Impact of Goal Directed Therapy in Head and Neck Oncological Surgery with Microsurgical Reconstruction: Free Flap Viability and Complications
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Demographic Data
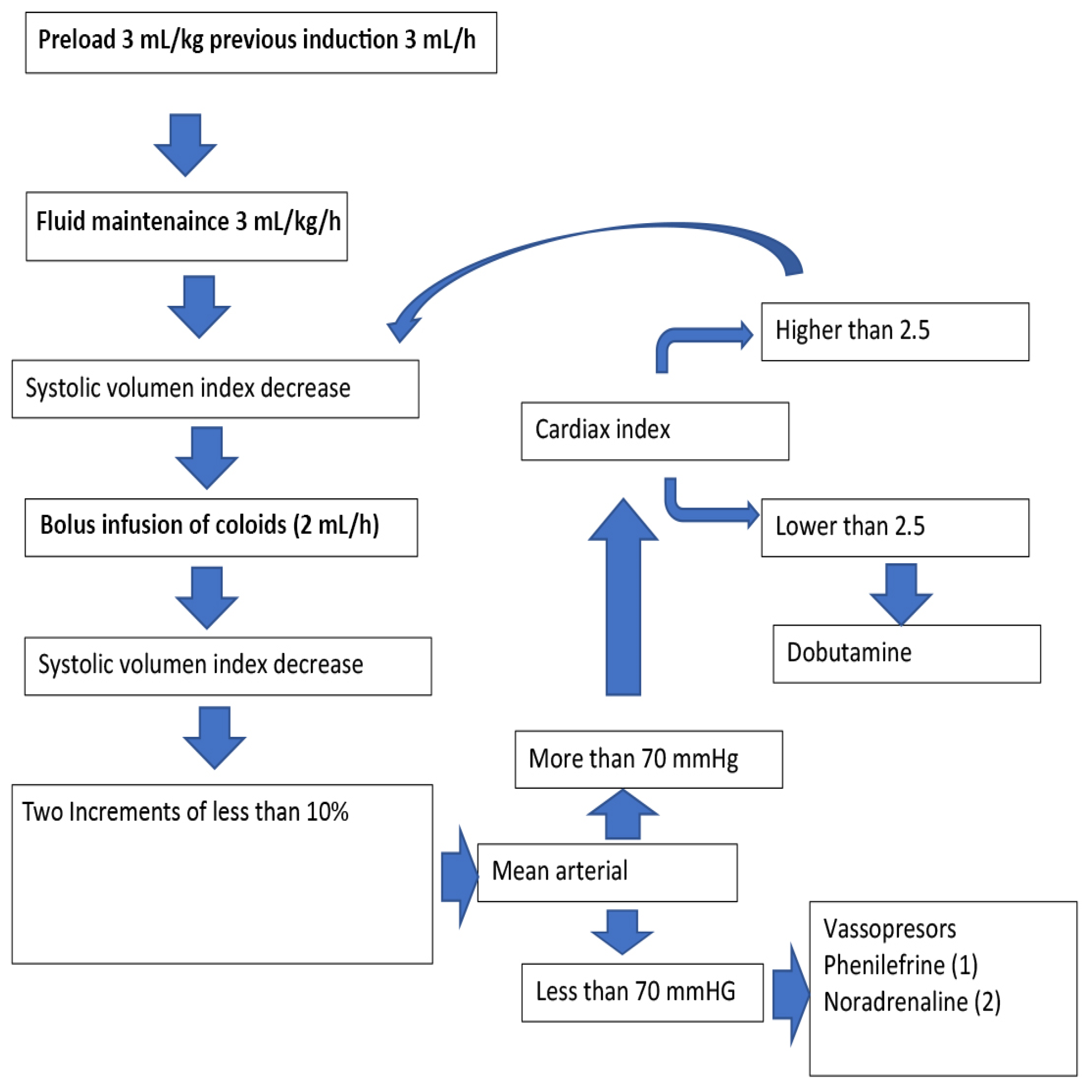
2.2. Surgical Data
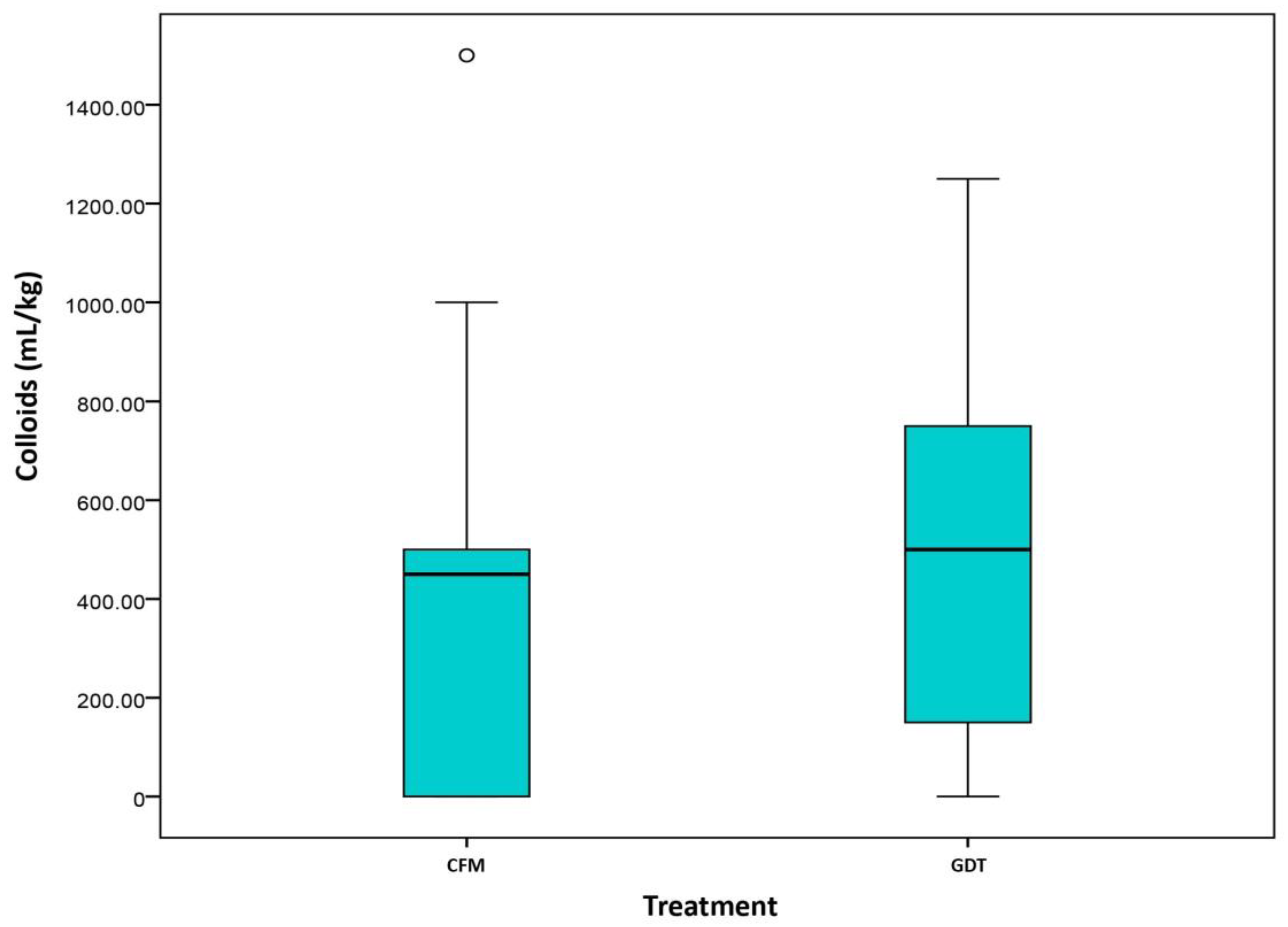
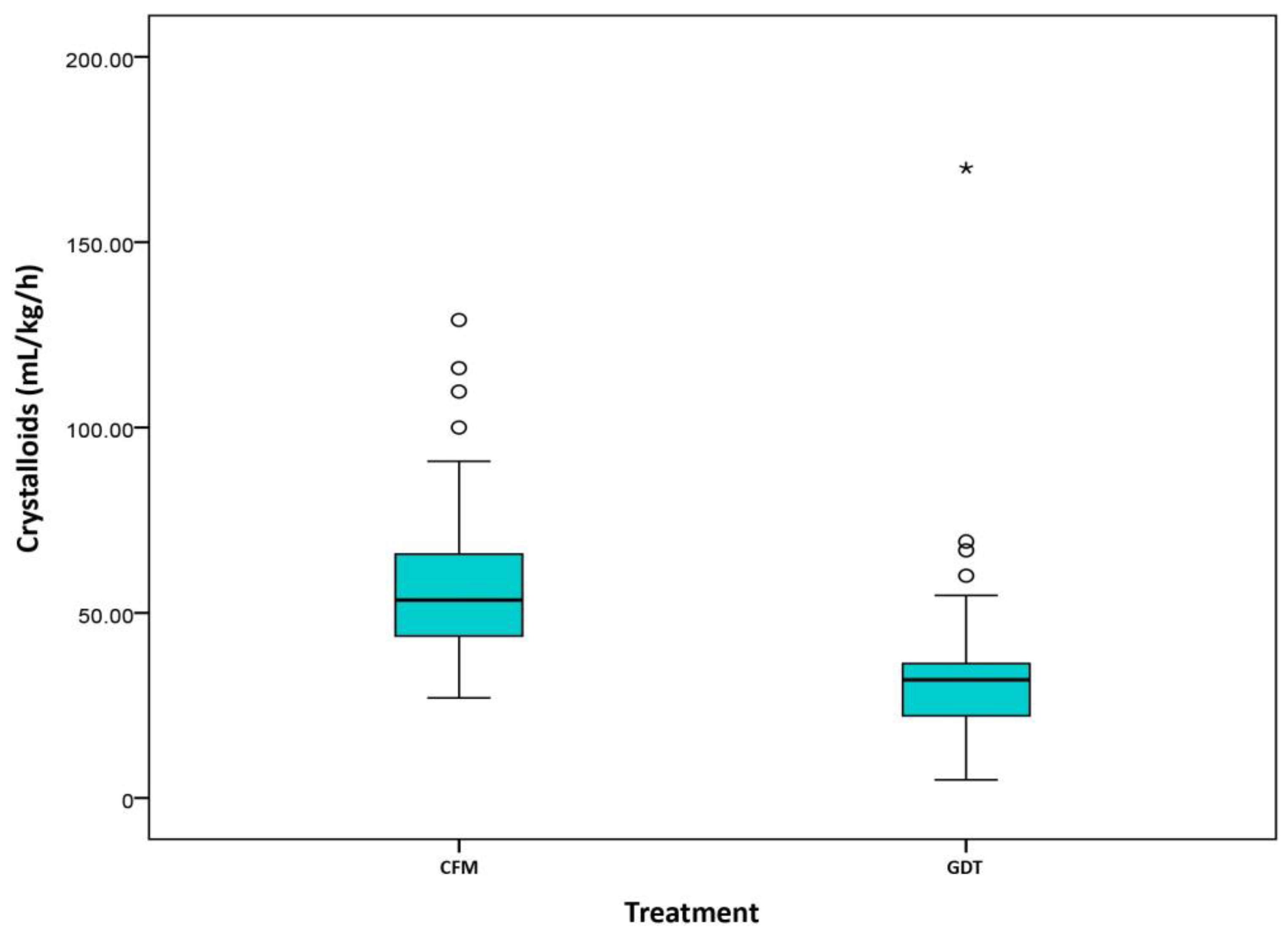
2.3. Fluid Management and Vasopressor Data
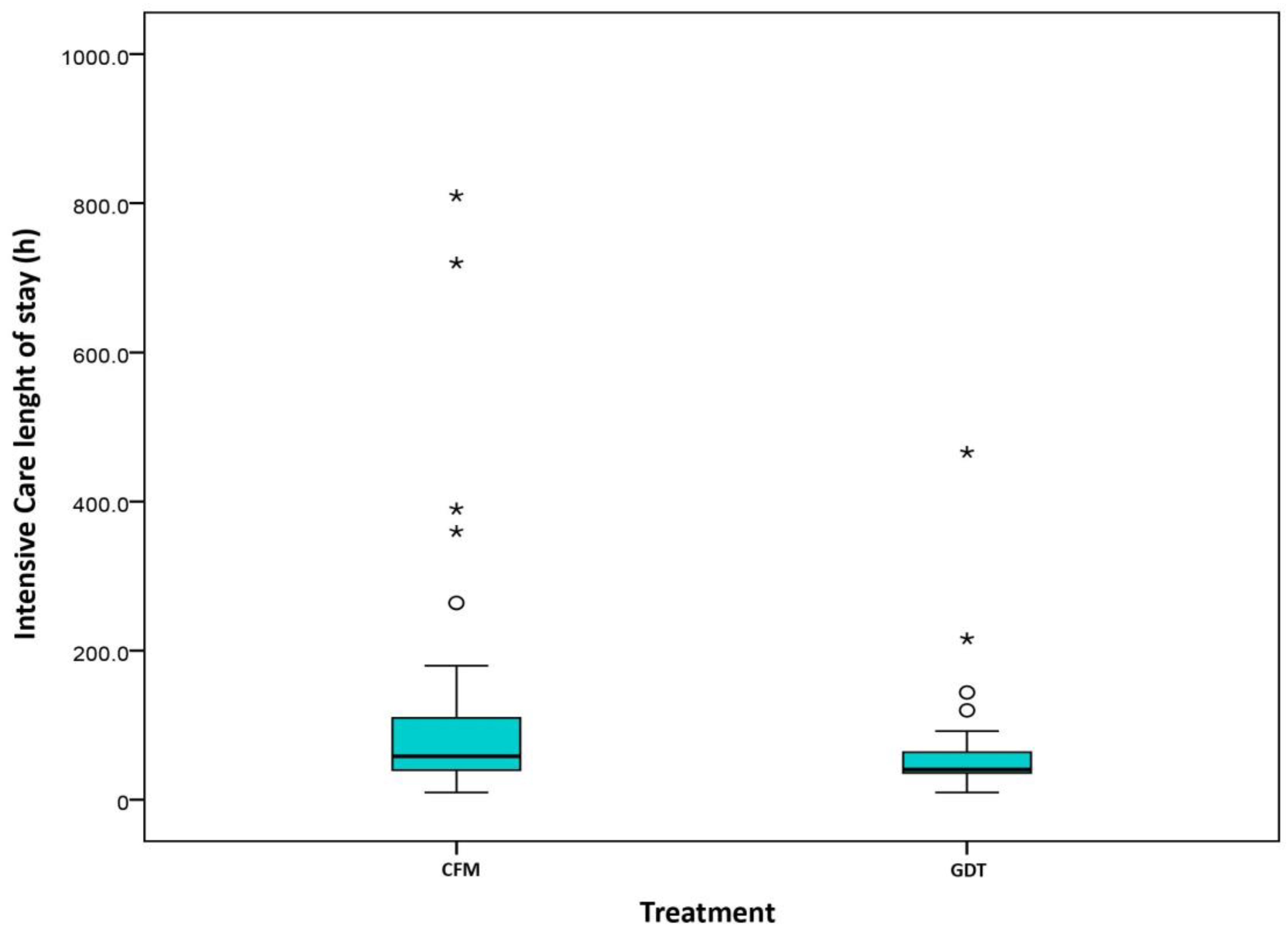
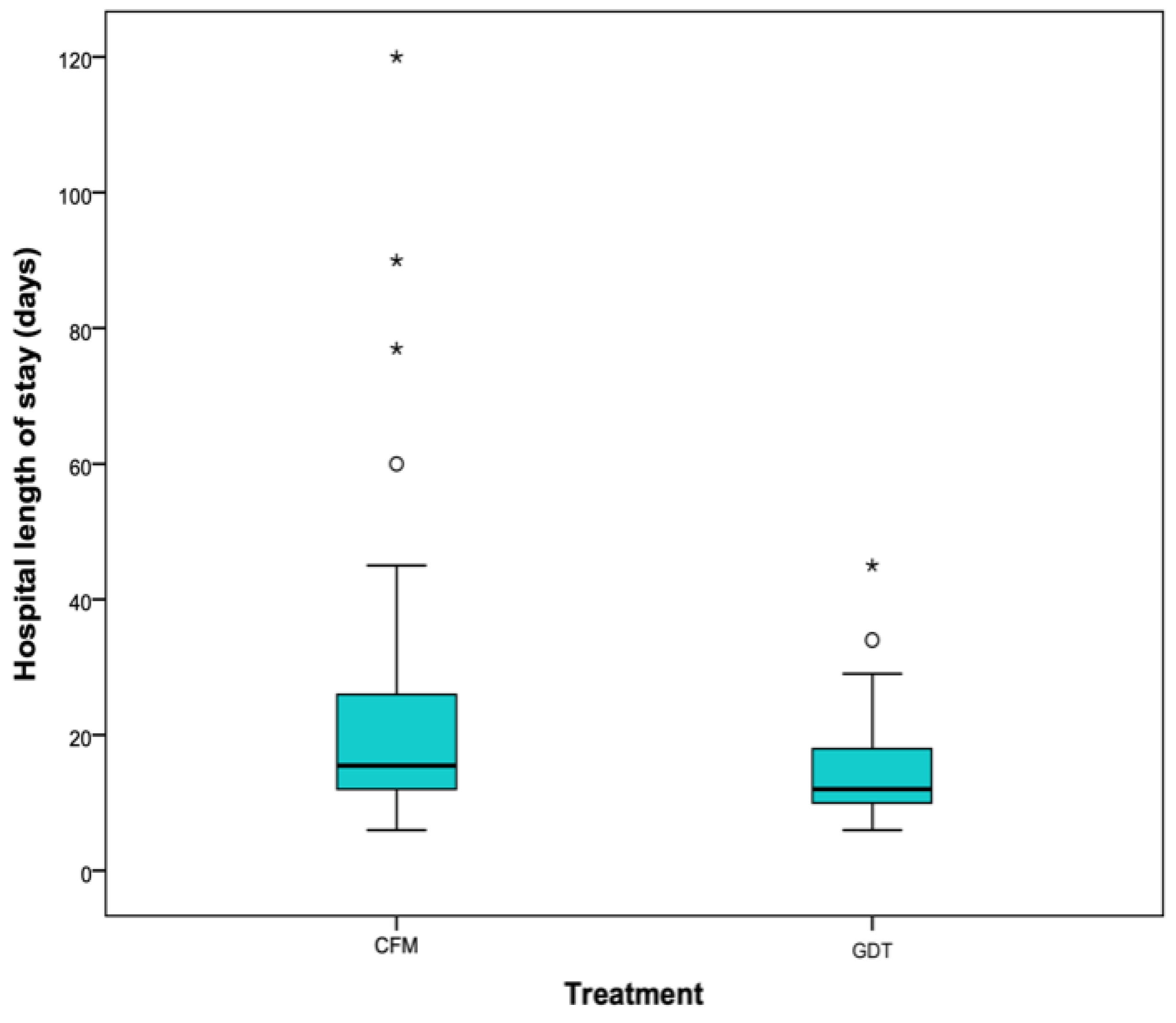
2.4. Length of Stay
2.5. Postoperative Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Parameters Collected
- Demographic data
- Age
- Weight
- Sex
- Preoperative factors
- Smoking
- High blood pressure
- Chronic obstructive pulmonary disease
- Alcoholism
- Dyslipidaemia
- Previous chemotherapy
- Previous radiation therapy
- Acute myocardial infarction
- History of arrhythmia
- Presence of hypoalbuminemia
- Diabetes mellitus
- ASA classification
- Haemoglobin level, presence of preoperative anaemia
- Background medication: antidepressants, benzodiazepines, antihypertensives (CC blockers, ACE inhibitors/ARBs, beta blockers)
- Anticoagulants/antiplatelet agents
- Intraoperative factors
- Use or non-use of FloTraq®
- Type of flap
- Bone/non-bone flap
- Surgical time
- Need for transfusion (volume)
- Tracheotomy
- Neck dissection
- Fluid therapy
- Crystalloids
- Colloids
- Vasopressors
- Postoperative factors
- PACU length of stay (hours)
- Hospital length of stay (days)
- Time on ventilator (hours)
- Twenty-four hour fluid therapy
- Crystalloids
- Colloids
- Need for transfusion
- Vasopressors
- Postoperative complications
- Bleeding
- Thrombosis
- Other complications
- Pneumonia
- Arrhythmia
- Ischaemic heart disease
- Pulmonary thromboembolism
- Deep vein thrombosis
- Congestive heart failure
- Confusion
- Cerebral vascular accident (CVA)
- Creatinine above 1.2 mg/mL
- Creatinine greater than 24 h per month
- Diuresis first 24 h
- Antiplatelet/anticoagulant medication at the time of pedicle section
- Heparin
- Lysine acelsalicylate
- Antithrombotic prophylaxis 12 h before the procedure
- 40 IU low molecular weight heparin (LMWH)/no
- Graft outcome
- Necrosis
- Partial (flaps rescued in a subsequent surgical review)
- Total
- Viability
- Survival
- Yes/No
4.3. Sample Size Calculation
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urken, M.L.; Weinberg, H.; Buchbinder, D.; Moscoso, J.F.; Lawson, W.; Catalano, P.J.; Biller, H.F. Microvascular free flaps in head and neck reconstruction. Report of 200 cases and review of complications. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 633–640. [Google Scholar] [CrossRef]
- McLean, D.H.; Buncke, H.J. Autotransplant of omentum to a large scalp defect, with microsurgical revascularization. Plast. Reconstr. Surg. 1972, 49, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hagau, N.; Longrois, D. Anesthesia for free vascularized tissue transfer. Microsurgery 2009, 29, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Khouri, R.K.; Cooley, B.C.; Kunselman, A.R.; Landis, J.R.; Yeramian, P.; Ingram, D.; Natarajan, N.; Benes, C.O.; Wallemark, C. A Prospective Study of Microvascular Free-Flap Surgery and Outcome. Plast. Reconstr. Surg. 1998, 102, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, J.N.; Derks, L.H.; Klimek, M.; Mureau, M.A.M. Perioperative Fluid Management and Use of Vasoactive and Antithrombotic Agents in Free Flap Surgery: A Literature Review and Clinical Recommendations. J. Reconstr. Microsurg. 2013, 1, 357–366. [Google Scholar]
- Quinlan, J. Anaesthesia for Reconstructive Free Flap Surgery. Anaesth. Intensive Care Med. 2003, 4, 87–90. [Google Scholar]
- Aya, H.D.; Cecconi, M.; Hamilton, M.; Rhodes, A. Goal-directed therapy in cardiac surgery: A systematic review and meta-analysis. Br. J. Anaesth. 2013, 110, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Watson, X.; Cecconi, M. Haemodynamic monitoring in the peri-operative period: The past, the present and the future. Anaesthesia 2017, 72, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Grocott, M.P.W.W.; Dushianthan, A.; Hamilton, M.A.; Mythen, M.G.; Harrison, D.; Rowan, K. Perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: A Cochrane Systematic Review. Br. J. Anaesth. 2013, 111, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Tapia, B.T.; Garrido, E.; Cebrian, J.L.; Del Castillo, J.L.; Alsina, E.; Gilsanz, F. New techniques and recommendations in the management of free flap surgery for head and neck defects in cancer patients. Minerva Anestesiol. 2020, 86, 861–871. [Google Scholar] [CrossRef]
- Michard, F.; Biais, M.; Lobo, S.M.; Futier, E. Perioperative hemodynamic management 4.0. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 247–255. [Google Scholar] [CrossRef]
- MacDonald, D.J.F. Anesthesia for microvascular surgery—A physiological approach. Br. J. Anaesth. 1985, 57, 904–912. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, A.; Turner, M.W.H.; Anand, R.; Puxeddu, R.; Brennan, P.A. Cardiac output monitoring to guide fluid replacement in head and neck microvascular free flap surgery-what is current practice in the UK? Br. J. Oral Maxillofac. Surg. 2012, 50, 500–503. [Google Scholar] [CrossRef]
- Aditianingsih, D.; Anaesthetist, C. Guiding principles of fluid and volume therapy. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 249–260. [Google Scholar] [CrossRef]
- Nolan, J.P.; Mythen, M.G. Hydroxyethyl starch: Here today, gone tomorrow. Br. J. Anaesth. 2013, 111, 321–324. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23946354 (accessed on 10 April 2014). [CrossRef] [PubMed] [Green Version]
- Chappell, D.; Jacob, M. Role of the glycocalyx in fluid management: Small things matter. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 227–234. [Google Scholar] [CrossRef]
- Chan, S.T.F.; Kapadia, C.R.; Johnson, A.W.; Radcliffe, A.G.; Dudley, H.A.F. Extracellular fluid volume expansion and third space sequestration at the site of small bowel anastomoses. Br. J. Surg. 1983, 70, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Voldby, A.W.; Brandstrup, B. Fluid therapy in the perioperative setting—A clinical review. J. Intensive Care 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noblett, S.E.; Snowden, C.P.; Shenton, B.K.; Horgan, A.F. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br. J. Surg. 2006, 93, 1069–1076. [Google Scholar] [CrossRef]
- Chong, M.A.; Wang, Y.; Berbenetz, N.M.; Mcconachie, I. Does goal-directed haemodynamic and fluid therapy improve perioperative outcomes? A systematic review and meta-analysis. Eur. J. Anaesthesiol. 2018, 35, 469–483. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E. Hemodynamic Goal-Directed Therapy in High-Risk Surgical Patients. JAMA 2014, 311, 2177. Available online: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2014.5306 (accessed on 10 April 2014). [CrossRef]
- Ettinger, K.S.; Arce, K.; Lohse, C.M.; Peck, B.W.; Reiland, M.D.; Bezak, B.J.; Moore, E.J. Higher perioperative fluid administration is associated with increased rates of complications following head and neck microvascular reconstruction with fibular free flaps. Microsurgery 2017, 37, 128–136. [Google Scholar] [CrossRef]
- Kruse, A.L.D.D.; Luebbers, H.T.; Grätz, K.W.; Obwegeser, J.A. Factors influencing survival of free-flap in reconstruction for cancer of the head and neck: A literature review. Microsurgery 2010, 30, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Rocca, G.D.; Pompei, L. Goal-directed therapy in anesthesia: Any clinical impact or just a fashion? Minerva Anestesiol. 2011, 77, 545–553. [Google Scholar]
- Mayer, J.; Boldt, J.; Poland, R.; Peterson, A.; Manecke, G.R. Continuous Arterial Pressure Waveform-Based Cardiac Output Using the FloTrac/Vigileo: A Review and Meta-analysis. J. Cardiothorac. Vasc. Anesth. 2009, 23, 401–406. [Google Scholar] [CrossRef]
- Bahlmann, H.; Halldestam, I.; Nilsson, L. Goal-directed therapy during transthoracic oesophageal resection does not improve outcome Randomised controlled trial. Eur. J. Anaesthesiol. 2019, 36, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Hallock, G. Critical threshold for tissue viability as determined by laser Doppler flowmetry. Ann. Plast. Surg. 1992, 28, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K. The effects of systemic phenylephrine and epinephrine on pedicle artery and microvascular perfusion in a pig model of myoadipocutaneous rotational flaps. Plast. Reconstr. Surg. 2007, 120, 1289–1299. [Google Scholar]
- Haughey, B.H.; Wilson, E.; Kluwe, L.; Piccirillo, J.; Fredrickson, J.; Sessions, D.; Spector, G. Free flap reconstruction of the head and neck: Analysis of 241 cases. Otolaryngol. Head Neck Surg. 2001, 125, 10–17. [Google Scholar] [CrossRef]
- Varadarajan, V.V.; Arshad, H.; Dziegielewski, P.T.T. Head and neck free flap reconstruction: What is the appropriate post-operative level of care? Oral Oncol. 2017, 75, 61–66. [Google Scholar] [CrossRef] [PubMed]


| Conventional Fluid Management (CFM) | Goal Directed Therapy (GDT) | |
|---|---|---|
| Gender (male) % | 49.1 | 57.9 |
| Age, years (median, interquartile range) | 58 (18/87) | 58.50 (18/81) |
| Weight, kilograms (median, interquartile range) | 65 (45/95) | 65 (46/127) |
| CFM | GDT | |
|---|---|---|
| Smoker (%) | 51.9 | 48.1 |
| Hypertension (%) | 48.9 | 51.1 |
| Chronic pulmonary disease (%) | 51.5 | 48.5 |
| Alcoholism (%) | 55.3 | 44.7 |
| Dyslipidaemia (%) | 52 | 48 |
| Ischaemic heart disease (%) | 1.4 | 8.5 |
| Arrhythmia (%) | 1.4 | 5.2 |
| Hypoalbuminemia (%) | 4.3 | 3.4 |
| Diabetes mellitus (%) | 1.4 | 6.8 |
| Anaemia (%) | 17.9 | 22.8 |
| CFM | GDT | |
|---|---|---|
| Antidepressants (%) | 11.6 | 8.5 |
| Benzodiazepines (%) | 23.2 | 18.6 |
| Calcium channels (CC) blockers (%) | 4.3 | 10.2 |
| Angiotensin-converting enzyme (ACE) inhibitors)/Angiotensin II receptor blockers (ARBs) (%) | 19.1 | 33.9 |
| Beta-blockers (%) | 8.7 | 6.9 |
| CFM | GDT | |
|---|---|---|
| Anterolateral thigh | 25% | 21.7% |
| Radial/cubital/forearm | 29.2% | 41.7% |
| Fibula | 36.1% | 21.7% |
| Iliac crest | 4.2% | 3.3% |
| Scapula bone | 1.4% | 0% |
| Others | 4.1% | 11.6% |
| Yes | No | p Value | |
|---|---|---|---|
| Free flap bleeding (%) | 12.5 | 52.4 | * |
| Free flap thrombosis (%) | 75.8 | 90 | * |
| Medical/surgical complications (%) | 25.6 | 20.3 | 0.633 |
| Renal failure (first 48h/30 days) | 14.7/13 | 14.1/3.2 | */* |
| Yes | No | p Value | |
|---|---|---|---|
| Free flap bleeding (%) | 42.9 | 46.7 | 1 |
| Free flap | 78,6 | 100 | * |
| thrombosis (%) | |||
| Medical/surgical complications (%) | 25.4 | 20.5 | 0.641 |
| Renal failure (%) | 15.9/6.5 | 14/9.8 | 1/* |
| (24 h/30 days) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia, B.; Garrido, E.; Cebrian, J.L.; Del Castillo, J.L.; Gonzalez, J.; Losantos, I.; Gilsanz, F. Impact of Goal Directed Therapy in Head and Neck Oncological Surgery with Microsurgical Reconstruction: Free Flap Viability and Complications. Cancers 2021, 13, 1545. https://doi.org/10.3390/cancers13071545
Tapia B, Garrido E, Cebrian JL, Del Castillo JL, Gonzalez J, Losantos I, Gilsanz F. Impact of Goal Directed Therapy in Head and Neck Oncological Surgery with Microsurgical Reconstruction: Free Flap Viability and Complications. Cancers. 2021; 13(7):1545. https://doi.org/10.3390/cancers13071545
Chicago/Turabian StyleTapia, Blanca, Elena Garrido, Jose Luis Cebrian, Jose Luis Del Castillo, Javier Gonzalez, Itsaso Losantos, and Fernando Gilsanz. 2021. "Impact of Goal Directed Therapy in Head and Neck Oncological Surgery with Microsurgical Reconstruction: Free Flap Viability and Complications" Cancers 13, no. 7: 1545. https://doi.org/10.3390/cancers13071545





