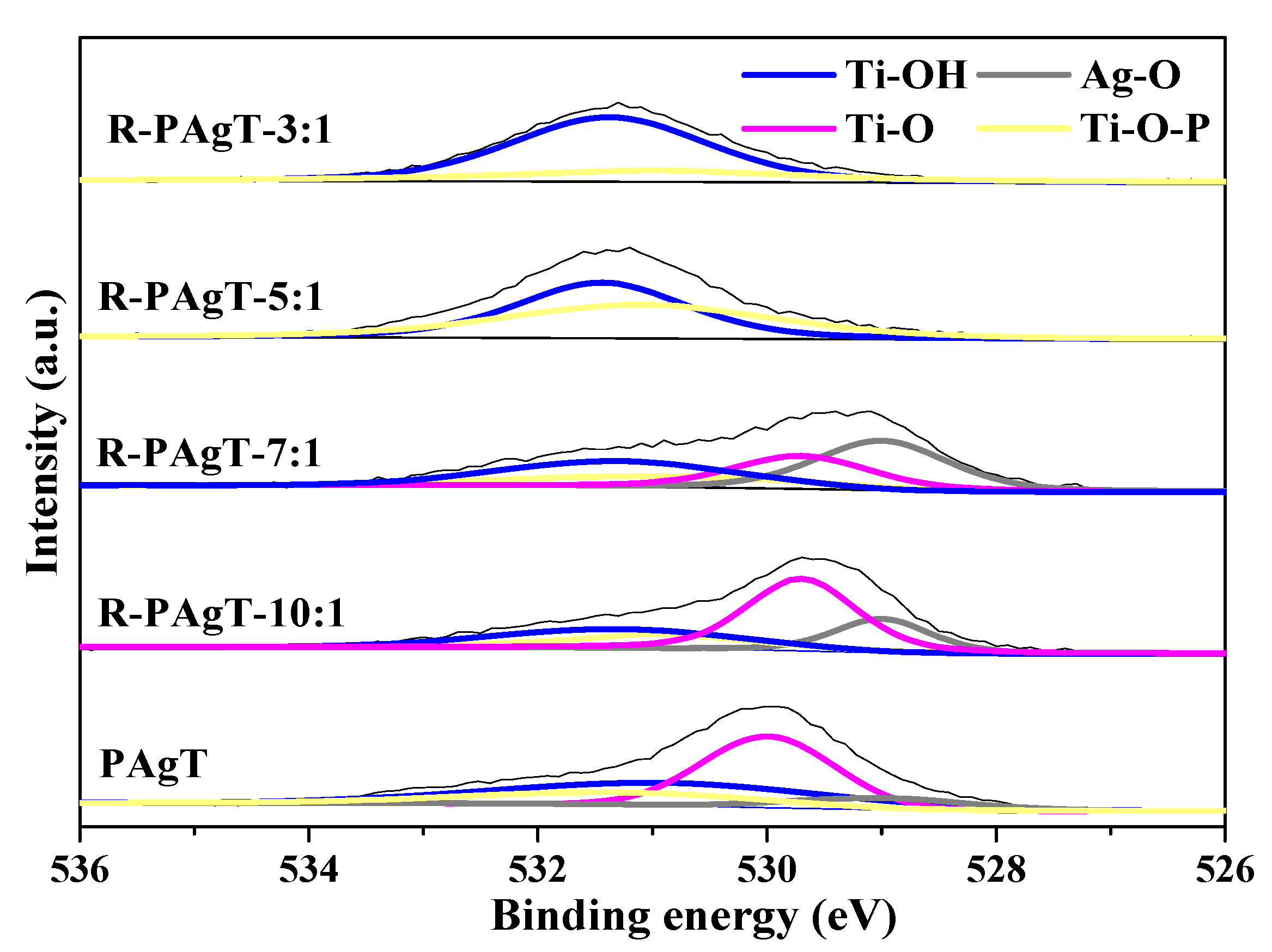
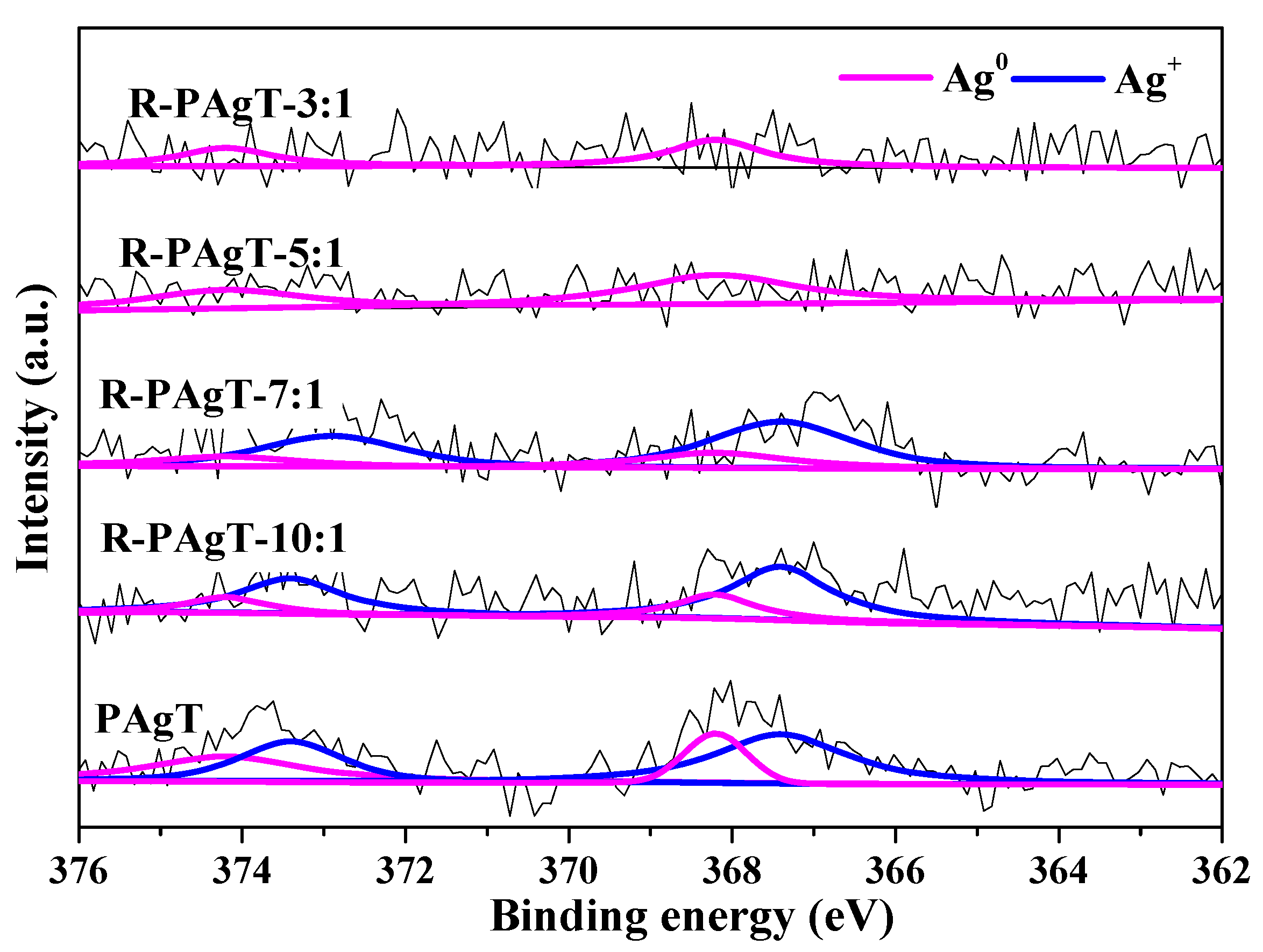
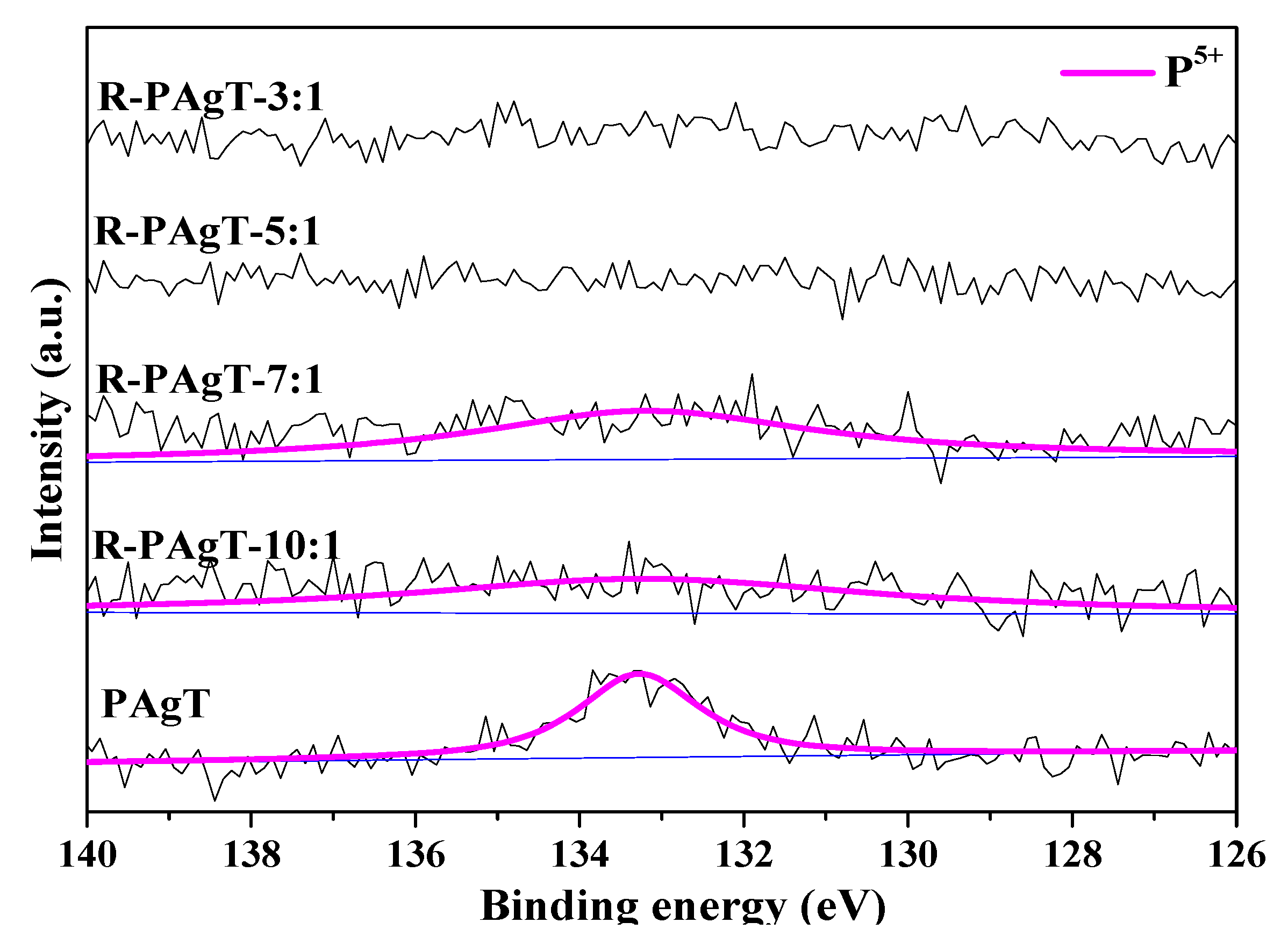
2.2.2. Chemical Bonds of R-PAgT Photocatalysts
2.2.3. Elemental Composition of R-PAgT Photocatalysts
2.3. Different Performances of R-PAgT-7:1 and R-PAgT-10:1
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the PAgT Photocatalyst
3.3. Preparation of R-PAgT Composite with Different Mass Ratios of PAgT to NaBH4
3.4. Characterizations
3.5. H2 Evolution Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Jiang, H.; Huo, P.; Edelmannová, M.F.; Čapek, L.; Kočí, K. Hydrogen production from methanol-water mixture over NiO/TiO2 nanorods structure photocatalysts. J. Environ. Chem. Eng. 2022, 10, 106908. [Google Scholar] [CrossRef]
- Subhasish, M.; Lopamudra, A.; Sharmila, S.; Kali, S.; Rashmi, A. Designing g-C3N4/NiFe2O4 S-scheme heterojunctions for efficient photocatalytic degradation of Rhodamine B and tetracycline hydrochloride. Appl. Surf. Sci. Adv. 2024, 24, 100647. [Google Scholar]
- Qiu, B.; Cai, L.; Zhang, N.; Tao, X.; Chai, Y. A ternary dumbbell structure with spatially separated catalytic sites for photocatalytic overall water splitting. Adv. Sci. 2020, 7, 1903568. [Google Scholar] [CrossRef]
- Akira, F.; Kenichi, H. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar]
- Roberto, F.; Marianna, B.; Salvatore, S.; Leonardo, P. Photocatalytic H2 production over inverse opal TiO2 catalysis. Catal. Today 2019, 312, 113–119. [Google Scholar]
- Devipriya, G.; Ashutosh, N.; Animes, G.; Nageswara, P. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45, 2729–2744. [Google Scholar]
- Shen, M.; Wang, M.; Wang, Q.; Tian, J.; Zhang, L.; Wang, L.; Shi, J. A Ti-OH bond breaking route for creating oxygen vacancy in titania towards efficient CO2 photoreduction. Chem. Eng. J. 2021, 425, 131513. [Google Scholar] [CrossRef]
- Rashmi, A.; Kulamani, P. A review on TiO2/g-C3N4 visible-light- responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar]
- Zhu, Q.; Na, L.; Ma, Q.; Sharma, A.; Nagai, D.; Sun, X.; Zhang, C.; Yang, Y. Sol-gel/hydrothermal two-step synthesis strategy for promoting Ag species–modified TiO2-based composite activity toward H2 evolution under solar light. Mater. Today Energy 2021, 20, 100648. [Google Scholar] [CrossRef]
- Bad’ura, Z.; Naldoni, A.; Qin, S.; Bakandritsos, A.; Kment, S.; Schmuki, P.; Zoppellaro, G. Light-Induced Migration of Spin Defects in TiO2 Nanosystems and their Contribution to the H2 Evolution Catalysis from Water. ChemSusChem 2021, 14, 4408–4414. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Guo, C.Y.; Liu, X.; Li, L.; Wang, L.; Xu, H.L.; Zhang, D.K.; Li, C.H.; Li, Q.; Wang, W.T. Ag nanoparticles anchored organic/inorganic Z-scheme 3DOMM-TiO2–based heterojunction for efficient photocatalytic and photoelectrochemical water splitting. Chin. J. Catal. 2022, 43, 1360–1370. [Google Scholar] [CrossRef]
- Shah, M.W.; Zhu, Y.; Fan, X.; Zhao, J.; Li, Y.; Asim, S.; Wang, C. Facile synthesis of defective TiO2-x nanocrystals with high surface area and tailoring bandgap for visible-light photocatalysis. Sci. Rep. 2015, 5, 15804. [Google Scholar]
- Huang, R.; Li, X.; Gao, W.; Zhang, X.; Liang, S.; Luo, M. Recent advances in photocatalytic nitrogen fixation: From active sites to ammonia quantification methods. RSC Adv. 2021, 11, 14844–14861. [Google Scholar] [CrossRef]
- Ariyanti, D.; Mills, L.; Dong, J.; Yao, Y.; Gao, W. NaBH4 modified TiO2: Defect site enhancement related to its photocatalytic activity. Mater. Chem. Phys. 2017, 199, 571–576. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Shi, R.; Wang, B.; Waterhouse, G.I.N.; Wu, L.; Tung, C.; Zhang, T. Tuning oxygen vacancies in ultrathin TiO2 nanosheets to boost photocatalytic nitrogen fixation up to 700 nm. Adv. Mater. 2019, 31, 1806482. [Google Scholar] [CrossRef]
- Andrea, L.; Patricia, R.; Carokina, G.; Rodrigo, S.; Patricio, V.; Paula, Z.; Pedro, O. FTIR and Raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Tobaldi, D.; Pullar, R.; Škapin, A.; Seabra, M.; Labrincha, J. Visible light activated photocatalytic behavior of rare earth modified commercial TiO2. Mater. Res. Bull. 2014, 50, 183–190. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, H.; Wang, H.; Xue, G.; Tian, Z.; Zhang, J.; Yuan, S.; Yu, T.; Zou, Z. The role of oxygen vacancy-Ti3+ states on TiO2 nanotubes’ surface in dye-sensitized solar cells. Mater. Lett. 2013, 100, 51–53. [Google Scholar] [CrossRef]
- Hayat, K.; Zhuoran, J.; Dimitrios, B. Molybdenum doped graphene/TiO2 hybrid photocatalyst for UV/visible photocatalytic applications. Solar Energy 2018, 162, 420–430. [Google Scholar]
- Jiang, X.; Zhang, Y.; Jiang, J.; Rong, Y.; Wang, Y.; Wu, Y.; Pan, C. Characterization of oxygen vacancy associates within hydrogenated TiO2: A Positron Annihilation Study. J. Phy. Chem. C 2012, 116, 22619–22624. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Q.; Wang, X.; Kawazoe, N.; Yang, Y. Nonmetal–metal–semiconductor-promoted P/Ag/Ag2O/Ag3PO4/TiO2 photocatalyst with superior photocatalytic activity and stability. J. Mater. Chem. A 2015, 3, 17858–17865. [Google Scholar] [CrossRef]
- Na, L.; Ming, J.; Sharma, A.; Sun, X.; Kawazoe, N.; Chen, G.; Yang, Y. Sustainable photocatalytic disinfection of four representative pathogenic bacteria isolated from real water environment by immobilized TiO2-based composite and its mechanism. Chem. Eng. J. 2021, 42, 6131217. [Google Scholar]
- Gao, Y.; Wang, T. Preparation of Ag2O/TiO2 nanocomposites by two-step method and study of its degradation of RHB. J. Mol. Struct. 2021, 1224, 129049. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Luo, Q.; An, J.; Li, X.; Yin, R.; Zhao, M. Enhanced visible-light photocatalytic performances of Ag3PO4 surface-modified with small amounts of TiO2 and Ag. Appl. Sur. Sci. 2014, 321, 439–446. [Google Scholar] [CrossRef]
- Sun, X.; Ming, J.; Ma, Q.; Zhang, C.; Zhu, Y.; An, G.; Chen, G.; Yang, Y. Fabrication of optimal oxygen vacancy amount in P/Ag/Ag2O/Ag3PO4/TiO2 through a green photoreduction process for sustainable H2 evolution under solar light. J. Colloid. Interf. Sci. 2023, 645, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Fu, X.; Chen, S.F.; Zhu, Y.F. Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl. Phys. Lett. 2011, 99, 191903. [Google Scholar] [CrossRef]
- Hu, X.; Ma, Q.; Wang, X.; Yang, Y. Layered Ag/Ag2O/BiPO4/Bi2WO6 heterostructures by two-step method for enhanced photocatalysis. J. Catal. 2020, 387, 28–38. [Google Scholar] [CrossRef]
- Guan, R.; Zhai, H.; Li, J.; Qi, Y. Reduced mesoporous TiO2 with Cu2S heterojunction and enhanced hydrogen production without noble metal cocatalyst. Appl. Surf. Sci. 2020, 507, 144772. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, Y.; Xu, Z.; Li, G.; Huang, X.; Hao, W. Direct Observation of Oxygen Vacancy Self-Healing on TiO2 Photocatalysts for Solar Water Splitting. Angewandte Chemie 2019, 58, 14229–14233. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Perng, T.-P. Fabrication and band structure of Ag3PO4–TiO2 heterojunction with enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 149–159. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhu, Y.; An, G.; Chen, G.; Yang, Y. Enhancing Photocatalytic Hydrogen Evolution with Oxygen Vacancy-Modified P/Ag/Ag2O/Ag3PO4/TiO2 by Using Optimized NaBH4 Reduction Strategy. Catalysts 2025, 15, 167. https://doi.org/10.3390/catal15020167
Sun X, Zhu Y, An G, Chen G, Yang Y. Enhancing Photocatalytic Hydrogen Evolution with Oxygen Vacancy-Modified P/Ag/Ag2O/Ag3PO4/TiO2 by Using Optimized NaBH4 Reduction Strategy. Catalysts. 2025; 15(2):167. https://doi.org/10.3390/catal15020167
Chicago/Turabian StyleSun, Xiang, Yunxin Zhu, Guangqi An, Guoping Chen, and Yingnan Yang. 2025. "Enhancing Photocatalytic Hydrogen Evolution with Oxygen Vacancy-Modified P/Ag/Ag2O/Ag3PO4/TiO2 by Using Optimized NaBH4 Reduction Strategy" Catalysts 15, no. 2: 167. https://doi.org/10.3390/catal15020167
APA StyleSun, X., Zhu, Y., An, G., Chen, G., & Yang, Y. (2025). Enhancing Photocatalytic Hydrogen Evolution with Oxygen Vacancy-Modified P/Ag/Ag2O/Ag3PO4/TiO2 by Using Optimized NaBH4 Reduction Strategy. Catalysts, 15(2), 167. https://doi.org/10.3390/catal15020167







