The Effect of Vacuum Annealing on the Structural, Electric, and Optical Properties, and Photocatalytic Activities of Sputtered TiO2 and Nb-Doped TiO2 Films
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Analysis
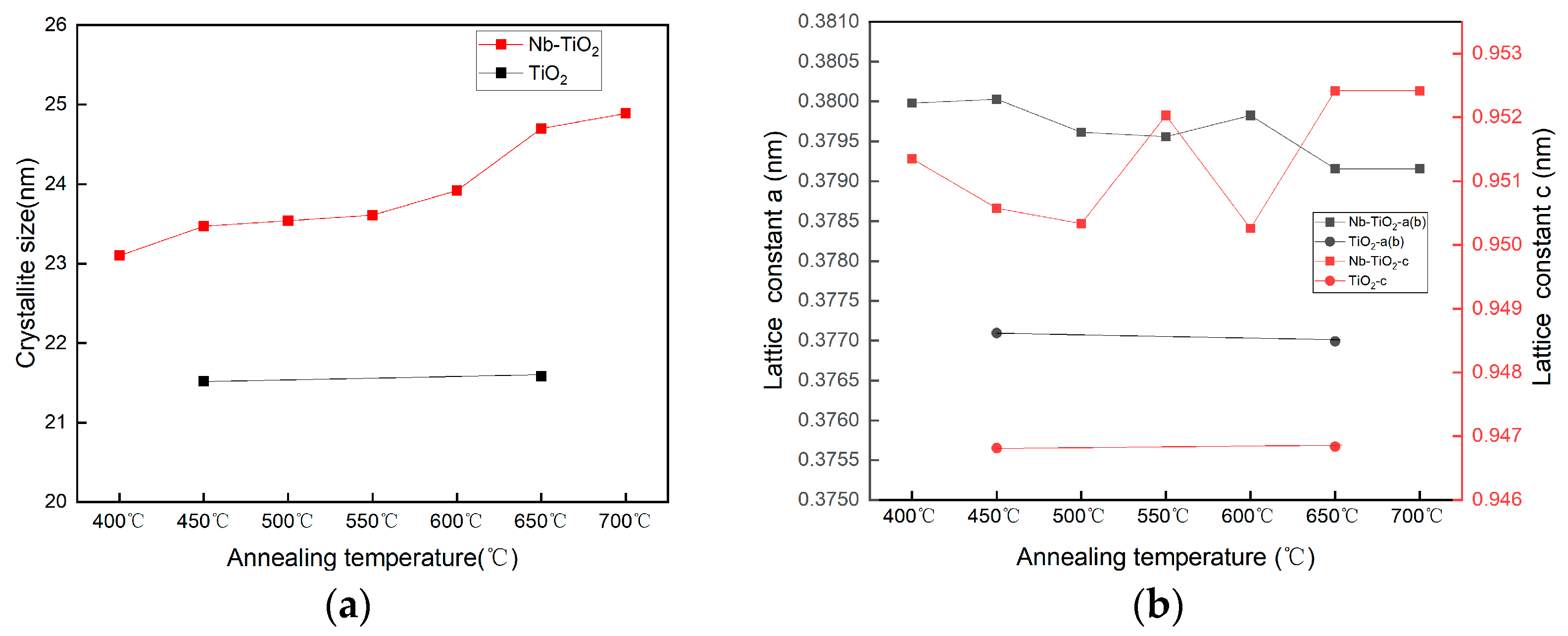
2.1.1. XRD Analysis
2.1.2. FE-SEM Analysis
2.1.3. Raman Analysis
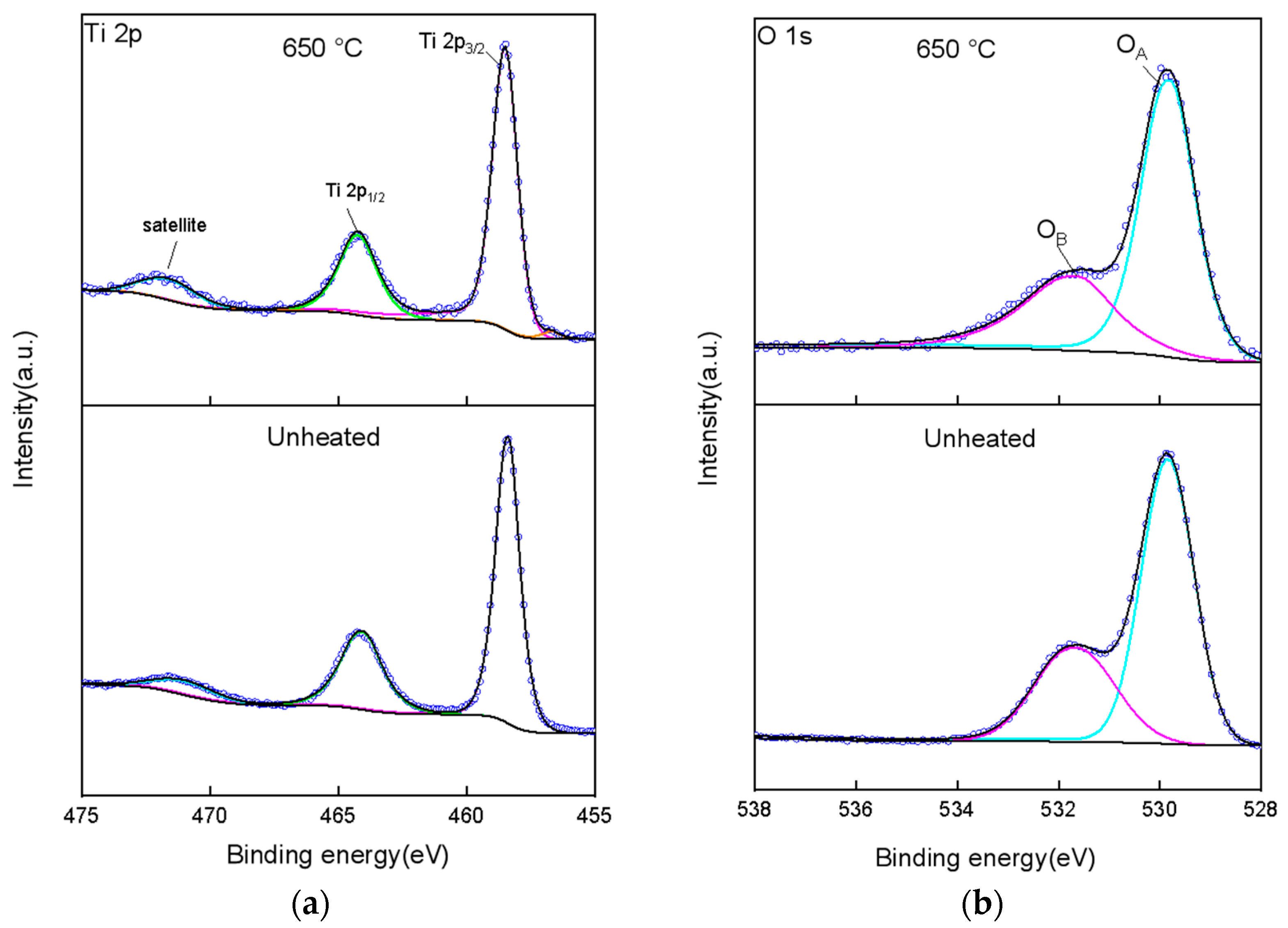
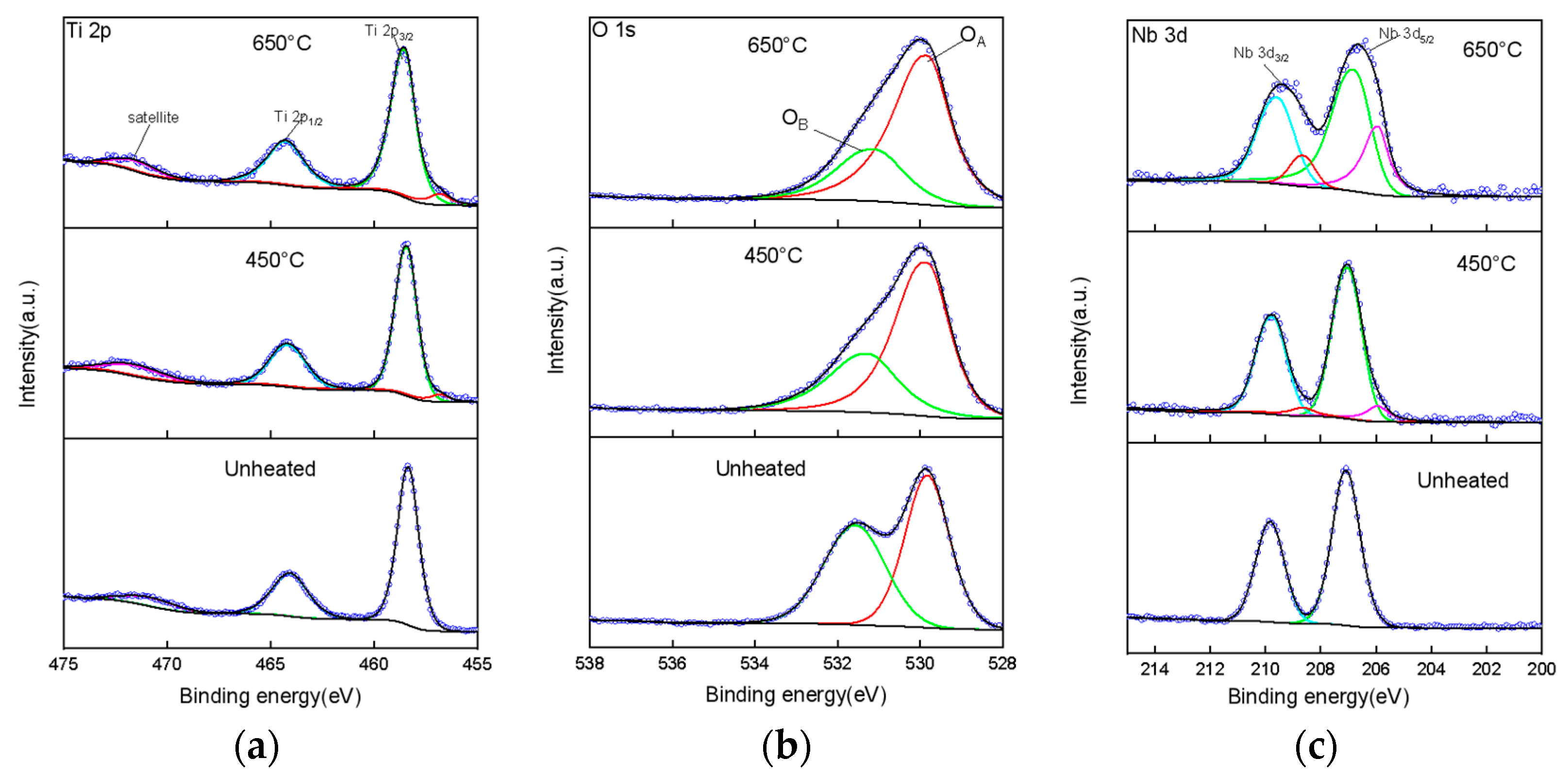
2.1.4. XPS Analysis
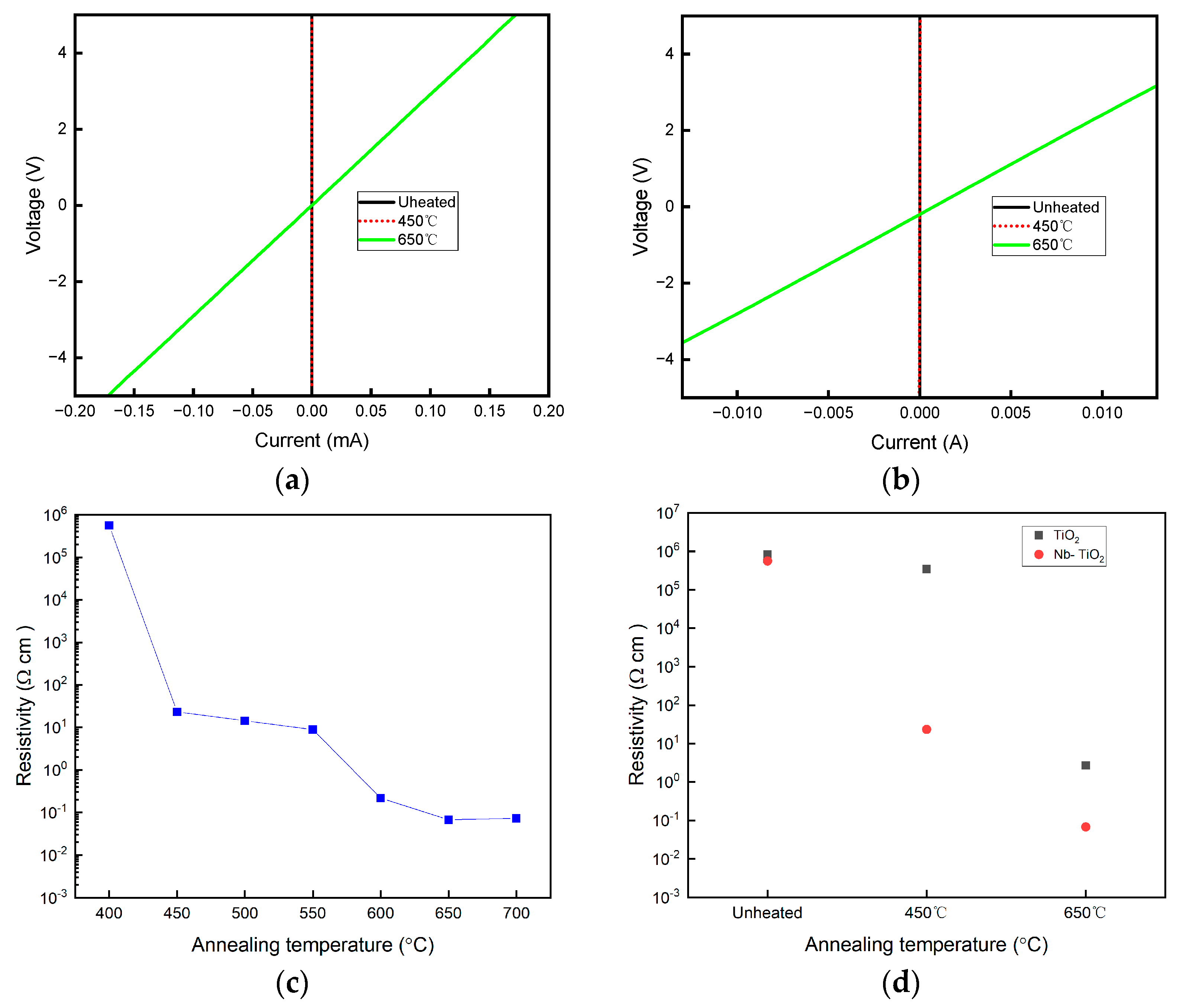
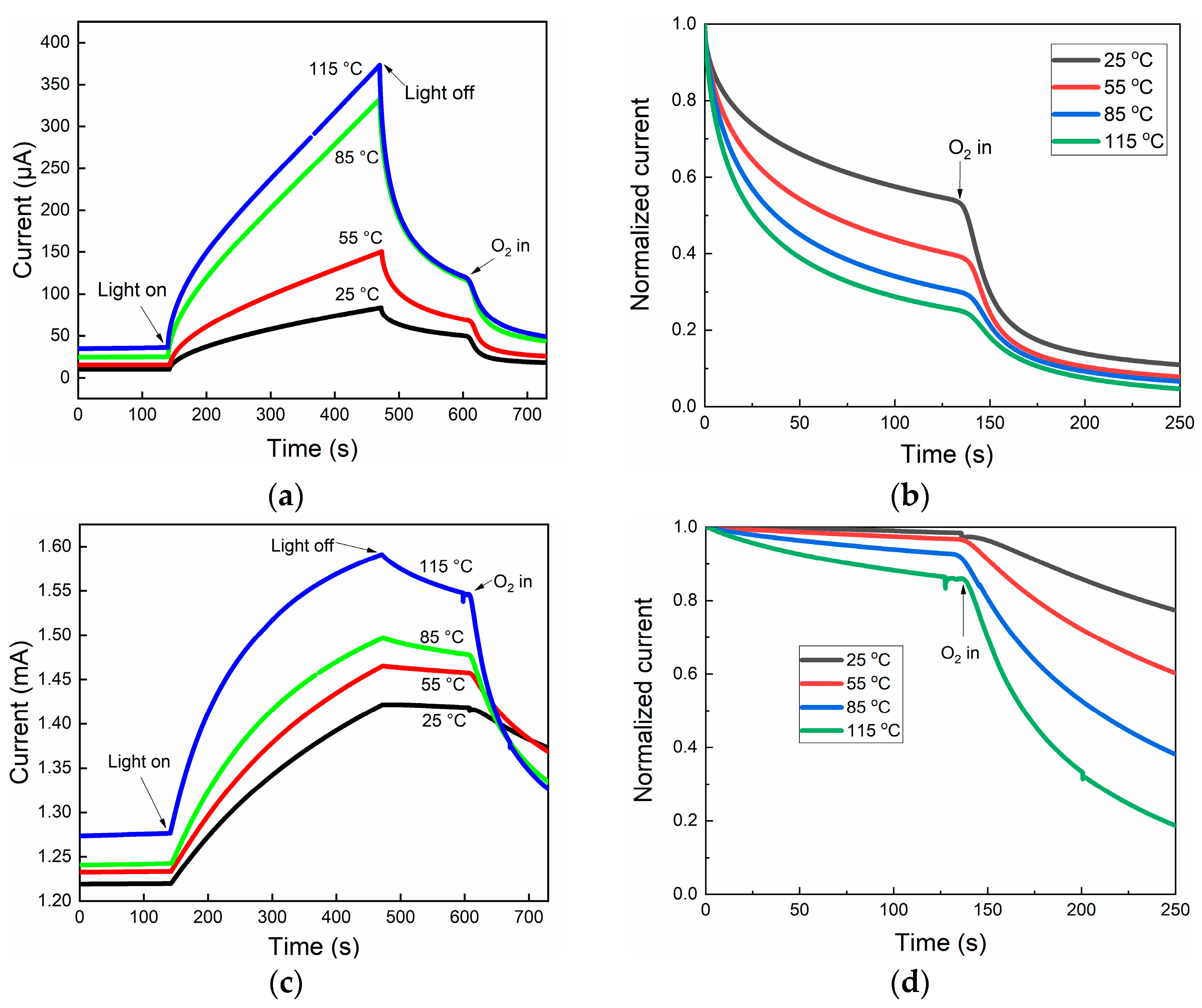
2.2. Electric Property Analysis
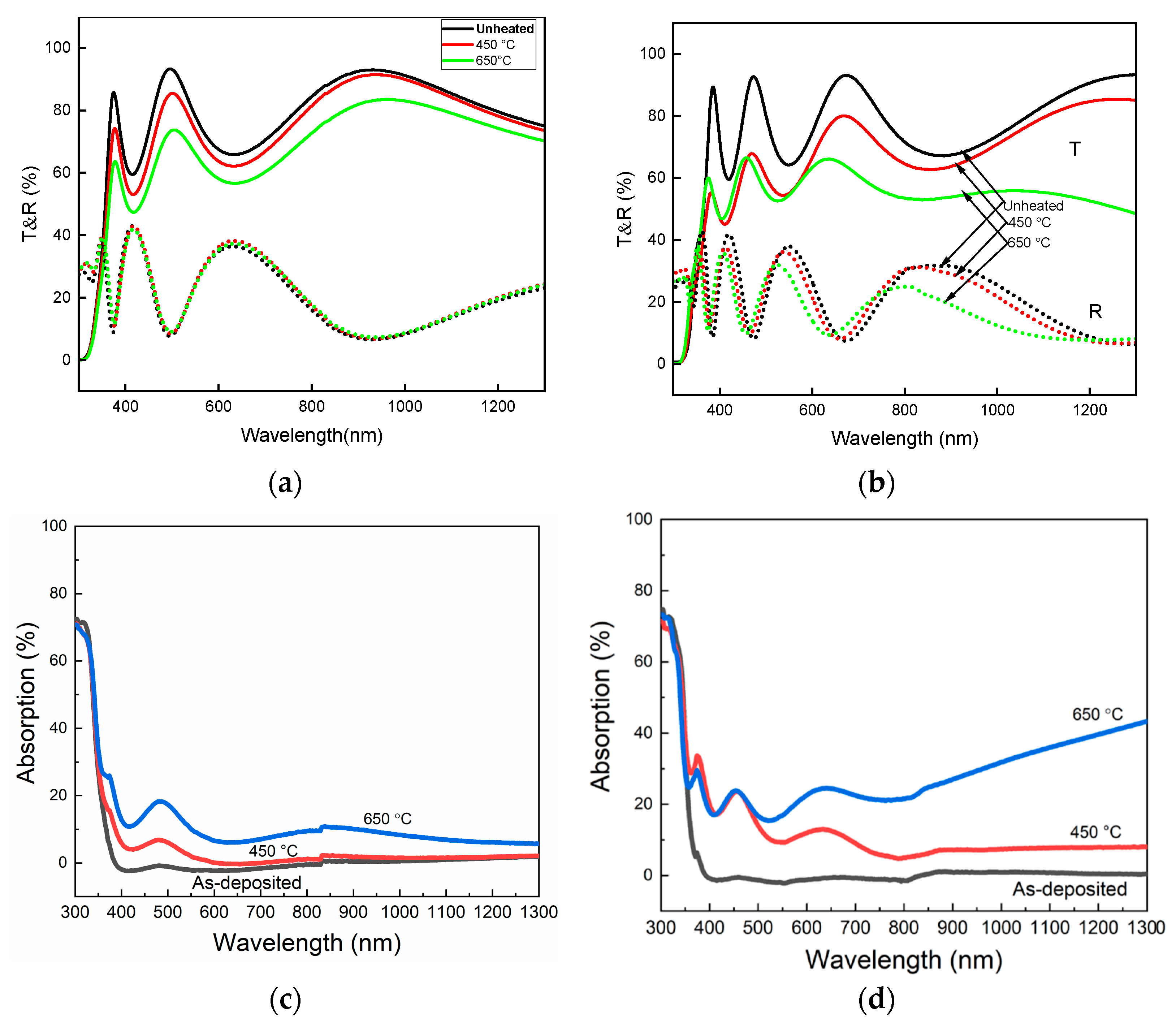
2.3. Optical Property Analysis
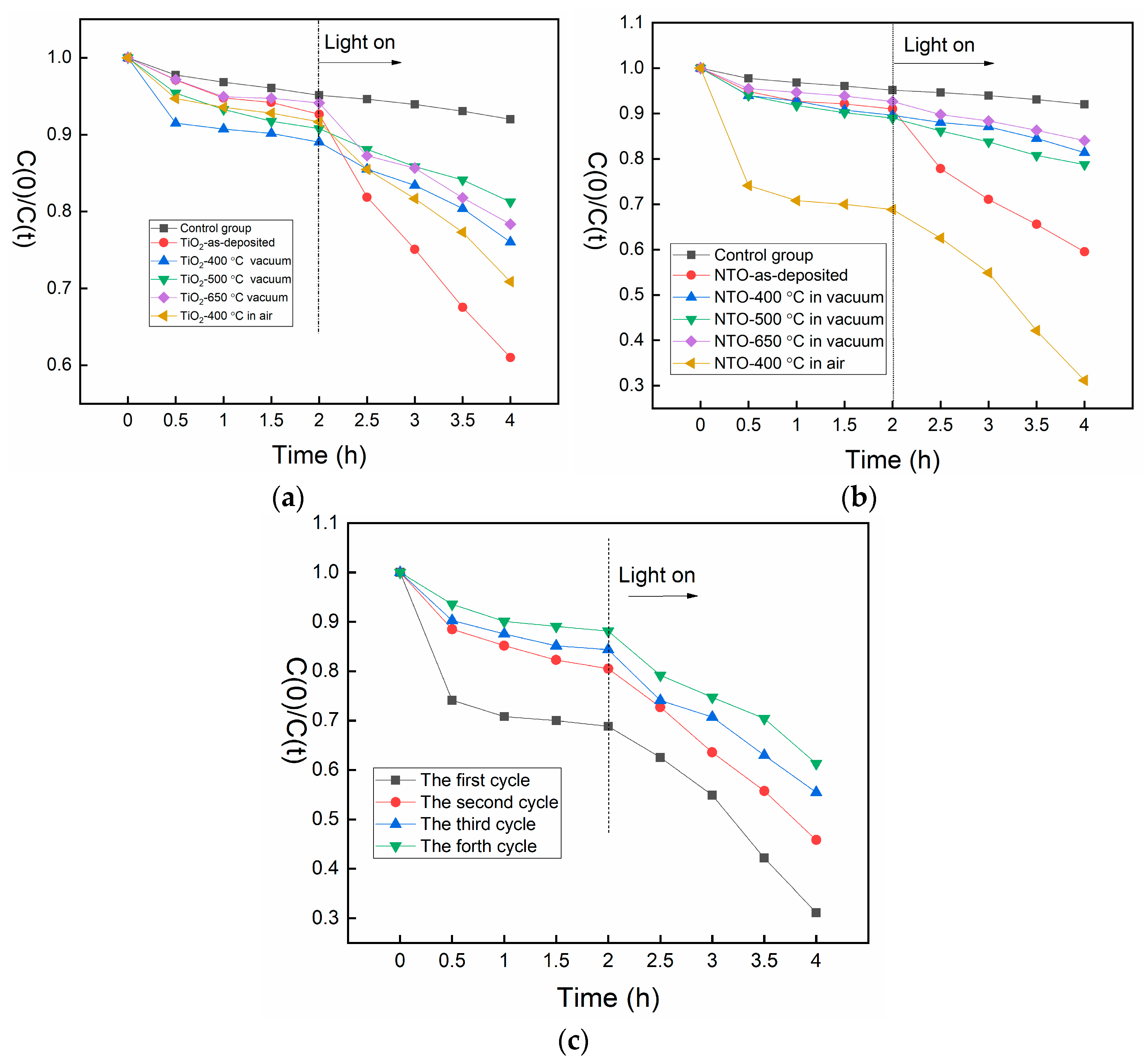
2.4. Photocatalytic Performance Analysis
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.Y.; Conte, M.; Anpo, M.; Tang, Z.R.; Xu, Y.J. Cooperative Coupling of Oxidative Organic Synthesis and Hydrogen Production over Semiconductor-Based Photocatalysts. Chem. Rev. 2021, 121, 13051–13085. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in Semiconductor-based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30, 1704696. [Google Scholar] [CrossRef]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 reduction. Nat. Rev. Methods Primers 2023, 3, 61. [Google Scholar] [CrossRef]
- Ge, M.; Cai, J.; Iocozzia, J.; Cao, C.; Huang, J.; Zhang, X.; Shen, J.; Wang, S.; Zhang, S.; Zhang, K.-Q.; et al. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrogen Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Hejazi, S.; Mohajernia, S.; Osuagwu, B.; Zoppellaro, G.; Andryskova, P.; Tomanec, O.; Kment, S.; Zboril, R.; Schmuki, P. On the Controlled Loading of Single Platinum Atoms as a Co-Catalyst on TiO2 Anatase for Optimized Photocatalytic H2 Generation. Adv. Mater. 2020, 32, 1908505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Gao, F.; Tan, X.; Cai, Y.; Hu, B.; Huang, Q.; Fang, M.; Wang, X. Application of MOFs and COFs for photocatalysis in CO2 reduction, H2 generation, and environmental treatment. EnergyChem 2022, 4, 100078. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Wang, L.; Yu, X.; Sun, J.; Tang, H. Unraveling the Roles of Hot Electrons and Cocatalyst toward Broad Spectrum Photocatalytic H2 Generation of g-C3N4 Nanotube. SolarRRL 2021, 5, 2000504. [Google Scholar]
- Yue, X.; Hou, J.; Zhao, H.; Wu, P.; Guo, Y.; Shi, Q.; Chen, L.; Peng, S.; Liu, Z.; Cao, G. Au-Ag alloy nanoparticles with tunable cavity for plasmon-enhanced photocatalytic H2 evolution. J. Energy Chem. 2020, 49, 1–7. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent development and future prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-,N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Cheng, C.; Fang, W.H.; Long, R.; Prezhdo, O.V. Water Splitting with a Single-Atom Cu/TiO2 Photocatalyst: Atomistic Origin of High Efficiency and Proposed Enhancement by Spin Selection. JACS Au 2021, 1, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Spanu, D.; Minguzzi, A.; Recchia, S.; Shahvardanfard, F.; Tomanec, O.; Zboril, R.; Schmuki, P.; Ghigna, P.; Altomare, M. An Operando X-ray Absorption Spectroscopy Study of a NiCu-TiO2 Photocatalyst for H2 Evolution. ACS Catal. 2010, 10, 8293–8302. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Kurenkova, A.Y.; Gerasimov, E.Y.; Gromov, N.V.; Medvedeva, T.B.; Saraev, A.A.; Kaichev, V.V. Comparative study of photoreforming of glycerol on Pt/TiO2 and CuOx/TiO2 photocatalysts under UV light. Mater. Lett. 2021, 283, 128901. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Rao, H.; Wang, C.; Tang, J. Platinum- and CuOx -Decorated TiO2 Photocatalyst for Oxidative Coupling of Methane to C2 Hydrocarbons in a Flow Reactor. Angew. Chem. Int. Ed. 2020, 59, 19702–19707. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, L.; Chen, C.; Huang, M.; Huang, Y.; Liu, S.; Li, B. Engineering Catalytic Interfaces in Cuδ+/CeO2-TiO2 Photocatalysts for Synergistically Boosting CO2 Reduction to Ethylene. ACS Nano 2022, 16, 2306–2318. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Mane, G.P.; Rane, V.; Tripathi, A.K.; Tyagi, A.K. Selective CO2 Photoreduction with Cu-Doped TiO2 Photocatalyst: Delineating the Crucial Role of Cu-Oxidation State and Oxygen Vacancies. J. Phys. Chem. C 2021, 125, 1793–1810. [Google Scholar] [CrossRef]
- Wang, R.; Che, G.; Wang, C.; Liu, C.; Liu, B.; Ohtani, B.; Liu, Y.; Zhang, X. Alcohol Plasma Processed Surface Amorphization for Photocatalysis. ACS Catal. 2022, 12, 12206–12216. [Google Scholar] [CrossRef]
- Safeen, K.; Micheli, V.; Bartali, R.; Gottardi, G.; Safeen, A.; Ullah, H.; Laidani, N. Influence of intrinsic defects on the electrical and optical properties of TiO2:Nb films sputtered at room temperature. Thin Solid Films 2018, 645, 173–179. [Google Scholar] [CrossRef]
- Naito, R.; Ariga, M.; Makiuchi, K.; Nakamura, A.; Sudare, T.; Nakayama, R.; Shimizu, R.; Kaneko, K.; Sato, Y.; Hitosugi, T.; et al. Fabrication of conductive Nb-doped anatase TiO2 thin films by mist chemical vapor deposition using aqueous solutions of water-soluble Ti and Nb compounds. Jpn. J. Appl. Phys. 2024, 63, 045504. [Google Scholar] [CrossRef]
- Tasaki, C.; Oka, N.; Yagi, T.; Taketoshi, N.; Baba, T.; Kamiyama, T.; Nakamura, S.-i.; Shigesato, Y. Thermophysical Properties of Transparent Conductive Nb-Doped TiO2 Films. Jpn. J. Appl. Phys. 2012, 51, 035802. [Google Scholar] [CrossRef]
- Akazawa, H. Transparent Conductive Properties of TiOx and Nb-Doped TiOx Films Produced by Reactive Co-Sputtering from Ti and Nb2O5 Targets. Jpn. J. Appl. Phys. 2011, 51, 015803. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Zheng, H.; Zhang, X.; Liu, Y. Defect-Induced Yellow Color in Nb-Doped TiO2 and Its Impact on Visible-Light Photocatalysis. J. Phys. Chem. C 2015, 119, 16623–16632. [Google Scholar] [CrossRef]
- Kato, M.; Najima, H.; Ozawa, T. Effects of Nb Doping on the Photocatalytic Performance of Rutile TiO2 Single Crystals. J. Electrochem. Soc. 2019, 166, H468–H472. [Google Scholar] [CrossRef]
- Miyagi, T.; Kamei, M.; Sakaguchi, I.; Mitsuhashi, T.; Yamazaki, A. Photocatalytic Property and Deep Levels of Nb-doped Anatase TiO2 Film Grown by Metalorganic Chemical Vapor Depostion. Jpn. J. Appl. Phys. 2004, 43, 775–776. [Google Scholar] [CrossRef]
- Gomes, G.H.M.; de Jesus, M.A.M.L.; Ferlauto, A.S.; Viana, M.M.; Mohallem, N.D.S. Characterization and application of niobium-doped titanium dioxide thin films prepared by sol-gel process. Appl. Phys. A 2021, 127, 641. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Sahdan, M.Z.; Nafarizal, N.; Adriyanto, F.; Saim, H.; Tawil, S.N.M.; Bakri, A.S. Optimization of oxygen on Nb-doped TiO2 using DC and RF magnetron sputtering using composite and metal target. AIP Conf. Proc. 2017, 1788, 030112. [Google Scholar]
- Berghuis, W.J.H.; Melskens, J.; Macco, B.; Basuvalingam, S.B.; Verheijen, M.A.; Kessels, W.M.M. Atomic layer deposition of Nb-doped TiO2: Dopant incorporation and effect of annealing. J. Vac. Sci. Technol. A 2020, 38, 022408. [Google Scholar] [CrossRef]
- Gardecka, A.J.; Goh, G.K.L.; Sankar, G.; Parkin, I.P. On the nature of niobium substitution in niobium doped titania thin films by AACVD and its impact on electrical and optical properties. J. Mater. Chem. A 2015, 3, 17755–17762. [Google Scholar] [CrossRef]
- Mattsson, A.; Leideborg, M.; Larsson, K.; Westin, G.; Osterlund, L. Adsorption and solar light decomposition of acetone on anatase TiO2 and niobium doped TiO2 thin films. J. Phys. Chem. B 2006, 110, 1210–1220. [Google Scholar] [CrossRef]
- Yang, X.; Min, Y.; Li, S.; Wang, D.; Mei, Z.; Liang, J.; Pan, F. Conductive Nb-doped TiO2 thin films with whole visible absorption to degrade pollutants. Catal. Sci. Technol. 2018, 8, 1357–1365. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Duan, L.; Cao, M.; Sun, H.; Shao, J.; Chen, S.; Xie, H.; Chang, X.; Chen, C. Influence of annealing process on conductive properties of Nb-doped TiO2 polycrystalline films prepared by sol–gel method. Appl. Surf. Sci. 2011, 257, 10156–10160. [Google Scholar] [CrossRef]
- Sato, Y.; Sanno, Y.; Tasaki, C.; Oka, N.; Kamiyama, T.; Shigesato, Y. Electrical and optical properties of Nb-doped TiO2 films deposited by dc magnetron sputtering using slightly reduced Nb-doped TiO2-x ceramic targets. J. Vac. Sci. Technol. A 2010, 28, 851–855. [Google Scholar] [CrossRef]
- Manwani, K.; Panda, E. Effect of annealing temperature on the microstructure and optoelectronic properties of niobium-doped anatase TiO2 thin films grown on soda-lime glass substrate. Bull. Mater. Sci. 2022, 45, 213. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Dho, J. Post-deposition annealing effects on the transparent conducting properties of anatase Nb:TiO2 films on glass substrates. Mater. Sci. Eng. B 2014, 182, 1–5. [Google Scholar] [CrossRef]
- Fallah, M.; Zamani-Meymian, M.-R.; Rahimi, R.; Rabbani, M. Effect of annealing treatment on electrical and optical properties of Nb doped TiO2 thin films as a TCO prepared by sol–gel spin coating method. Appl. Surf. Sci. 2014, 316, 456–462. [Google Scholar] [CrossRef]
- Manwani, K.; Panda, E. Annealing effected Nb dopant activation and optoelectronic properties in anatase thin films. J. Mater. Sci. Mater. Electron. 2021, 32, 3273–3285. [Google Scholar] [CrossRef]
- Zheng, H.; Li, L.; Sun, Z.; Yu, S.; Luo, W. Preferential orientation, microstructure and functional properties of SnO2:Sb thin film: The effects of post-growth annealing. Appl. Surf. Sci. 2016, 362, 230–236. [Google Scholar] [CrossRef]
- Wang, F.; Wu, M.Z.; Wang, Y.Y.; Yu, Y.M.; Wu, X.M.; Zhuge, L.J. Influence of thickness and annealing temperature on the electrical, optical and structural properties of AZO thin films. Vacuum 2013, 89, 127–131. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. Optical, electrical and structural characteristics of Al:ZnO thin films with various thicknesses deposited by DC sputtering at room temperature and annealed in air or vacuum. Vacuum 2010, 84, 924–929. [Google Scholar] [CrossRef]
- Lü, X.; Yang, W.; Quan, Z.; Lin, T.; Bai, L.; Wang, L.; Huang, F.; Zhao, Y. Enhanced Electron Transport in Nb-Doped TiO2 Nanoparticles via Pressure-Induced Phase Transitions. J. Am. Chem. Soc 2013, 136, 419–426. [Google Scholar] [CrossRef]
- Lim, H.-D.; Seong, W.M.; Kim, J.; Lee, B.; Kim, D.H.; Kang, K. Nb-doped TiO2 air-electrode for advanced Li-air batteries. J. Asian Ceram. 2018, 3, 77–81. [Google Scholar] [CrossRef]
- Pati, S.; Banerji, P.; Majumder, S.B. Properties of indium doped nanocrystalline ZnO thin films and their enhanced gas sensing performance. RSC Adv. 2015, 5, 61230–61238. [Google Scholar] [CrossRef]
- Nikam, R.M. Simple Calculation based Method for Lattice Parameters in Tetragonal System using Powder X-Ray Diffraction Data. Int. J. Res. Appl. Sci. Eng. Technol. 2021, 9, 85–88. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, J.; Zhu, H.; Liu, L.; Liu, Z.; Tu, J. Effects of Nb concentration on Nb-doped anatase TiO2: DFT + U calculations. Phys. Scr. 2022, 97, 055819. [Google Scholar] [CrossRef]
- Quynh, L.M.; Tien, N.T.; Thanh, P.V.; Hieu, N.M.; Doanh, S.C.; Thuat, N.T.; Tuyen, N.V.; Luong, N.H.; Hoang, N.L.H. Optical and electrical responses of magnetron-sputtered amorphous Nb-doped TiO2 thin films annealed at low temperature. Phys. B Condens. Matter 2018, 532, 200–203. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, V.; Sachdev, K. Investigation of effect of doping concentration in Nb-doped TiO2 thin films for TCO applications. J. Mater. Sci. 2017, 52, 11580–11591. [Google Scholar] [CrossRef]
- Ohsaka, T.; Yamaoka, S.; Shimomura, O. Effect of hydrostatic pressure on the Raman spectrum of anatase TiO2. Solid State Commun. 1979, 30, 345–347. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Z.; Zhang, M. Investigation of structural transformations in nanophase titanium dioxide by Raman spectroscopy. Appl. Phys. A-Mater. 1998, 66, 621–627. [Google Scholar] [CrossRef]
- Meng, J.; Zou, G.; Ma, Y.; Wang, X.; Zhao, M. Temperature-induced phase transition and morphotropic phase boundary in the nanocrystalline Pb1-xSrxTiO3 system. J. Phys. Condens. Matter. 1994, 6, 6549. [Google Scholar] [CrossRef]
- Su, H.; Huang, Y.-T.; Chang, Y.-H.; Zhai, P.; Hau, N.Y.; Cheung, P.C.H.; Yeh, W.-T.; Wei, T.-C.; Feng, S.-P. The Synthesis of Nb-doped TiO2 Nanoparticles for Improved-Performance Dye Sensitized Solar Cells. Electrochim. Acta 2015, 182, 230–237. [Google Scholar] [CrossRef]
- Potlog, T.; Dumitriu, P.; Dobromir, M.; Manole, A.; Luca, D. Nb-doped TiO2 thin films for photovoltaic applications. Mater. Design 2015, 85, 558–563. [Google Scholar] [CrossRef]
- Cheng, G.; Akhtar, M.S.; Yang, O.B.; Stadler, F.J. Novel preparation of anatase TiO2@reduced graphene oxide hybrids for high-performance dye-sensitized solar cells. ACS Appl. Mater. Inter. 2013, 5, 6635–6642. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.-C.; Chiu, M.-C.; Tsai, D.-C.; Huang, C.-J.; Shieu, F.-S. Effect of Annealing on the Nb-Doped TiO2 Films Prepared by DC/RF Cosputtering. J. Electrochem. Soc. 2008, 155, G173–G179. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, X.; Zhou, X.; He, H.; Xu, X.; Lin, Q.; Zhou, H.; Zhang, S.; Zhang, H.; Liu, Q. Structure and stoichiometry evolution of sputtered Nb doped TiO2 films induced by O2 pressure variation during postannealing process. J. Vac. Sci. Technol. A 2016, 34, 051512. [Google Scholar] [CrossRef]
- Zhao, W.H.; Wen, L.P.; Liu, B.S. Atmosphere-Dependent Electron Relaxation of the Ag-Decorated TiO2 and the Relations with Photocatalytic Properties. Catalysts 2023, 13, 970. [Google Scholar] [CrossRef]
- Krol, R.v.d.; Goossens, A.; Meulenkamp, E.A. Electrical and optical properties of TiO2 in accumulation and of lithium titanate Li0.5TiO2. J. Appl. Phys. 2001, 90, 2235. [Google Scholar] [CrossRef]
- Park, J.-H.; Choi, Y.-Y.; Kim, H.-K.; Lee, H.H.; Na, S.-I. The effects of rapid thermal annealing on the electrical, optical, and structural properties of Nb:TiO2 multilayer electrodes with an inserted nanoscale Ag layer for organic solar cells. J. Appl. Phys. 2010, 108, 083509. [Google Scholar] [CrossRef]
- Manifacier, J.C.; Gasiot, J.; Fillard, J.P. A simple method for the determination of the optical constants n, k and the thickness of a weakly absorbing thin film. J. Phys. E Sci. Instrum. 1976, 9, 1002. [Google Scholar] [CrossRef]
- Park, S.-M.; Ikegami, T.; Ebihara, K.; Shin, P.-K. Structure and properties of transparent conductive doped ZnO films by pulsed laser deposition. Appl. Surf. Sci. 2006, 253, 1522–1527. [Google Scholar] [CrossRef]
- Aghamalyan, N.R.; Kafadaryan, E.A.; Hovsepyan, R.K.; Petrosyan, S.I. Absorption and reflection analysis of transparent conductive Ga-doped ZnO films. Semicond. Sci. Tech. 2005, 20, 80–85. [Google Scholar] [CrossRef]
- Hou, Q.-Y.; Lv, Z.-Y.; Zhao, C.-W. Effects of Nb doping concentration on TiO2 electricel conductivity and optical performance. Acta Phys. Sin. 2015, 64, 017201. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Fang, Y.; Liu, B. The Effect of Vacuum Annealing on the Structural, Electric, and Optical Properties, and Photocatalytic Activities of Sputtered TiO2 and Nb-Doped TiO2 Films. Catalysts 2025, 15, 166. https://doi.org/10.3390/catal15020166
Li M, Fang Y, Liu B. The Effect of Vacuum Annealing on the Structural, Electric, and Optical Properties, and Photocatalytic Activities of Sputtered TiO2 and Nb-Doped TiO2 Films. Catalysts. 2025; 15(2):166. https://doi.org/10.3390/catal15020166
Chicago/Turabian StyleLi, Mengna, Yingying Fang, and Baoshun Liu. 2025. "The Effect of Vacuum Annealing on the Structural, Electric, and Optical Properties, and Photocatalytic Activities of Sputtered TiO2 and Nb-Doped TiO2 Films" Catalysts 15, no. 2: 166. https://doi.org/10.3390/catal15020166
APA StyleLi, M., Fang, Y., & Liu, B. (2025). The Effect of Vacuum Annealing on the Structural, Electric, and Optical Properties, and Photocatalytic Activities of Sputtered TiO2 and Nb-Doped TiO2 Films. Catalysts, 15(2), 166. https://doi.org/10.3390/catal15020166





